Spatial Conservation Assessment for Native Fishes in the Lahontan and Central Nevada Basins, USA
Abstract
:1. Introduction
2. Materials and Methods
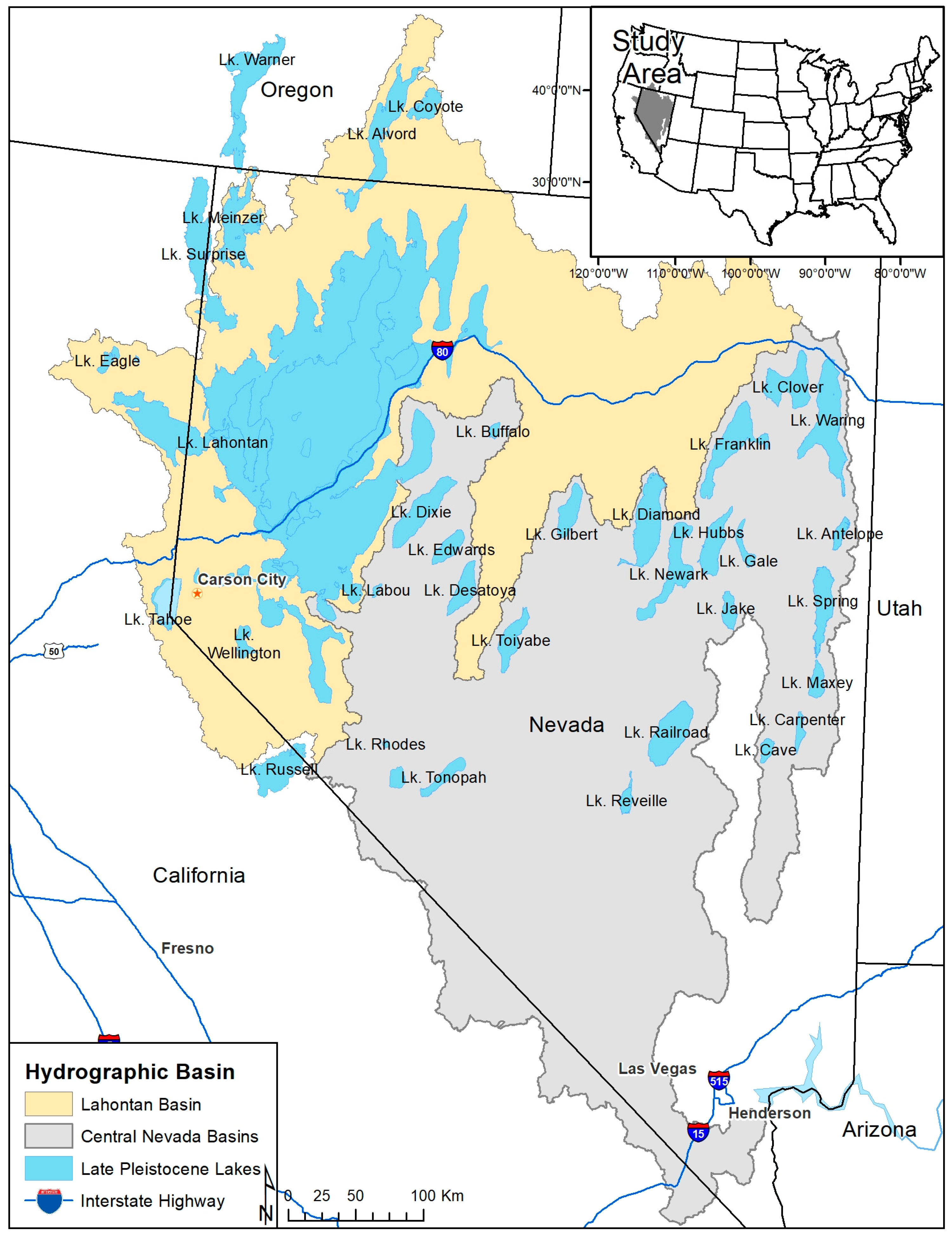
2.1. Study Area
2.2. The Approach: Native Fish Conservation Value of Subwatersheds
2.3. Core-Area Zonation
2.4. Planning Units
2.5. Fish Species Data
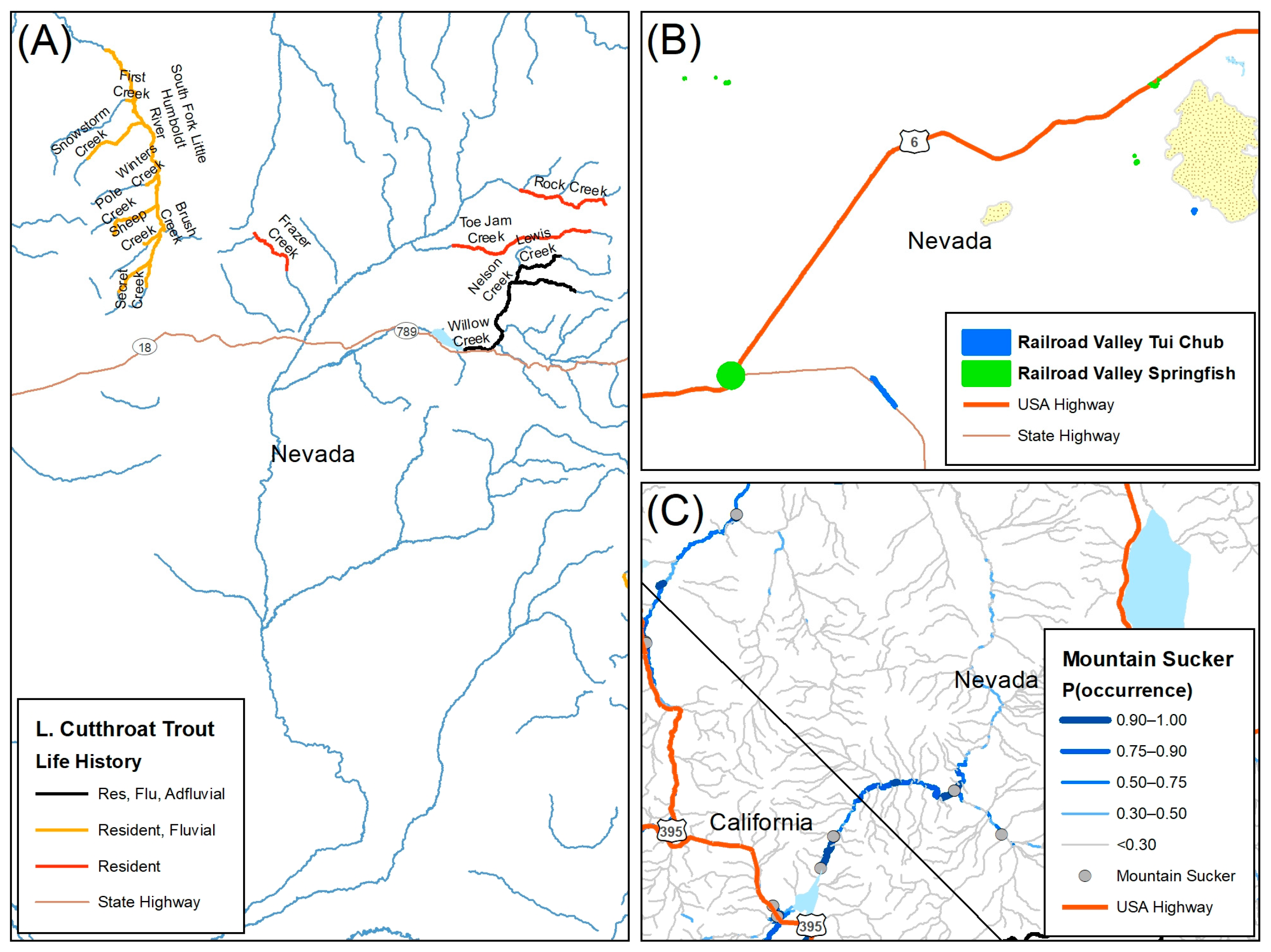
2.5.1. The 3 Rs for Native Trout
2.5.2. Nevada Division of Natural Heritage Database
2.5.3. Species Distribution Models
2.6. Distribution Discounting
2.7. Aquatic Connectivity
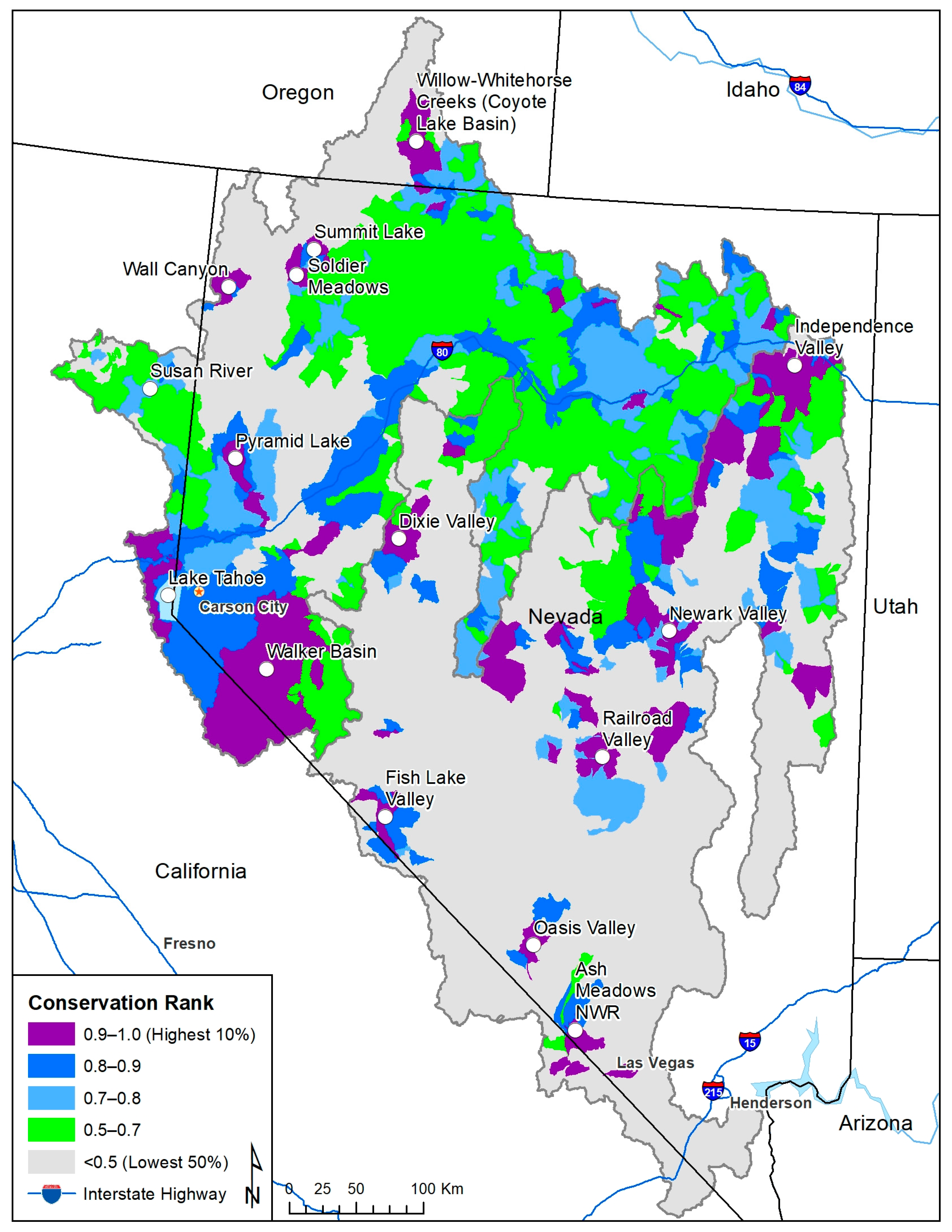
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isbell, F.; Balvanera, P.; Mori, A.S.; He, J.-S.; Bullock, J.M.; Regmi, G.R.; Seabloom, E.W.; Ferrier, S.; Sala, O.E.; Guerrero-Ramírez, N.R.; et al. Expert perspectives on global biodiversity loss and its drivers and impacts on people. Front. Ecol. Environ. 2023, 21, 94–103. [Google Scholar] [CrossRef]
- Harrison, I.; Abell, R.; Darwall, W.; Thieme, M.L.; Tickner, D.; Timboe, I. The freshwater biodiversity crisis. Science 2018, 362, 1369. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.E. Multispecies conservation: Bringing efficiency to the science of native fish conservation. In Multispecies and Watershed Approaches to Freshwater Fish Conservation; Dauwalter, D.C., Birdsong, T.W., Garrett, G.P., Eds.; American Fisheries Society: Bethesda, MD, USA, 2019; pp. 1–9. [Google Scholar]
- Darwall, W.R.T.; Freyhof, J. Lost fishes, who is counting? The extent of the threat to freshwater fish biodiversity. In Conservation of Freshwater Fishes; Closs, G.P., Krkosek, M., Olden, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 1–36. [Google Scholar]
- Closs, G.P.; Angermeier, P.L.; Darwall, W.R.T.; Balcombe, S.R. Why are freshwater fishes so imperiled? In Conservation of Freshwater Fishes; Closs, G.P., Krkosek, M., Olden, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 37–75. [Google Scholar]
