Optimizing the Flocculation Effect of Cationic Polyacrylamide Prepared with UV-Initiated Polymerization by Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Preparation
3. Single-Factor Flocculation Test, Results and Discussion
3.1. Single Factor Flocculation Test Design
3.2. Results and Discussion of the Single-Factor Flocculation Test
3.2.1. Impact of Wastewater pH on the Flocculation Effect of CPAM
3.2.2. Impacts of the Dosage and Intrinsic Viscosity of CPAM on Its Flocculation Effect
3.2.3. Impact of the Dosage and Cationic Degree of CPAM on Its Flocculation Effect
3.2.4. Impact of Stirring Time on Flocculation Properties of CPAM
3.2.5. Impact of the Settling Time on CPAM Flocculation Efficiency
4. RSM Flocculation Test, Results and Discussion
4.1. RSM Flocculation Test Design
4.2. Results and Discussion of the RSM Flocculation Test
4.2.1. Discussion of RSM Test Results
4.2.2. Model Fitting
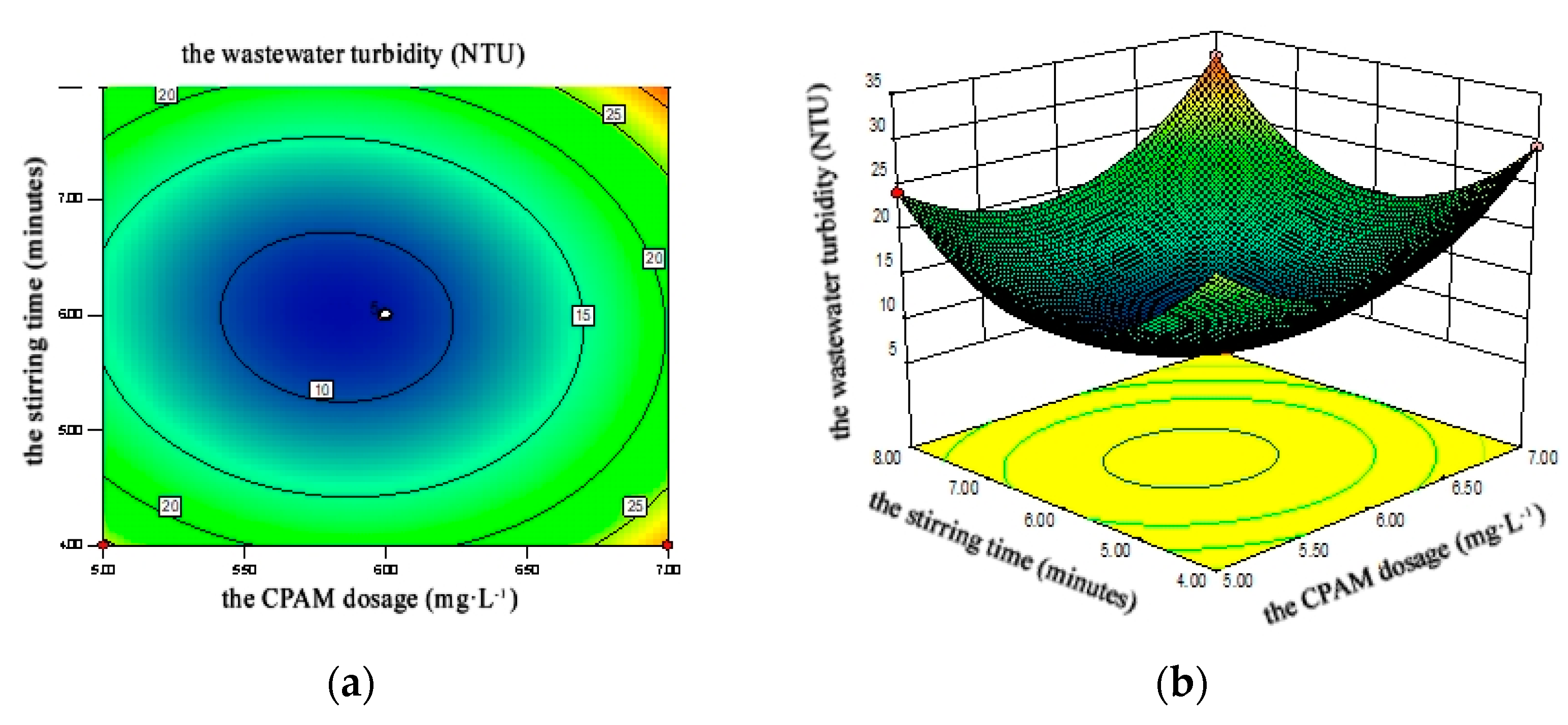
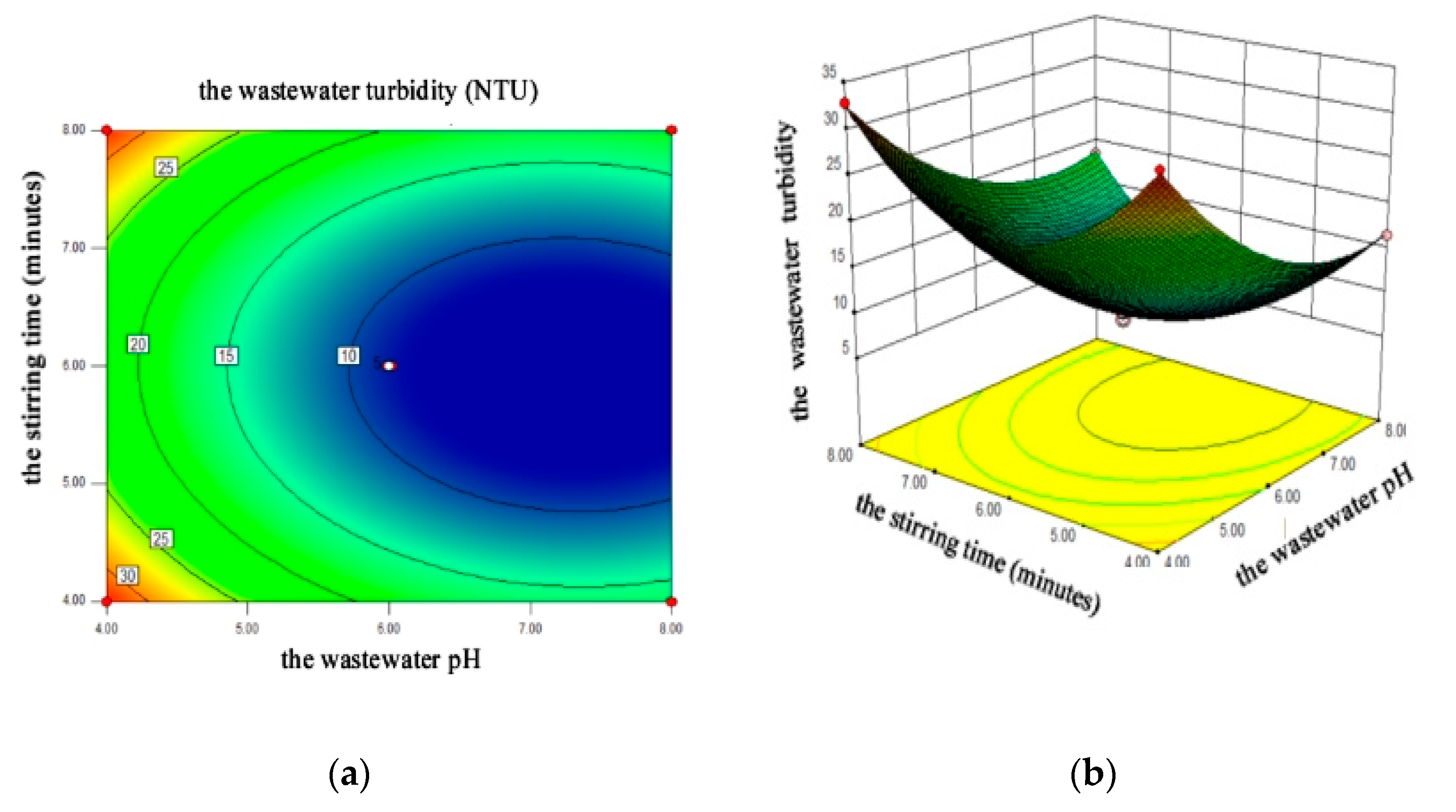
4.2.3. Response Surface Analysis
4.2.4. Flocculating Optimization and Model Validation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Imran, M.; Saeed, Z.; Pervaiz, M.; Mehmood, K.; Ejaz, R.; Younas, U.; Nadeem, H.A.; Hussain, S. Enhanced visible light photocatalytic activity of TiO2 co-doped with Fe, Co, and S for degradation of Cango red. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 1196–1204. [Google Scholar] [CrossRef]
- Shaheen, S.; Saeed, Z.; Ahmad, A.; Pervaiz, M.; Younas, U.; Khan, R.R.M.; Luque, R.; Rajendran, S. Green synthesis of graphene-based metal nanocomposite for electro and photocatalytic activity; recent advancement and future prospective. Chemosphere 2023, 311, 136982. [Google Scholar] [CrossRef]
- Chi, N.; Liu, J.; Lei, M.; Feng, L. Preparation of amphiphilic cationic polyacrylamide (CPAM) with cationic microblock structure to enhance printing and dyeing sludge dewatering and condition performance. Environ. Sci. Pollut. Res. 2023, 30, 13079–13093. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, J.; Yao, C. Cationic polyacrylamide emulsion with ultra-high concentration as a flocculant for paper mill wastewater treatment. BioResources 2020, 152, 3173–3189. