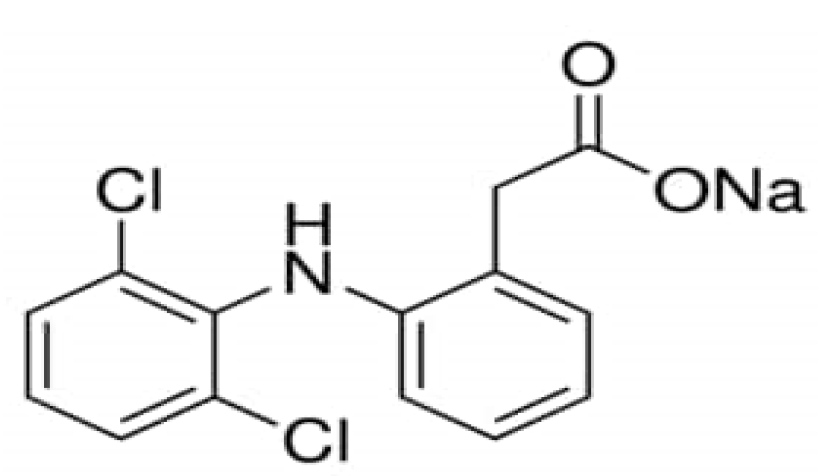
The Catalytic Degradation of the Inflammatory Drug Diclofenac Sodium in Water by Fe2+/Persulfate, Fe2+/Peroxymonosulfate and Fe2+/H2O2 Processes: A Comparative Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Degradation Experiment
2.3. Analytical Methods
3. Results and Discussion
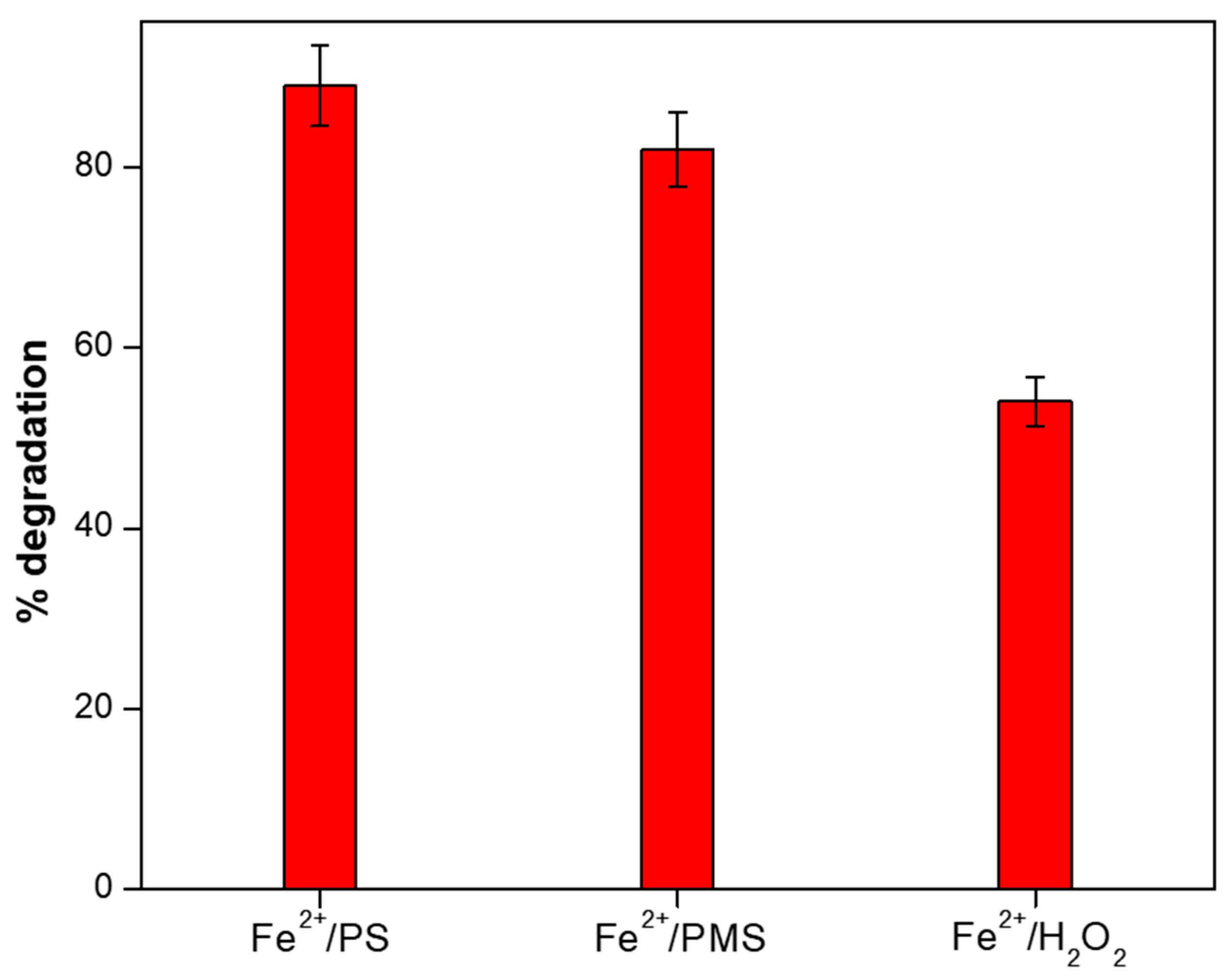
3.1. Degradation of DCF Sodium by Fe2+/PS, Fe2+/PMS and Fe2+/H2O2 Systems
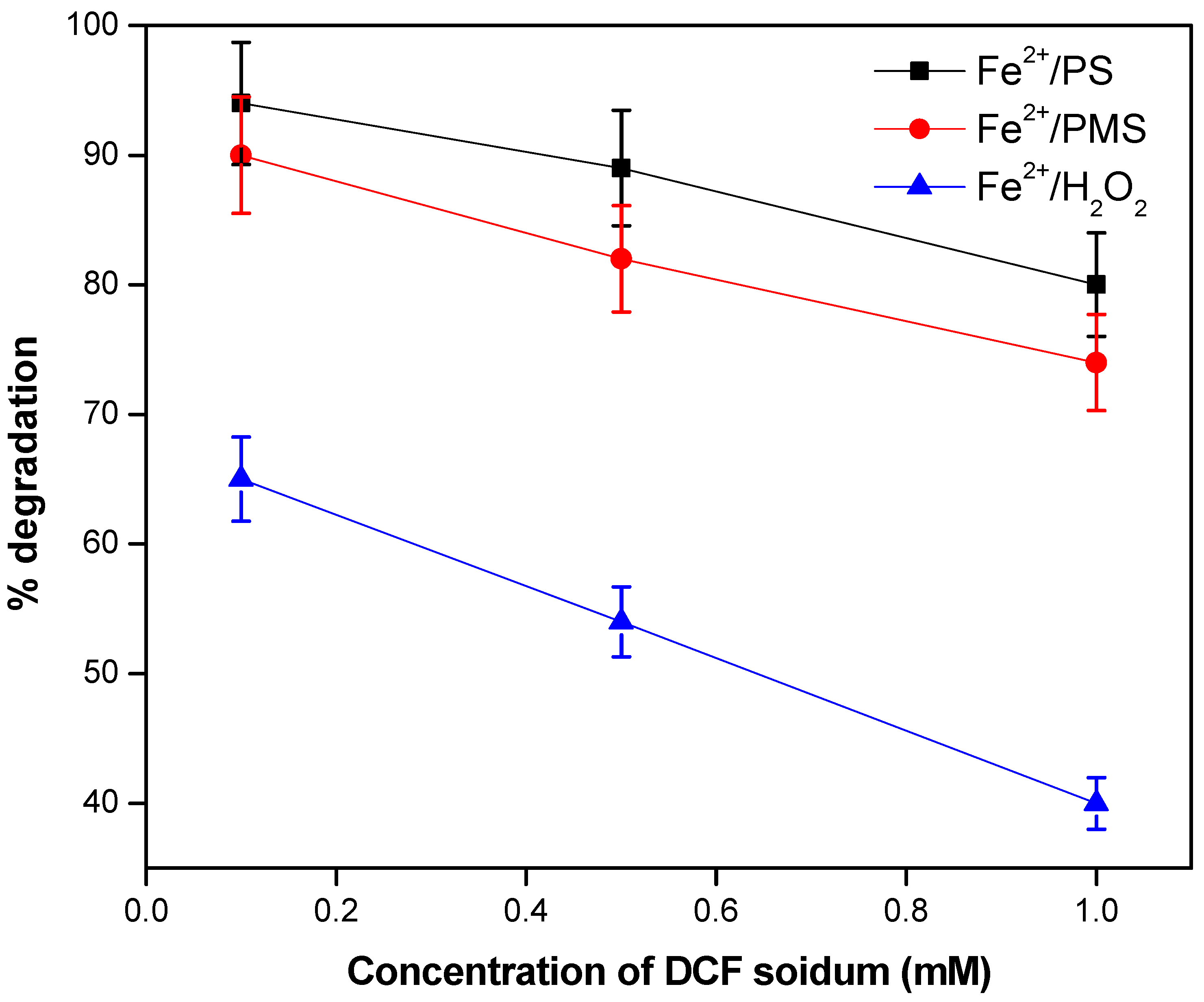
3.2. The effect of the Initial Concentration of DCF Sodium
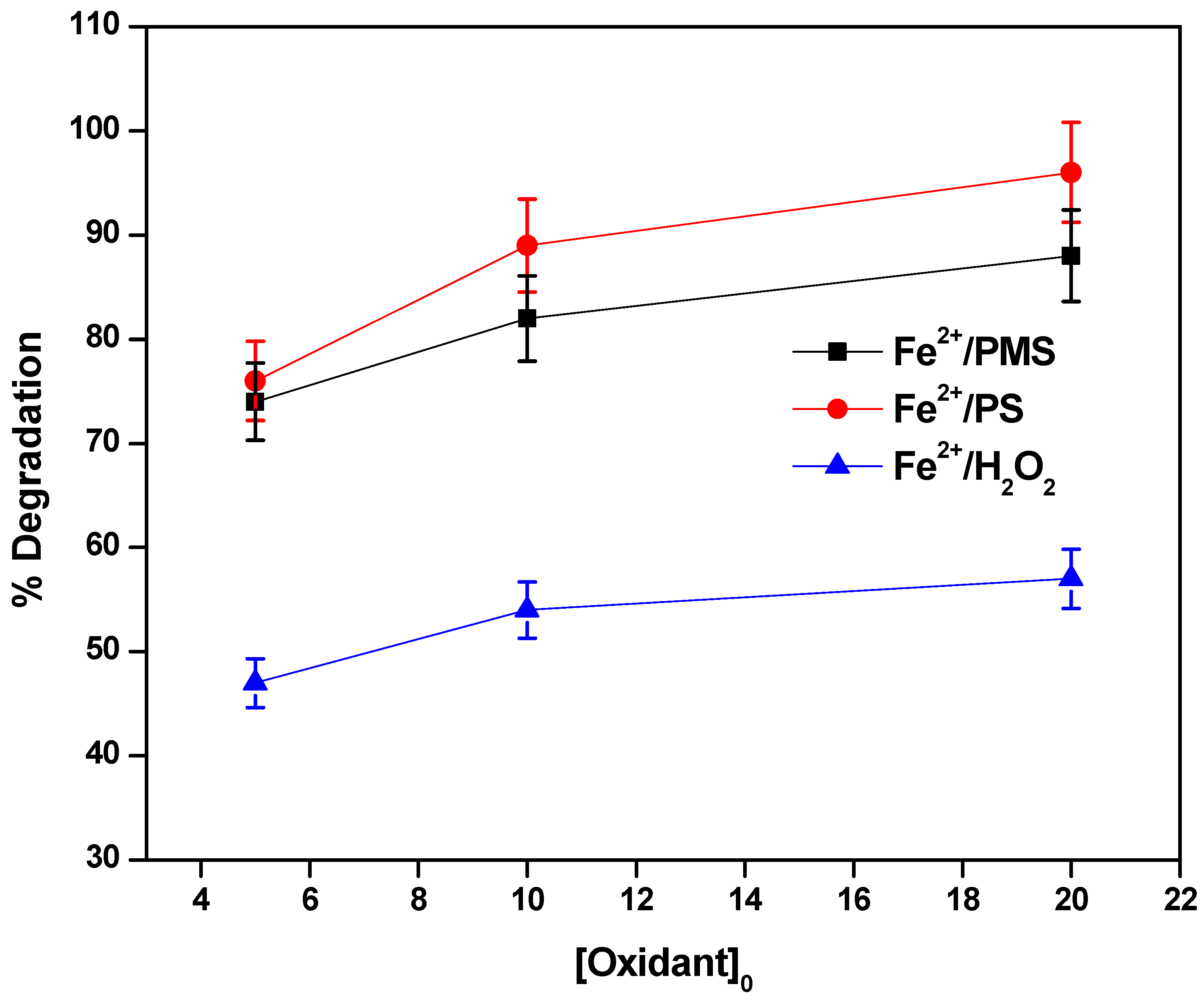
3.3. The Effect of Concentrations of H2O2, PS and PMS
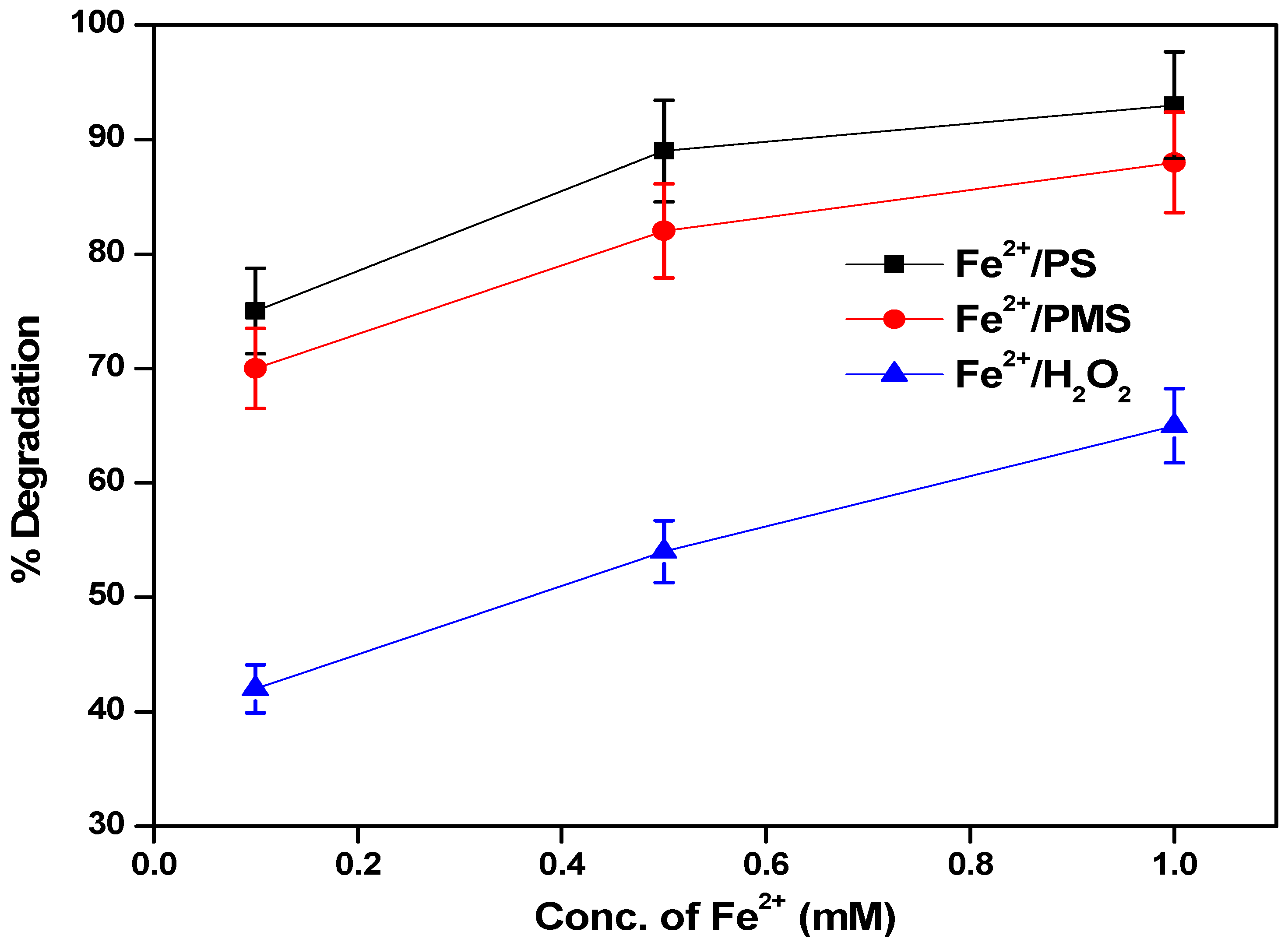
3.4. The Effect of Concentrations of Fe2+
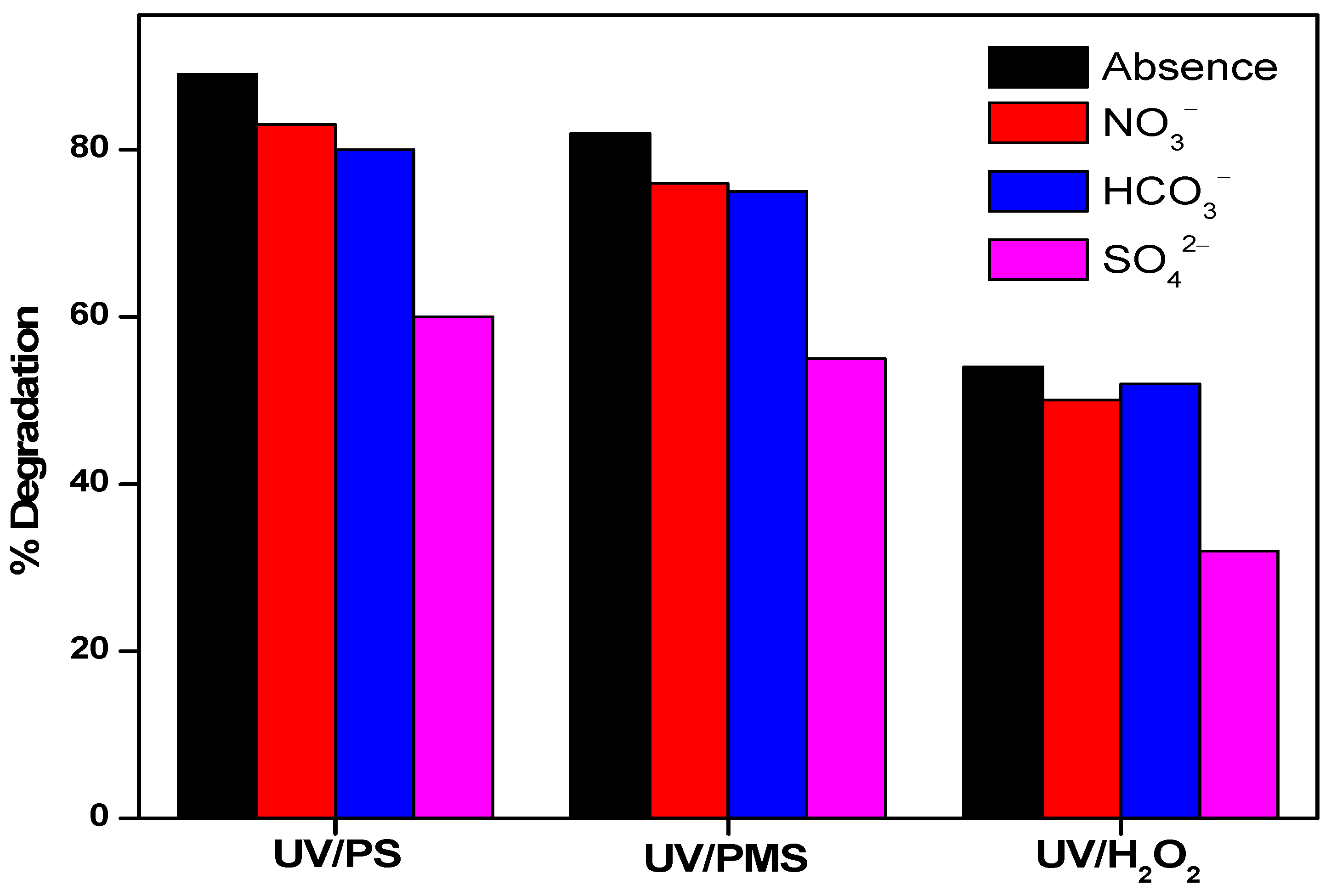
3.5. The Effect of Inorganic Anions
3.6. FTIR Studies of DCF SODIUM Degradation
3.7. Mineralization Studies
3.8. Implications and Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vatovec, C.; Kolodinsky, J.; Callas, P.; Hart, C.; Gallagher, K. Pharmaceutical pollution sources and solutions: Survey of human and veterinary. J. Environ. Manag. 2021, 285, 112106. [Google Scholar] [CrossRef]
- Vatovec, C.; Kolodinsky, J.; Callas, P.; Hart, C.; Gallagher, K. Medication purchasing, use, and disposal. J. Environ. Manag. 2021, 285, 112106. [Google Scholar] [CrossRef]
- Ptneedi, C.B.; Prasadu, K.D. Impact of pharmaceutical waste on human life and environment. Rasayan J. Chem. 2015, 8, 67–70. [Google Scholar]
- Khan, H.; Rehman, M.; Malik, R. Fate and toxicity of pharmaceuticals in water environment: An insight on their occurrence in South Asia. J. Environ. Manag. 2020, 271, 111030. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Rodrigues, J.; Coelho, M.; Martins, A.; Cardoso, E.; Cardoso, V.; Benoliel, M.; Almeida, C. Occurrence of pharmaceutical active compounds in sewage sludge from two urban wastewater treatment plants and their potential behaviour in agricultural soils. Environ. Sci. Water Res. Technol. 2021, 7, 969–982. [Google Scholar]
- Khan, J.A.; Sayed, M.; Khan, S.; Shah, N.S.; Dionysiou, D.D.; Boczkaj, G. Advanced oxidation processes for the treatment of contaminants of emerging concern. In Contaminants of Emerging Concern in Water and Wastewater; Elsevier: Amsterdam, The Netherlands, 2020; pp. 299–365. [Google Scholar]
