Fluorine-Rich Shallow Groundwater in Weigan River Basin (Xinjiang): Enrichment Factors and Spatial Distribution
Abstract
1. Introduction
2. Materials and Methods
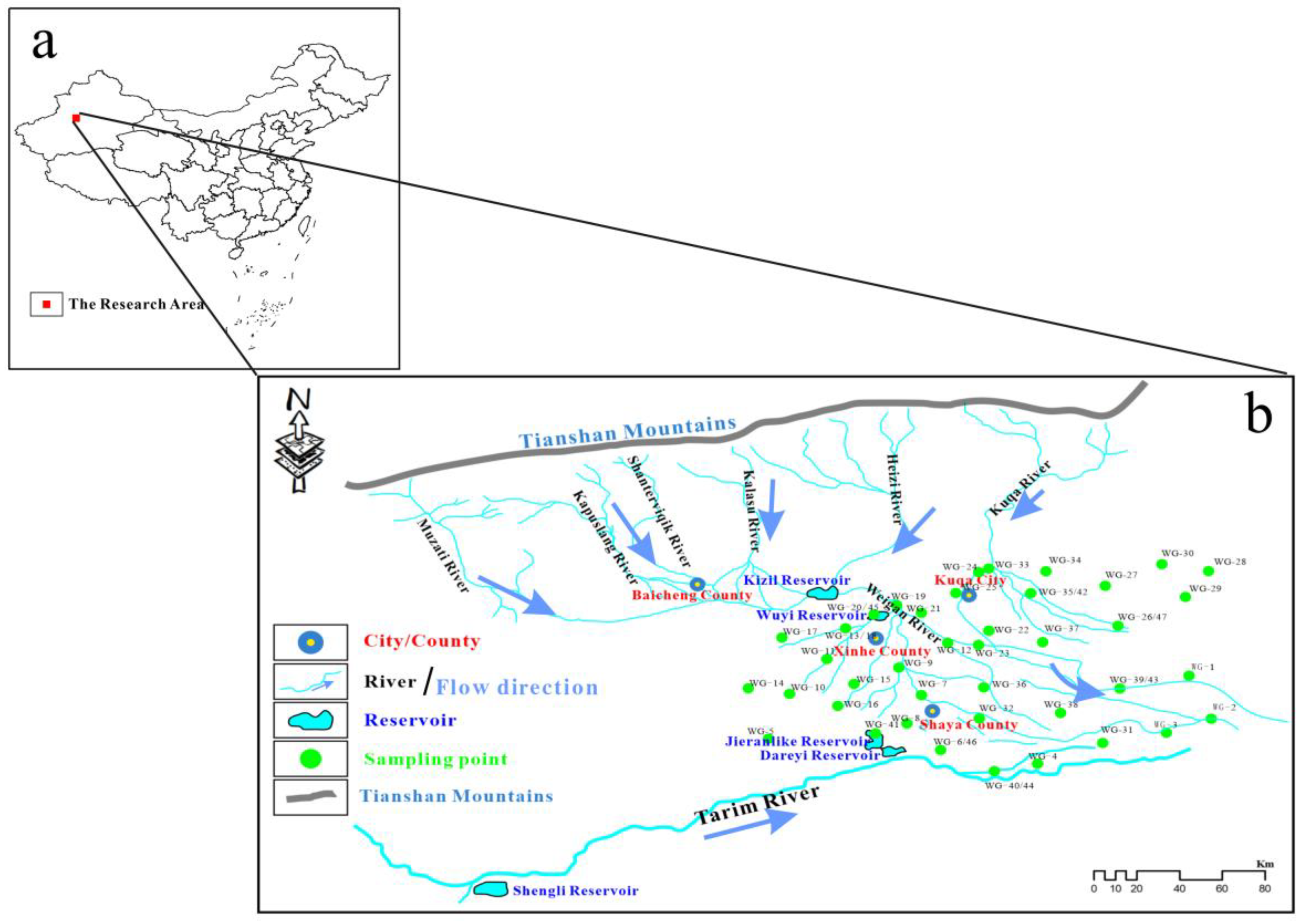
2.1. Overview of the Research Area
2.2. Sample Collection and Tests
3. Results
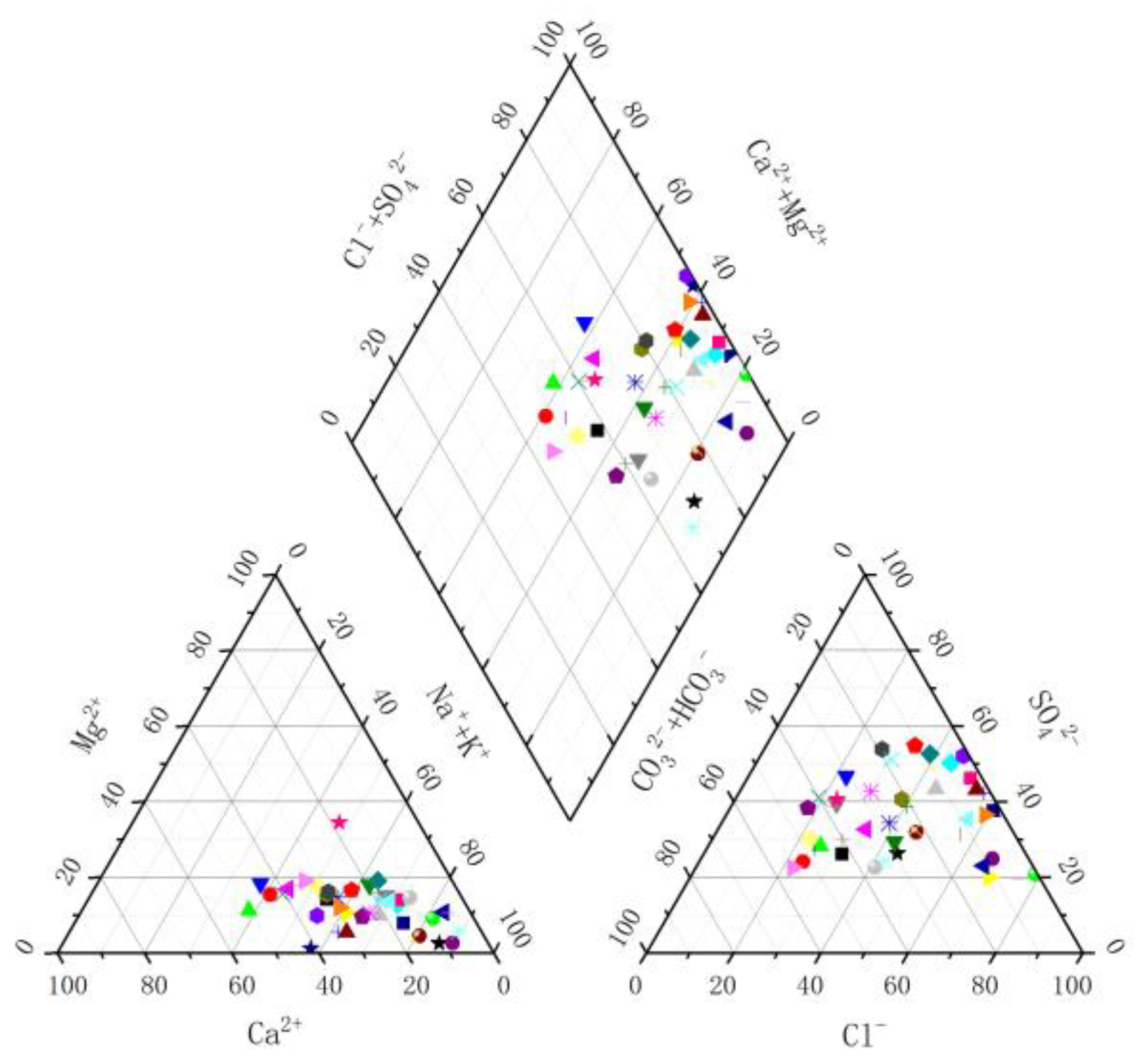
3.1. Hydrochemical Characteristics
3.2. Fluoride Distribution Law in the Groundwater of the Research Area
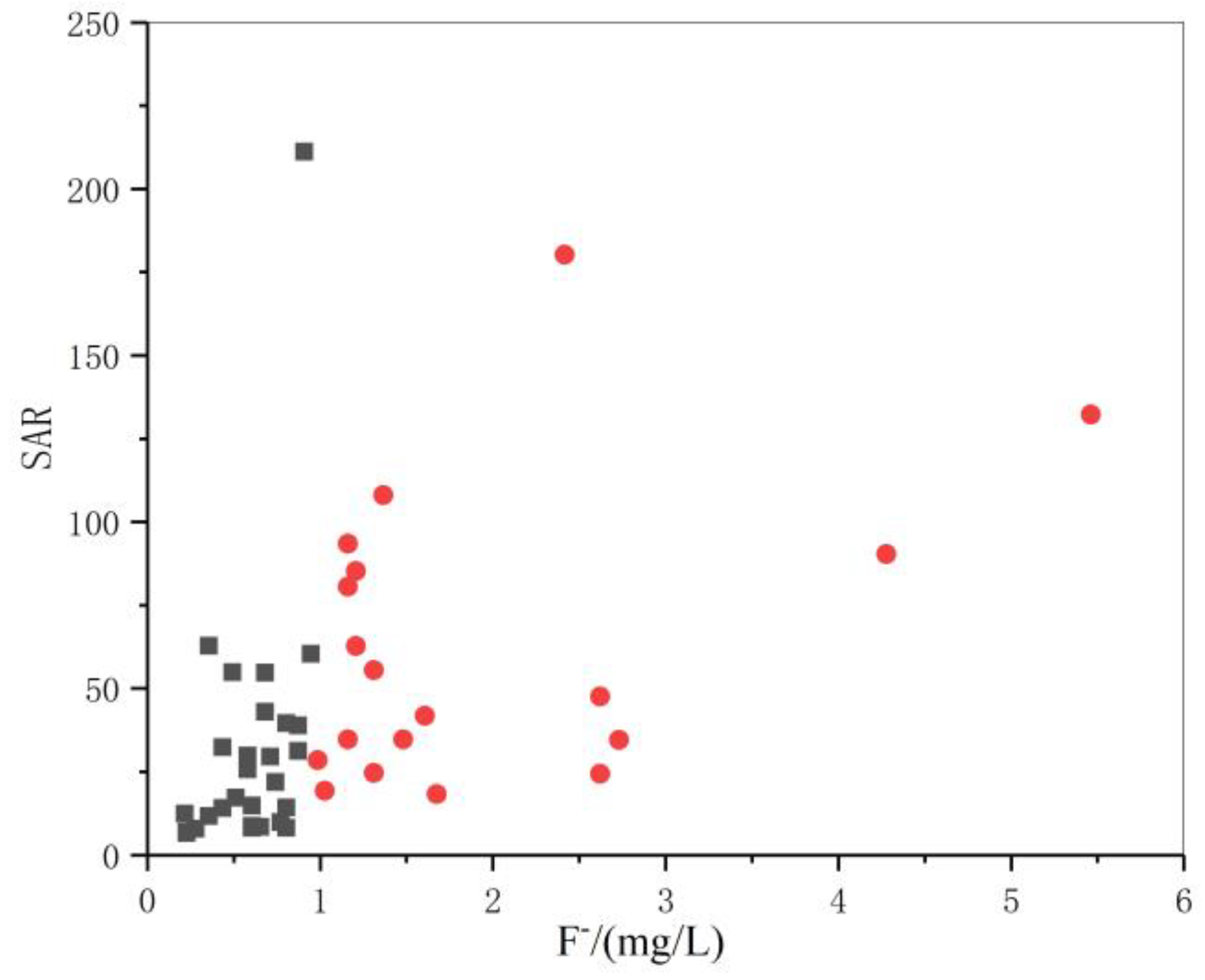
3.3. Relationship between the Fluoride Ion Concentration and the Hydrochemical Index
3.3.1. Relationship between ρ[F−] and pH Values
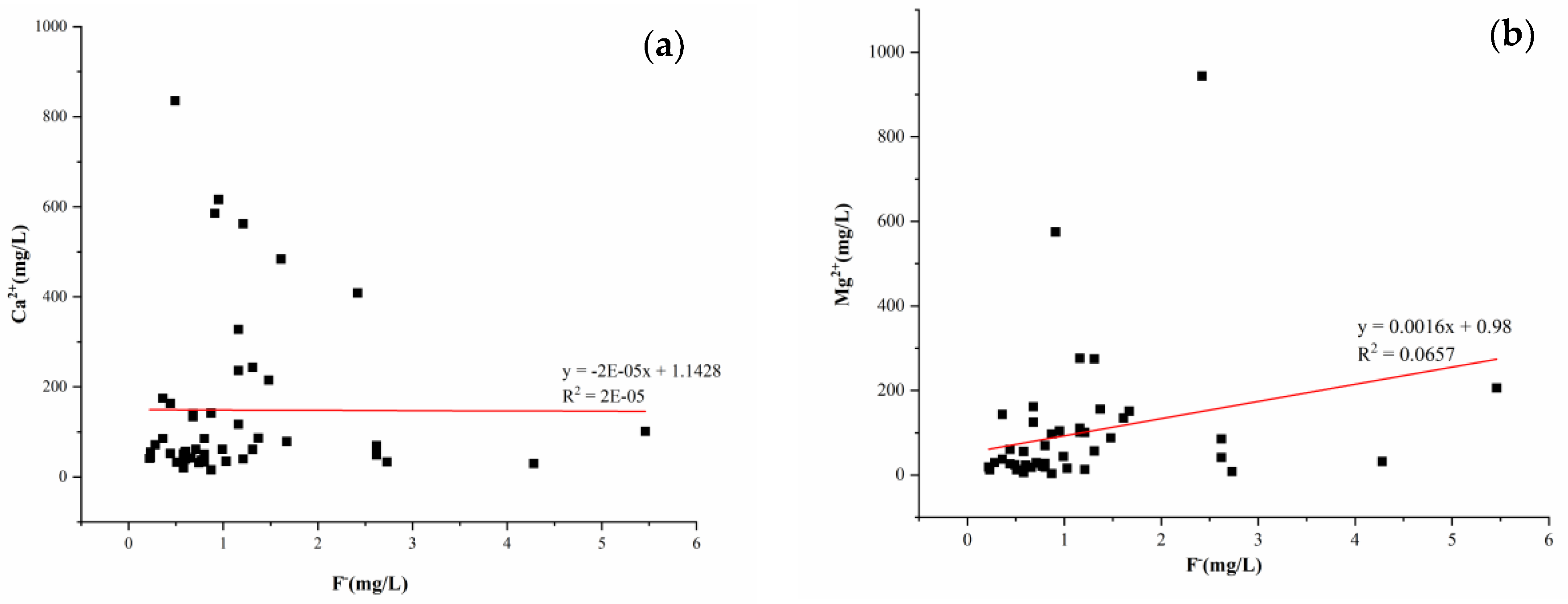
3.3.2. Relationship between the ρ[F−] Value and the Calcium and Magnesium Ion Mass Concentrations
3.3.3. Relationship between ρ[F−] and the F−/Cl− Ratio
3.3.4. Cation Exchange
3.4. Saturation Indices of Fluorite, Dolomite, Calcite, and Halite in the Groundwater
3.5. Analyses of the Main Ion Sources
3.6. Analyses of the Main Constituents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample ID | pH | EH | CaCO3 | CODMn | TDS | Ca2+ | K+ | Mg2+ | Na+ | SO42− | Cl− | HCO3− | F− | NO3− + NO2− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 | 7.95 | 123 | 2001.6 | 3.20 | 7543.0 | 327.79 | 65.23 | 276.23 | 1623.48 | 2354.00 | 1952.59 | 445.45 | 1.16 | 93.23 |
| H2 | 8.25 | 128 | 224.4 | 1.40 | 424.0 | 56.52 | 6.11 | 21.57 | 54.82 | 138.16 | 63.14 | 125.09 | 0.60 | 1.25 |
| H3 | 7.94 | 132 | 651.3 | 1.56 | 1802.0 | 163.23 | 7.79 | 60.70 | 342.24 | 234.34 | 804.01 | 131.19 | 0.44 | 5.09 |
| H4 | 8.03 | 124 | 805.6 | 1.04 | 1500.0 | 79.02 | 11.34 | 150.87 | 195.78 | 468.25 | 268.00 | 372.22 | 1.67 | 2.49 |
| H5 | 8.67 | 118 | 154.0 | 0.64 | 460.0 | 34.83 | 2.79 | 15.97 | 97.32 | 134.38 | 94.30 | 82.38 | 1.03 | 0.03 |
| H6 | 7.67 | 128 | 4974.0 | 5.40 | 19,653.0 | 408.57 | 23.99 | 943.57 | 4683.93 | 3630.00 | 8509.42 | 183.06 | 2.42 | 2.37 |
| H7 | 8.46 | 98 | 344.2 | 0.64 | 972.0 | 61.76 | 5.95 | 43.91 | 206.51 | 341.44 | 203.48 | 115.94 | 0.99 | 0.03 |
| H8 | 8.03 | 143 | 844.3 | 2.24 | 2968.0 | 133.99 | 12.83 | 125.12 | 622.66 | 861.96 | 957.86 | 48.82 | 0.68 | 1.08 |
| H9 | 8.42 | 110 | 200.2 | 0.52 | 568.0 | 31.54 | 6.24 | 27.77 | 119.07 | 164.12 | 99.26 | 146.45 | 0.74 | 0.05 |
| H10 | 8.15 | 137 | 492.5 | 0.76 | 1642.0 | 85.43 | 2.99 | 69.51 | 348.34 | 373.30 | 590.60 | 85.43 | 0.80 | 0.12 |
| H11 | 8.21 | 113 | 277.6 | 0.56 | 925.0 | 61.80 | 2.05 | 29.27 | 199.26 | 263.21 | 270.52 | 70.17 | 0.71 | 0.06 |
| H12 | 8.10 | 116 | 170.2 | 0.64 | 364.0 | 37.30 | 6.18 | 20.80 | 53.55 | 85.03 | 63.14 | 128.14 | 0.77 | 0.73 |
