Trace and Rare Earth Element (REE) Geochemistry of Recently Formed Stromatolites at Lake Salda, SW Turkey
Abstract
:1. Introduction
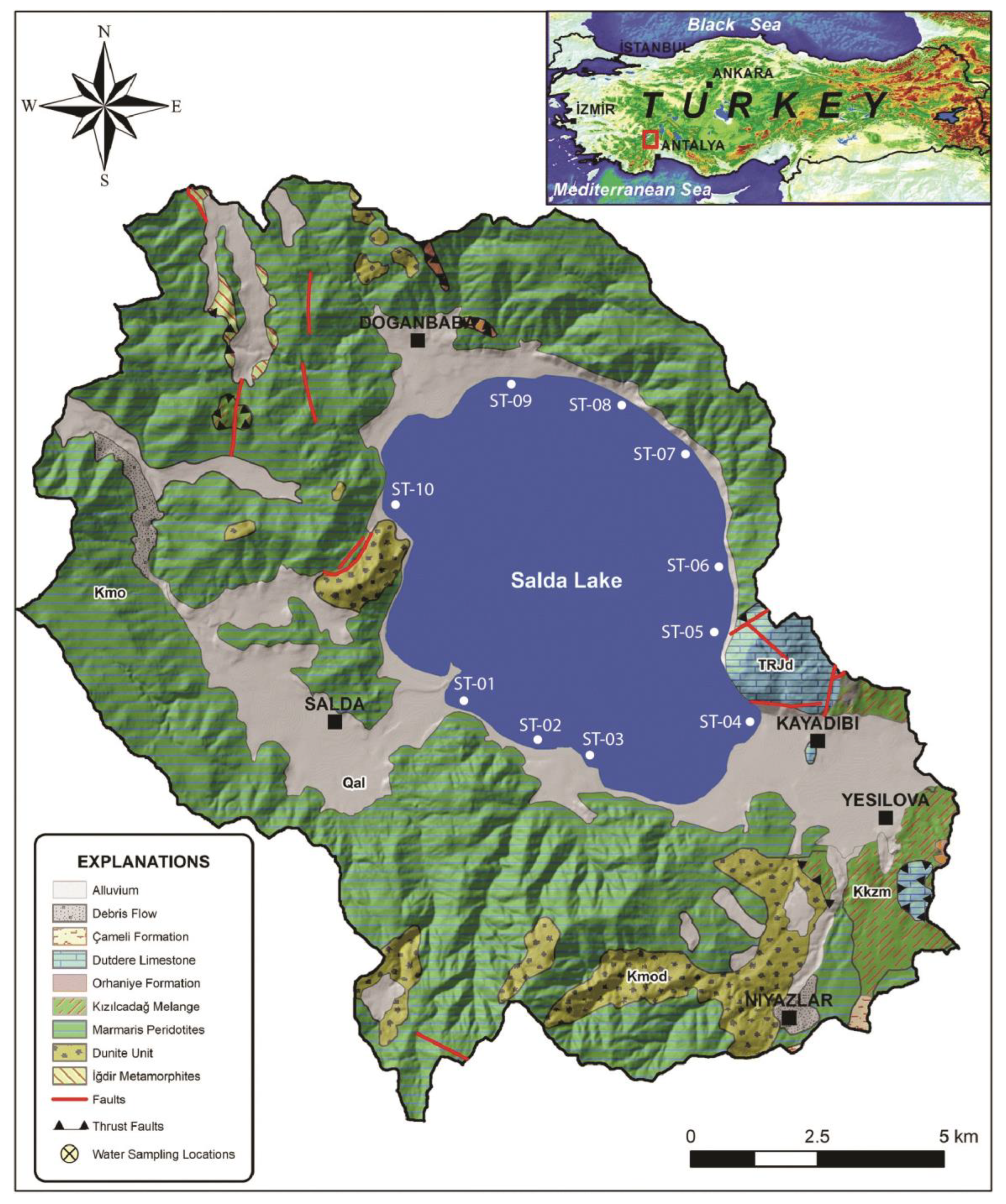
2. Geological Setting
3. Sampling and Analytical Method
4. Results
4.1. Salda Lake Water
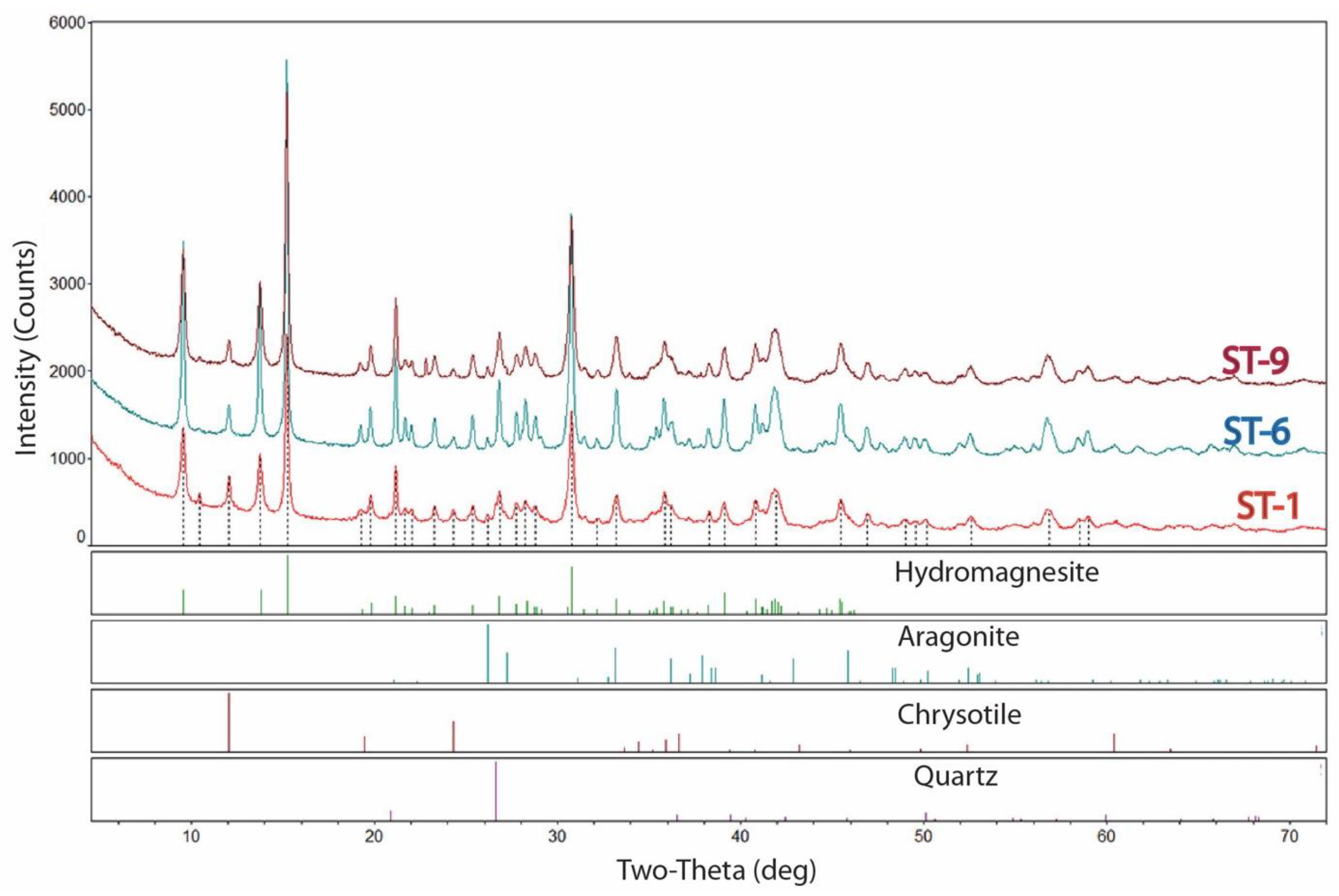
4.2. XRD Data
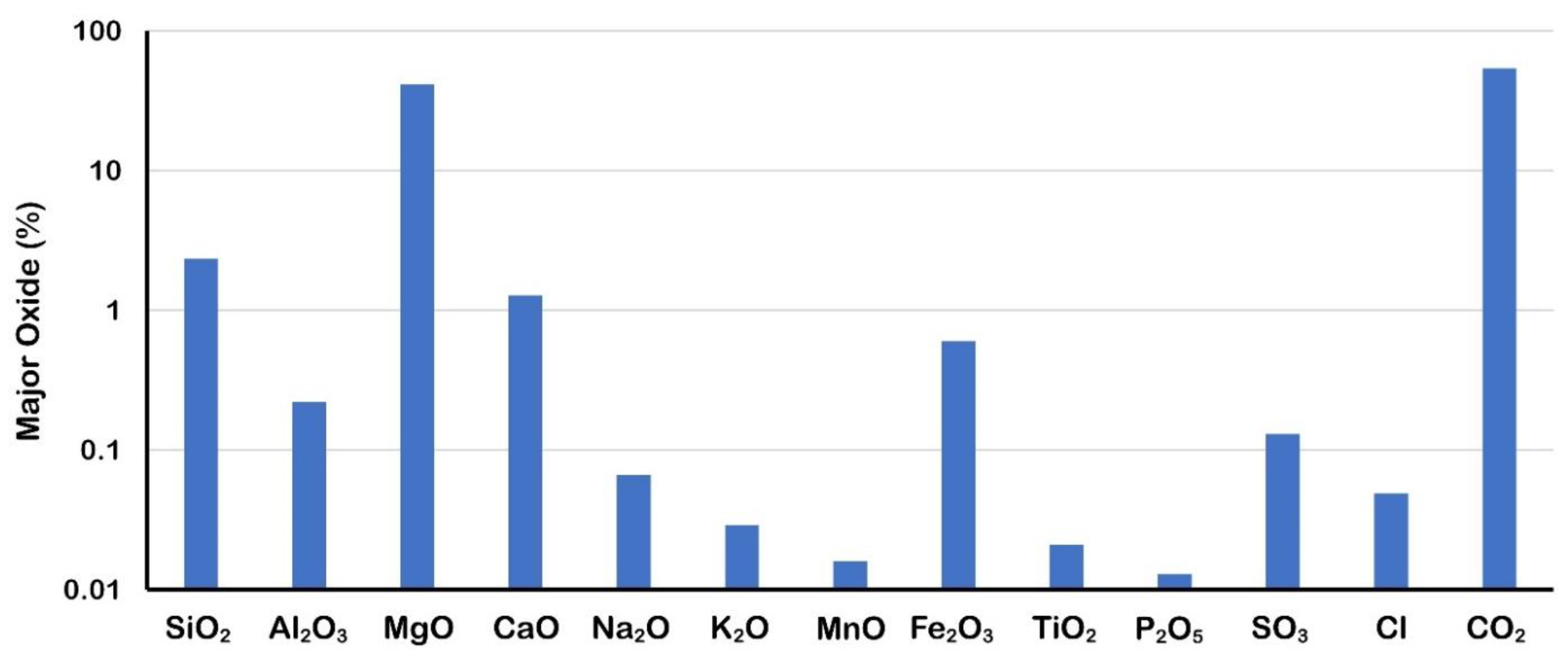
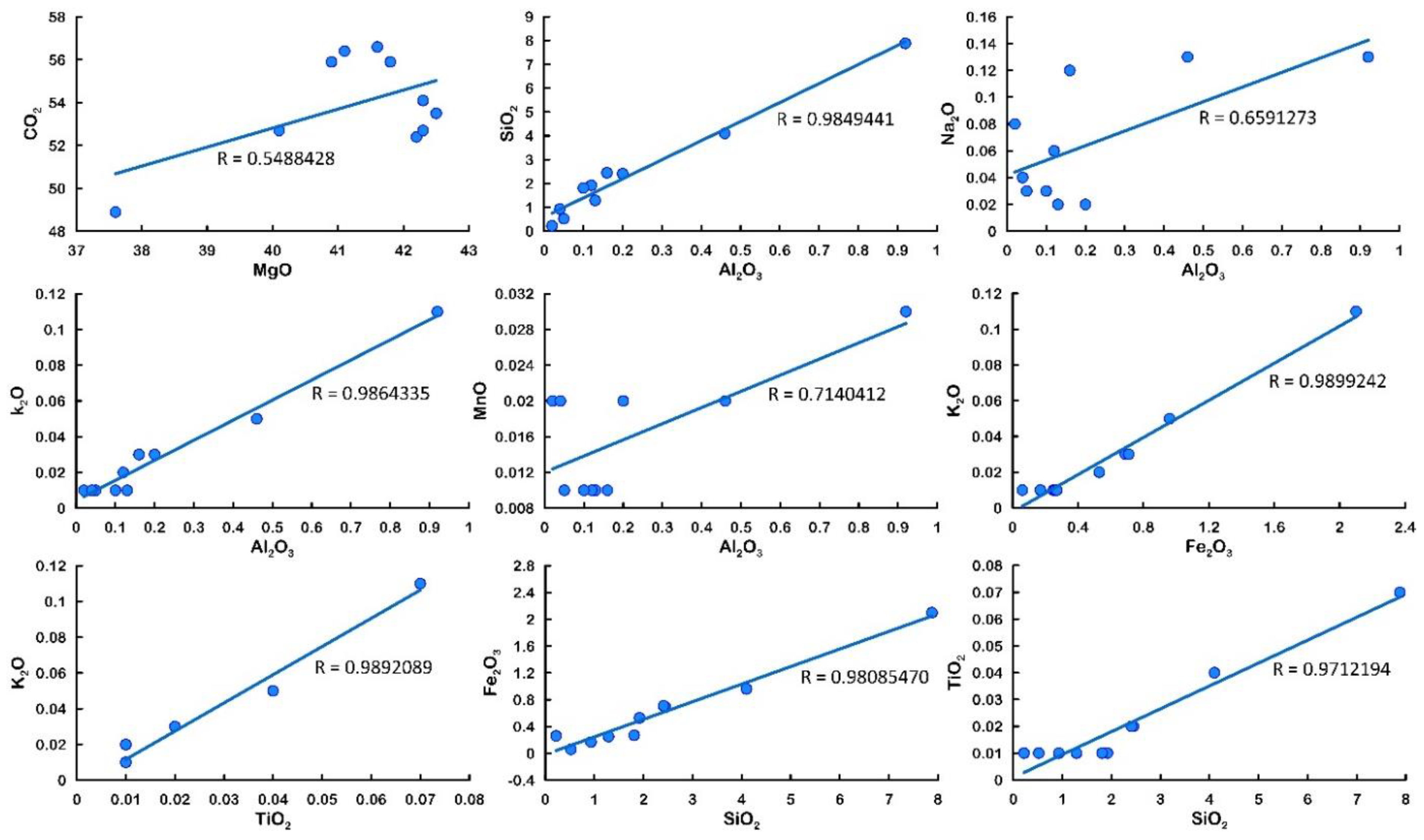
4.3. Major Oxides
4.4. Trace Elements
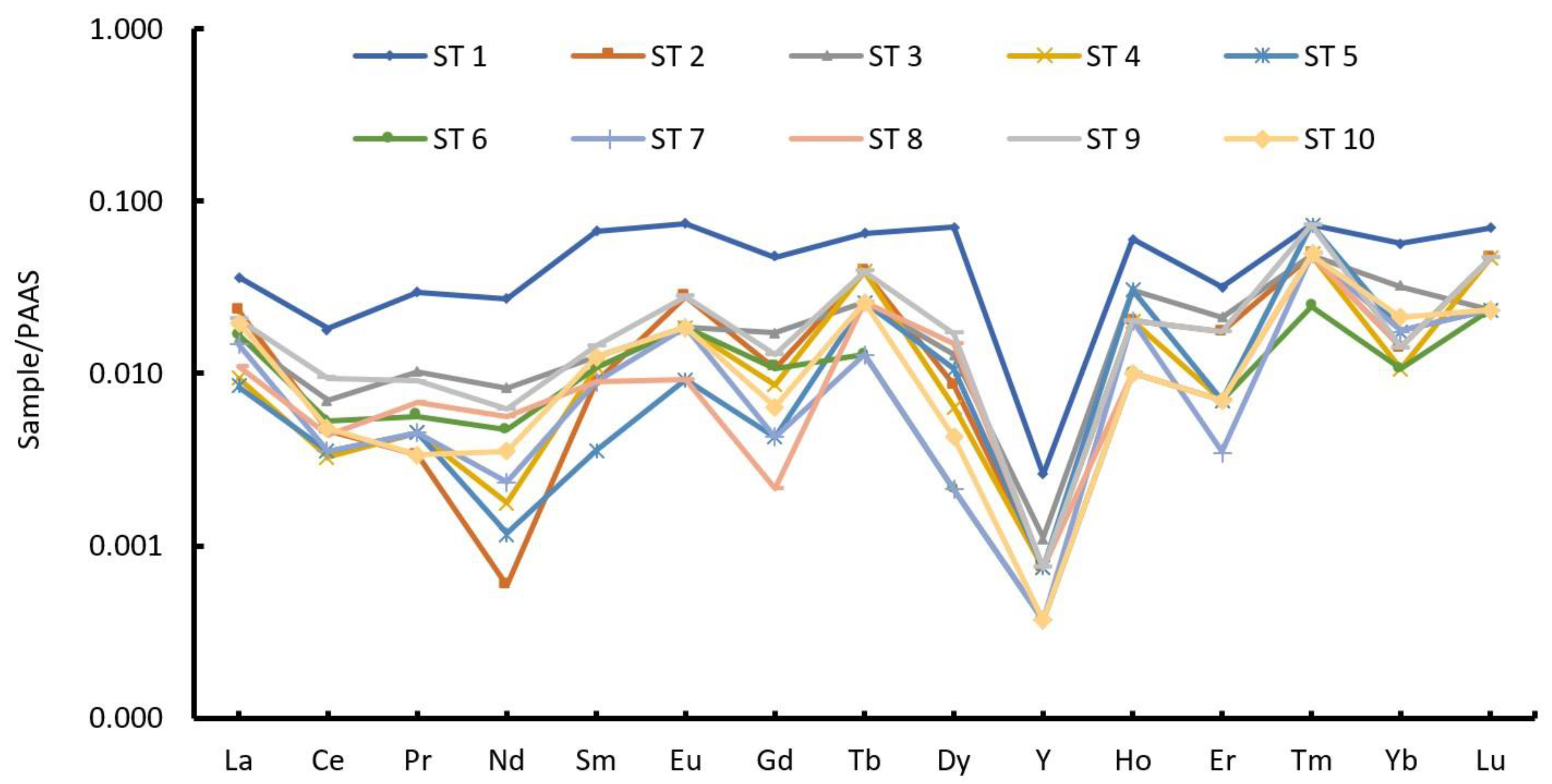
4.5. Rare Earth Elements
5. Discussion
5.1. Major Oxides
5.2. Trace Elements
5.3. Rare Earth Elements
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goudge, T.A.; Aureli, K.L.; Head, J.W.; Fassett, C.I.; Mustard, J.F. Classification and analysis of candidate impact crater-hosted closed-basin lakes on Mars. Icarus 2015, 260, 346–367. [Google Scholar] [CrossRef]
- Goudge, T.A.; Mustard, J.F.; Head, J.W.; Fassett, C.I.; Wiseman, S.M. Assessing the mineralogy of the watershed and fan deposits of the Jezero crater paleolake system, Mars. J. Geophys. Res. Planets 2015, 120, 775–808. [Google Scholar] [CrossRef]
- Garczynski, B.; Horgan, B.; Kah, L.; Balci, N.; Gunes, Y. Searching for potential biosignatures in Jezero Crater with Mars 2020—A spectral investigation of terrestrial lacustrine carbonate analogs. In Proceedings of the Ninth International Conference on Mars, Pasadena, CA, USA, 22–25 July 2019; p. 6302. [Google Scholar]
- Horgan, B.H.; Anderson, R.B.; Dromart, G.; Amador, E.S.; Rice, M.S. The mineral diversity of Jezero crater: Evidence for possible lacustrine carbonates on Mars. Icarus 2020, 339, 113526. [Google Scholar] [CrossRef]
- Karaman, M. Comparison of thresholding methods for shoreline extraction from Sentinel-2 and Landsat-8 imagery: Extreme Lake Salda, track of Mars on Earth. J. Environ. Manag. 2021, 298, 113481. [Google Scholar] [CrossRef]
- Riu, L.; Carter, J.; Poulet, F. The M3 project: 3–Global abundance distribution of hydrated silicates at Mars. Icarus 2022, 374, 114809. [Google Scholar] [CrossRef]
- Petryshyn, V.A.; Junkins, E.N.; Stamps, B.W.; Bailey, J.V.; Stevenson, B.S.; Spear, J.R.; Corsetti, F.A. Builders, tenants, and squatters: The origins of genetic material in modern stromatolites. Geobiology 2021, 19, 261–277. [Google Scholar] [CrossRef]
- Allwood, A.C.; Walter, M.R.; Kamber, B.S.; Marshall, C.P.; Burch, I.W. Stromatolite reef from the Early Archaean era of Australia. Nature 2006, 441, 714–718. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Knoll, A.H. Stromatolites in Precambrian carbonates: Evolutionary mileposts or environmental dipsticks? Annu. Rev. Earth Planet. Sci. 1999, 27, 313–358. [Google Scholar] [CrossRef]
- Visscher, P.T.; Reid, R.P.; Bebout, B.M. Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites. Geology 2000, 28, 919–922. [Google Scholar] [CrossRef]
