Allometric Growth Patterns and Ontogenetic Development during Early Larval Stages of Schizothorax waltoni Regan and Percocypris retrodorslis in Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source and Culture Conditions of the Larvae
2.2. Feeding Management
2.3. Morphological and Morphometric Features of Larval Developmental Stages
2.4. Allometric Growth
3. Results
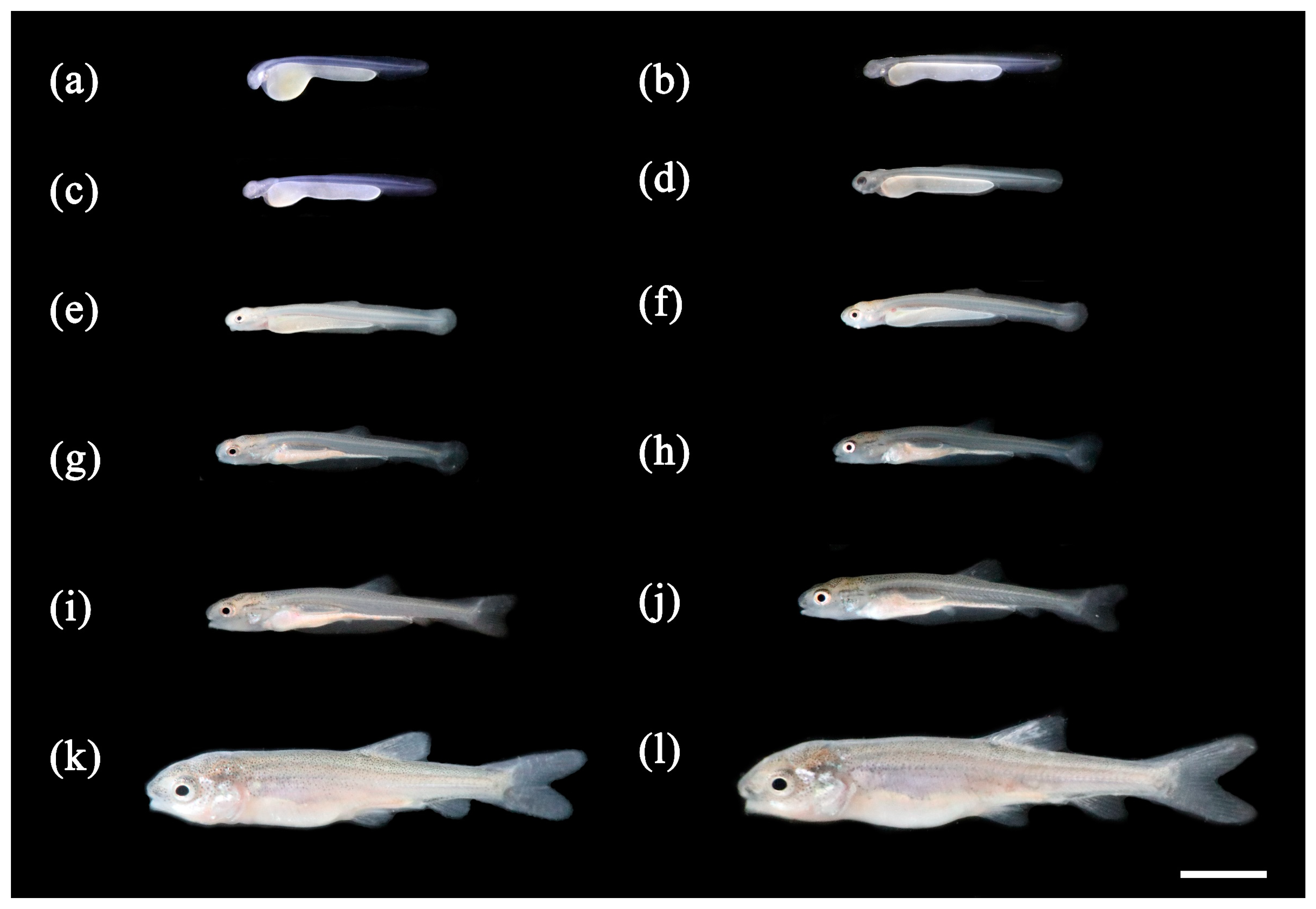
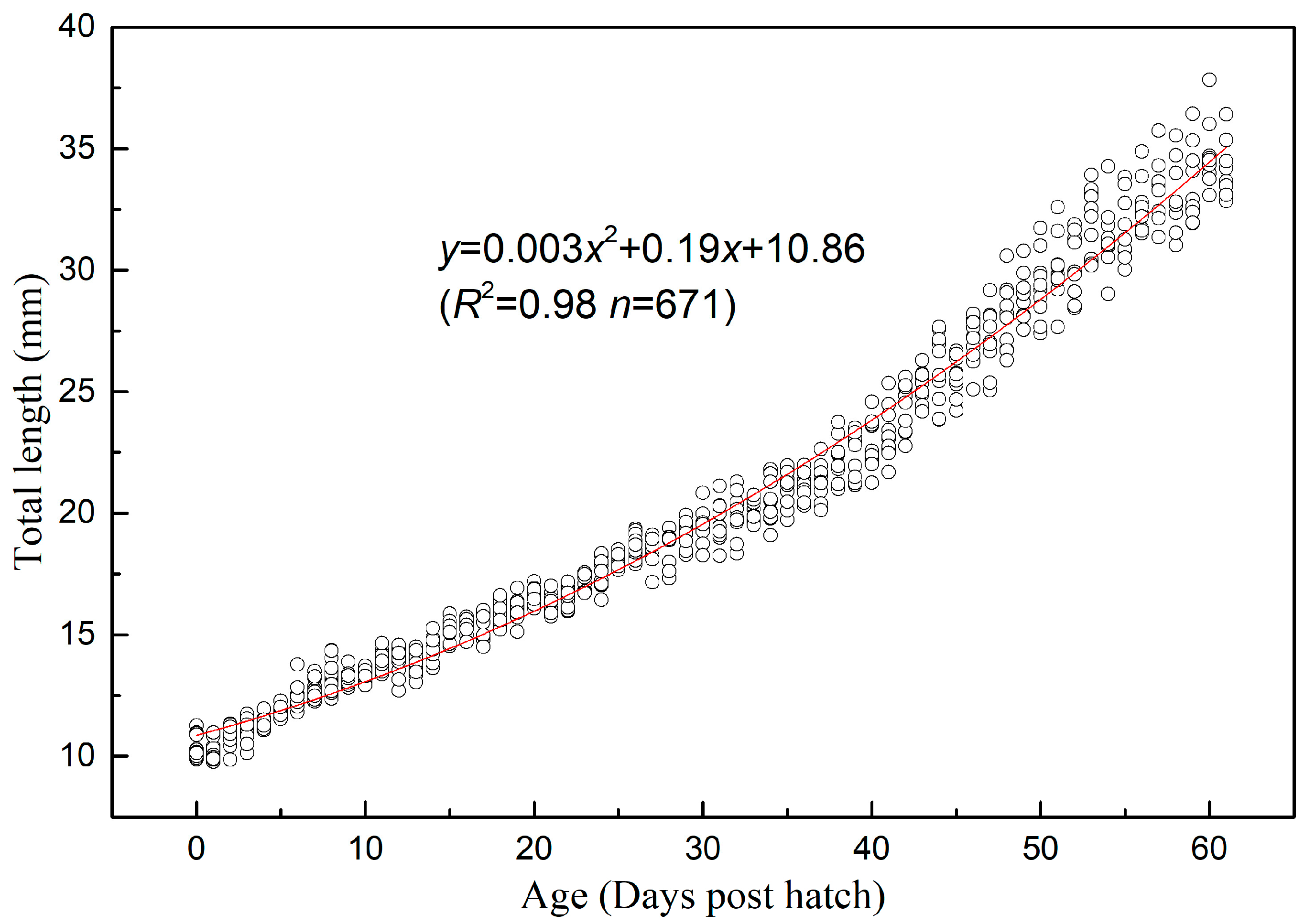
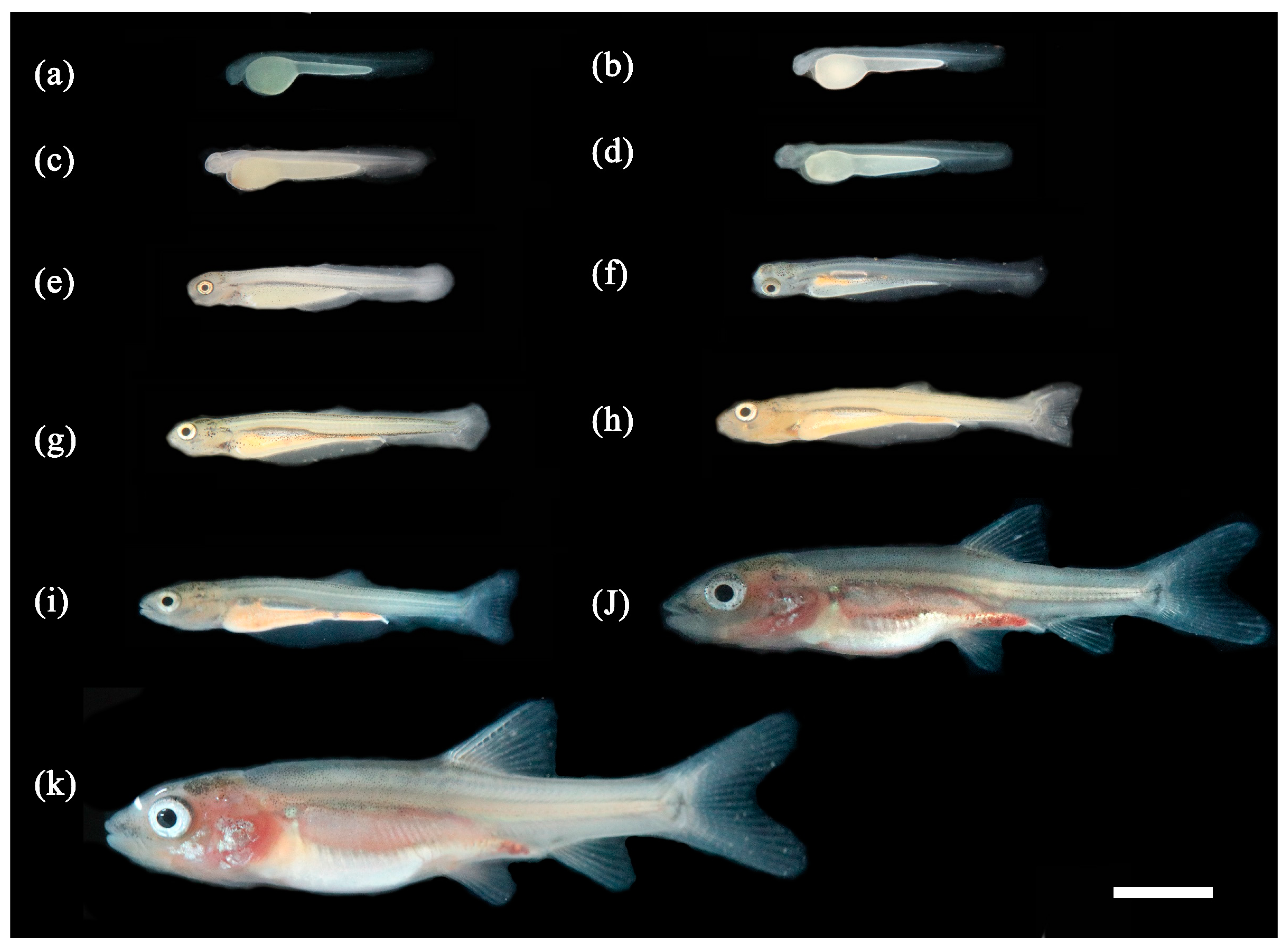
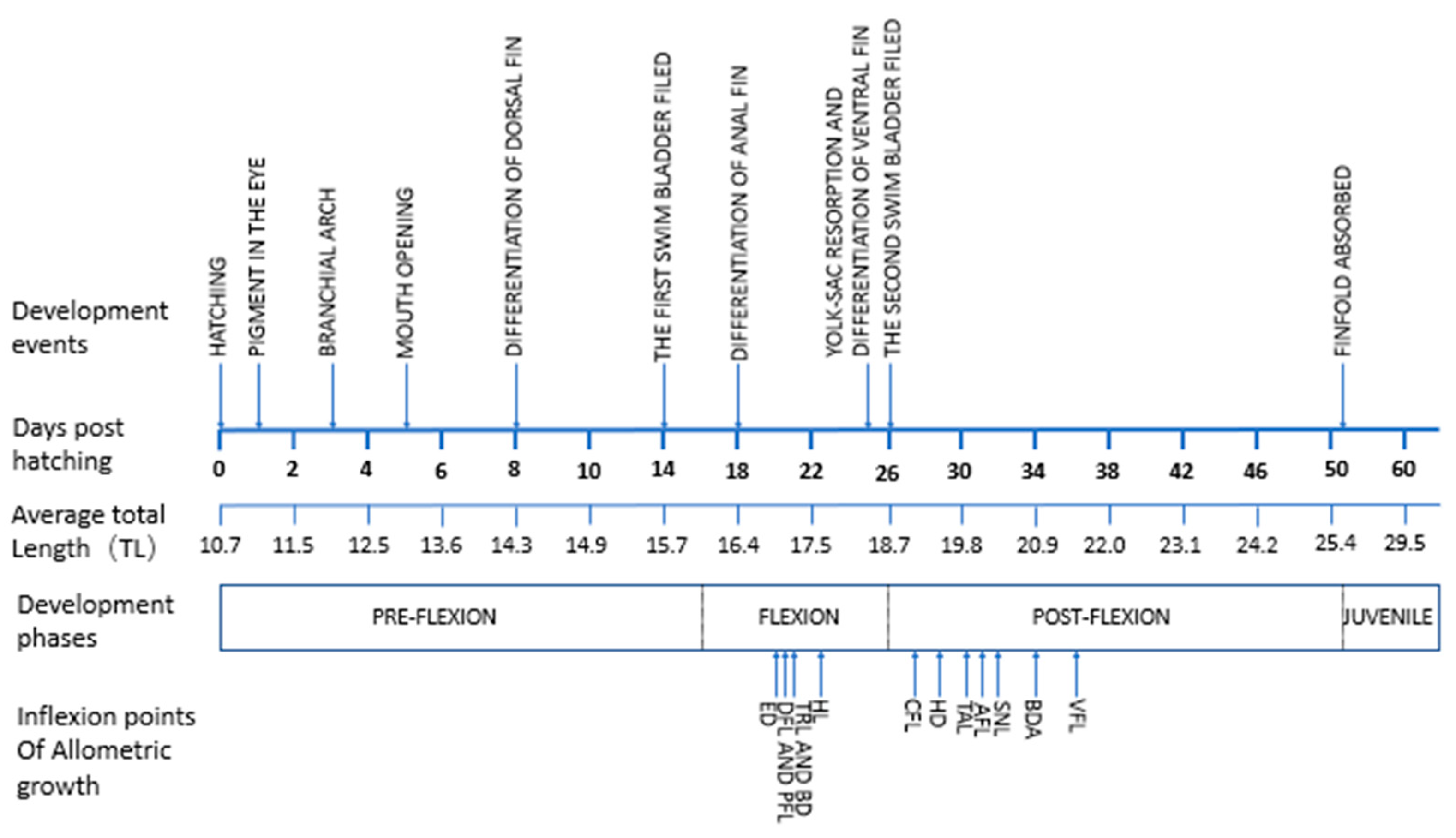
3.1. Ontogenetic Development
3.1.1. Ontogenetic Development of Schizothorax waltoni
3.1.2. Ontogenetic Development of Percocypris retrodorslis
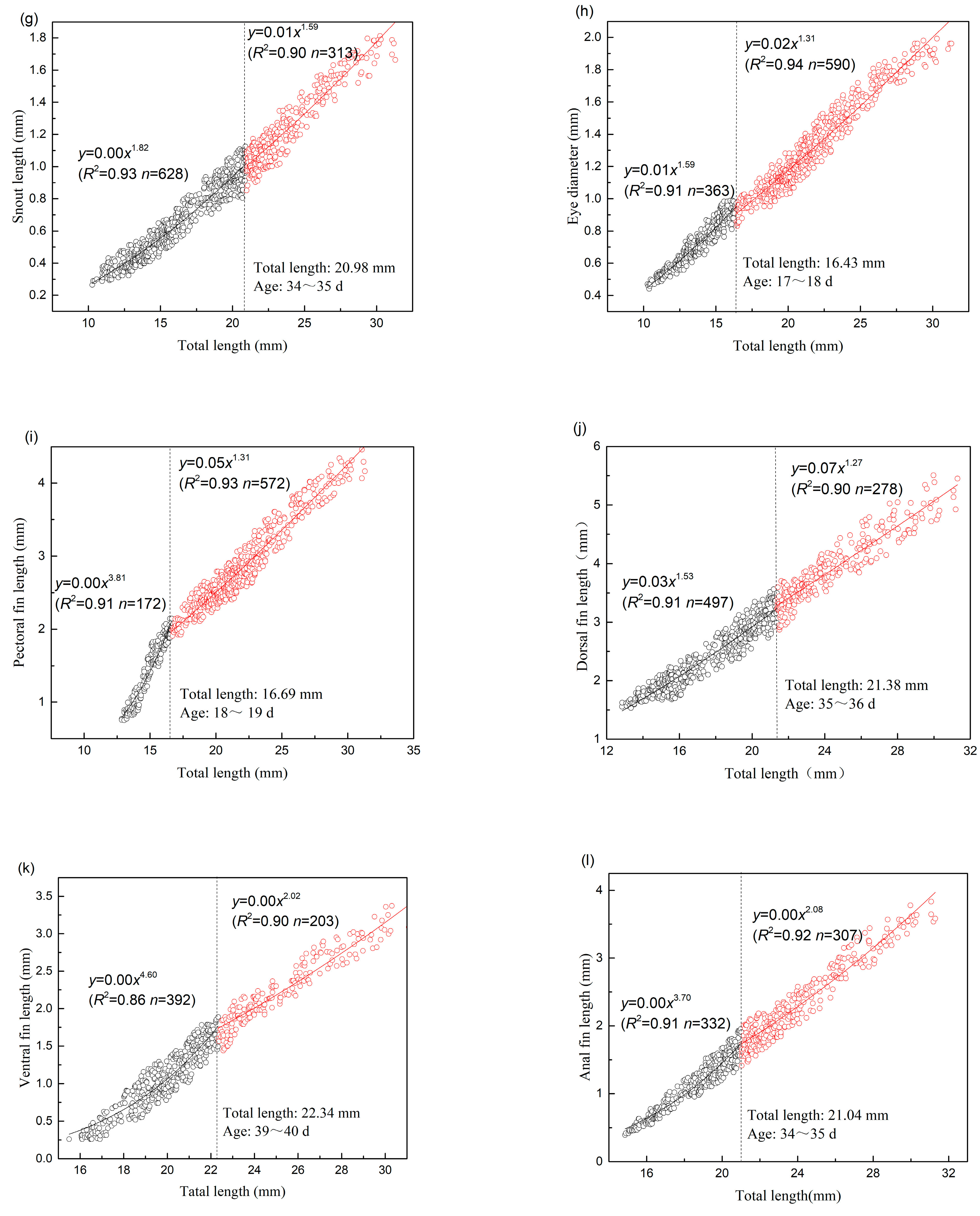
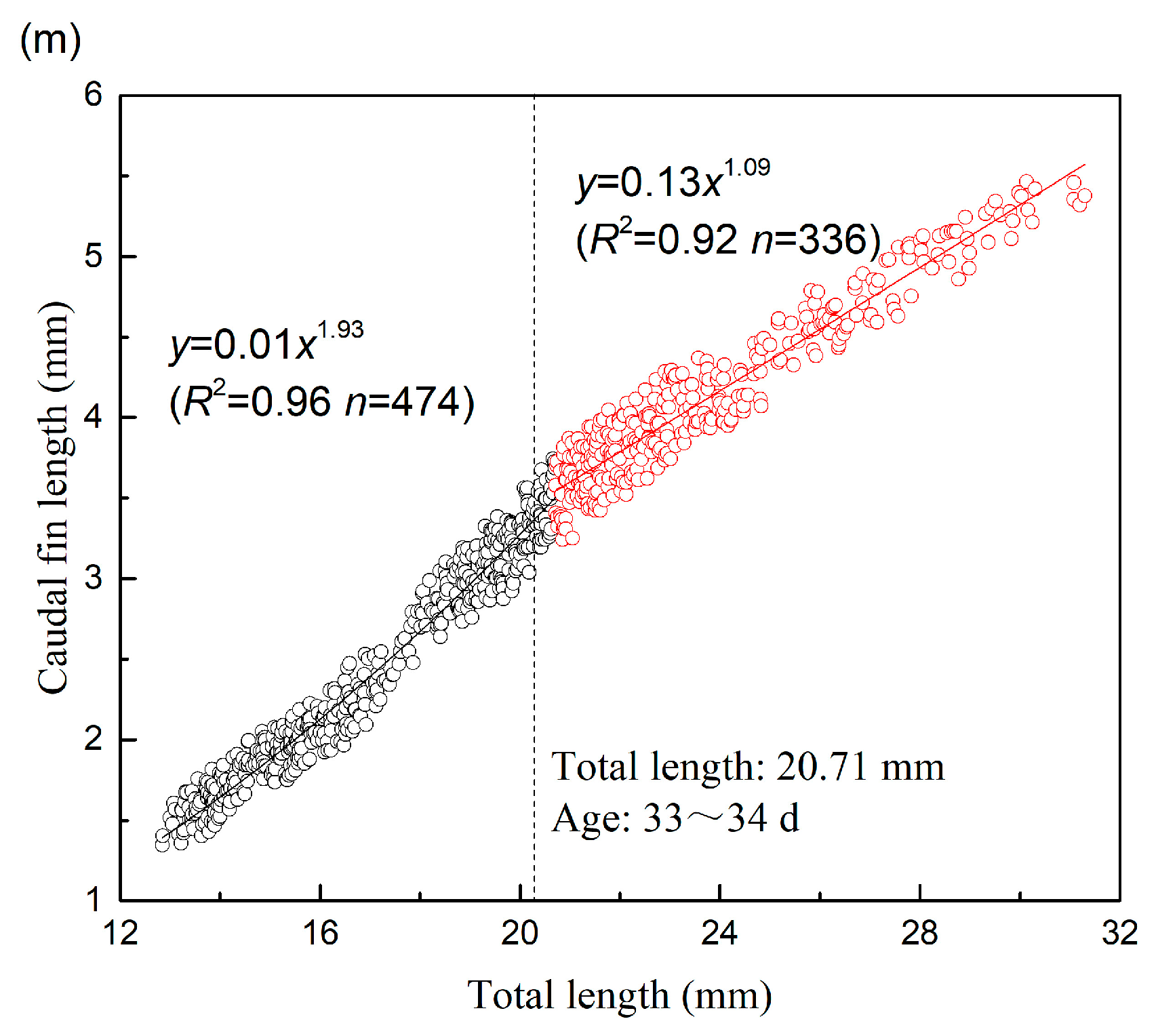
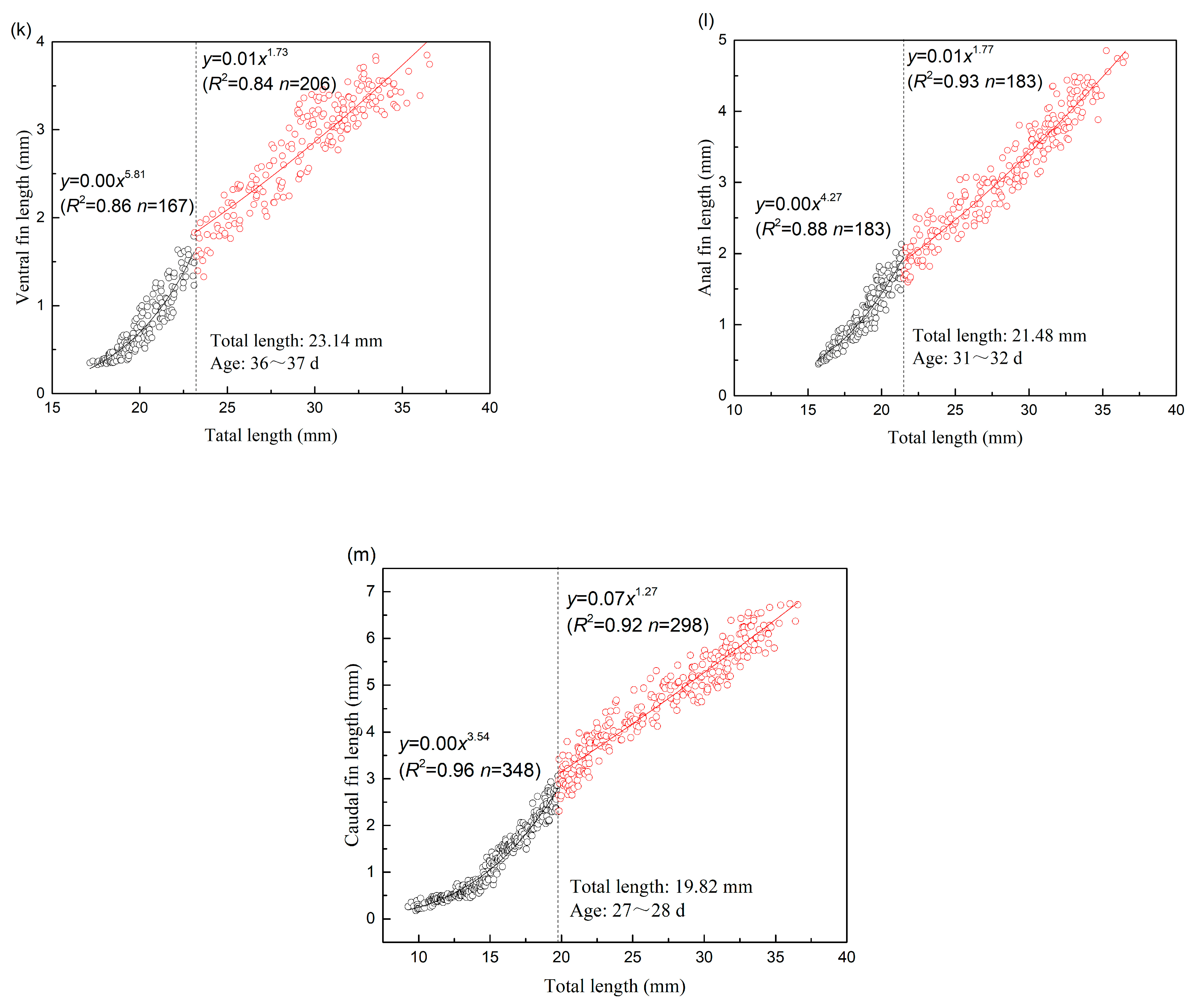
3.2. Allometric Growth Pattern
3.2.1. Allometric Growth Pattern of Schizothorax waltoni
3.2.2. Allometric Growth Pattern of Percocypris retrodorslis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, K.; Houde, E.D. Predation on eggs and larvae of marine fish and the recruitment problem. Adv. Mar. Biol. 1989, 25, 1–83. [Google Scholar]
- Peña, R.; Dumas, S. Effect of delayed first feeding on development and feeding ability of Paralabrax maculatofasciatus larvae. J. Fish Biol. 2005, 67, 640–651. [Google Scholar] [CrossRef]
- Osse, J.W.; van den Boogaart, J.G.M.; van Snik, G.M.J.; van der Sluys, L. Priorities during early growth of fish larvae. Aquaculture 1997, 155, 249–258. [Google Scholar] [CrossRef]
- Osse, J.W.; van den Boogaart, J.G.M. Fish larvae, development, allometric growth, and the aquatic environment. Int. Counc. Exp. Sea. Mar. Sci. Symp. 1995, 201, 21–34. [Google Scholar]
- van Snik, G.M.J.; van den Boogaart, J.G.M.; Osse, J.W.M. Larval growth patterns in Cyprinus carpio and Clarias gariepinus with attention to the fanfold. J. Fish Biol. 1997, 50, 1339–1352. [Google Scholar] [CrossRef]