| H13 | 7.88 | 129 | 3860.1 | 6.32 | 18,567.0 | 585.46 | 41.41 | 575.04 | 5086.34 | 2320.56 | 8822.80 | 48.82 | 0.91 | 0.66 |
| H14 | 8.15 | 111 | 400.3 | 0.36 | 956.0 | 61.31 | 7.10 | 56.88 | 189.13 | 201.87 | 289.46 | 180.01 | 1.31 | 0.35 |
| H15 | 8.10 | 108 | 193.2 | 0.44 | 352.0 | 42.59 | 4.73 | 18.13 | 46.54 | 77.73 | 69.48 | 125.09 | 0.66 | 3.42 |
| H16 | 8.30 | 111 | 715.6 | 0.56 | 1924.0 | 116.93 | 13.51 | 101.12 | 362.80 | 404.79 | 723.18 | 146.45 | 1.16 | 0.03 |
| H17 | 8.06 | 109 | 164.1 | 1.16 | 302.0 | 33.12 | 4.83 | 18.76 | 41.61 | 54.80 | 54.59 | 125.09 | 0.80 | 0.72 |
| H18 | 7.96 | 115 | 217.2 | 1.28 | 1634.0 | 29.78 | 6.78 | 32.23 | 502.68 | 288.65 | 501.40 | 344.76 | 4.28 | 0.37 |
| H19 | 8.26 | 116 | 870.7 | 1.88 | 4124.0 | 86.39 | 13.92 | 155.93 | 1188.64 | 609.99 | 1735.63 | 259.34 | 1.37 | 0.48 |
| H20 | 8.15 | 105 | 544.4 | 1.60 | 1745.0 | 69.45 | 9.78 | 85.51 | 419.13 | 298.00 | 539.97 | 463.75 | 2.62 | 0.74 |
| H21 | 8.33 | 127 | 154.0 | 1.48 | 1426.0 | 40.13 | 2.70 | 13.27 | 440.13 | 221.11 | 593.01 | 70.17 | 1.21 | 0.11 |
| H22 | 8.15 | 120 | 214.2 | 0.64 | 472.0 | 45.17 | 3.54 | 23.37 | 86.97 | 119.75 | 134.00 | 88.48 | 0.60 | 0.14 |
| H23 | 8.57 | 112 | 51.3 | 0.80 | 416.0 | 15.83 | 0.78 | 3.65 | 121.52 | 76.71 | 129.19 | 82.38 | 0.87 | 0.03 |
| H24 | 8.38 | 122 | 77.1 | 0.60 | 404.0 | 19.95 | 1.20 | 5.98 | 107.58 | 87.86 | 126.27 | 57.97 | 0.58 | 0.03 |
| H25 | 8.10 | 119 | 243.2 | 0.56 | 556.0 | 52.18 | 3.64 | 26.55 | 88.93 | 158.31 | 148.89 | 79.33 | 0.44 | 0.03 |
| H26 | 9.91 | 70 | 1976.6 | 2.64 | 5412.0 | 616.14 | 14.25 | 104.19 | 1145.05 | 1452.00 | 1928.48 | 36.61 | 0.95 | 8.75 |
| H27 | 10.77 | 45 | 2110.7 | 3.52 | 6023.0 | 835.47 | 23.50 | 24.20 | 1137.85 | 1463.44 | 2362.39 | 30.51 | 0.49 | 10.08 |
| H28 | 8.35 | 116 | 1786.4 | 1.52 | 4468.0 | 484.06 | 11.12 | 134.82 | 735.33 | 1479.28 | 1321.01 | 33.56 | 1.61 | 6.01 |
| H29 | 8.34 | 102 | 1796.4 | 2.00 | 4726.0 | 243.39 | 22.42 | 274.59 | 892.69 | 1699.28 | 1245.71 | 256.28 | 1.31 | 25.87 |
| H30 | 8.46 | 92 | 1020.8 | 1.52 | 2768.0 | 140.91 | 12.62 | 161.73 | 527.96 | 811.36 | 898.30 | 207.47 | 0.68 | 3.94 |
| H31 | 8.68 | 118 | 144.5 | 0.56 | 384.0 | 32.83 | 2.30 | 12.49 | 82.18 | 117.22 | 54.59 | 128.14 | 0.51 | 3.44 |
| H32 | 8.42 | 121 | 252.2 | 0.48 | 562.0 | 50.70 | 2.53 | 27.61 | 89.60 | 207.24 | 104.22 | 70.17 | 0.80 | 0.18 |
| H33 | 8.13 | 122 | 775.6 | 0.80 | 1964.0 | 141.83 | 5.35 | 97.00 | 341.10 | 693.97 | 431.78 | 128.14 | 0.87 | 47.29 |