- Reid, R.P.; Visscher, P.T.; Decho, A.W.; Stolz, J.F.; Bebout, B.; Dupraz, C.; Macintyre, I.; Paerl, H.; Pinckney, J.; Prufert-Bebout, L. The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature 2000, 406, 989–992. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Braithwaite, C.; Zedef, V. Hydromagnesite stromatolites and sediments in an alkaline lake, Salda Golu, Turkey. J. Sediment. Res. 1996, 66, 991–1002. [Google Scholar]
- Northup, E.; Kathleen, H.; Lavoie, D. Geomicrobiology of caves: A review. Geomicrobiol. J. 2001, 18, 199–222. [Google Scholar]
- Erum, B.; Shahid, N.; Tabinda, A.; Khula, S. Characteristics of ultramafic rocks and associated magnesite deposits, Nal Area, Khuzdar, Balochistan, Pakistan. J. Geol. Min. Res. 2009, 1, 034–041. [Google Scholar]
- Cangemi, M.; Censi, P.; Reimer, A.; D’Alessandro, W.; Hause-Reitner, D.; Madonia, P.; Oliveri, Y.; Pecoraino, G.; Reitner, J. Carbonate precipitation in the alkaline lake Specchio di Venere (Pantelleria Island, Italy) and the possible role of microbial mats. Appl. Geochem. 2016, 67, 168–176. [Google Scholar] [CrossRef]
- Oskierski, H.; Dlugogorski, B.; Oliver, T.; Jacobsen, G. Chemical and isotopic signatures of waters associated with the carbonation of ultramafic mine tailings, Woodsreef Asbestos Mine, Australia. Chem. Geol. 2016, 436, 11–23. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, M.; Ye, C. Hydromagnesite precipitation in the Alkaline Lake Dujiali, central Qinghai-Tibetan Plateau: Constraints on hydromagnesite precipitation from hydrochemistry and stable isotopes. Appl. Geochem. 2017, 78, 139–148. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Harrison, A.L.; Dipple, G.M.; McCutcheon, J.; Southam, G.; Kenward, P.A. A depositional model for hydromagnesite–magnesite playas near Atlin, British Columbia, Canada. Sedimentology 2014, 61, 1701–1733. [Google Scholar] [CrossRef]
- Riding, R.E.; Awramik, S.M. Microbial Sediments; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Balci, N.; Demirel, C.; Kurt, M. Geomicrobiology of Lake Salda and microbial influences on present-day stromatolite formation. Bull. Earth Sci. Appl. Res. Cent. Hacet. Univ. 2018, 39, 19–40. [Google Scholar]
- Kazanci, N.; Girgin, S.; Dügel, M. On the limnology of Salda Lake, a large and deep soda lake in southwestern Turkey: Future management proposals. Aquat. Conserv. Mar. Freshw. Ecosyst. 2004, 14, 151–162. [Google Scholar] [CrossRef]
- Zeng, L.-Q.; Yi, H.-S.; Xia, G.-Q.; Simon, K.; Heim, C.; Arp, G. Palaeoenvironmental setting of lacustrine stromatolites in the Miocene Wudaoliang Group, northern Tibetan Plateau. J. Palaeogeogr. 2019, 8, 1–15. [Google Scholar] [CrossRef]
- Franchi, F. Petrographic and geochemical characterization of the Lower Transvaal Supergroup stromatolitic dolostones (Kanye Basin, Botswana). Precambrian Res. 2018, 310, 93–113. [Google Scholar] [CrossRef]
- Bonales, L.; Muñoz-Iglesias, V.; Santamaría-Pérez, D.; Caceres, M.; Fernandez-Remolar, D.; Prieto-Ballesteros, O. Quantitative Raman spectroscopy as a tool to study the kinetics and formation mechanism of carbonates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 26–30. [Google Scholar] [CrossRef]