- Fuiman, L.A. Growth gradients in fish larvae. J. Fish Biol. 1983, 23, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Xu, S.; Liu, Q.; Wang, X.; Yang, J.; Song, Z.; Li, J. Osteological ontogeny and allometric growth in larval and juvenile turbot (Scophthalmus maximus). Aquaculture 2018, 498, 351–363. [Google Scholar] [CrossRef]
- Gisbert, E.; Merino, G.; Muguet, J.B.; Bush, D.; Piedrahita, R.H.; Conklin, D.E. Morphological development and allometric growth patterns in hatchery-reared California halibut larvae. J. Fish Biol. 2002, 61, 1217–1229. [Google Scholar] [CrossRef]
- Kupren, K.; Żarski, D.; Kucharczyk, D. Early development and allometric growth patterns in ide Leuciscus idus (Linnaeus 1758). J. Appl. Ichthyol. 2015, 31, 509–517. [Google Scholar] [CrossRef]
- Eagderi, S.; Moshayedi, F.; Mousavi-Sabet, H. Allometric growth pattern and morphological changes of Pterophyllum scalare (Schultze, 1823) during the early development. Iran. J. Sci. Technol. Trans. A 2017, 41, 965–970. [Google Scholar] [CrossRef]
- Zhou, X.J.; Xie, C.X.; Huo, B.; Duan, Y.J.; Yang, X.; Ma, B.S. Reproductive biology of schizothorax waltoni (cyprinidae: Schizothoracinae) in the yarlung zangbo river in Tibet, China. Environ. Biol. Fish. 2015, 98, 597–609. [Google Scholar] [CrossRef]
- Lin, Y.H.; Miao, A.; Jiang, H.B. Study on morphological differences among three Schizothorax species. Guizhou Agric. Sci. 2010, 38, 121–126. [Google Scholar]
- Wang, M.; Yang, J.X.; and Chen, X.Y. Molecular Phylogeny and Biogeography of Percocypris (Cyprinidae, Teleostei). PLoS ONE 2013, 8, e61827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.Q.; Yue, X.J. The complete mitochondrial genome of Percocypris retrodorsalis (Teleostei, Cypriniformes) in Nujiang River: Characterization and phylogenetic position. Mitochondrial DNA Part B 2020, 5, 3375–3377. [Google Scholar] [CrossRef]