| H34 | 8.11 | 114 | 1045.8 | 1.28 | 3516.0 | 174.85 | 9.32 | 143.42 | 792.31 | 1146.64 | 1017.42 | 109.84 | 0.36 | 8.89 |
| H35 | 8.34 | 111 | 174.9 | 0.44 | 296.0 | 42.62 | 3.09 | 15.10 | 37.12 | 54.97 | 54.59 | 112.89 | 0.23 | 4.68 |
| H36 | 8.30 | 113 | 200.6 | 0.52 | 342.0 | 55.11 | 3.21 | 12.26 | 38.70 | 75.13 | 67.99 | 118.99 | 0.23 | 7.07 |
| H37 | 8.35 | 110 | 311.8 | 0.76 | 510.0 | 71.40 | 4.30 | 29.45 | 56.12 | 175.65 | 84.37 | 109.84 | 0.28 | 6.58 |
| H38 | 8.32 | 121 | 182.5 | 0.56 | 402.0 | 41.24 | 4.38 | 18.74 | 68.39 | 81.11 | 99.26 | 125.09 | 0.22 | 2.91 |
| H39 | 8.10 | 123 | 382.2 | 0.52 | 684.0 | 85.66 | 6.08 | 37.69 | 92.34 | 167.46 | 173.71 | 161.70 | 0.36 | 11.60 |
| H40 | 7.89 | 135 | 912.7 | 1.00 | 2262.0 | 214.91 | 3.68 | 87.81 | 427.24 | 544.37 | 883.41 | 57.97 | 1.48 | 1.67 |
| H41 | 9.42 | 101 | 1070.9 | 3.00 | 4326.0 | 236.37 | 12.86 | 110.73 | 1060.88 | 1053.36 | 1702.31 | 45.76 | 1.16 | 2.07 |
| H42 | 10.06 | 80 | 1886.5 | 2.40 | 5724.0 | 562.33 | 14.49 | 100.71 | 1141.39 | 1571.68 | 1965.35 | 5.10 | 1.21 | 9.55 |
| H43 | 8.39 | 101 | 120.1 | 0.80 | 562.0 | 33.45 | 1.99 | 8.04 | 157.34 | 126.10 | 173.71 | 82.38 | 2.73 | 0.47 |
| H44 | 8.11 | 111 | 1115.9 | 3.28 | 5768.0 | 101.38 | 10.76 | 206.19 | 1640.05 | 734.36 | 2844.51 | 170.86 | 5.46 | 1.05 |
| H45 | 8.17 | 99 | 373.3 | 0.56 | 914.0 | 52.05 | 6.25 | 55.73 | 188.93 | 246.75 | 258.08 | 125.09 | 0.58 | 0.15 |
| H46 | 8.20 | 105 | 171.1 | 1.92 | 344.0 | 39.68 | 6.21 | 19.48 | 44.24 | 100.67 | 61.19 | 94.58 | 0.60 | 0.04 |
| H47 | 8.14 | 119 | 304.2 | 1.00 | 772.0 | 48.76 | 8.47 | 41.62 | 163.64 | 173.18 | 173.71 | 216.62 | 2.62 | 1.94 |
References
- Kut, K.; Sarswat, A.; Srivastava, A.; Pittman, C.U.; Mohan, D. A review of fluoride in african groundwater and local reme-diation methods. Groundw. Sustain. Dev. 2016, 2, 190–212. [Google Scholar] [CrossRef]
- Demelash, H.; Beyene, A.; Abebe, Z.; Melese, A. Fluoride concentration in ground water and prevalence of dental fluorosis in Ethiopian Rift Valley: Systematic review and meta-analysis. BMC Public Health 2019, 19, 163. [Google Scholar] [CrossRef]
- Battaleb-Looie, S.; Moore, F.; Jacks, G.; Ketabdari, M.R. Geological sources of fluoride and acceptable intake of fluoride in an endemic fluorosis area, southern Iran. Environ. Geochem. Health 2012, 34, 641–650. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Nanthakumar, K.; Velmurugan, P.; Tamilarasi, S.; Lakshmanaperumalsamy, P. Prevalence of certain inorganic constituents in groundwater samples of Erode district, Tamilnadu, India, with special emphasis on fluoride, fluorosis and its remedial measures. Environ. Monit. Assess. 2008, 160, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Kumar, S.; Pham, Q.B.; Gupta, N.; Rezania, S.; Kamyab, H.; Yadav, S.; Vymazal, J.; Kumar, V.; Tri, D.Q.; et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review. Ecotoxicol. Environ. Saf. 2019, 182, 109362. [Google Scholar] [CrossRef]
