Does Methane Contribute to Growth of Invertebrate Communities in Drinking Water?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Full-Scale Waterworks
2.1.1. Filter Sand Sampling
2.1.2. Water Sampling
2.2. Pilot Columns
2.2.1. Columns without Methane Removal (Setup and Sampling)
2.2.2. Columns with Methane Removal (Setup and Sampling)
2.3. Microscopy
2.4. Analyses of Specific Water Quality Parameters
2.5. Molecular Analyses
3. Results
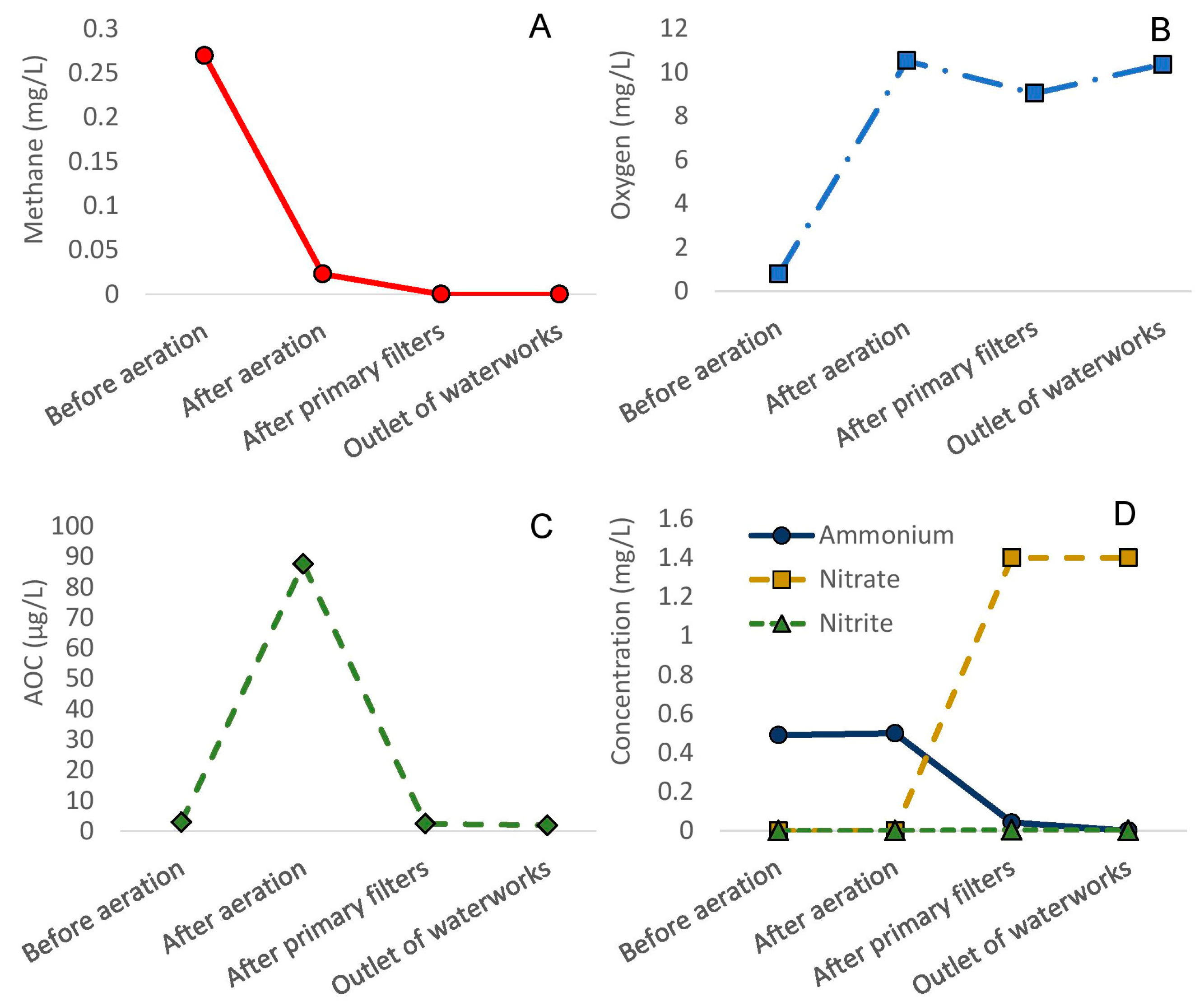
3.1. Efficiency of Full-Scale Water Treatment
3.2. Organisms in Rapid Sand Filters
3.2.1. Microscopy
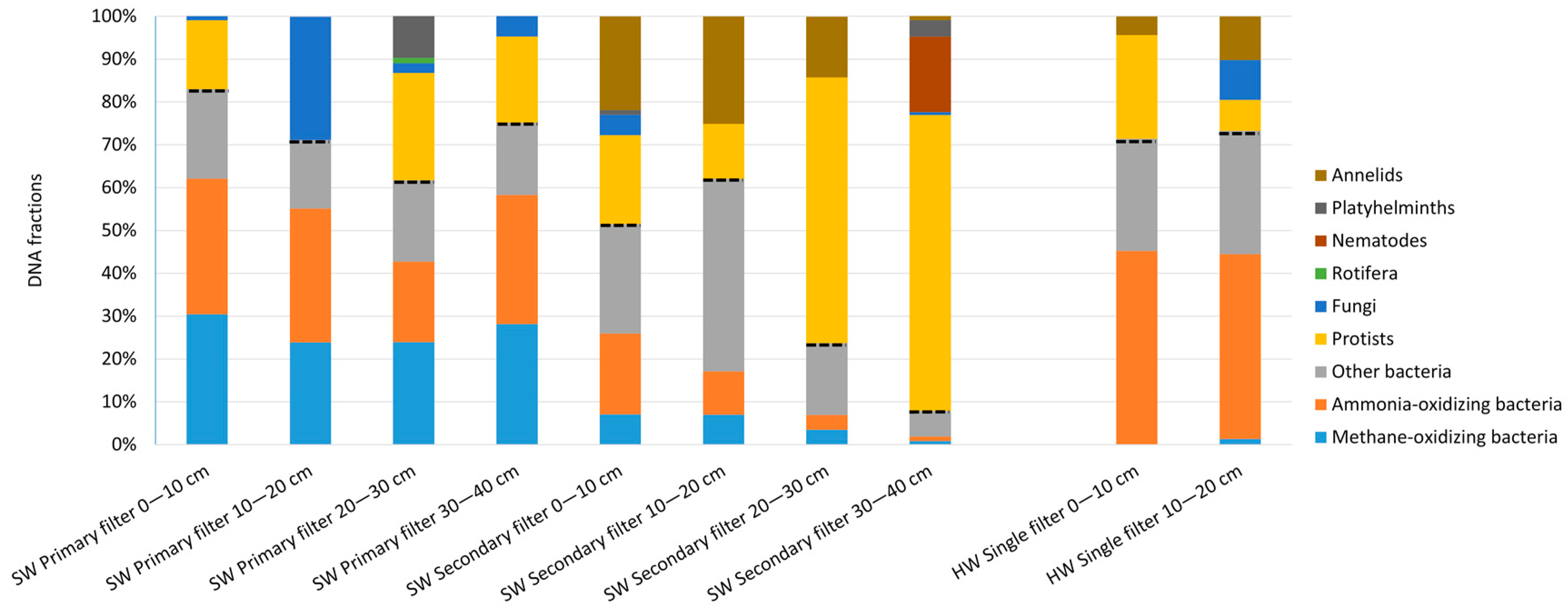
3.2.2. Molecular Analyses
3.3. Estimation of Suitable Methane Levels
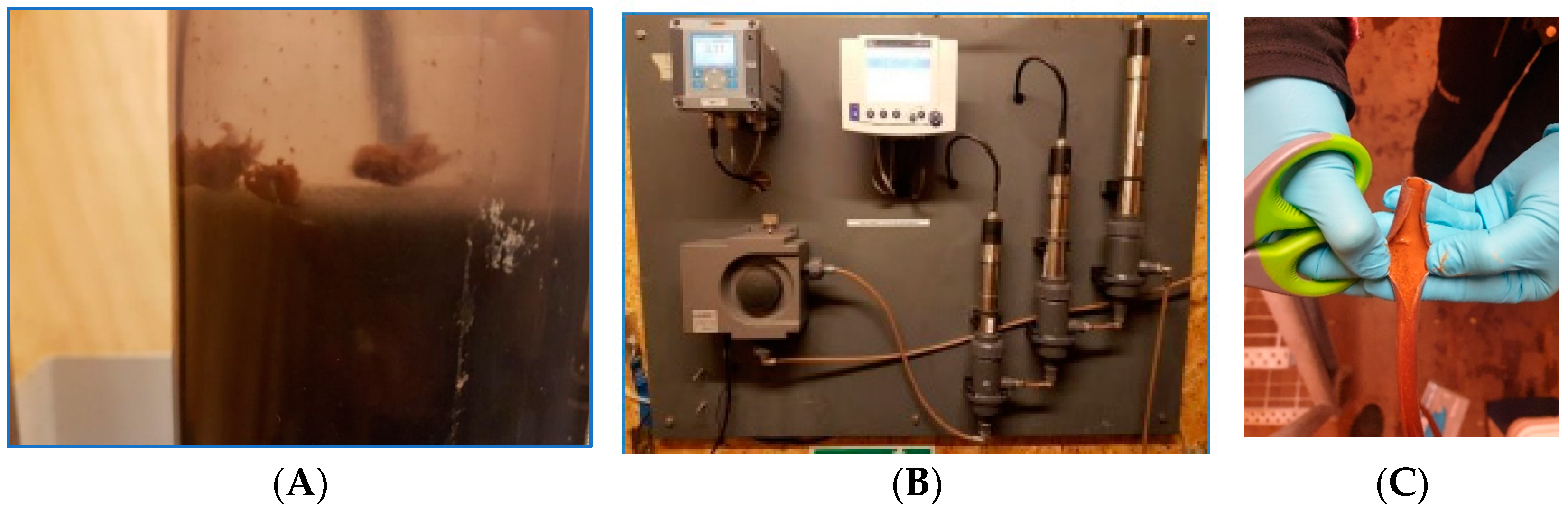
3.3.1. Inspection of Visible Growth
3.3.2. Molecular Analyses of Prokaryotes and Eukaryotes
3.3.3. AOC Analyses
4. Discussion
4.1. Effect of Methane on Invertebrate Populations
4.2. Which Methane Concentration to Aim for?
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prest, E.; Hammes, F.; van Loosdrecht, M.; Vrouwenvelder, J.S. Biological Stability of Drinking Water: Controlling Factors, Methods, and Challenges. Front. Microbiol. 2016, 7, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Chakhtoura, J.; Prest, E.I.; Saikaly, P.E.; van Loosdrecht, M.C.M.; Hammes, F.; Vrouwenvelder, J.S. Dynamics of bacterial communities before and after distribution in a full-scale drinking water network. Water Res. 2015, 74, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, M.J.; Arvin, E.; Corfitzen, C.B.; Albrechtsen, H.-J. Mecoprop (MCPP) removal in full-scale rapid sand filters at a groundwater-based waterworks. Sci. Total Environ. 2014, 499, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.O.; Boe-Hansen, R.; Musovic, S.; Smets, B.F.; Albrechtsen, H.-J.; Binning, P.J. Effects of dynamic operating conditions on nitrification in biological rapid sand filters for drinking water treatment. Water Res. 2014, 64, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Gülay, A.; Musovic, S.; Albrechtsen, H.-J.; Smets, B.F. Neutrophilic iron-oxidizing bacteria: Occurrence and relevance in biological drinking water treatment. Water Sci. Technol. Water Supply. 2013, 13, 1295–1301. [Google Scholar] [CrossRef]