- Elnakeeb, M.; Naiel, M.; Vasilyeva, L.M. Paddlefish, Polyodon spathula: Historical, current status and future aquaculture prospects in Russia. Int. Aquat. Res. 2021, 13, 89–107. [Google Scholar]
- National Forestry and Grassland Administration. Wildlife under Special State Protection List. 2021. Available online: http://www.forestry.gov.cn/html/main/main_3954/20210225160347342521589/file/20210225160401102702964.pdf (accessed on 11 June 2022). (In Chinese)
- Saemi-Komsari, M.; Mousavi-Sabet, H.; Kratochwil, C.; Sattari, F.; Eagderi, M. Early developmental and allometric patterns in the electric yellow cichlid Labidochromis caeruleus. J. Fish Biol. 2018, 92, 1888–1901. [Google Scholar] [CrossRef] [Green Version]
- Kupren, K.; Palińska-Żarska, K.; Krejszeff, S.; Żarski, D. Early development and allometric growth in hatchery-reared Eurasian perch, Perca fluviatilis L. Aquac. Res. 2019, 50, 2528–2536. [Google Scholar] [CrossRef]
- Kendall, A.W.; Ahlstrom, E.H.; Moser, H.G. Early life history stages of fishes and their characters. In Ontogeny and Systematics of Fishes; Moser, H.G., Richards, W.J., Cohen, D.M., Fahay, M.P., Kendall, A.W., Richardson, S.L., Eds.; American Society of Ichthyologists and Herpetologists, Special Publication No. 1, 2; Allen Press Inc.: Lawrence, KA, USA, 1984; pp. 11–12. [Google Scholar]
- Nowosad, J.; Kupren, K.; Biegaj, M.; Kucharczyk, D. Allometric and ontogenetic larval development of common barbel during rearing under optimal conditions. Animal 2021, 15, 100107. [Google Scholar] [CrossRef]
- Peña, R.; Dumas, S. Development and allometric growth patterns during early larval stages of the spotted sand bass Paralabrax maculatofasciatus (Percoidei: Serranidae). Sci. Mar. 2009, 73, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Gisbert, E. Early development and allometric growth patterns in Siberian sturgeon and their ecological significance. J. Fish Biol. 1999, 54, 852–862. [Google Scholar] [CrossRef]