- Adimalla, N.; Qian, H.; Nandan, M. Groundwater chemistry integrating the pollution index of groundwater and evaluation of potential human health risk: A case study from hard rock terrain of south India. Ecotoxicol. Environ. Saf. 2020, 206, 111217. [Google Scholar] [CrossRef]
- Brindha, K.; Rajesh, R.; Murugan, R.; Elango, L. Fluoride contamination in groundwater in parts of Nalgonda District, Andhra Pradesh, India. Environ. Monit. Assess. 2010, 172, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, V.; Nwabisa, D.P.; Rajmohan, N. Evaluation of high fluoride contaminated fractured rock aquifer in South Africa—Geochemical and chemometric approaches. Chemosphere 2019, 235, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Rivera, J.; Cardona, A.; Edmunds, W. Use of abstraction regime and knowledge of hydrogeological conditions to control high-fluoride concentration in abstracted groundwater: San Luis Potosí basin, Mexico. J. Hydrol. 2002, 261, 24–47. [Google Scholar] [CrossRef]
- Li, D.; Gao, X.; Wang, Y.; Luo, W. Diverse mechanisms drive fluoride enrichment in groundwater in two neighboring sites in northern China. Environ. Pollut. 2018, 237, 430–441. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Liu, Y.; Wang, Y. Impact of anthropogenic activities on the enrichment of fluoride and salinity in groundwater in the Yuncheng Basin constrained by Cl/Br ratio, δ18O, δ2H, δ13C and δ7Li isotopes. J. Hydrol. 2019, 579, 124211. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, P.; Qian, H.; Yang, F. Hydrogeochemistry and fluoride contamination in Jiaokou Irrigation District, Central China: Assessment based on multivariate statistical approach and human health risk. Sci. Total. Environ. 2020, 741, 140460. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, Y.; Xing, L.; Jia, Y. Spatial variation in arsenic and fluoride concentrations of shallow groundwater from the town of Shahai in the Hetao basin, Inner Mongolia. Appl. Geochem. 2012, 27, 2187–2196. [Google Scholar] [CrossRef]