- Skovhus, T.L.; Søborg, D.A.; Braga, F.; Højris, B.; Kristensen, K.B.; Hansen, K.L. Effects of early biofilm formation on water quality during commissioning of new polyethylene pipes. Env. Sci. Water Res. Tech. 2022, 8, 1992–2005. [Google Scholar] [CrossRef]
- Favere, J.; Barbosa, R.G.; Sleutels, T.; Verstraete, W.; De Gusseme, B.; Boon, N. Safeguarding the microbial water quality from source to tap. NPJ Clean Water. 2021, 4, 28. [Google Scholar] [CrossRef]
- Bucheli-Witschel, M.; Kötzsch, S.D.; Widler, R.; Egli, T. A new method to assess the influence of migration from polymeric materials on the biostability of drinking water. Water Res. 2012, 46, 4246–4260. [Google Scholar] [CrossRef]
- van der Kooij, D. Assimilable Organic Carbon as an Indicator of Bacterial Regrowth. J. Am. Water Works Ass. 1992, 84, 57–65. [Google Scholar] [CrossRef]
- Prest, E.I.; Martijn, B.J.; Rietveld, M.; Lin, Y.; Schaap, P.G. (Micro)Biological Sediment Formation in a Non-Chlorinated Drinking Water Distribution System. Water 2023, 15, 214. [Google Scholar] [CrossRef]
- Christensen, S.C.B.; Nissen, E.; Arvin, E.; Albrechtsen, H.-J. Distribution of Asellus aquaticus and microinvertebrates in a non-chlorinated drinking water supply system, effects of pipe material and sedimentation. Water Res. 2011, 45, 3215–3224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christopher, S.; Michels, U.; Gunkel, G. Paratanytarsus grimmii (Chironomidae) Larvae in Drinking Water Distribution Systems: Impairment or disaster? Water 2023, 15, 377. [Google Scholar] [CrossRef]
- Walker, A.P. The microscopy of consumer complaints. J. Inst. Water Eng. Sci. 1983, 37, 200–214. [Google Scholar]
- Grimont, F.; Grimont. The Genus Serratia. In P.A.D. Prokaryotes, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 219–244. [Google Scholar]