- Su, L.; Luo, S.; Qiu, N.; Xu, C.; Hou, M.; Xiong, X.; Wang, J. Comparative allometric growth of rare minnow (Gobiocypris rarus) in two culture environments. Hydrobiologia 2020, 847, 2083–2095. [Google Scholar] [CrossRef]
- Pepe-Victoriano, R.; Miranda, L.; Ortega, A.; Merino, G.E. Descriptive morphology and allometric growth of the larval development of Sarda chiliensis chiliensis (Cuvier, 1832) in a hatchery in northern Chile. Aquac. Rep. 2021, 19, 100576. [Google Scholar] [CrossRef]
- Sado, T.; Kimura, S. Developmental morphology of the cyprinid fish Horadandia atukorali. Ichthyol. Res. 2005, 52, 152–157. [Google Scholar] [CrossRef]
- Sado, T.; Kimura, S. Developmental morphology of the cyprinid fish Chela dadiburjori. Ichthyol. Res. 2005, 52, 20–26. [Google Scholar] [CrossRef]
- Kupren, K.; Rams, I.; Żarski, D.; Kucharczyk, D. Early development and allometric growth patterns of rheophilic cyprinid common dace Leuciscus leuciscus (Cyprinidae: Leuciscinae). Ichthyol. Res. 2016, 63, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Kupren, K.; Nowosad, J.; Żarski, D.; Targońska, K.; Hakuć-Błażowska, A.; Kucharczyk, D. Early development and allometric growth in laboratory reared European chub Leuciscus cephalus (Linnaeus, 1758). Turk. J. Fish. Aquat. Sc. 2015, 15, 385–392. [Google Scholar]
- Mendiola, D.; Ibaibarriaga, L.; Alvarez, P. Thermal effects on growth and time to starvation during the yolk-sac larval period of Atlantic mackerel Scomber scombrus L. J. Fish Biol. 2007, 70, 895–910. [Google Scholar] [CrossRef]
- Nakamura, M. Cyprinid Fishes of Japan; Spec Publ Res Inst Nat Resourc No. 4.; Shigen Kagaku Kenkyusyo: Tokyo, Japan, 1969. (In Japanese) [Google Scholar]