- Censi, P.; Cangemi, M.; Madonia, P.; Saiano, F.; Brusca, L.; Zuddas, P. Discrimination between effects induced by microbial activity and water-rock interactions under hydrothermal conditions according to REE behaviour. Procedia Earth Planet. Sci. 2013, 7, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Bolhar, R.; Van Kranendonk, M.J. A non-marine depositional setting for the northern Fortescue Group, Pilbara Craton, inferred from trace element geochemistry of stromatolitic carbonates. Precambrian Res. 2007, 155, 229–250. [Google Scholar] [CrossRef]
- Frimmel, H.E. Trace element distribution in Neoproterozoic carbonates as palaeoenvironmental indicator. Chem. Geol. 2009, 258, 338–353. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Zheng, Y.-F.; Chen, F. Trace element and strontium isotope constraints on sedimentary environment of Ediacaran carbonates in southern Anhui, South China. Chem. Geol. 2009, 265, 345–362. [Google Scholar] [CrossRef]
- Corkeron, M.; Webb, G.E.; Moulds, J.; Grey, K. Discriminating stromatolite formation modes using rare earth element geochemistry: Trapping and binding versus in situ precipitation of stromatolites from the Neoproterozoic Bitter Springs Formation, Northern Territory, Australia. Precambrian Res. 2012, 212, 194–206. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Telfeyan, K.; Chevis, D.A.; Rosenheim, B.E.; Leybourne, M.I. Rare earth elements in stromatolites—1. Evidence that modern terrestrial stromatolites fractionate rare earth elements during incorporation from ambient waters. In Evolution of Archean Crust and Early Life; Springer: Dordrecht, The Netherlands, 2014; pp. 385–411. [Google Scholar]
- Kamber, B.S.; Webb, G.E.; Gallagher, M. The rare earth element signal in Archaean microbial carbonate: Information on ocean redox and biogenicity. J. Geol. Soc. 2014, 171, 745–763. [Google Scholar] [CrossRef]
- Censi, P.; Cangemi, M.; Brusca, L.; Madonia, P.; Saiano, F.; Zuddas, P. The behavior of rare-earth elements, Zr and Hf during biologically-mediated deposition of silica-stromatolites and carbonate-rich microbial mats. Gondwana Res. 2015, 27, 209–215. [Google Scholar] [CrossRef]
- Wang, S.; Magalhães, V.H.; Pinheiro, L.M.; Liu, J.; Yan, W. Tracing the composition, fluid source and formation conditions of the methane-derived authigenic carbonates in the Gulf of Cadiz with rare earth elements and stable isotopes. Mar. Pet. Geol. 2015, 68, 192–205. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, M.; Ye, C.; Power, I.M. Trace and rare earth element geochemistry of Holocene hydromagnesite from Dujiali Lake, central Qinghai–Tibetan Plateau, China. Carbonates Evaporites 2019, 34, 1265–1279. [Google Scholar] [CrossRef]
- Debruyne, D.; Hulsbosch, N.; Muchez, P. Unraveling rare earth element signatures in hydrothermal carbonate minerals using a source–sink system. Ore Geol. Rev. 2016, 72, 232–252. [Google Scholar] [CrossRef]