- Muhlfeld, C.C.; Dauwalter, D.C.; D’Angelo, V.S.; Ferguson, A.; Giersch, J.J.; Impson, N.D.; Kiozumi, I.; Kovach, R.P.; McGinnity, P.; Schoeffmann, J.; et al. Global status of freshwater trout and char: Conservation challenges in the 21st Century. In Status and Conservation of Trout and Char Worldwide; Kershner, J.L., Williams, J.E., Gresswell, R.E., Lobon-Cervia, J., Eds.; American Fisheries Society: Bethesda, MD, USA, 2019; pp. 717–760. [Google Scholar]
- Dauwalter, D.C.; Duchi, A.; Epifanio, J.; Gandolfi, A.; Gresswell, R.; Juanes, F.; Kershner, J.; Lobón-Cerviá, J.; McGinnity, P.; Meraner, A.; et al. A call for global action to conserve native trout in the 21st century and beyond. Ecol. Freshw. Fish 2020, 29, 429–432. [Google Scholar] [CrossRef]
- Higgins, J.; Zablocki, J.; Newsock, A.; Krolopp, A.; Tabas, P.; Salama, M. Durable freshwater protection: A framework for establishing and maintaining long-term protection for freshwater ecosystems and the values they sustain. Sustainability 2021, 13, 1950. [Google Scholar] [CrossRef]
- Roni, P.; Beechie, T.J.; Bilby, R.E.; Leonetti, F.E.; Pollock, M.M.; Pess, G.R. A review of stream restoration techniques and a hierarchical strategy for prioritizing restoration in Pacific Northwest watersheds. N. Am. J. Fish. Manag. 2002, 22, 1–20. [Google Scholar] [CrossRef]
- Perry, D.; Harrison, I.; Fernandes, S.; Burnham, S.; Nichols, A. Global analysis of durable policies for free-flowing river protections. Sustainability 2021, 13, 2347. [Google Scholar] [CrossRef]
- Acreman, M.; Hughes, K.A.; Arthington, A.H.; Tickner, D.; Dueñas, M.-A. Protected areas and freshwater biodiversity: A novel systematic review distils eight lessons for effective conservation. Conserv. Lett. 2020, 13, e12684. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Palmer, M.A.; Allan, J.D.; Alexander, G.; Barnas, K.; Brooks, S.; Carr, J.; Clayton, S.; Dahm, C.; Follstad-Shah, J.; et al. Synthesizing U.S. river restoration efforts. Science 2005, 308, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, E.S.; Sudduth, E.B.; Palmer, M.A.; Allan, J.D.; Meyer, J.L.; Alexander, G.; Follastad-Shah, J.; Hassett, B.; Jenkinson, R.; Lave, R.; et al. Restoring rivers one reach at a time: Results from a survey of U.S. river restoration practitioners. Restor. Ecol. 2007, 15, 482–493. [Google Scholar] [CrossRef] [Green Version]
- Roni, P.; Åberg, U.; Weber, C. A review of approaches for monitoring the effectiveness of regional river habitat restoration programs. N. Am. J. Fish. Manag. 2018, 38, 1170–1186. [Google Scholar] [CrossRef] [Green Version]
- Beechie, T.; Pess, G.; Morley, S.; Butler, L.; Downs, P.; Maltby, A.; Skidmore, P.; Clayton, S.; Muhlfeld, C.; Hanson, K. Watershed assessments and identification of restoration needs. In Stream and Watershed Restoration; Roni, P., Beechie, T., Eds.; John Wiley & Sons: Chichester, UK, 2013; pp. 50–113. [Google Scholar]
- Williams, J.E.; Williams, R.N.; Thurow, R.F.; Elwell, L.; Philipp, D.P.; Harris, F.A.; Kershner, J.L.; Martinez, P.J.; Miller, D.; Reeves, G.H.; et al. Native Fish Conservation Areas: A vision for large-scale conservation of native fish communities. Fisheries 2011, 36, 267–277. [Google Scholar] [CrossRef]
- Birdsong, T.W.; Garrett, G.P.; Labay, B.J.; Bean, M.; Bean, P.; Botros, J.; Casarez, M.; Cohen, A.; Heger, T.; Kalmback, A.; et al. Texas Native Fish Conservation Areas Network: Strategic investments in restoration and preservation of freshwater fish diversity. In Multispecies and Watershed Approaches to Freshwater Fish Conservation; Dauwalter, D.C., Birdsong, T.W., Garrett, G.P., Eds.; American Fisheries Society: Bethesda, MD, USA, 2019; pp. 183–229. [Google Scholar]
- Garrett, G.P.; Birdsong, T.W.; Bean, M.G.; Labay, B.J. Chihuahuan Desert Native Fish Conservation Areas: A multispecies and watershed approach to preservation of freshwater fish diversity. In Multispecies and Watershed Approaches to Freshwater Fish Conservation; Dauwalter, D.C., Birdsong, T.W., Garrett, G.P., Eds.; American Fisheries Society Symposium 91: Bethesda, MD, USA, 2019; pp. 231–252. [Google Scholar]