- Osse, J.W.; van den Boogaart, J.G.M. Allometric growth in Fish Larvae: Timing and Function. In The Development of Form and Function in Fishes and the Question of Larval Adaptation; Govoni, J.J., Ed.; Symposium 40; American Fisheries Society: Bethesda, MD, USA, 2004; pp. 167–194. [Google Scholar]
- Koumoundouros, G.; Divanach, P.; Kentouri, M. Ontogeny and allometric plasticity of Dentex dentex (Osteichthyes: Sparidae) in rearing conditions. Mar. Biol. 1999, 135, 561–572. [Google Scholar] [CrossRef]
- Brown, A.L.; Busby, M.S.; Mier, K.L. Walleye Pollock Theragra chalcogramma during transformation from the larval to juvenile stage: Otolith and osteological development. Mar. Biol. 2001, 139, 845–851. [Google Scholar]
- Li, J.; Han, Y.; Xu, L.; Ma, Q.; Song, T.; Dong, Y.; Liu, X.; Pang, Y.; Li, Q. Embryological stages and allometric growth during Yolk-sac larvae of Lampetra japonica (Martens). Acta Hydrobiol. Sin. 2017, 41, 1207–1217. [Google Scholar]
- Xi, D.; Zhang, X.; Lv, H.; Zhang, P.; Zhang, Z. Studies on the early allometric growth pattern of black rockfish Sebastes schlegelii. Period. Ocean. Univ. China 2014, 12, 28–34. [Google Scholar] [CrossRef]
- He, T.; Xiao, Z.; Liu, Q.; Li, J. Allometric growth in rock bream larvae (Oplegnathus fasciatus Temminck et Schlegel 1844). J. Fish. China 2012, 36, 1242–1248. [Google Scholar] [CrossRef]
- Carter, J.E.; Sporre, M.A.; Eytan, R.I. Larviculture, allometric growth patterns, and gape morphology of the florida blenny, Chasmodes saburrae. Aquaculture 2022, 554, 738153. [Google Scholar] [CrossRef]
- Alvarez-González, C.A. Actividad enzimática digestiva y evaluación de dietas para el destete de larvas de la cabrilla arenera Paralabrax maculatofasciatus (Percoidei: Serranidae). Ph. D. Thesis, Centro Interdisciplinario de Ciencias Marinas, La Paz, Mexico, 2003. [Google Scholar]