- Franchi, F.; Hofmann, A.; Cavalazzi, B.; Wilson, A.; Barbieri, R. Differentiating marine vs hydrothermal processes in Devonian carbonatemounds using rare earth elements (Kess Kess mounds, Anti-Atlas, Morocco). Chem. Geol. 2015, 409, 69–86. [Google Scholar] [CrossRef]
- Wang, S.; Yan, W.; Chen, Z.; Zhang, N.; Chen, H. Rare earth elements in cold seep carbonates from the southwestern Dongsha area, northern South China Sea. Mar. Pet. Geol. 2014, 57, 482–493. [Google Scholar] [CrossRef]
- Li, R.; Jones, B. Evaluation of carbonate diagenesis: A comparative study of minor elements, trace elements, and rare-earth elements (REE + Y) between Pleistocene corals and matrices from Grand Cayman, British West Indies. Sediment. Geol. 2014, 314, 31–46. [Google Scholar] [CrossRef]
- Loope, G.R.; Kump, L.R.; Arthur, M.A. Shallow water redox conditions from the Permian–Triassic boundary microbialite: The rare earth element and iodine geochemistry of carbonates from Turkey and South China. Chem. Geol. 2013, 351, 195–208. [Google Scholar] [CrossRef]
- Himmler, T.; Bach, W.; Bohrmann, G.; Peckmann, J. Rare earth elements in authigenic methane-seep carbonates as tracers for fluid composition during early diagenesis. Chem. Geol. 2010, 277, 126–136. [Google Scholar] [CrossRef]
- Sasmaz, A.; Sasmaz, B.; Hein, J.R. Geochemical approach to the genesis of the Oligocene-stratiform manganese-oxide deposit, Chiatura (Georgia). Ore Geol. Rev. 2021, 128, 103910. [Google Scholar] [CrossRef]
- Jirsa, F.; Gruber, M.; Stojanovic, A.; Omondi, S.O.; Mader, D.; Körner, W.; Schagerl, M. Major and trace element geochemistry of Lake Bogoria and Lake Nakuru, Kenya, during extreme draught. Geochemistry 2013, 73, 275–282. [Google Scholar] [CrossRef]
- Barrat, J.; Boulegue, J.; Tiercelin, J.; Lesourd, M. Strontium isotopes and rare-earth element geochemistry of hydrothermal carbonate deposits from Lake Tanganyika, East Africa. Geochim. Cosmochim. Acta 2000, 64, 287–298. [Google Scholar] [CrossRef]
- Yi, H.; Lin, J.; Zhao, X.; Zhou, K.; Li, J.; Huang, H. Geochemistry of rare earth elements and origin of positive europium anomaly in Miocene-Oligocene lacustrine carbonates from Tuotuohe Basin of Tibetan Plateau. Acta Sedimentol. Sin. 2008, 26, 1. [Google Scholar]
- Akkuş, A. Salda gölünün jeomorfolojisi. İstanbul Üniversitesi Coğrafya Derg. 1987, 2, 109–115. [Google Scholar]
- Şenel, M.; Selçuk, H.; Bilgin, Z.; Şen, A.; Karaman, T.; Dinçer, M.; Durukan, E.; Arbas, A.; Örçen, S.; Bilgi, C. Çameli (Denizli)-Yeşilova (Burdur)-Elmalı (Antalya) ve kuzeyinin jeolojisi. MTA Rap 1989, 9429, 344. [Google Scholar]
- Russell, M.J.; Ingham, J.K.; Zedef, V.; Maktav, D.; Sunar, F.; Hall, A.J.; Fallick, A.E. Search for signs of ancient life on Mars: Expectations from hydromagnesite microbialites, Salda Lake, Turkey. J. Geol. Soc. 1999, 156, 869–888. [Google Scholar] [CrossRef]
- Doyen, A.; Comlekciler, F.; Kocak, K. Stratigraphic features of the Yesilova ophiolite, Burdur, south-western Turkey. In STRATI 2013; Springer: Cham, Switzerland, 2014; pp. 493–498. [Google Scholar]