- Khan, A.; Aziz, H.; Khan, N.; Hasan, M.; Ahmed, S.; Farooqi, I.; Dhingra, A.; Vambol, V.; Changani, F.; Yousefi, M. Impact, disease outbreak and the eco-hazards associated with pharmaceutical residues: A critical review. Int. J. Environ. Sci. Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Li, H.; Helm, P.A.; Metcalfe, C.D. Sampling in the great lakes for pharmaceuticals, personal care products, and endocrine-disrupting substances using passive polar organic chemical integrative sampler. Environ. Toxicol. Chem. 2010, 29, 751–762. [Google Scholar] [CrossRef]
- Srain, H.; Beazley, K.; Walker, T. Pharmaceuticals and personal care products and their sublethal and lethal effects in aquatic organisms. Environ. Rev. 2021, 29, 142–181. [Google Scholar] [CrossRef]
- JShaheen, F.; Sizirici, B.; Yildiz, I. Fate, transport, and risk assessment of widely prescribed pharmaceuticals in terrestrial and aquatic systems: A review. Emerg. Contam. 2022, 8, 216–228. [Google Scholar] [CrossRef]
- Nguyen, P.Y.; Carvalho, G.; Reis, M.A.; Oehmen, A. A review of the biotransformations of priority pharmaceuticals in biological wastewater treatment processes. Water Res. 2021, 188, 116446. [Google Scholar] [CrossRef]
- Shalauddin, M.; Akhter, S.; Basirun, W.J.; Bagheri, S.; Anuar, N.S.; Johan, M.R. Hybrid nanocellulose/f-MWCNTs nanocomposite for the electrochemical sensing of diclofenac sodium in pharmaceutical drugs and biological fluids. Electrochim. Acta 2019, 304, 323–333. [Google Scholar] [CrossRef]
- Galloni, M.; Cerrato, G.; Giordana, A.; Falletta, E.; Bianchi, C. Sustainable Solar Light Photodegradation of Diclofenac by Nano-and Micro-Sized SrTiO3. Catalysts 2022, 12, 804. [Google Scholar] [CrossRef]
- Bahadori, Y.; Razmi, H. Design of an electrochemical platform for the determination of diclofenac sodium utilizing a graphenized pencil graphite electrode modified with a Cu–Al layered double hydroxide/chicken feet yellow membrane. New J. Chem. 2021, 45, 14616–14625. [Google Scholar] [CrossRef]
- Khalaf, S.; Shoqeir, J.H.; Lelario, F.; Scrano, L.; Karaman, R.; Bufo, S.A. Removal of diclofenac sodium from aqueous environment using heterogeneous photocatalysis treatment. Imp. J. Interdiscip. Res. 2017, 3, 766–772. [Google Scholar]
- Rehman, F.; Ahmad, W.; Sayed, M. Mechanistic investigations on the removal of diclofenac sodium by UV/S2O82−/Fe2+, UV/HSO5−/Fe2+ and UV/H2O2/Fe2+-based advanced oxidation processes. Environ. Technol. 2021, 42, 3995–4005. [Google Scholar] [CrossRef]