- Wang, Y.; Shvartsev, S.L.; Su, C. Genesis of arsenic/fluoride-enriched soda water: A case study at Datong, northern China. Appl. Geochem. 2009, 24, 641–649. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, Q.; Zhang, F.; Huang, Y.; Liu, J.; Wang, X.; Yang, Y.; Liu, X. Geochemistry of iodine-rich groundwater in the Taiyuan Basin of central Shanxi Province, North China. J. Geochem. Explor. 2013, 135, 117–123. [Google Scholar] [CrossRef]
- Dai, S.; Li, W.; Tang, Y.; Zhang, Y.; Feng, P. The sources, pathway, and preventive measures for fluorosis in Zhijin County, Guizhou, China. Appl. Geochem. 2007, 22, 1017–1024. [Google Scholar] [CrossRef]
- Su, H.; Kang, W.; Kang, N.; Liu, J.; Li, Z. Hydrogeochemistry and health hazards of fluoride-enriched groundwater in the Tarim Basin, China. Environ. Res. 2021, 200, 111476. [Google Scholar] [CrossRef]
- Wang, G.Q.; Xiao, B.Y.; Huang, Y.Z.; Yao, H.; Hu, Y.; Qian, R.C.; Zhang, C.; Liu, K.T.; Gu, Y.L. Epidemiological studies on endemic fluorosis and arsenism in xinjiang. Chin. J. Prev. Med. 1995, 1, 30–33. [Google Scholar]
- Ma, P.J.; Wang, T.T.; Xia, R.X.; Lin, Q. Review and Prospect of endemic disease control in Xinjiang. Dis. Prev. Control. Bull. 2020, 35, 86–89. [Google Scholar] [CrossRef]
- Meng, L.; Ding, J.; Wang, J.; Ge, X. Spatial distribution of soil salinity in Ugan-Kuqa River delta oasis based on environmental variables. Trans. Chin. Soc. Agric. Eng. (Trans. CSAE) 2019, 36, 175–181. [Google Scholar]
- Liu, C.; Cao, Y.; Yang, H.; Jiao, P.; Gu, Q. Discussion on Paleogene-Neogene Environmental Change of Salt Lakes in Kuqa Foreland Basin and Its Potash-forming Effect. Acta Geosci. Sin. 2013, 34, 12. [Google Scholar] [CrossRef]
- Hu, S.; Song, Y.; Tian, C.; Li, Y.; Li, X.; Chen, X. Suitable scale of Weigan River plain oasis. Sci. China Ser. D Earth Sci. 2007, 50, 56–64. [Google Scholar] [CrossRef]
- Pan, H.Y.; Zhou, C.J.; Bi, J.B.; Liu, Y.D.; Huang, L.W. Hydrochemical characteristics and fluoride enrichment mechanisms of high-fluoride grounderwater in a typical piedmont proluvial fan in aksu area, xinjiang, China. Bull. Geol. Sci. Technol. 2021, 40, 194–203. [Google Scholar] [CrossRef]
- Li, Q.; Jia, R.L.; Zhou, J.L.; Wang, Y.P. Analysis of chemical characteristics of high-fluoride groundwater in Aksu prefecture, Xinjiang. J. Arid. Land Resour. Environ. 2013, 27, 87–92. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.L.; Nai, W.H.; Zeng, Y.Y.; Chen, Y.F. Characteristics of high fluoride groundwater in plain of Yarkant river basin in Xinjiang. J. Arid. Land Resour. Environ. 2020, 34, 100–106. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Y.Y.; Luan, F.J.; Zhou, J.L.; Tan, P.F.; Wang, X.C.; Li, J.Z.; Feng, J.H. The Enrichment Factors of High Fluoride Ground-water in the Kashgar River Basin, Xinjiang. Ground Water 2020, 42, 1–3. [Google Scholar] [CrossRef]