- Christensen, S.C.B.; Albrechtsen, H.-J. Overlevelse af coliforme bakterier i drikkevand (Survival of Coliform Bacteria in Drinking Water); DTU Environment, Technical University of Denmark: Kongens Lyngby, Denmark, 2015. (In Danish) [Google Scholar]
- Danish Drinking Water Act. BEK Number 1383 of 03/10/2022. Miljømin., j.nr. 2022—9820, Lovtidende A, Danish EPA, Copenhagen, Denmark, 2022. Available online: https://www.retsinformation.dk/eli/lta/2022/1383 (accessed on 1 February 2023). (In Danish).
- Jørgensen, C.; Albrechtsen, H.-J.; Arvin, E.; Corfitzen, C.B. Undersøgelse af bakterieantal og eftervækstpotentiale i Vandværksvand; No. 719; Miljøstyrelsen: Copenhagen, Denmark, 2002. (In Danish) [Google Scholar]
- Christensen, S.C.B.; Larsen, S.L.; Asmussen, O.W.; Boe-Hansen, R.; Nava, S.B.; Afshar, S.V.; Albrechtsen, H.-J. Ormebekæmpelse i vandværksfiltre—Forekomst og Bekæmpelsesteknologi; Naturstyrelsen: Copenhagen, Denmark, 2015. (In Danish) [Google Scholar]
- Hedegaard, M.J.; Schliemann-Haug, M.A.M.; Milanovic, N.; Lee, C.O.; Boe-Hansen, R.; Albrechtsen, H.-J. Importance of Methane oxidation for Microbial Degradation of the Herbicide Bentazone in Drinking Water Production. Front. Environ. Sci. 2020, 8, 79. [Google Scholar] [CrossRef]
- Harder, C.B.; Albers, C.N.; Rosendahl, S.; Aamand, J.; Ellegaard-Jensen, L.; Ekelund, F. Successional trophic complexity and biogeographical structure of eukaryotic communities in waterworks’ rapid sand filters. FEMS Microbiol. Ecol. 2019, 95, 11. [Google Scholar]
- van der Kooij, D.; Veenendaal, H.R. Determination of the concentration of easily assimilable organic carbon in drinking water with growth measurements using pure bacterial cultures. In The AOC Manual; SWE 95.022; Kiwa: Nieuwegein, Holland, 1995. [Google Scholar]
- Andersen, L.O.B.; Röser, D.; Nejsum, P.; Nielsen, H.V.; Stensvold, C.R. Is supplementary bead beating for DNA extraction from nematode eggs by use of the nuclisens easymag protocol necessary. J. Clin. Microbiol. 2013, 51, 1345–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krogsgaard, L.R.; Andersen, L.O.; Johannesen, T.B.; Engsbro, A.L.; Stensvold, C.R.; Nielsen, H.V.; Bytzer, P. Characteristics of the bacterial microbiome in association with common intestinal parasites in irritable bowel syndrome. Clin. Transl. Gastroenterol. 2018, 19, 161. [Google Scholar] [CrossRef]