- Cunha, I.; Planas, M. Optimal prey size for early turbot larvae (Scophthalmus maximus L.) based on mouth and ingested prey size. Aquaculture 1999, 175, 103–110. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, D.; Zhou, S.; Ma, Z.; Hu, J.; Yang, R. Study on the Allometric Growth of Larvae and Juvenile Lates calarifer. Mar. Fish. 2018, 40, 179–188. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Z.; Liu, Q.; Zhai, J.; Pang, Z.; Ma, W.; Ma, D.; Xu, S.; Xiao, Y.; Li, J. Allometric growth pattern during early ontogeny of spotted knifejaw (Oplegnathus punctatus). Mar. Sci. 2016, 40, 43–48. [Google Scholar]
- Yang, Q.; Ma, Z.; Cheng, D.; Jiang, S.; Li, Y.; Chen, M. Allometric growth in larvae and juvenile golden pompano (Trachinotus ovatus). Fish. Sci. 2017, 36, 259–266. [Google Scholar] [CrossRef]
- Song, H.; Liu, W.; Wang, L.; Tang, F. Allometric growth during Yolk-sac larvae of chum salmon (Oncorhynchus keta Walbaum) and consequent ecological significance. Acta Hydrobiol. Sin. 2013, 37, 329–335. [Google Scholar]
- Ortíz-Galindo, J.L.; Peña, R.; Perezgomez-Alvarez, L.; Castro-Aguirre, J.L. Desarrollo osteológico de la cabrilla arenera Paralabrax maculatofasciatus (Steindachner, 1868) (Percoidei: Serranidae). In Memorias del VII Congreso Nacional de Ictiología; 2000; pp. 277–288. [Google Scholar]
- Johnston, I.A.; Hall, T.E. Mechanisms of muscle development and responses to temperature change in fish larvae. In The Development of Form and Function in Fishes and the Question of Larval Adaptation; Govoni, J.J., Ed.; Symposium 40: American Fisheries Society: Bethesda, MD, USA, 2004; pp. 85–116. [Google Scholar]
- Xie, C.X.; Huo, B.; Wei, K.J.; Ma, B.S.; Qin, J.H. Biology and Resource Conservation of Schizothoracinae Fishes in the Middle Rcaches of Yarlung Zangbo River; Science Press: Beijing, China, 2019. [Google Scholar]
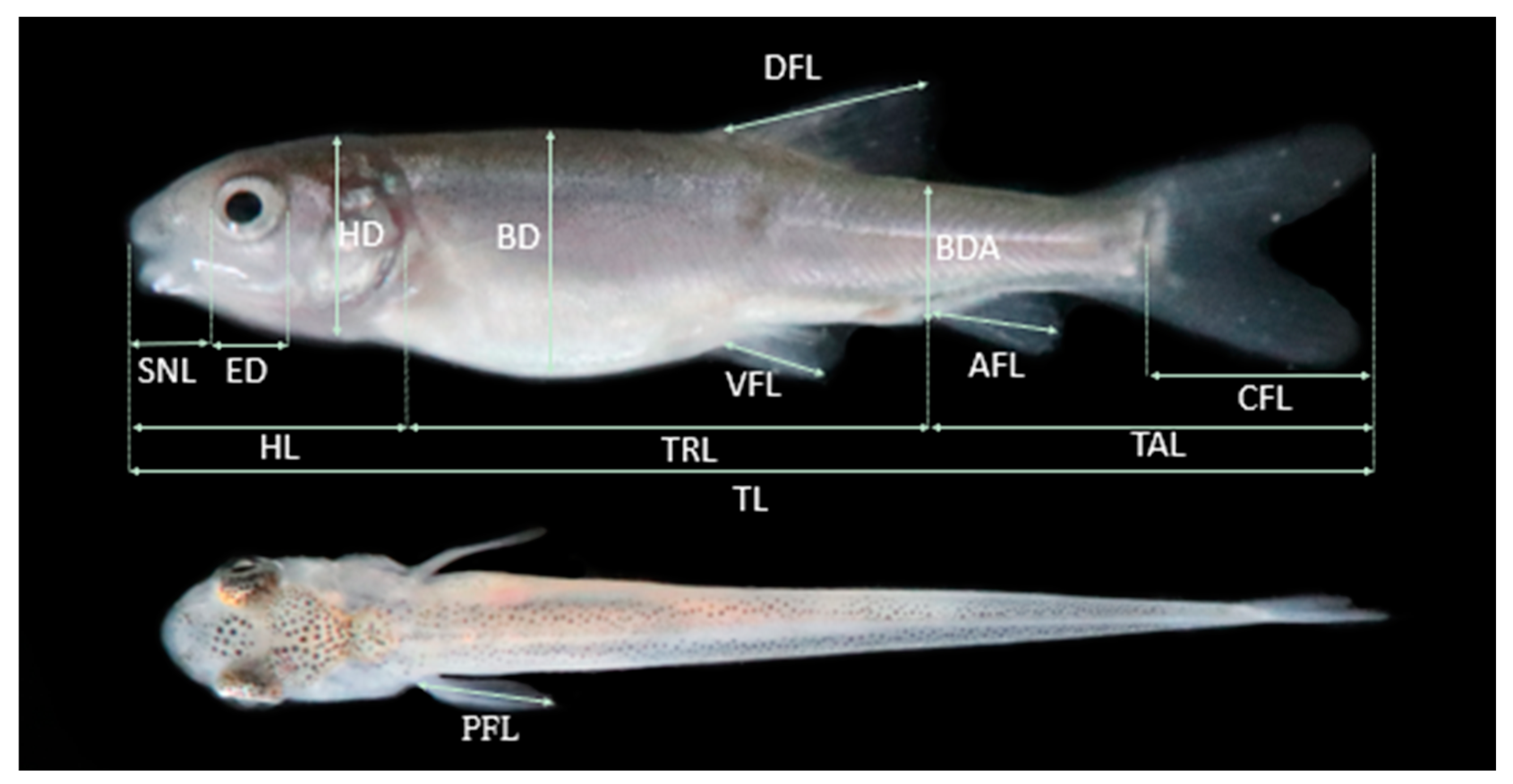





| Character | SL: Inflection Point (mm) | Before Inflexion Point | After Inflexion Point | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | b | a | R2 | p Value | n | b | a | R2 | p Value | ||