- Li, L.; Zhou, J.L.; Qi, W.Q.; Chen, F.; Zeng, Y.Y.; Chen, Y.F. Distribution and formation process of fluorine in groundwater in oasis area of Hotan river basin. J. Arid. Land Resour. Environ. 2019, 33, 112–118. [Google Scholar] [CrossRef]
- He, J.; An, Y.; Zhang, F. Geochemical characteristics and fluoride distribution in the groundwater of the Zhangye Basin in Northwestern China. J. Geochem. Explor. 2013, 135, 22–30. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y. Geochemical characteristics of shallow groundwater in Datong basin, northwestern China. J. Geochem. Explor. 2005, 87, 109–120. [Google Scholar] [CrossRef]
- Su, C.; Wang, Y.; Xie, X.; Li, J. Aqueous geochemistry of high-fluoride groundwater in Datong Basin, Northern China. J. Geochem. Explor. 2013, 135, 79–92. [Google Scholar] [CrossRef]
- Olaka, L.A.; Wilke, F.D.; Olago, D.O.; Odada, E.O.; Mulch, A.; Musolff, A. Groundwater fluoride enrichment in an active rift setting: Central Kenya Rift case study. Sci. Total. Environ. 2016, 545–546, 641–653. [Google Scholar] [CrossRef]
- Vasanthavigar, M.; Srinivasamoorthy, K.; Prasanna, M.V. Evaluation of groundwater suitability for domestic, irrigational, and industrial purposes: A case study from Thirumanimuttar river basin, Tamilnadu, India. Environ. Monit. Assess. 2011, 184, 405–420. [Google Scholar] [CrossRef]
- Mao, R.Y.; Guo, H.M.; Jia, Y.F.; Jiang, X.Y.; Cao, Y.S.; Zhao, W.G.; Wang, Z. Distribution characteristics and genesis of fluoride grounderwater in the Hetao basin, Inner Mongolia. Earth Sci. Front. 2016, 23, 9. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, L.; Qiao, D.; Feng, D.; Wang, H. Vacuum tribological performance of phosphonium-based ionic liquids as lubricants and lubricant additives of multialkylatedcyclopentanes. Tribol. Int. 2013, 66, 289–295. [Google Scholar] [CrossRef]
- Ye, C.Y.; Zheng, J.P. Study on existence forms of chemical compositions and saturation indexes of waters from Gasikule Salt Lake, Qinghai Province. Sci. Technol. Rev. 2016, 34, 11. [Google Scholar]
- Fu, W. Analyses of chemical components in water for endemic fluorosis region in basin of Aksu river. J. Shaanxi No Rmal Univ. (Nat. Sci. Ed.) 2002, 4, 96–101. [Google Scholar]
- Mountaineering Scientific Expedition Team of the Chinese Academy of Sciences. In Textbook of Geology and Paleontology of the Tomur Peak Area in Tianshan; Xinjiang People’s Publishing House: Urumqi, Xinjiang, China, 1985; pp. 45–50.