- Ziylan, A.; Dogan, S.; Agopcan, S.; Kidak, R.; Aviyente, V.; Ince, N. Sonochemical degradation of diclofenac: Byproduct assessment, reaction mechanisms and environmental considerations. Environ. Sci. Pollut. Res. 2014, 21, 5929–5939. [Google Scholar] [CrossRef]
- Khayyat, M.H.; Lai, E.P.C.; Kollu, K.; Ormeci, B. Degradation of diclofenac in molecularly imprinted polymer submicron particles by UV light irradiation and HCl acid treatment. Water Resour. Prot. 2011, 3, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Fedrizzi, D.; Kosfeld, V.; Schlechtriem, C.; Ganz, V.; Derrer, S.; Rentsch, D.; Hollender, J. Biotransformation changes bioaccumulation and toxicity of diclofenac in aquatic organisms. Environ. Sci. Technology. 2020, 54, 4400–4408. [Google Scholar] [CrossRef]
- Gupta, K.R.; Keche, K.; Ganorkar, A.V. Stability indicating RP-HPLC method for determination of eperisone hydrochloride and diclofenac sodium in tablets dosage form. Int. J. Pharm. Chem. Anal. 2016, 3, 205–218. [Google Scholar]
- Naddeo, V.; Ricco, D.; Scannapieco, D.; Belgiorno, V. Degradation of antibiotics in wastewater during sonolysis, ozonattion, and their simultaneous application: Operating conditions effects and processes evaluation. Int. J. Photoenergy 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity-a review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Bendová, H.; Kamenická, B.; Weidlich, T.; Beneš, L.; Vlček, M.; Lacina, P.; Švec, P. Application of Raney Al-Ni alloy for simple hydrodehalogenation of Diclofenac and other halogenated biocidal contaminants in alkaline aqueous solution under ambient conditions. Materials 2022, 15, 3939. [Google Scholar] [CrossRef]
- Park, Y.; Kim, S.; Kim, J.; Khan, S.; Han, C. UV/TiO2 Photocatalysis as an Efficient Livestock Wastewater Quaternary Treatment for Antibiotics Removal. Water 2022, 14, 958. [Google Scholar] [CrossRef]
- Sultana, S.; Hayat, A.; Sayed, M.; Rehan, I.; Rehan, K.; Tabassum, S.; Amin, N.U.; Khan, S.; Khan, A.; Shah, L.A.; et al. Photo-Fenton oxidation of dichlorophene in aqueous solution: Kinetics investigation and effects of operational parameters. Desalination Water Treat. 2021, 222, 295–301. [Google Scholar] [CrossRef]
- Khan, S.; Sayed, M.; Sohail, M.; Shah, L.A.; Raja, M.A. Advanced oxidation and reduction processes. Adv. Water Purif. Tech. 2019, 47, 135–164. [Google Scholar]