- Birdsong, T.W.; Allen, M.S.; Claussen, J.E.; Garrett, G.P.; Grabowski, T.B.; Graham, J.; Harris, F.; Hartzog, A.; Hendrickson, D.A.; Krause, R.A.; et al. Native Black Bass Initiative: Implementing watershed-scale approaches to conservation of endemic black bass and other native fishes in the southern United States. In Black Bass Diversity: Multidisciplinary Science for Conservation; Tringali, M.D., Long, J.M., Birdsong, T.W., Allen, M.S., Eds.; American Fisheries Society: Bethesda, MD, USA, 2015; pp. 363–378. [Google Scholar]
- Ferrier, S.; Wintle, B. Quantitative approaches to spatial conservation prioritization: Matching the solution to the need. In Spatial Conservation Prioritization; Moilanen, A., Wilson, K.A., Possingham, H.P., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 1–15. [Google Scholar]
- Moilanen, A.; Leathwick, J.; Elith, J. A method for spatial freshwater conservation prioritization. Freshw. Biol. 2008, 53, 577–592. [Google Scholar] [CrossRef]
- Hermoso, V.; Linke, S.; Prenda, J.; Possingham, H.P. Addressing longitudinal connectivity in the systematic conservation planning of fresh waters. Freshw. Biol. 2011, 56, 57–70. [Google Scholar] [CrossRef]
- Dauwalter, D.C.; Vail-Muse, S.L.; Thompson, T.R.; Whittier, J.B.; Johnson, K.M.; Bean, M.G. Partnering on multispecies aquatic assessments to inform efficient conservation delivery. In Multispecies and Watershed Approaches to Freshwater Fish Conservation; Dauwalter, D.C., Birdsong, T.W., Garrett, G.P., Eds.; American Fisheries Society, Symposium 91: Bethesda, MD, USA, 2019; pp. 11–32. [Google Scholar]
- Reheis, M. Extent of Pleistocene Lakes in the Western Great Basin: USGS Miscellaneous Field Studies Map. 1999. Available online: https://pubs.er.usgs.gov/publication/mf2323 (accessed on 7 January 2023).