- Zeng, J.H. Types of saline soil and some characteristics of salt accumulation in arid areas of China. Chin. J. Soil Sci. 1963, 1, 35–42. [Google Scholar] [CrossRef]
- Jia, C.S. Textbook of Studies om origins and migration direction of petroleum in shaya uplift of tarim basin; China University of mining and Technology (Beijing): Beijing, China, 2012; pp. 41–44. [Google Scholar]
- Yang, S.Y. Late Hydrocarbon Generation in Baichengsag of the Tarim Basin; China University of mining and Technology (Beijing): Beijing, China, 2020. [Google Scholar]
- Wang, J.; Han, H.D.; Xu, J.L.; Li, Y.S. Hydrochemical characteristics of the mountain runoff in Tarim River Basin, China. China Environ. Sci. 2021, 41, 1576–1587. [Google Scholar] [CrossRef]
- Dedzo, M.G.; Tsozué, D.; Mimba, M.E.; Teddy, F.; Nembungwe, R.M.; Linida, S. Importance of Rocks and Their Weathering Products on Groundwater Quality in Central-East Cameroon. Hydrology 2017, 4, 23. [Google Scholar] [CrossRef]
- Bisht, H.; Arya, P.C.; Kumar, K. Hydro-chemical analysis and ionic flux of meltwater runoff from Khangri Glacier, West Kameng, Arunachal Himalaya, India. Environ. Earth Sci. 2018, 77, 598. [Google Scholar] [CrossRef]



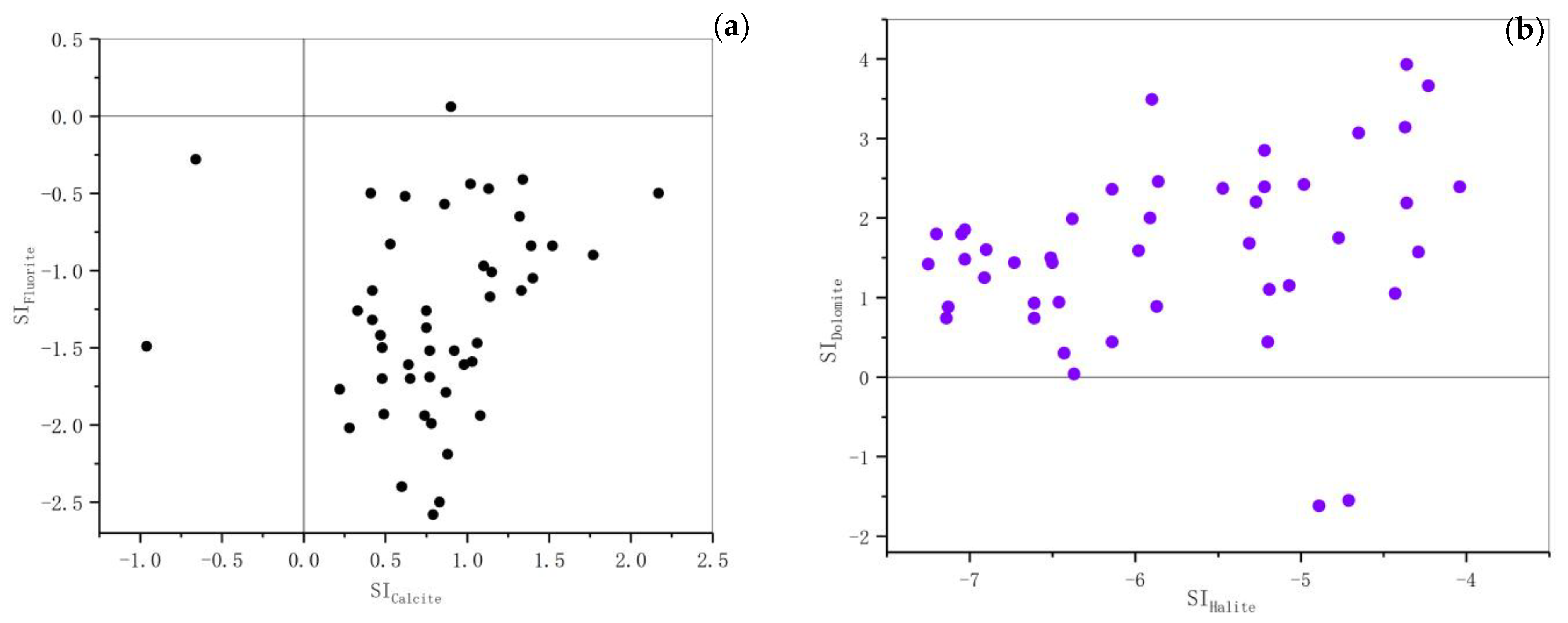
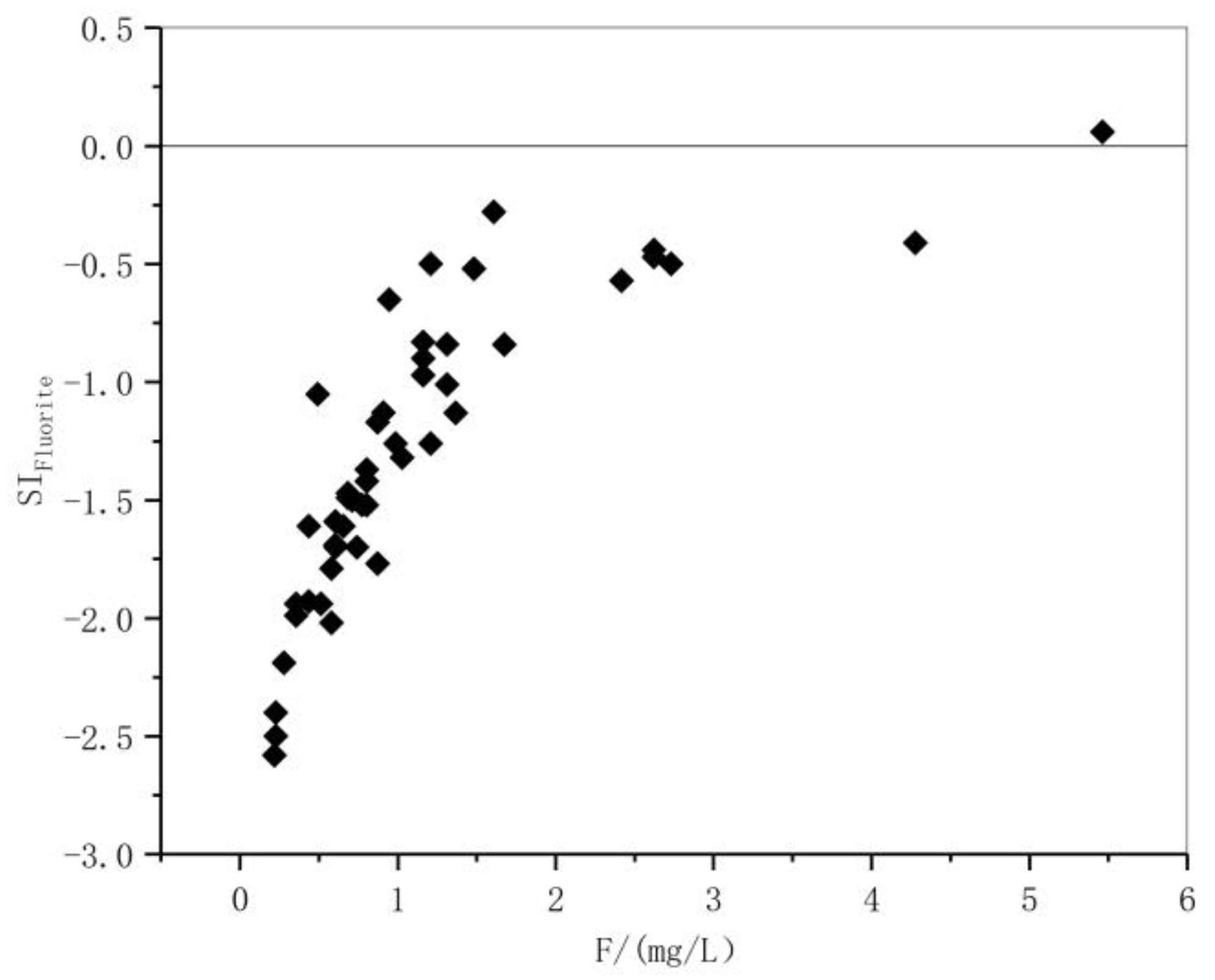



| ρ[F−] < 1.0 mg/L | ρ[F−] ≥ 1.0 mg/L | All Sets of Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Ave | Min | Max | Ave | Min | Max | Ave | SE | |
| pH | 7.88 | 10.8 | 8.38 | 7.67 | 10.1 | 8.35 | 7.67 | 10.8 | 8.37 | 0.08 |
| Eh | 45 | 142 | 113 | 80 | 135 | 113 | 45 | 142 | 112 | 2.46 |
| TH | 51.3 | 3860 | 600 | 120 | 4974 | 1062 | 51.3 | 4974 | 786 | 0.52 |
| TDS | 296 | 18,567 | 1924 | 460 | 19,653 | 2827 | 296 | 19,653 | 2647 | 583 |
| Na+ + K+ | 72.8 | 1159 | 435 | 212 | 1650 | 862 | 72.8 | 1651 | 607 | 149 |
| Ca2+ | 15.83 | 835 | 134 | 29.8 | 562 | 171 | 15.8 | 835 | 149 | 27.1 |
| Mg2+ | 3.65 | 575 | 63.8 | 8.04 | 944 | 150 | 3.65 | 944 | 98.4 | 23.4 |
| F− | 0.22 | 0.95 | 0.6 | 1 | 5.46 | 1.94 | 0.22 | 5.46 | 1.14 | 0.15 |