| HL | 17.61 | 411 | 2.313 | 0.005 | 0.955 | 0.022 | 542 | 1.252 | 0.098 | 0.947 | 0.039 |
| TRL | 20.40 | 592 | 0.436 | 2.327 | 0.865 | 0.019 | 361 | 0.752 | 0.894 | 0.899 | 0.045 |
| TAL | 21.91 | 725 | 1.387 | 0.11 | 0.982 | 0.041 | 228 | 1.127 | 0.244 | 0.964 | 0.039 |
| HD | 19.36 | 501 | 1.664 | 0.018 | 0.962 | 0.008 | 452 | 1.264 | 0.038 | 0.906 | 0.041 |
| BD | 19.81 | 538 | 1.261 | 0.064 | 0.941 | 0.011 | 415 | 1.506 | 0.032 | 0.905 | 0.046 |
| BDA | 20.43 | 452 | 1.263 | 0.036 | 0.916 | 0.004 | 342 | 1.642 | 0.012 | 0.916 | 0.014 |
| SNL | 20.98 | 628 | 1.823 | 0.004 | 0.927 | 0.004 | 313 | 1.591 | 0.008 | 0.895 | 0.006 |
| ED | 16.43 | 363 | 1.594 | 0.011 | 0.907 | 0.002 | 590 | 1.311 | 0.023 | 0.935 | 0.005 |
| PFL | 16.69 | 172 | 3.813 | 0.000 | 0.907 | 0.016 | 572 | 1.312 | 0.049 | 0.932 | 0.021 |
| DFL | 21.38 | 497 | 1.531 | 0.030 | 0.908 | 0.026 | 278 | 1.272 | 0.067 | 0.903 | 0.041 |
| VFL | 22.34 | 392 | 4.603 | 0.000 | 0.857 | 0.024 | 203 | 2.019 | 0.003 | 0.902 | 0.021 |
| AFL | 21.04 | 332 | 3.700 | 0.000 | 0.905 | 0.014 | 307 | 2.084 | 0.003 | 0.919 | 0.027 |
| CFL | 20.71 | 474 | 1.934 | 0.010 | 0.961 | 0.017 | 336 | 1.092 | 0.128 | 0.915 | 0.024 |
| Character | TL: Inflection Point (mm) | Before Inflexion Point | After Inflexion Point | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | b | a | R2 | p Value | n | b | a | R2 | p Value | ||
| HL | 18.06 | 308 | 2.374 | 0.004 | 0.960 | 0.033 | 363 | 1.106 | 0.172 | 0.929 | 0.015 |
| TRL | 17.15 | 284 | 0.458 | 2.155 | 0.846 | 0.022 | 387 | 0.750 | 0.893 | 0.926 | 0.038 |
| TAL | 21.23 | 417 | 1.256 | 0.160 | 0.905 | 0.016 | 254 | 1.156 | 0.214 | 0.946 | 0.011 |
| HD | 20.16 | 386 | 1.757 | 0.015 | 0.958 | 0.016 | 285 | 1.283 | 0.061 | 0.950 | 0.045 |
| BD | 17.18 | 285 | 1.293 | 0.060 | 0.925 | 0.011 | 386 | 1.446 | 0.040 | 0.954 | 0.037 |