- Sigler, W.F.; Sigler, J.W. Fishes of the Great Basin: A Natural History; University of Nevada Press: Reno, NV, USA, 1987. [Google Scholar]
- Reheis, M.C.; Sarna-Wojcicki, A.M.; Reynolds, R.L.; Repenning, C.A.; Mifflin, M.D. Pliocene to middle Pleistocene lakes in the western Great Basin: Ages and connections. In Great Basin Aquatic Systems History; Hershler, R., Madsen, D.B., Currey, D.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2002; pp. 53–108. [Google Scholar]
- Peacock, M.M.; Neville, H.M.; Finger, A.J. The Lahontan Basin evolutionary lineage of Cutthroat Trout. In Cutthroat Trout: Evolutionary Biology and Taxonomy; Trotter, P.C., Bisson, P.A., Schultz, L.D., Roper, B.B., Eds.; American Fisheries Society: Bethesda, MD, USA, 2018; pp. 231–259. [Google Scholar]
- Overpeck, J.T.; Bonar, S.A. Southwestern fish and aquatic systems: The climate challenge. In Standing between Life and Extinction; Propst, D.L., Williams, J.E., Bestgen, K.R., Hoagstrom, C.W., Eds.; University of Chicago Press: Chicago, IL, USA, 2021; pp. 137–152. [Google Scholar]
- Moilanen, A.; Pouzols, F.M.; Meller, L.; Veach, V.; Arponen, A.; Leppänen, J.; Kujala, H. Zonation: Spatial Conservation Planning Methods and Software, Version 4.0; University of Helsinki: Helsinki, Finland, 2014. [Google Scholar]
- DFHP. Framework for Strategic Conservation of Desert Fishes; Desert Fish Habitat Partnership (DFHP): Salt Lake City, UT, USA, 2015; p. 31. [Google Scholar]
- Di Minin, E.; Veach, V.; Lehtomaki, J.; Montesino Pouzols, F.; Moilanen, A. A Quick Introduction to Zonation; University of Helsinki: Helsinki, Finland, 2014; Available online: https://researchportal.helsinki.fi/en/publications/a-quick-introduction-to-zonation (accessed on 7 January 2023).
- USGS. Watershed Boundary Dataset; U.S. Geological Survey: Washington, DC, USA, 2019.
- Haak, A.L.; Williams, J.E. Spreading the risk: Native trout management in an warmer and less-certain future. N. Am. J. Fish. Manag. 2012, 32, 387–401. [Google Scholar] [CrossRef]
- USFWS. Lahontan Cutthroat Trout (Oncorhynchus Clarkii Henshawi) 5-Year Review: Summary and Evaluation; United States Fish and Wildlife Service (USFWS): Reno, NV, USA, 2009. [Google Scholar]
- May, B.; Albeke, S. Proposed Protocol—Lahontan Cutthroat Trout Range-Wide Database Update: Historical Range, Current Status, Risk and Population Health Determinations and Population Restoration Potential Protocols; Unpublished report; 2008; p. 31. [Google Scholar]
- NatureServe. Element Occurrence Data Standard; NatureServe: Arlington, VA, USA, 2002; p. 201. [Google Scholar]
- Billman, E.J.; Lee, J.B.; Young, D.O.; McKell, M.D.; Evans, R.P.; Shiozawa, D.K. Phylogenetic divergence in a desert fish: Differentiation of speckled dace within the Bonneville, Lahontan, and Upper Snake River basins. West. N. Am. Nat. 2010, 70, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Moyle, P.B.; Campbell, M.A.; Finger, A.J.; O’Rourke, S.M.; Baumsteiger, J.; Miller, M.R. Population genomic analysis of the Speckled Dace species complex identifies three distinct lineages in California. Trans. Am. Fish. Soc. 2022, 151, 695–710. [Google Scholar] [CrossRef]
- Deacon, J.E.; Williams, J.E. Annotated list of the fishes of Nevada. Proc. Biol. Soc. Wash. 1984, 97, 103–118. [Google Scholar]
- Houston, D.D.; Evans, R.P.; Shiozawa, D.K. Evaluating the genetic status of a Great Basin endemic minnow: The relict dace (Relictus solitarius). Conserv. Genet. 2012, 13, 727–742. [Google Scholar] [CrossRef]
- Finger, A.J.; Benjamin, A.; Crookshanks, C.; Campbell, M.A.; Sağlam, İ.K. Broad- and fine-scale structure across the distribution of the relict dace (Relictus solitarius) in the Great Basin desert, USA. Conserv. Sci. Pract. 2022, 4, e12672. [Google Scholar] [CrossRef]
- Al-Chokhachy, R.; Heki, L.; Loux, T.; Peka, R. Return of a giant: Coordinated conservation leads to the first wild reproduction of Lahontan Cutthroat Trout in the Truckee River in nearly a century. Fisheries 2020, 45, 63–73. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- GBIF.org. GBIF Occurrence Download. 2020. Available online: https://doi.org/10.15468/dl.txanmk (accessed on 19 December 2020).