- Ring, H.C.; Thorsen, J.; Saunte, D.M.; Lilje, B.; Bay, L.; Riis, P.T.; Larsen, N.; Andersen, L.O.; Nielsen, H.V.; Miller, I.M.; et al. The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol. 2017, 153, 897–905. [Google Scholar] [CrossRef]
- Castaldelli, G.; Mantovani, S.; Benvenuti, M.R.; Rossi, R.; Fano, E.A. Invertebrate colonisation of GAC filters in a potabilization plant treating groundwater. J. Water Supply Res. Technol.-Aqua 2005, 54, 561–568. [Google Scholar] [CrossRef]
- Schreiber, H.; Schoenen, D.; Traunspurger, W. Invertebrate colonization of granular activated carbon filters. Water Res. 1997, 31, 743–748. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Liu, L.; Zhang, J.; Wang, Q. Invertebrate community characteristics in biologically active carbon filter. J. Environ. Sci. 2010, 22, 648–655. [Google Scholar] [CrossRef]
- van Lieverloo, J.H.M.; van Buuren, R.; Veenendaal, G.; van der Kooij, D. Controlling invertebrates in distribution systems with zero or low disinfectant residual. Water Suppl. 1998, 16, 199–204. [Google Scholar]
- Gunkel, G.; Michels, U.; Scheideler, M. Climate Change: Water Temperature and Invertebrate Propagation in Drinking-Water Distribution Systems, Effects, and Risk Assessment. Water 2022, 14, 1246. [Google Scholar] [CrossRef]
- Mrkajic, N.S.; Hama, J.R.; Strobel, B.W.; Hansen, H.C.B.; Rasmussen, L.H.; Pedersen, A.-K.; Christensen, S.C.B.; Hedegaard, M.J. Removal of phytotoxins in filter sand used for drinking water treatment. Water Res. 2021, 205, 117610. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Zhang, T.-Y.; Wang, H.; Hu, C.-Y.; Tang, Y.-L.; Xu, B. Occurrence of fungal spores in drinking water: A review of pathogenicity, odor, chlorine resistance and control strategies. Sci. Tot. Environ. 2022, 853, 158626. [Google Scholar] [CrossRef] [PubMed]