- Ara, A.; Khattak, R.; Khan, M.S.; Begum, B.; Khan, S.; Han, C. Synthesis, Characterization, and Solar Photo-Activation of Chitosan-Modified Nickel Magnetite Bio-Composite for Degradation of Recalcitrant Organic Pollutants in Water. Catalysts 2022, 12, 983. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Sayed, M.; Tabassum, S.; Shah, N.S.; Khan, J.A.; Shah, L.A.; Rehman, F.; Khan, S.U.; Khan, H.M.; Ullah, M. Acid fuchsin dosimeter: A potential dosimeter for food irradiation dosimetry. J. Food Meas. Charact. 2019, 13, 707–715. [Google Scholar] [CrossRef]
- Covinich, L.G.; Benboechea, D.I.; Fenoglio, R.J.; Area, M.C. Advanced oxidation processes for wastewater treatment in the pulp and paper industry. Am. J. Environ. Eng. 2014, 4, 56–70. [Google Scholar] [CrossRef] [Green Version]
- M’Arimi, M.; Mecha, C.; Kiprop, A.; Ramkat, R. Recent trends in applications of advanced oxidation processes (AOPs) in bioenergy production. Renew. Sustain. Energy Rev. 2020, 121, 109669. [Google Scholar] [CrossRef]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.; Mantzavinos, D.; Fatta-Kassinos, D. Factors affecting diclofenac decomposition in water by UV-A/TiO2 photocatalysis. Chem. Eng. J. 2010, 161, 53–59. [Google Scholar] [CrossRef]
- Hashim, N.; Natarajan, P.; Ray, A. Intrinsic kinetic study for photocatalytic degradation of diclofenac under UV and visible light. Ind. Eng. Chem. Res. 2014, 53, 18637–18646. [Google Scholar] [CrossRef]
- Hartmann, J.; Bartels, P.; Mau, U.; Witter, M.; Tümpling, W.; Hofmann, J.; Nietzschmann, E. Degradation of the drug diclofenac in water by sonolysis in presence of catalysts. Chemosphere 2008, 70, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Meroni, D.; Jiménez-Salcedo, M.; Falletta, E.; Bresolin, B.; Kait, C.; Boffito, D.; Bianchi, C.; Pirola, C. Sonophotocatalytic degradation of sodium diclofenac using low power ultrasound and micro sized TiO2. Ultrason. Sonochemistry 2020, 67, 105123. [Google Scholar] [CrossRef]
- Dhawle, R.; Mantzavinos, D.; Lianos, P. UV/H2O2 degradation of diclofenac in a photocatalytic fuel cell. Appl. Catal. B: Environ. 2021, 299, 120706. [Google Scholar] [CrossRef]
- Jabbari, F.; Eslami, A.; Mahmoudian, J. Degradation of diclofenac in water using the O3/UV/S2O8 advanced oxidation process. Health Scope 2020, 9, e99436. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, J. Degradation of diclofenac in aqueous solution by ionizing radiation in the presence of humic acid. Sep. Purif. Technol. 2020, 234, 116079. [Google Scholar] [CrossRef]
- Skvortsova, L.; Bolgaru, K.; Sherstoboeva, M.; Dychko, K. Degradation of diclofenac in aqueous solutions under conditions of combined homogeneous and heterogeneous photocatalysis. Russ. J. Phys. Chem. A 2020, 94, 1248–1253. [Google Scholar] [CrossRef]