- Liu, C.; Newell, G.; White, M. The effect of sample size on the accuracy of species distribution models: Considering both presences and pseudo-absences or background sites. Ecography 2019, 42, 535–548. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.N.; Dauwalter, D.C.; Thurow, R.F.; Philipp, D.P.; Williams, J.E.; Walser, C.A. Identification and utility of native fish conservation areas in the Upper Snake River basin. In Multispecies and Watershed Approaches to Freshwater Fish Conservation; Dauwalter, D.C., Birdsong, T.W., Garrett, G.P., Eds.; American Fisheries Society: Bethesda, MD, USA, 2019; pp. 135–159. [Google Scholar]
- Nagal, D.; Peterson, E.; Isaak, D.; Ver Hoef, J.; Horan, D. National Stream Internet Protocol and User Guide; US Forest Service, Rocky Mountain Research Station Air, Water, and Aquatic Environments Program: Boise, ID, USA, 2015; p. 46. Available online: https://www.fs.usda.gov/rm/boise/AWAE/projects/NationalStreamInternet.html (accessed on 7 January 2023).
- Isaak, D.J.; Wenger, S.J.; Peterson, E.E.; Ver Hoef, J.M.; Nagel, D.E.; Luce, C.H.; Hostetler, S.W.; Dunham, J.B.; Roper, B.B.; Wollrab, S.P.; et al. The NorWeST summer stream temperature model and scenarios for the western U.S.: A crowd-sourced database and new geospatial tools foster a user-community and predict broad climate warming of rivers and streams. Water Resour. Res. 2017, 53, 9181–9205. [Google Scholar] [CrossRef] [Green Version]
- Moilanen, A.; Wintle, B.A.; Elith, J.; Burgman, M. Uncertainty analysis for regional-scale reserve selection. Conserv. Biol. 2006, 20, 1688–1697. [Google Scholar] [CrossRef]
- Crawford, S.; Whelan, G.; Infante, D.M.; Blackhart, K.; Daniel, W.M.; Fuller, P.L.; Birdsong, T.; Wieferich, D.J.; McClees-Funinan, R.; Stedman, S.M.; et al. Through a Fish’s Eye: The Status of Fish Habitats in the United States 2015; National Fish Habitat Partnership: Washington, DC, USA, 2016. Available online: https://pubs.er.usgs.gov/publication/70200345 (accessed on 7 January 2023).
- Wang, L.; Infante, D.; Esselman, P.; Cooper, A.; Wu, D.; Taylor, W.; Beard, D.; Whelan, G.; Ostroff, A. A hierarchical spatial framework and database for the National River Fish Habitat Condition Assessment. Fisheries 2011, 36, 436–449. [Google Scholar] [CrossRef] [Green Version]
- Whelan, G.E. The really big picture for fish habitat: The conceptual underpinnings and vision for the National Fish Habitat Partnership’s National Fish Habitat Assessment. In Multispecies and Watershed Approaches to Freshwater Fish Conservation; Dauwalter, D.C., Birdsong, T.W., Garrett, G.P., Eds.; American Fisheries Society: Bethesda, MD, USA, 2019; pp. 33–55. [Google Scholar]
- Carlson, A.J.; Rahel, F.J. Annual intrabasin movement and mortality of adult Bonneville Cutthroat Trout among complementary riverine habitats. Trans. Am. Fish. Soc. 2010, 139, 1360–1371. [Google Scholar] [CrossRef]
- USACE. National Inventory of Dams; U.S. Army Corps of Engineers: Hanover, NH, USA, 2008.
- La Rivers, I. Fishes and Fisheries of Nevada; Nevada State Fish and Game Commission: Reno, NV, USA, 1962. [Google Scholar]
- Deacon, J.E.; Williams, A.E.; Deacon Williams, C.; Williams, J.E. Fueling population growth in Las Vegas: How large-scale groundwater withdrawal could burn regional biodiversity. BioScience 2007, 57, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Minckley, W.L.; Deacon, J.E. Southwestern fishes and the enigma of “endangered species”. Science 1968, 159, 1424–1432. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.L.; Strayer, D.L.; Wallace, J.B.; Eggert, S.L.; Helfman, G.S.; Leonard, N.E. The contribution of headwater streams to biodiversity in river networks. J. Am. Water Resour. Assoc. 2007, 43, 86–103. [Google Scholar] [CrossRef] [Green Version]
- Dauwalter, D.C. Fish assemblage associations and thresholds with existing and projected oil and gas development. Fish. Manag. Ecol. 2013, 20, 289–301. [Google Scholar] [CrossRef]