- Wessels, P.H. A Novel Approach to Anaerobic Groundwater Treatment: Mitigating the Effect of Methane on the Biological Stability of Drinking Water. Master’s Thesis, TU Delft, Delft University of Technology, Delft, The Netherlands, 2014. [Google Scholar]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. IPCC, 2021: Climate Change 2021: The Physical Science Basi; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; 2391p. [Google Scholar]

| Parameter | Vacuum Stripping without N2 Dosing | Vacuum Stripping with 0.8 g/h N2 Dosing | ||
|---|---|---|---|---|
| Inlet | Outlet | Inlet | Outlet | |
| Methane (average) (mg/L) | 0.36 | 0.03 | 0.36 | 0.018 |
| AOC (µg/L) | 5.7 | 5.2 | 8.4 | 6.7 |
| Average Inlet Conc. (mg CH4/L) | Average Conc. after CH4 Removal (mg CH4/L) | Water Source | Bacterial Growth | |
|---|---|---|---|---|
| Hvidovre waterworks with stair aerator | <0.01 | <0.01 | Anaerobic groundwater | No visible growth |
| Slangerup waterworks with aeration in closed cassettes | 0.25 | 0.023 | Anaerobic groundwater | Methane-oxidizing bacteria |
| Column without methane removal | <0.01 | <0.01 | Aerobic drinking water | No visible growth |
| Column without methane removal | 0.24 | 0.22 | Anaerobic groundwater | Rapid visible growth |
| Column with vacuum stripping | 0.36 | 0.03 | Anaerobic groundwater | No visible growth and no methane-oxidizing bacteria |
| Column with vacuum stripping and N2 dosing | 0.36 | 0.018 | Anaerobic groundwater | No visible growth and no methane-oxidizing bacteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, S.C.B.; Lopato, L.; Quinzanos, S.; Hedegaard, M.J. Does Methane Contribute to Growth of Invertebrate Communities in Drinking Water? Water 2023, 15, 1044. https://doi.org/10.3390/w15061044
Christensen SCB, Lopato L, Quinzanos S, Hedegaard MJ. Does Methane Contribute to Growth of Invertebrate Communities in Drinking Water? Water. 2023; 15(6):1044. https://doi.org/10.3390/w15061044
Chicago/Turabian StyleChristensen, Sarah C. B., Laure Lopato, Sonsoles Quinzanos, and Mathilde J. Hedegaard. 2023. "Does Methane Contribute to Growth of Invertebrate Communities in Drinking Water?" Water 15, no. 6: 1044. https://doi.org/10.3390/w15061044