| BDA | 22.42 | 324 | 1.336 | 0.033 | 0.940 | 0.006 | 221 | 1.472 | 0.022 | 0.948 | 0.017 |
| SNL | 21.63 | 429 | 2.344 | 0.000 | 0.927 | 0.005 | 242 | 1.529 | 0.011 | 0.906 | 0.015 |
| ED | 16.92 | 278 | 1.440 | 0.019 | 0.905 | 0.003 | 393 | 0.945 | 0.081 | 0.947 | 0.007 |
| PFL | 17.01 | 139 | 2.644 | 0.001 | 0.827 | 0.013 | 392 | 1.242 | 0.049 | 0.926 | 0.042 |
| DFL | 16.94 | 235 | 4.678 | 0.000 | 0.915 | 0.017 | 264 | 1.479 | 0.029 | 0.927 | 0.035 |
| VFL | 23.14 | 167 | 5.816 | 0.000 | 0.859 | 0.019 | 206 | 1.727 | 0.008 | 0.836 | 0.039 |
| AFL | 21.48 | 183 | 4.274 | 0.000 | 0.883 | 0.021 | 183 | 1.774 | 0.008 | 0.928 | 0.044 |
| CFL | 19.82 | 348 | 3.538 | 0.000 | 0.956 | 0.025 | 298 | 1.266 | 0.071 | 0.916 | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Li, D.; Wei, K.; Zhu, X.; Xu, J.; Ma, B. Allometric Growth Patterns and Ontogenetic Development during Early Larval Stages of Schizothorax waltoni Regan and Percocypris retrodorslis in Southwest China. Water 2023, 15, 824. https://doi.org/10.3390/w15040824
Xu B, Li D, Wei K, Zhu X, Xu J, Ma B. Allometric Growth Patterns and Ontogenetic Development during Early Larval Stages of Schizothorax waltoni Regan and Percocypris retrodorslis in Southwest China. Water. 2023; 15(4):824. https://doi.org/10.3390/w15040824
Chicago/Turabian StyleXu, Bin, Dapeng Li, Kaijin Wei, Xiangyun Zhu, Jin Xu, and Baoshan Ma. 2023. "Allometric Growth Patterns and Ontogenetic Development during Early Larval Stages of Schizothorax waltoni Regan and Percocypris retrodorslis in Southwest China" Water 15, no. 4: 824. https://doi.org/10.3390/w15040824
APA StyleXu, B., Li, D., Wei, K., Zhu, X., Xu, J., & Ma, B. (2023). Allometric Growth Patterns and Ontogenetic Development during Early Larval Stages of Schizothorax waltoni Regan and Percocypris retrodorslis in Southwest China. Water, 15(4), 824. https://doi.org/10.3390/w15040824