- Williams, J.E.; Macdonald, C.A.; Deacon Williams, C.; Weeks, H.; Lampman, G.; Sada, D.W. Prospects for recovering endemic fishes pursuant to the U.S. Endangered Species Act. Fisheries 2005, 30, 24–29. [Google Scholar] [CrossRef]
- Booth, D.B.; Roy, A.H.; Smith, B.; Capps, K.A. Global perspectives on the urban stream syndrome. Freshw. Sci. 2016, 35, 412–420. [Google Scholar] [CrossRef] [Green Version]
- O’Neal, S.; Hughes, R.M. Fisheries and Hard Rock Mining: AFS Symposium Synopsis. Fisheries 2012, 37, 54–55. [Google Scholar] [CrossRef]
- Dauwalter, D.C.; Fesenmyer, K.A.; Miller, S.W.; Porter, T. Response of riparian vegetation, instream habitat, and aquatic biota to riparian grazing exclosures. N. Am. J. Fish. Manag. 2018, 38, 1187–1200. [Google Scholar] [CrossRef] [Green Version]
- Fesenmyer, K.A.; Dauwalter, D.C.; Evans, C.; Allai, T. Livestock management, beaver, and climate influences on riparian vegetation in a semi-arid landscape. PLoS ONE 2018, 13, e0208928. [Google Scholar] [CrossRef]
- Hughes, R.M.; Vadas, R.L. Agricultural effects on streams and rivers: A western USA focus. Water 2021, 13, 1901. [Google Scholar] [CrossRef]
- Dauwalter, D.C.; Baker, M.A.; Baker, S.M.; Lee, R.; Walrath, J.D. Physical habitat complexity partially offsets the negative effect of Brook Trout on Yellowstone Cutthroat Trout in the peripheral Goose Creek subbasin. West. N. Am. Nat. 2022, 82, 660–676. [Google Scholar] [CrossRef]
- Swanson, S.; Wyman, S.; Evans, C. Practical grazing management to maintain or restore riparian functions and values on rangelands. J. Rangel. Appl. 2015, 2, 1–28. [Google Scholar]
- Haak, A.L.; Williams, J.E. Using native trout restoration to jumpstart freshwater conservation planning in the Interior West. J. Conserv. Plan. 2013, 9, 38–52. [Google Scholar]
- Al-Chokhacky, R.; Peacock, M.; Heki, L.G.; Thiede, G. Evaluating the reintroduction potential of Lahontan cutthroat trout in Fallen Leaf Lake, California. N. Am. J. Fish. Manag. 2009, 29, 1296–1313. [Google Scholar] [CrossRef]
- Dauwalter, D.C.; Sanderson, J.S.; Williams, J.E.; Sedell, J.R. Identification and implementation of Native Fish Conservation Areas in the Upper Colorado River Basin. Fisheries 2011, 36, 278–288. [Google Scholar] [CrossRef]
- Armstrong, J.B.; Fullerton, A.H.; Jordan, C.E.; Ebersole, J.L.; Bellmore, J.R.; Arismendi, I.; Penaluna, B.E.; Reeves, G.H. The importance of warm habitat to the growth regime of cold-water fishes. Nat. Clim. Chang. 2021, 11, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.A.; Cabeza, M.; Klein, C.J. Fundamental concepts of spatial conservation prioritization. In Spatial Conservation Prioritization; Moilanen, A., Wilson, K.A., Possingham, H.P., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 16–27. [Google Scholar]


| Common Name | Scientific Name | Heritage Rank | Model Input | Curve (Up, Down) | DFHP Rank (Weight) |
|---|---|---|---|---|---|
| Devils Hole Pupfish | Cyprinodon diabolis | G1S1 | NDNH (0 or 1) | 1, 1 | 1.89 |
| Ash Meadows Amargosa Pupfish | Cyprinodon nevadensis mionectes | G2T2S2 | NDNH (0 or 1) | 2, 2 | 1.67 |
| Warm Springs Amargosa Pupfish | Cyprinodon nevadensis pectoralis | G2T1S1 | NDNH (0 or 1) | 2, 2 | 2.22 |
| Desert Dace | Eremichthys acros | G1S1 | NDNH (0 or 1) | 2, 2 | 2.22 |
| Relict Dace | Relictus solitarius | G2G3S2S3 | NDNH (0 or 1) | 2, 2 | 2.56 |
| Ash Meadows Speckled Dace | Rhinichthys osculus nevadensis | G5T1S1 | NDNH (0 or 1) | 2, 2 | 2.00 |
| Big Smoky Valley Speckled Dace | Rhinichthys osculus lariversi | G5T1S1 | NDNH (0 or 1) | 2, 1 | 1.89 |
| Clover Valley Speckled Dace | Rhinichthys osculus oligoporus | G5T1S1 | NDNH (0 or 1) | 2, 1 | 1.89 |
| Diamond Valley Speckled Dace | Rhinichthys osculus ssp. | G5THSH | NDNH (0 or 1) | 2, 1 | 2.33 |
| Grass Valley Speckled Dace | Rhinichthys osculus reliquus | G5T5SX | NDNH (0 or 1) | 2, 1 | 2.11 |
| Independence Valley Speckled Dace | Rhinichthys osculus lethoporus | G5T1S1 | NDNH (0 or 1) | 2, 1 | 1.89 |
| Lahontan Basin Speckled Dace | Rhinichthys osculus | G5S5 | SDM (0–1) | 2, 1 | 1.00 |