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, H.; Sun, Y.; Zhao, C.; Zhou, Y.; Tang, X.; Zhao, C. A combined process of chemical precipitation and flocculation for treating phosphating wastewater. Desalination Water Treat. 2016, 57, 25520–25531. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, X.; Dong, X.; Feng, Z.; Dong, Y. Characterisation of floc size, effective density and sedimentation under various flocculation mechanisms. Water Sci. Technol. 2020, 827, 1261–1271. [Google Scholar] [CrossRef]
- Sang, Y.; Lin, A.; Liu, X. Population balance modeling of cationic polyacrylamide (CPAM) induced flocculation process for lignin recovery from the pre-hydrolysis liquor of kraft pulping process. Sep. Purif. Technol. 2019, 221, 152–158. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Guo, J.; Li, F.; Fan, W.; Liao, Y.; Guan, Q. Characterization and Evaluation of Dewatering Properties of PADB, a Highly Efficient Cationic Flocculant. Ind. Eng. Chem. Res. 2014, 537, 2572–2582. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, H.; Huang, F.; Li, X.; He, S.; Zhao, C. Template Polymerization of a Novel Cationic Polyacrylamide: Sequence Distribution, Characterization, and Flocculation Performance. Ind. Eng. Chem. Res. 2016, 55, 9819–9828. [Google Scholar] [CrossRef]
- Shang, H.Z.; Liu, J.P.; Zheng, Y.B.; Wang, L.G. Synthesis, characterization, and flocculation properties of poly(acrylamide-methacryloxyethyltrimethyl ammonium chloride-methacryloxypropyl-trimethoxy silane). J. Appl. Polym. Sci. 2009, 111, 1594–1599. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Zhu, C.; Guo, J.; Zhao, C.; Liao, Y.; Guan, Q. UV-initiated polymerization of hydrophobically associating cationic flocculants: Synthesis, characterization, and dewatering properties. Chem. Eng. J. 2013, 234, 318–326. [Google Scholar] [CrossRef]
- Daifa, M.; Shmoeli, E.; Domb, A.J. Enhanced flocculation activity of polyacrylamide-based flocculant for purification of industrial wastewater. Polym. Adv. Technol. 2019, 30, 2636–2646. [Google Scholar] [CrossRef]