- Kirkayak, Y. Salda Gölü Mars Gezegenini Keşfetme ve Anlama Görevinde Ilham Kaynagi Oldu; TMMOB Jeoloji Mühendisleri Odası Yayınları: Ankara, Turkey, 2021. [Google Scholar]
- Davraz, A.; Varol, S.; Sener, E.; Sener, S.; Aksever, F.; Kırkan, B.; Tokgözlü, A. Assessment of water quality and hydrogeochemical processes of Salda alkaline lake (Burdur, Turkey). Environ. Monit. Assess. 2019, 191, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Wiley–Blackwell: Hoboken, NJ, USA, 1985. [Google Scholar]
- Sokal, R.R.; Rohlf, F. Biometry: The Principles and Practice of Statistics in Biological Research; W.H. Freeman and Co.: New York, NY, USA, 1995; p. 880. [Google Scholar]
- Jones, B.; Manning, D.A. Comparison of geochemical indices used for the interpretation of palaeoredox conditions in ancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Balci, N.; Gunes, Y.; Kaiser, J.; On, S.A.; Eris, K.; Garczynski, B.; Horgan, B.H. Biotic and abiotic imprints on Mg-rich stromatolites: Lessons from lake Salda, SW Turkey. Geomicrobiol. J. 2020, 37, 401–425. [Google Scholar] [CrossRef]
- Olivier, N.; Boyet, M. Rare earth and trace elements of microbialites in Upper Jurassic coral-and sponge-microbialite reefs. Chem. Geol. 2006, 230, 105–123. [Google Scholar] [CrossRef]
- Cangemi, M.; Bellanca, A.; Borin, S.; Hopkinson, L.; Mapelli, F.; Neri, R. The genesis of actively growing siliceous stromatolites: Evidence from Lake Specchio di Venere, Pantelleria Island, Italy. Chem. Geol. 2010, 276, 318–330. [Google Scholar] [CrossRef]
- Nothdurft, L.D.; Webb, G.E.; Kamber, B.S. Rare earth element geochemistry of Late Devonian reefal carbonates, Canning Basin, Western Australia: Confirmation of a seawater REE proxy in ancient limestones. Geochim. Cosmochim. Acta 2004, 68, 263–283. [Google Scholar] [CrossRef]
- Bau, M.; Alexander, B. Preservation of primary REE patterns without Ce anomaly during dolomitization of Mid-Paleoproterozoic limestone and the potential re-establishment of marine anoxia immediately after the “Great Oxidation Event”. South Afr. J. Geol. 2006, 109, 81–86. [Google Scholar] [CrossRef]
- Kamber, B.S.; Bolhar, R.; Webb, G.E. Geochemistry of late Archaean stromatolites from Zimbabwe: Evidence for microbial life in restricted epicontinental seas. Precambrian Res. 2004, 132, 379–399. [Google Scholar] [CrossRef]
- Shields, G.A. The marine carbonate and chert isotope records and their implications for tectonics, life and climate on the early earth. Dev. Precambrian Geol. 2007, 15, 971–983. [Google Scholar]
- Riding, R.; Fralick, P.; Liang, L. Identification of an Archean marine oxygen oasis. Precambrian Res. 2014, 251, 232–237. [Google Scholar] [CrossRef]
- Allwood, A.C.; Kamber, B.S.; Walter, M.R.; Burch, I.W.; Kanik, I. Trace elements record depositional history of an Early Archean stromatolitic carbonate platform. Chem. Geol. 2010, 270, 148–163. [Google Scholar] [CrossRef]