- Calza, P.; Sakkas, V.; Medana, C.; Baiocchi, C.; Dimou, A.; Pelizzetti, E.; Albanis, T. Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl. Catal. B: Environ. 2006, 67, 197–205. [Google Scholar] [CrossRef]
- Steven, T.; Nawaz, R.; Sahrin, N.; Lee, K.; Bianchi, C.; Kait, C. H2O2-assisted sonophotocatalytic degradation of diclofenac using a visible light-active flower-like micron-sized TiO2 photocatalyst. Malays. J. Chem. 2021, 23, 108–125. [Google Scholar]
- Qiang, C.; Li, N.; Zuo, S.; Guo, Z.; Zhan, W.; Li, Z.; Ma, J. Microwave-assisted synthesis of RuTe2/black TiO2 photocatalyst for enhanced diclofenac degradation: Performance, mechanistic investigation and intermediates analysis. Sep. Purif. Technol. 2022, 283, 120214. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Deng, H. Ag modified g-C3N4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J. Mol. Catal. A: Chem. 2016, 423, 270–276. [Google Scholar] [CrossRef]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Photocatalytic activity of ZnO-WO3 for diclofenac degradation under visible light irradiation. J. Photochem. Photobiol. A: Chem. 2019, 383, 111993. [Google Scholar]
- Meroni, D.; Bianchi, C.; Boffito, D.; Cerrato, G.; Bruni, A.; Sartirana, M. Falletta. Piezo-enhanced photocatalytic diclofenac mineralization over ZnO. Ultrason. Sonochemistry 2021, 75, 105615. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.; Iervolino, G.; Imparato, C.; Rea, I.; Borbone, F.; De Stefano, L.; Aronne, A.; Vaiano, V. F-doped ZnO nano-and meso-crystals with enhanced photocatalytic activity in diclofenac degradation. Sci. Total Environ. 2021, 762, 143066. [Google Scholar] [CrossRef] [PubMed]
- Fuku, K.; Kanai, H.; Todoroki, M.; Mishima, N.; Akagi, T.; Kamegawa, T.; Ikenaga, N. Heterogeneous Fenton Degradation of Organic Pollutants in Water Enhanced by Combining Iron-type Layered Double Hydroxide and Sulfate. Chem. Asian J. 2021, 16, 1887–1892. [Google Scholar] [CrossRef]
- Quang, H.; Dinh, N.; Thi, T.; Bao, L.; Yuvakkumar, R.; Nguyen, V.-H. Fe2+, Fe3+, Co2+ as highly efficient cocatalysts in the homogeneous electro-Fenton process for enhanced treatment of real pharmaceutical wastewater. J. Water Process Eng. 2022, 46, 102635. [Google Scholar] [CrossRef]