| Cl− | 54.6 | 8822 | 712 | 94.3 | 8509 | 1354 | 54.6 | 8823 | 971 | 261 |
| HCO3− | 30.5 | 207.5 | 104 | 5.1 | 464 | 186 | 5.1 | 464 | 137 | 14.7 |
| CO32− | 0 | 12 | 6.22 | 0 | 96.0 | 21.2 | 0 | 96.03 | 12.3 | 2.51 |
| SO42− | 54.8 | 2321 | 422 | 126 | 3630 | 860 | 54.8 | 3630 | 599 | 110 |
| EOF1 | EOF2 | EOF3 | EOF4 | |
|---|---|---|---|---|
| pH | −0.05 | 0.95 | −0.04 | −0.07 |
| EH | 0.07 | −0.87 | 0.04 | −0.13 |
| CODMn | 0.92 | 0.2 | 0.09 | 0.15 |
| TDS | 0.98 | 0.08 | 0.11 | 0.09 |
| Ca2+ | 0.64 | 0.69 | 0.21 | −0.05 |
| K+ | 0.62 | 0.11 | 0.66 | 0.2 |
| Mg2+ | 0.93 | −0.18 | 0.11 | 0.14 |
| Na+ | 0.98 | 0.02 | 0.05 | 0.1 |
| SO42− | 0.87 | 0.22 | 0.35 | 0.11 |
| Cl− | 0.99 | 0.04 | −0.02 | 0.06 |
| CO32− | 0.05 | 0.33 | 0.38 | 0.63 |
| HCO3− | −0.06 | −0.36 | 0.4 | 0.72 |
| F− | 0.21 | −0.1 | −0.24 | 0.82 |
| NO3− | 0.08 | 0 | 0.96 | 0.1 |
| NO2− | 0.31 | 0.81 | 0.36 | 0.02 |
| Se | 0.16 | 0.13 | 0.93 | 0.06 |
| As | −0.17 | −0.14 | −0.08 | −0.3 |
| Variance contribution rate % | 37.46 | 18.91 | 17.17 | 10.72 |
| Accumulative contribution rate % | 37.46 | 56.38 | 73.55 | 84.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Shao, F.; Zhang, Z.; Li, T. Fluorine-Rich Shallow Groundwater in Weigan River Basin (Xinjiang): Enrichment Factors and Spatial Distribution. Water 2023, 15, 926. https://doi.org/10.3390/w15050926
Liu T, Shao F, Zhang Z, Li T. Fluorine-Rich Shallow Groundwater in Weigan River Basin (Xinjiang): Enrichment Factors and Spatial Distribution. Water. 2023; 15(5):926. https://doi.org/10.3390/w15050926
Chicago/Turabian StyleLiu, Tianchao, Fengjun Shao, Zizhao Zhang, and Tong Li. 2023. "Fluorine-Rich Shallow Groundwater in Weigan River Basin (Xinjiang): Enrichment Factors and Spatial Distribution" Water 15, no. 5: 926. https://doi.org/10.3390/w15050926
APA StyleLiu, T., Shao, F., Zhang, Z., & Li, T. (2023). Fluorine-Rich Shallow Groundwater in Weigan River Basin (Xinjiang): Enrichment Factors and Spatial Distribution. Water, 15(5), 926. https://doi.org/10.3390/w15050926