- Petrash, D.; Robbins, L.; Shapiro, R.; Mojzsis, S.; Konhauser, K. Chemical and textural overprinting of ancient stromatolites: Timing, processes, and implications for their use as paleoenvironmental proxies. Precambrian Res. 2016, 278, 145–160. [Google Scholar] [CrossRef]
- Franchi, F.; Turetta, C.; Cavalazzi, B.; Corami, F.; Barbieri, R. Trace elements and REE geochemistry of Middle Devonian carbonate mounds (Maïder Basin, Eastern Anti-Atlas, Morocco): Implications for early diagenetic processes. Sediment. Geol. 2016, 343, 56–71. [Google Scholar] [CrossRef]
- Zheng, M. An Introduction to Saline Lakes on the Qinghai—Tibet Plateau; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997; Volume 76. [Google Scholar]
- Braithwaite, C.; Zedef, V. Living hydromagnesite stromatolites from Turkey. Sediment. Geol. 1994, 92, 1–5. [Google Scholar] [CrossRef]
- Kazmierczak, J.; Altermann, W.; Kremer, B.; Kempe, S.; Eriksson, P.G. Mass occurrence of benthic coccoid cyanobacteria and their role in the production of Neoarchean carbonates of South Africa. Precambrian Res. 2009, 173, 79–92. [Google Scholar] [CrossRef]
- Zedef, V.; Russell, M.J.; Fallick, A.E.; Hall, A.J. Genesis of vein stockwork and sedimentary magnesite and hydromagnesite deposits in the ultramafic terranes of southwestern Turkey: A stable isotope study. Econ. Geol. 2000, 95, 429–445. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Thom, J.M.; Dipple, G.M.; Gabites, J.E.; Southam, G. The hydromagnesite playas of Atlin, British Columbia, Canada: A biogeochemical model for CO2 sequestration. Chem. Geol. 2009, 260, 286–300. [Google Scholar] [CrossRef]
- Green, D.; Young, B. Hydromagnesite and dypingite from the Northern Pennine Orefield, northern England. Proc. Yorks. Geol. Soc. 2006, 56, 151–154. [Google Scholar] [CrossRef]
- Zhao, Y.; Nie, F.-j.; Hou, Z.-q.; Li, Z.-q.; Zhao, X.-t.; Ma, Z.-b. Geochemistry of Targejia hot spring type cesium deposit in Tibet. Miner. Depos. Beijing 2007, 26, 163. [Google Scholar]
- Zhao, Y.-y.; Cui, Y.-b.; Zhao, X.-t. Geological and geochemical features and significance of travertine in travertine-island from Zhabuye salt lake, Tibet, China. Geol. Bull. China 2010, 29, 124–141. [Google Scholar]
- Feng, J.-L.; Zhao, Z.-H.; Chen, F.; Hu, H.-P. Rare earth elements in sinters from the geothermal waters (hot springs) on the Tibetan Plateau, China. J. Volcanol. Geotherm. Res. 2014, 287, 1–11. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the earth’s crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Boveiri Monji, A.; Javad Ahmadi, S.; Zolfonoun, E. Selective biosorption of zirconium and hafnium from acidic aqueous solutions by rice bran, wheat bran and platanus orientalis tree leaves. Sep. Sci. Technol. 2008, 43, 597–608. [Google Scholar] [CrossRef]
- Censi, P.; Saiano, F.; Zuddas, P.; Nicosia, A.; Mazzola, S.; Raso, M. Authigenic phase formation and microbial activity control Zr, Hf, and rare earth element distributions in deep-sea brine sediments. Biogeosciences 2014, 11, 1125–1136. [Google Scholar] [CrossRef]