- Fijałkowska, G.; Szewczuk-Karpisz, K.; Wiśniewska, M. Chromium (VI) and lead (II) accumulation at the montmorillonite/aqueous solution interface in the presence of polyacrylamide containing quaternary amine groups. J. Mol. Liq. 2019, 293, 111514. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Chibowski, S.; Urban, T.; Terpiłowski, K. Investigations of chromium (III) oxide removal from the aqueous suspension using the mixed flocculant composed of anionic and cationic polyacrylamides. J. Hazard. Mater. 2019, 368, 378–385. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Fijałkowska, G.; Wiśniewska, M.; Wójcik, G. Chromium (VI) reduction and accumulation on the kaolinite surface in the presence of cationic soil flocculant. J. Soils Sediments 2020, 20, 3688–3697. [Google Scholar] [CrossRef]
- Harif, S.; Aboulhassan, M.A.; Bammou, L. Multi-response optimization for color removal from cardboard wastewater using polyaluminum chloride and cationic polyacrylamide. Int. J. Environ. Sci. Technol. 2023, 20, 4281–4292. [Google Scholar] [CrossRef]
- Khan, S.; Zheng, H.; Sun, Q.; Liu, Y.; Li, H.; Ding, W.; Navarro, A. Synthesis and characterization of a novel cationic polyacrylamide-based flocculants to remove Congo red efficiently in acid aqueous environment. J. Mater. Sci. Mater. Electron. 2020, 31, 18832–18843. [Google Scholar] [CrossRef]
- Lapointe, M.; Farner, J.M.; Hernandez, L.M.; Tufenkji, N. Understanding and improving microplastic removal during water treatment: Impact of coagulation and flocculation. Environ. Sci. Technol. 2020, 54, 8719–8727. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Wang, D.; Wu, Y.; Yang, Q.; Liu, Y.; Wang, Q.; Li, X.; Li, H.; Zeng, G.; et al. Unveiling the mechanisms of how cationic polyacrylamide affects short-chain fatty acids accumulation during long-term anaerobic fermentation of waste activated sludge. Water Res. 2019, 155, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, Q.; Feng, B.; Feng, S.; Tian, C.; Jiang, X.; Wu, X.; Xiao, B. Improving the performance of shipboard rotary drum filters in the removal of cyanobacterial blooms by cationic polyacrylamide flocculation. Sep. Purif. Technol. 2019, 215, 660–669. [Google Scholar] [CrossRef]
- Park, J.B.K.; Meerman, C.; Craggs, R. Continuous low dosing of cationic polyacrylamide (PAM) to enhance algal harvest from a hectare-scale wastewater treatment high rate algal pond. N. Z. J. Bot. 2019, 57, 112–124. [Google Scholar] [CrossRef]