- Wang, D.; Suo, M.; Lai, S.; Deng, L.; Liu, J.; Yang, J.; Chen, S.; Wu, M.-F.; Zou, J.-P. Photoinduced acceleration of Fe3+/Fe2+ cycle in heterogeneous FeNi-MOFs to boost peroxodisulfate activation for organic pollutant degradation. Appl. Catal. B: Environ. 2023, 321, 122054. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Giannakis, S.; Marjanovic, M.; Kohantorabi, M.; Gholami, M.; Grandjean, D.; de Alencastro, L.F.; Pulgarín, C. Solar-assisted bacterial disinfection and removal of contaminants of emerging concern by Fe2+-activated HSO5-vs. S2O82-in drinking water. Appl. Catal. B: Environ. 2019, 248, 62–72. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S. Photo-Fenton applied to the removal of pharmaceutical and other pollutants of emerging concern. Curr. Opin. Green Sustain. Chem. 2021, 29, 100458. [Google Scholar] [CrossRef]
- Ganzenko, O.; Trellu, C.; Oturan, N.; Huguenot, D.; Pechaud, Y.; van Hullebusch, E.; Oturan, M. Electro-Fenton treatment of a complex pharmaceutical mixture: Mineralization efficiency and biodegradability enhancement. Chemosphere 2020, 253, 126659. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Singh, A. Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh district, Uttar Pradesh. J Geol Soc India 2014, 83, 329–343. [Google Scholar] [CrossRef]
- Singh, K.; Tewari, G.; Kumar, S. Evaluation of groundwater quality for suitability of irrigation purposes: A case study in the Udham Singh Nagar, Uttarakhand. J. Chem. 2020, 2020, 1–15. [Google Scholar] [CrossRef]
- Song, Q.; Feng, Y.; Liu, G.; Lv, W. Degradation of the flame retardant triphenyl phosphate by ferrous ion-activated hydrogen peroxide and persulfate: Kinetics, pathways, and mechanisms. Chem. Eng. J. 2019, 361, 929–936. [Google Scholar] [CrossRef]
- Khan, S.; He, X.; Khan, H.M.; Boccelli, D.; Dionysiou, D.D. Efficient degradation of lindane in aqueous solution by iron (II) and/or UV activated peroxymonosulfate. J. Photochem. Photobiol. A: Chem. 2016, 316, 37–43. [Google Scholar] [CrossRef]
- Pourzamani, H.; Mengelizadeh, N.; Hajizadeh, Y.; Mohammadi, H. Electrochemical degradation of diclofenac using three-dimensional electrode reactor with multi-walled carbon nanotubes. Environ. Sci. Pollut. Res. 2018, 25, 24746–24763. [Google Scholar] [CrossRef]