- Hatch, J.; Leventhal, J. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) Stark Shale Member of the Dennis Limestone, Wabaunsee County, Kansas, USA. Chem. Geol. 1992, 99, 65–82. [Google Scholar] [CrossRef]
- Armstrong-Altrin, J.S.; Machain-Castillo, M.L.; Rosales-Hoz, L.; Carranza-Edwards, A.; Sanchez-Cabeza, J.-A.; Ruíz-Fernández, A.C. Provenance and depositional history of continental slope sediments in the Southwestern Gulf of Mexico unraveled by geochemical analysis. Cont. Shelf Res. 2015, 95, 15–26. [Google Scholar] [CrossRef]
- Roy, A.; Chakrabarti, G.; Shome, D. Geochemistry of the Neoproterozoic Narji limestone, Cuddapah Basin, Andhra Pradesh, India: Implication on palaeoenvironment. Arab. J. Geosci. 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Armstrong-Altrin, J.; Verma, S.P.; Madhavaraju, J.; Lee, Y.I.; Ramasamy, S. Geochemistry of upper Miocene Kudankulam limestones, southern India. Int. Geol. Rev. 2003, 45, 16–26. [Google Scholar] [CrossRef]
- Ramos-Vázquez, M.A.; Armstrong-Altrin, J.S.; Rosales-Hoz, L.; Machain-Castillo, M.L.; Carranza-Edwards, A. Geochemistry of deep-sea sediments in two cores retrieved at the mouth of the Coatzacoalcos River delta, western Gulf of Mexico, Mexico. Arab. J. Geosci. 2017, 10, 1–19. [Google Scholar] [CrossRef]
- Mitra, R.; Chakrabarti, G.; Shome, D. Geochemistry of the Palaeo–Mesoproterozoic Tadpatri shales, Cuddapah Basin, India: Implications on provenance, paleoweathering and paleoredox conditions. Acta Geochim. 2018, 37, 715–733. [Google Scholar] [CrossRef]
- Sen, S.; Mishra, M. Geochemistry of Rohtas limestone from Vindhyan Supergroup, Central India: Evidences of detrital input from felsic source. Geochem. Int. 2015, 53, 1107–1122. [Google Scholar] [CrossRef]
- Devi, K.R.; Duarah, B.P. Geochemistry of Ukhrul limestone of Assam-Arakan subduction basin, Manipur, Northeast India. J. Geol. Soc. India 2015, 85, 367–376. [Google Scholar] [CrossRef]
- Tobia, F.H. Stable isotope and rare earth element geochemistry of the Baluti carbonates (Upper Triassic), Northern Iraq. Geosci. J. 2018, 22, 975–987. [Google Scholar] [CrossRef]
- Nagarajan, R.; Madhavaraju, J.; Armstrong-Altrin, J.S.; Nagendra, R. Geochemistry of neoproterozoic limestones of the Shahabad formation, Bhima basin, Karnataka, southern India. Geosci. J. 2011, 15, 9–25. [Google Scholar] [CrossRef]
- Álvaro, J.J.; Ezzouhairi, H.; Ayad, N.A.; Charif, A.; Solá, R.; Ribeiro, M.L. Alkaline lake systems with stromatolitic shorelines in the Ediacaran volcanosedimentary Ouarzazate Supergroup, Anti-Atlas, Morocco. Precambrian Res. 2010, 179, 22–36. [Google Scholar] [CrossRef]
- Bau, M.; Moeller, P. Rare earth element fractionation in metamorphogenic hydrothermal calcite, magnesite and siderite. Mineral. Petrol. 1992, 45, 231–246. [Google Scholar] [CrossRef]