- Tajbakhsh, S.F.; Mohmmadipour, R.; Janani, H. One-pot production of a graft copolymer of cationic starch and cationic polyacrylamide applicable as flocculant for wastewater treatment. J. Macromol. Sci. Part A 2022, 59, 698–710. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.; Huang, F.; Li, X.; Shang, J.; Lai, J.; Liao, Y. Nitrogen and Phosphorus Removal by Activated Sludge Process: A Review. Mini-Rev. Org. Chem. 2017, 14, 99–106. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Fijałkowska, G.; Szewczuk-Karpisz, K.; Urban, T.; Nosal-Wiercińska, A.; Wójcik, G. Comparison of adsorption affinity of anionic and cationic polyacrylamides for montmorillonite surface in the presence of chromium (VI) ions. Adsorption 2019, 25, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Bu, X.; Alheshibri, M.; Zhan, H.; Xie, G. Floc structure and dewatering performance of kaolin treated with cationic polyacrylamide degraded by hydrodynamic cavitation. Chem. Eng. Commun. 2022, 209, 798–807. [Google Scholar] [CrossRef]
- Agbovi, H.K.; Wilson, L.D. Flocculation optimization of orthophosphate with FeCl3 and alginate using the Box–Behnken response surface methodology. Ind. Eng. Chem. Res. 2017, 56, 3145–3155. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Alam, M. Simultaneous modelling of coagulant recovery and reuse by response surface methodology. J. Environ. Manag. 2021, 285, 112139. [Google Scholar] [CrossRef]
- Birjandi, N.; Younesi, H.; Bahramifar, N.; Ghafari, S.; Zinatizadeh, A.A.; Sethupathi, S. Optimization of coagulation-flocculation treatment on paper-recycling wastewater: Application of response surface methodology. J. Environ. Sci. Health Part A 2013, 48, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mao, Y.; Wang, C.; Ke, Q.; Zhao, M.; Wang, Q. Application of a combined response surface methodology (RSM)-artificial neural network (ANN) for multiple target optimization and prediction in a magnetic coagulation process for secondary effluent from municipal wastewater treatment plants. Environ. Sci. Pollut. Res. 2022, 29, 36075–36087. [Google Scholar] [CrossRef]