- Tian, Y.; Jia, N.; Zhou, L.; Lei, J.; Wang, L.; Zhang, J.; Liu, Y. Photo-Fenton-like degradation of antibiotics by inverse opal WO3 co-catalytic Fe2+/PMS, Fe2+/H2O2 and Fe2+/PDS processes: A comparative study. Chemosphere 2022, 88, 132627. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Comparative study on sulfamethoxazole degradation by Fenton and Fe (II)-activated persulfate process. Rsc Advances. 2017, 7, 48670–48677. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, J. Trimethoprim degradation by Fenton and Fe (II)-activated persulfate processes. Chemosphere 2018, 191, 97–105. [Google Scholar] [CrossRef]
- Khan, S.; Sohail, M.; Han, C.; Khan, J.A.; Khan, H.M.; Dionysiou, D.D. Degradation of highly chlorinated pesticide, lindane, in water using UV/persulfate: Kinetics and mechanism, toxicity evaluation, and synergism by H2O2. J. Hazard. Mater. 2021, 402, 123558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, L.; Yang, Y.; Sun, P. Degradation of Norfloxacin in an Aqueous Solution by the Nanoscale Zero-Valent Iron-Activated Persulfate Process. J. Nanomater. 2020, 2020, 3286383. [Google Scholar] [CrossRef]
- Rivas, J.; Olga, G.; Maria, C.; Borralho, T.; Fernando, B. Influence of oxygen and free radicals promoters on UV–254 nm photolysis of diclofenac. Chem. Eng. J. 2010, 163, 35–40. [Google Scholar] [CrossRef]
- Devi, L.G.; Raju, K.S.A.; Kumar, S.G.; Rajashekhar, K.E. Photo-degradation of di azo dye Bismarck Brown by advanced photo-Fenton process: Influence of inorganic anions and evaluation of recycling efficiency of iron powder. J. Taiwan Inst. Chem. Eng. 2011, 42, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; He, X.; Khan, J.A.; Khan, H.M.; Boccelli, D.L.; Dionysiou, D.D. Kinetics and mechanism of sulfate radical-and hydroxyl radical-induced degradation of highly chlorinated pesticide lindane in UV/peroxymonosulfate system. Chem. Eng. J. 2017, 318, 135–142. [Google Scholar] [CrossRef]

| Sr. No | Transmittance at Wavenumber (cm−1) | Group Species | Vibration Mode | Compound Class |
|---|---|---|---|---|
| 1 | 724, 696, 673 | C=C | Bending | Alkene |
| 2 | 843, 787, 762 | C-Cl | Stretching | Halo compound |
| 3 | 1662, 1643, 1626 | C=C | Stretching | Alkene |
| 4 | 2112, 2034, 2000 | C=C=N | Stretching | Ketenimine |
| 5 | 2260, 2196, 2105 | C≡C | Stretching | Alkyne |
| 6 | 2275, 2269, 2263, 2250 | O=C=O | Stretching | Carbon dioxide |
| 7 | 3200, 2890, 2850, 2700 | O-H | Stretching | Alcohol |
| Sr. No | Transmittance at Wavenumber (cm−1) | Group Species | Vibration Mode | Compound Class |
|---|---|---|---|---|
| 1 | 724, 696, 673 | C=C | Bending | Alkene |
| 2 | 843, 787, 762 | C-Cl | Stretching | Halo compound |
| 3 | 1662, 1643, 1626 | C=C | Stretching | Alkene |