| Monitor Valley Speckled Dace | Rhinichthys osculus spp. | G5T1S1 | NDNH (0 or 1) | 2, 1 | 2.44 |
| Oasis Valley Speckled Dace | Rhinichthys osculus spp. | G5T1S1 | NDNH (0 or 1) | 2, 1 | 2.11 |
| Lahontan Redside | Richardsonius egregius | G5SNR | SDM (0–1) | 1, 1 | 1.56 |
| Big Smoky Valley Tui Chub | Siphateles bicolor ssp. 8 | G4T1S1 | NDNH (0 or 1) | 2, 1 | 2.33 |
| Charnock Springs Tui Chub | Siphateles bicolor ssp. 10 | G4T1QS1 | NDNH (0 or 1) | 2, 1 | 2.33 |
| Dixie Valley Tui Chub | Siphateles bicolor ssp. 9 | G4T1QS1 | NDNH (0 or 1) | 2, 1 | 2.33 |
| Duckwater Creek Tui Chub | Siphateles bicolor ssp. 3 | G4T1S1 | NDNH (0 or 1) | 2, 1 | 2.33 |
| Fish Creek Springs Tui Chub | Siphateles bicolor euchila | G4T1QS1 | NDNH (0 or 1) | 2, 1 | 2.33 |
| Fish Lake Valley Tui Chub | Siphateles bicolor ssp. 4 | G4T1QS1 | NDNH (0 or 1) | 2, 1 | 2.44 |
| High Rock Spring Tui Chub | Siphateles bicolor ssp. 11 | G4TXSX | NDNH (0 or 1) | 2, 1 | 2.22 |
| Hot Creek Valley Tui Chub | Siphateles bicolor ssp. 5 | G4T1QS1 | NDNH (0 or 1) | 2, 1 | 2.33 |
| Independence Valley Tui Chub | Siphateles bicolor isolata | G4T1QS1 | NDNH (0 or 1) | 2, 1 | 2.33 |
| Lahontan Creek Tui Chub | Siphateles bicolor obesa | G4T4S4 | NDNH (0 or 1) | 2, 1 | 1.44 |
| Little Fish Lake Valley Tui Chub | Siphateles bicolor ssp. 6 | G4T1S1 | NDNH (0 or 1) | 2, 1 | 2.33 |
| Newark Valley Tui Chub | Siphateles bicolor newarkensis | G1T1S1 | NDNH (0 or 1) | 2, 1 | 2.11 |
| Railroad Valley Tui Chub | Siphateles bicolor ssp. 7 | G4T1QS1 | NDNH (0 or 1) | 2, 1 | 2.00 |
| Railroad Valley Springfish | Crenichthys nevadae | G2S2 | NDNH (0 or 1) | 1, 1 | 1.89 |
| Ash Meadows Poolfish | Empetrichthys merriami | GXSX | NDNH (0 or 1) | 1, 1 | 2.11 |
| Pahrump Poolfish | Empetrichthys latos latos | G1T1S1 | NDNH (0 or 1) | 1, 1 | 2.11 |
| Big Spring Spinedace | Lepidomeda bicolor pratensis | G2T1S1 | NDNH (0 or 1) | 2, 2 | 1.89 |
| Paiute Sculpin | Cottus beldingii | G5SNR | SDM (0–1) | 2, 2 | 1.33 |
| * Shorthead Sculpin | Cottus confusus | G5S1 | NDNH (0 or 1) | 1.33 * | |
| Meadow Valley Wash Desert Sucker | Catostomus clarkii spp. 2 | G3G4T2S2 | NDNH (0 or 1) | 2, 2 | 2.22 |
| Mountain Sucker | Catostomus platyrhynchus | G5SNR | SDM (0–1) | 2, 2 | 0.89 |
| Tahoe Sucker | Catostomus tahoensis | G5S5 | SDM (0–1) | 2, 2 | 1.89 |
| Wall Canyon Sucker | Catostomus sp. | G1S1 | NDNH (0 or 1) | 2, 2 | 2.67 |
| Cui-ui | Chasmistes cujus | G1S1 | NDNH (0 or 1) | 2, 2 | 1.56 |
| Mountain Whitefish | Prosopium williamsoni | G5S3 | SDM (0–1) | 4, 4 | 1.44 |
| Lahontan Cutthroat Trout | Oncorhynchus clarkii henshawi | G4T3S3 | Rangewide DB | (2.25) * | |
| Western Lahontan UIEU Resident | #/km | 3, 3 | 0.250 | ||
| Adfluvial | #/km | 5, 5 | 0.250 | ||
| NW Lahontan UIEU Resident | #/km | 3, 3 | 0.250 | ||
| Adfluvial | #/km | 5, 5 | 0.250 | ||
| Eastern Lahontan UIEU Resident | #/km | 3, 3 | 0.167 | ||
| Fluvial | #/km | 4, 4 | 0.167 | ||
| Adfluvial | #/km | 5, 5 | 0.167 | ||
| Coyote Lakes UIEU Resident | #/km | 3, 3 | 0.500 | ||
| Historical Streams | SDM (0–1) | 3, 3 | 0.125 | ||
| Lakes | Presence = 1 | 4, 3 | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauwalter, D.C.; Miskow, E.; Crookshanks, C. Spatial Conservation Assessment for Native Fishes in the Lahontan and Central Nevada Basins, USA. Water 2023, 15, 1087. https://doi.org/10.3390/w15061087
Dauwalter DC, Miskow E, Crookshanks C. Spatial Conservation Assessment for Native Fishes in the Lahontan and Central Nevada Basins, USA. Water. 2023; 15(6):1087. https://doi.org/10.3390/w15061087
Chicago/Turabian StyleDauwalter, Daniel C., Eric Miskow, and Chris Crookshanks. 2023. "Spatial Conservation Assessment for Native Fishes in the Lahontan and Central Nevada Basins, USA" Water 15, no. 6: 1087. https://doi.org/10.3390/w15061087
APA StyleDauwalter, D. C., Miskow, E., & Crookshanks, C. (2023). Spatial Conservation Assessment for Native Fishes in the Lahontan and Central Nevada Basins, USA. Water, 15(6), 1087. https://doi.org/10.3390/w15061087