- Dbik, A.; El Messaoudi, N.; Bentahar, S.; El Khomri, M.; Lacherai, A.; Faska, N. Optimization of Methylene Blue Adsorption on Agricultural Solid Waste Using Box–Behnken Design (BBD) Combined with Response Surface Methodology (RSM) Modeling. Biointerface Res. Appl. Chem. 2022, 12, 4567–4583. [Google Scholar]
- Ezemagu, I.G.; Ejimofor, M.I.; Menkiti, M.C.; Nwobi-Okoye, C.C. Modeling and optimization of turbidity removal from produced water using response surface methodology and artificial neural network. S. Afr. J. Chem. Eng. 2021, 35, 78–88. [Google Scholar] [CrossRef]
- Gökçek, Ö.B.; Özdemir, S. Optimization of the coagulation–flocculation process for slaughterhouse wastewater using response surface methodology. CLEAN–Soil Air Water 2020, 48, 2000033. [Google Scholar] [CrossRef]
- Heidari, M.; Vosoughi, M.; Sadeghi, H.; Dargahi, A.; Mokhtari, S.A. Degradation of diazinon from aqueous solutions by electro-Fenton process: Effect of operating parameters, intermediate identification, degradation pathway, and optimization using response surface methodology (RSM). Sep. Sci. Technol. 2021, 56, 2287–2299. [Google Scholar] [CrossRef]
- Kim, S.-C. Application of response surface method as an experimental design to optimize coagulation–flocculation process for pre-treating paper wastewater. J. Ind. Eng. Chem. 2016, 38, 93–102. [Google Scholar] [CrossRef]
- Luo, S.; Wu, X.; Jiang, H.; Yu, M.; Liu, Y.; Min, A.; Li, W.; Ruan, R. Edible fungi-assisted harvesting system for efficient microalgae bio-flocculation. Bioresour. Technol. 2019, 282, 325–330. [Google Scholar] [CrossRef]
- Ma, C.; Yu, H.; Gao, Y.; Xu, W.; Xu, T.; Wang, L.; Zhao, B.; Zhang, Z.; Xu, J. Operation parameters optimization of a hybrid dead-end/cross-flow forward osmosis system for microalgae dewatering by response surface methodology. Process Saf. Environ. Prot. 2020, 143, 14–24. [Google Scholar] [CrossRef]
- Nourani, M.; Baghdadi, M.; Javan, M.; Bidhendi, G.N. Production of a biodegradable flocculant from cotton and evaluation of its performance in coagulation-flocculation of kaolin clay suspension: Optimization through response surface methodology (RSM). J. Environ. Chem. Eng. 2016, 4, 1996–2003. [Google Scholar] [CrossRef] [Green Version]