| 4 | 2112, 2034, 2000 | C=C=N | Stretching | Ketenimine |
| 5 | 2260, 2196, 2105 | C≡C | Stretching | Alkyne |
| 6 | 2230, 2243, 2256 | C≡N | Stretching | Nitrile |
| 7 | 2275, 2269, 2263, 2250 | O=C=O | Stretching | Carbon dioxide |
| 8 | 3200, 2890, 2850, 2700 | O-H | Stretching | Alcohol |
| 9 | 2987, 2913, 2845 | N-H | Stretching | Amine |
| Sr. No | Transmittance at Wavenumber (cm−1) | Group Species | Vibration Mode | Compound Class |
|---|---|---|---|---|
| 1 | 730, 710, 688, 665 | C=C | Bending | Alkene |
| 2 | 810, 774, 734 | C-Cl | Stretching | Halo compound |
| 3 | 1662, 1643, 1626 | C=C | Stretching | Alkene |
| 4 | 2112, 2034, 2000 | C=C=N | Stretching | Ketenimine |
| 5 | 2275, 2269, 2263, 2250 | C=C=O | Stretching | Isothiocyanate |
| 6 | 3200, 2890, 2850, 2700 | O-H | Stretching | Alcohol |
| 7 | 3015, 3085, 2923 | N-H | Stretching | Amine |
| Reaction Time (min) | TC (mg/L) Removal from DCF Sodium | ||
|---|---|---|---|
| Fe2+/PS | Fe2+/PMS | Fe2+/H2O | |
| 0 | 0.85 | 0.85 | 0.85 |
| 10 | 0.73 | 0.78 | 0.78 |
| 20 | 0.64 | 0.72 | 0.71 |
| 30 | 0.52 | 0.67 | 0.66 |
| 40 | 0.43 | 0.62 | 0.63 |
| 50 | 0.37 | 0.54 | 0.59 |
| 60 | 0.33 | 0.47 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, F.; Ahmad, W.; Parveen, N.; Zakir, S.K.; Khan, S.; Han, C. The Catalytic Degradation of the Inflammatory Drug Diclofenac Sodium in Water by Fe2+/Persulfate, Fe2+/Peroxymonosulfate and Fe2+/H2O2 Processes: A Comparative Analysis. Water 2023, 15, 885. https://doi.org/10.3390/w15050885
Rehman F, Ahmad W, Parveen N, Zakir SK, Khan S, Han C. The Catalytic Degradation of the Inflammatory Drug Diclofenac Sodium in Water by Fe2+/Persulfate, Fe2+/Peroxymonosulfate and Fe2+/H2O2 Processes: A Comparative Analysis. Water. 2023; 15(5):885. https://doi.org/10.3390/w15050885
Chicago/Turabian StyleRehman, Faiza, Waqas Ahmad, Nazish Parveen, Syed Khuram Zakir, Sanaullah Khan, and Changseok Han. 2023. "The Catalytic Degradation of the Inflammatory Drug Diclofenac Sodium in Water by Fe2+/Persulfate, Fe2+/Peroxymonosulfate and Fe2+/H2O2 Processes: A Comparative Analysis" Water 15, no. 5: 885. https://doi.org/10.3390/w15050885
APA StyleRehman, F., Ahmad, W., Parveen, N., Zakir, S. K., Khan, S., & Han, C. (2023). The Catalytic Degradation of the Inflammatory Drug Diclofenac Sodium in Water by Fe2+/Persulfate, Fe2+/Peroxymonosulfate and Fe2+/H2O2 Processes: A Comparative Analysis. Water, 15(5), 885. https://doi.org/10.3390/w15050885