- Onukwuli, O.D.; Nnaji, P.C.; Menkiti, M.C.; Anadebe, V.C.; Oke, E.O.; Ude, C.N.; Ude, C.J.; Okafor, N.A. Dual-purpose optimization of dye-polluted wastewater decontamination using bio-coagulants from multiple processing techniques via neural intelligence algorithm and response surface methodology. J. Taiwan Inst. Chem. Eng. 2021, 125, 372–386. [Google Scholar] [CrossRef]
- Rezania, N.; Hasani Zonoozi, M.; Saadatpour, M. Coagulation-flocculation of turbid water using graphene oxide: Simulation through response surface methodology and process characterization. Environ. Sci. Pollut. Res. 2021, 28, 14812–14827. [Google Scholar] [CrossRef]
- Singh, H.M.; Tyagi, V.V.; Ahmad, S.; Kothari, R. Optimization of flocculation efficiency of Chlorella pyrenoidosa with CaCl2 using the Box-Behnken design of response surface methodology: A cost effective statistical investigation. Biomass Convers. Biorefin. 2022, 21, 1–13. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, L.; Lin, X.; Xu, Z.; Luo, W.; Luo, L. Response surface methodology to optimize self-flocculation harvesting of microalgae Desmodesmus sp. CHX1. Environ. Technol. 2021, 43, 2647–2655. [Google Scholar] [CrossRef] [PubMed]
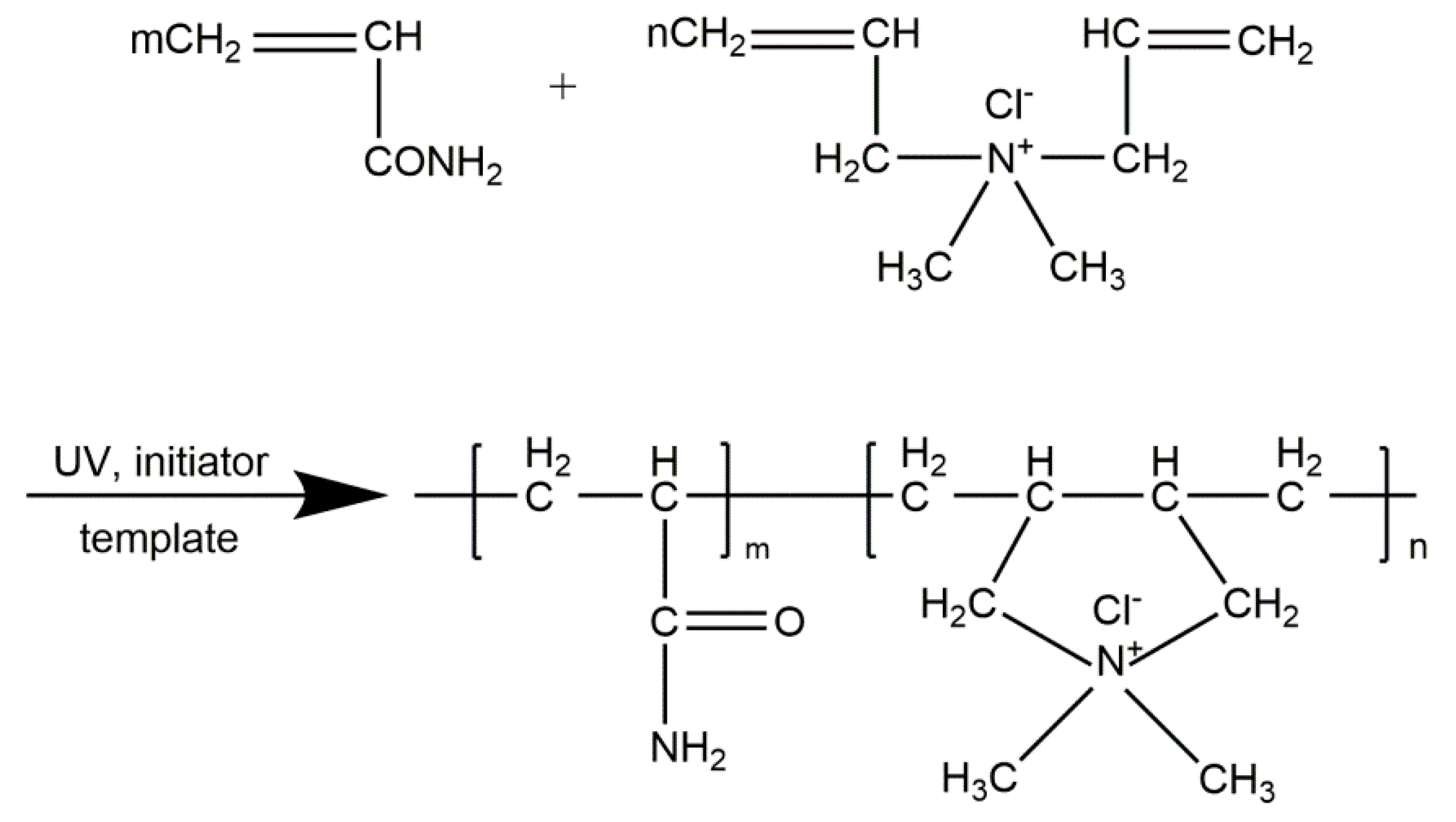

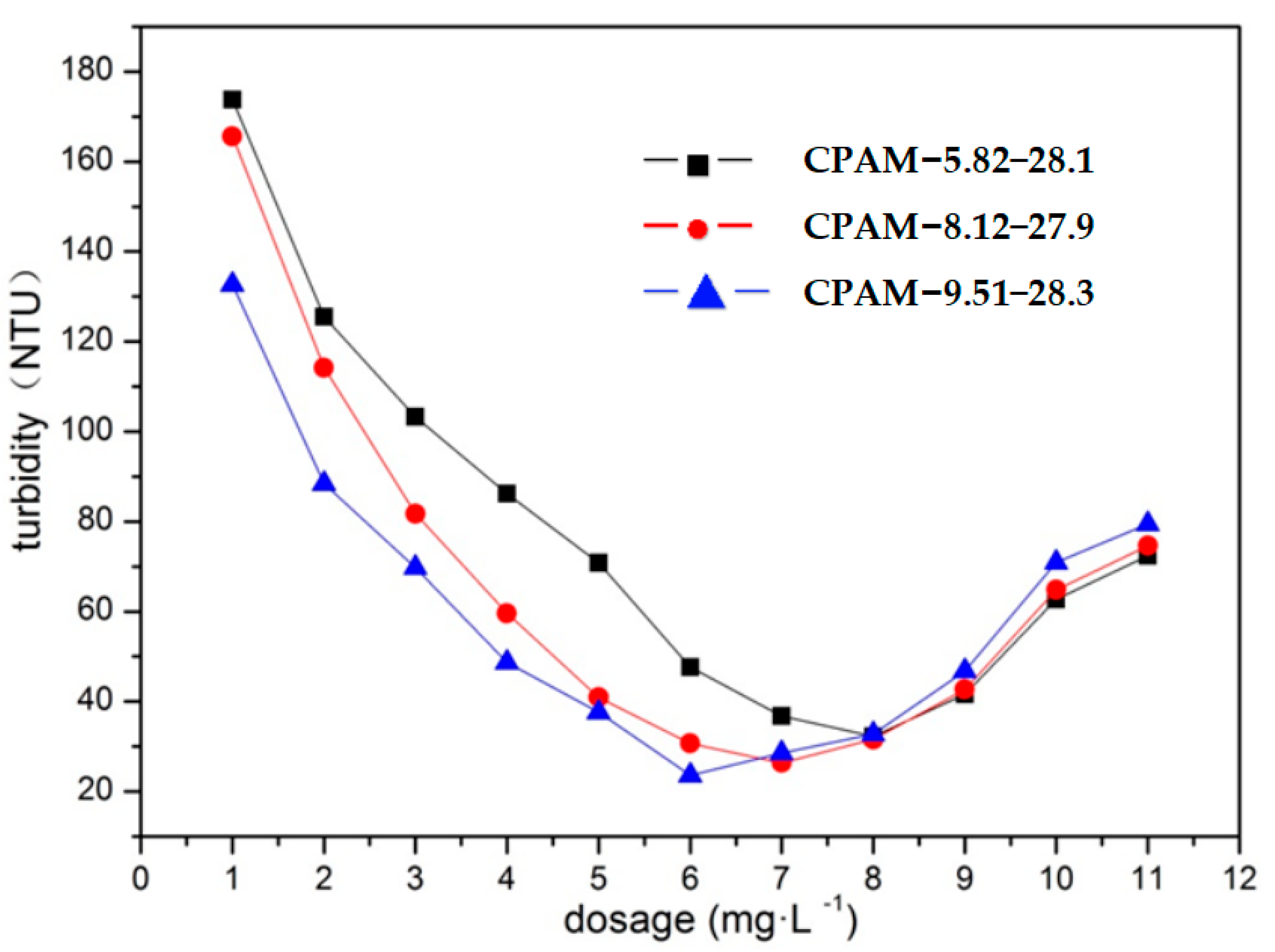
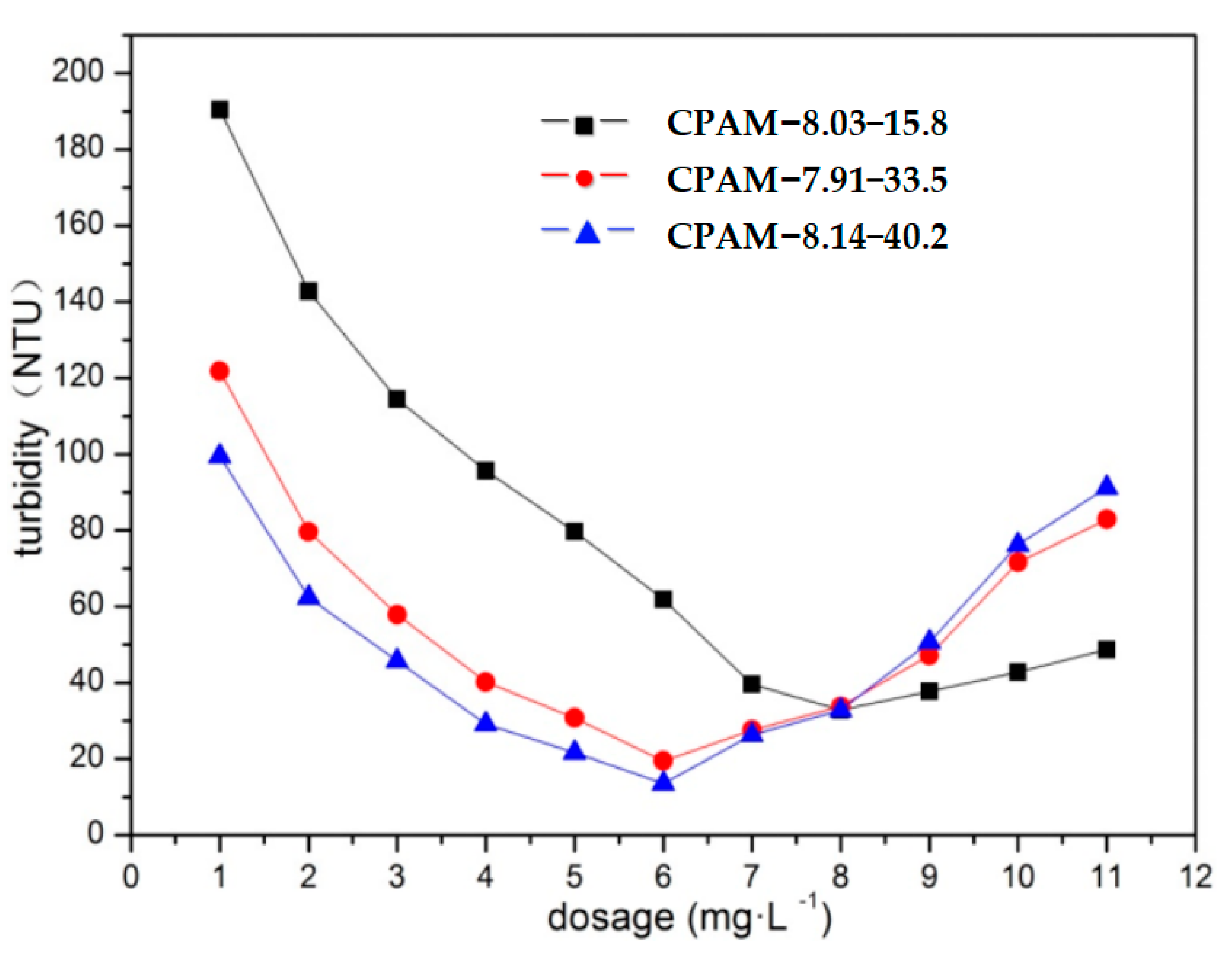
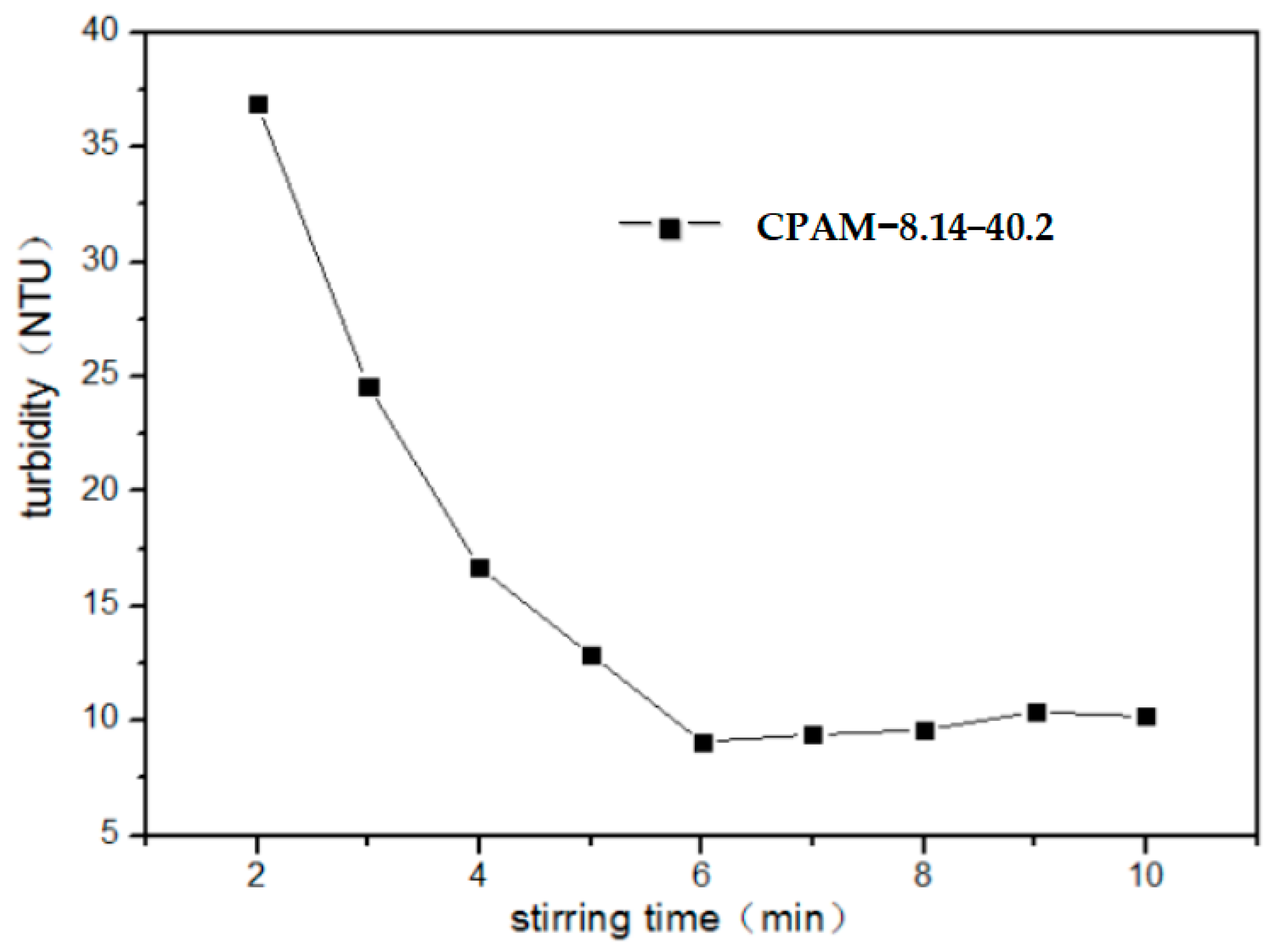

| CPAM Number | Intrinsic Viscosity (dL·g−1) | Cationic Degree (%) | The Main Parameters of the Polymerization Process | ||
|---|---|---|---|---|---|
| Molar Ratio of AM and DMD | Dosage of V-50 Initiator (%) | Total Monomer Content (%) | |||
| CPAM-8.03-15.8 | 8.03 | 15.8 | 7:3 | 0.08 | 30 |
| CPAM-7.91-33.5 | 7.91 | 33.5 | 5:5 | 0.05 | 30 |
| CPAM-8.14-40.2 | 8.14 | 40.2 | 4:6 | 0.03 | 30 |
| CPAM-5.82-28.1 | 5.82 | 28.1 | 5:5 | 0.13 | 30 |
| CPAM-8.12-27.9 | 8.12 | 27.9 | 5:5 | 0.04 | 30 |
| CPAM-9.51-28.3 | 9.51 | 28.3 | 5:5 | 0.03 | 30 |
| Variable Code | Variables | Variable Levels and Corresponding Values | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Z1 | CPAM dosage (mg·L−1) | 5 | 6 | 7 |
| Z2 | Wastewater pH | 4 | 6 | 8 |
| Z3 | Stirring time (minutes) | 4 | 6 | 8 |
| Run | CPAM Dosage (mg·L−1) | Wastewater pH | Stirring Time (Minutes) | Response Value of Turbidity (NTU) | ||
|---|---|---|---|---|---|---|
| Actual | Predicted | |||||
| Equation (2) | Equation (3) | |||||
| 1 | 6.0 | 6.0 | 6.0 | 8.90 | 8.82 | 8.82 |
| 2 | 6.0 | 4.0 | 8.0 | 32.80 | 32.45 | 32.45 |
| 3 | 7.0 | 4.0 | 6.0 | 33.30 | 33.50 | 32.43 |
| 4 | 6.0 | 6.0 | 6.0 | 8.80 | 8.82 | 8.82 |
| 5 | 6.0 | 4.0 | 4.0 | 33.10 | 32.85 | 32.85 |
| 6 | 6.0 | 6.0 | 6.0 | 8.10 | 8.82 | 8.82 |
| 7 | 6.0 | 8.0 | 4.0 | 16.50 | 16.85 | 16.85 |
| 8 | 5.0 | 8.0 | 6.0 | 13.00 | 12.80 | 12.73 |
| 9 | 5.0 | 6.0 | 8.0 | 24.30 | 24.25 | 24.25 |
| 10 | 5.0 | 6.0 | 4.0 | 25.80 | 26.65 | 25.65 |
| 11 | 6.0 | 6.0 | 6.0 | 9.20 | 8.82 | 8.82 |
| 12 | 5.0 | 4.0 | 6.0 | 27.10 | 27.50 | 27.58 |
| 13 | 7.0 | 6.0 | 8.0 | 32.10 | 32.25 | 32.25 |
| 14 | 7.0 | 8.0 | 6.0 | 18.9 | 18.50 | 18.58 |
| 15 | 6.0 | 6.0 | 6.0 | 9.10 | 8.82 | 8.82 |
| 16 | 7.0 | 6.0 | 4.0 | 29.3 | 29.35 | 29.35 |
| 17 | 6.0 | 8.0 | 8.0 | 18.5 | 18.75 | 18.75 |
| Source | Sum of Squares | Df | Mean Squares | F Value | p Value Prob > F | Remark | |
|---|---|---|---|---|---|---|---|
| Model | Equation (2) | 1532.076706 | 9 | 170.2307451 | 759.9586835 | <0.0001 | significant |
| Equation (3) | 1532.054206 | 8 | 191.5067757 | 963.2531945 | <0.0001 | significant | |
| Z1-the CPAM dosage(mg·L−1) | Equation (2) | 68.445 | 1 | 68.445 | 305.5580357 | <0.0001 | |
| Equation (3) | 68.445 | 1 | 68.445 | 344.2690978 | <0.0001 | ||
| Z2-the wastewater pH | Equation (2) | 441.045 | 1 | 441.045 | 1968.950893 | <0.0001 | |
| Equation (3) | 441.045 | 1 | 441.045 | 2218.396731 | <0.0001 | ||
| Z3-the stirring time(minutes) | Equation (2) | 1.125 | 1 | 1.125 | 5.022321429 | 0.06 | |
| Equation (3) | 1.125 | 1 | 1.125 | 5.658597925 | 0.0446 | ||
| Z12 | Equation (2) | 0.0225 | 1 | 0.0225 | 0.100446429 | 0.7605 | |
| Equation (3) | -- | -- | -- | -- | -- | ||
| Z13 | Equation (2) | 4.6225 | 1 | 4.6225 | 20.63616071 | 0.0027 | |
| Equation (3) | 4.6225 | 1 | 4.6225 | 23.25055014 | 0.0013 | ||
| Z23 | Equation (2) | 1.3225 | 1 | 1.3225 | 5.904017857 | 0.0454 | |
| Equation (3) | 1.3225 | 1 | 1.3225 | 6.651996228 | 0.0327 | ||
| Z12 | Equation (2) | 300.8200263 | 1 | 300.8200263 | 1342.946546 | <0.0001 | |
| Equation (3) | 300.8200263 | 1 | 300.8200263 | 1513.084068 | <0.0001 | ||
| Z22 | Equation (2) | 141.7642368 | 1 | 141.7642368 | 632.8760573 | <0.0001 | |
| Equation (3) | 141.7642368 | 1 | 141.7642368 | 713.054948 | <0.0001 | ||
| Z32 | Equation (2) | 473.3179211 | 1 | 473.3179211 | 2113.026433 | <0.0001 | |
| Equation (3) | 473.3179211 | 1 | 473.3179211 | 2380.725161 | <0.0001 | ||
| Residual | Equation (2) | 1.568 | 7 | 0.224 | |||
| Equation (3) | 1.5905 | 8 | 0.1988125 | ||||
| Lack of fit | Equation (2) | 0.82 | 3 | 0.273333333 | 1.461675579 | 0.3512 | not significant |
| Equation (3) | 0.8425 | 4 | 0.210625 | 1.126336898 | 0.4555 | not significant | |
| Pure error | Equation (2) | 0.748 | 4 | 0.187 | |||
| Equation (3) | 0.748 | 4 | 0.187 | ||||
| Cor total | Equation (2) | 1533.644706 | 16 | ||||
| Equation (3) | 1533.644706 | 16 | |||||
| R2 | Equation (2) | 0.9938 | |||||
| Equation (3) | 0.9907 | ||||||
| R2adj | Equation (2) | 0.9979 | |||||
| Equation (3) | 0.9977 | ||||||
| Flocculation Conditions | Wastewater Turbidity (NTU) | ||||
|---|---|---|---|---|---|
| CPAM Dosage (mg·L−1) | Wastewater pH | Stirring Time (Minutes) | Settling Time (Minutes) | Average of Measured Value | Predicted Value |
| 5.83 | 7.28 | 5.95 | 30 | 6.49 | 6.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Zhang, Z.; Li, Y.; Li, L.; Wang, H.; Liu, S.; Hua, X.; Li, B. Optimizing the Flocculation Effect of Cationic Polyacrylamide Prepared with UV-Initiated Polymerization by Response Surface Methodology. Water 2023, 15, 1200. https://doi.org/10.3390/w15061200
Fu C, Zhang Z, Li Y, Li L, Wang H, Liu S, Hua X, Li B. Optimizing the Flocculation Effect of Cationic Polyacrylamide Prepared with UV-Initiated Polymerization by Response Surface Methodology. Water. 2023; 15(6):1200. https://doi.org/10.3390/w15061200
Chicago/Turabian StyleFu, Chaochen, Zhengan Zhang, Yuying Li, Lin Li, Hongtian Wang, Shaobo Liu, Xia Hua, and Bailian Li. 2023. "Optimizing the Flocculation Effect of Cationic Polyacrylamide Prepared with UV-Initiated Polymerization by Response Surface Methodology" Water 15, no. 6: 1200. https://doi.org/10.3390/w15061200
APA StyleFu, C., Zhang, Z., Li, Y., Li, L., Wang, H., Liu, S., Hua, X., & Li, B. (2023). Optimizing the Flocculation Effect of Cationic Polyacrylamide Prepared with UV-Initiated Polymerization by Response Surface Methodology. Water, 15(6), 1200. https://doi.org/10.3390/w15061200