- Bau, M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Mao, L.; Mo, D.; Yang, J.; Guo, Y.; Lv, H. Rare earth elements geochemistry in surface floodplain sediments from the Xiangjiang River, middle reach of Changjiang River, China. Quat. Int. 2014, 336, 80–88. [Google Scholar] [CrossRef]
- German, C.R.; Elderfield, H. Application of the Ce anomaly as a paleoredox indicator: The ground rules. Paleoceanography 1990, 5, 823–833. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Xie, G.; Shen, Y.; Liu, S.; Hao, W. Trace and rare earth element (REE) characteristics of mudstones from Eocene Pinghu Formation and Oligocene Huagang Formation in Xihu Sag, East China Sea Basin: Implications for provenance, depositional conditions and paleoclimate. Mar. Pet. Geol. 2018, 92, 20–36. [Google Scholar] [CrossRef]
- Tostevin, R.; Shields, G.A.; Tarbuck, G.M.; He, T.; Clarkson, M.O.; Wood, R.A. Effective use of cerium anomalies as a redox proxy in carbonate-dominated marine settings. Chem. Geol. 2016, 438, 146–162. [Google Scholar] [CrossRef]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Mazumdar, A.; Tanaka, K.; Takahashi, T.; Kawabe, I. Characteristics of rare earth element abundances in shallow marine continental platform carbonates of Late Neoproterozoic successions from India. Geochem. J. 2003, 37, 277–289. [Google Scholar] [CrossRef]
- Polgári, M.; Hein, J.; Vigh, T.; Szabó-Drubina, M.; Fórizs, I.; Bíró, L.; Müller, A.; Tóth, A. Microbial processes and the origin of the Úrkút manganese deposit, Hungary. Ore Geol. Rev. 2012, 47, 87–109. [Google Scholar] [CrossRef]
- Worash, G.; Valera, R. Rare earth element geochemistry of the Antalo Supersequence in the Mekele Outlier (Tigray region, northern Ethiopia). Chem. Geol. 2002, 182, 395–407. [Google Scholar] [CrossRef]
- Abedini, A.; Calagari, A.A. Rare earth element geochemistry of the Upper Permian limestone: The Kanigorgeh mining district, NW Iran. Turk. J. Earth Sci. 2015, 24, 365–382. [Google Scholar] [CrossRef]
- Chen, L.; Ma, T.; Du, Y.; Xiao, C. Dissolved rare earth elements of different waters in Qaidam Basin, northwestern China. Procedia Earth Planet. Sci. 2017, 17, 61–64. [Google Scholar] [CrossRef]
- Schwinn, G.; Markl, G. REE systematics in hydrothermal fluorite. Chem. Geol. 2005, 216, 225–248. [Google Scholar] [CrossRef]
- Zeyen, N.; Benzerara, K.; Beyssac, O.; Daval, D.; Muller, E.; Thomazo, C.; Tavera, R.; López-García, P.; Moreira, D.; Duprat, E. Integrative analysis of the mineralogical and chemical composition of modern microbialites from ten Mexican lakes: What do we learn about their formation? Geochim. Et Cosmochim. Acta 2021, 305, 148–184. [Google Scholar] [CrossRef]
- Qu, C.; Liu, G.; Zhao, Y. Experimental study on the fractionation of yttrium from holmium during the coprecipitation with calcium carbonates in seawater solutions. Geochem. J. 2009, 43, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Chengli, Q.; Bo, L.; Gang, L. Enrichment of lanthanides in aragonite. J. Rare Earths 2009, 27, 1062–1065. [Google Scholar]

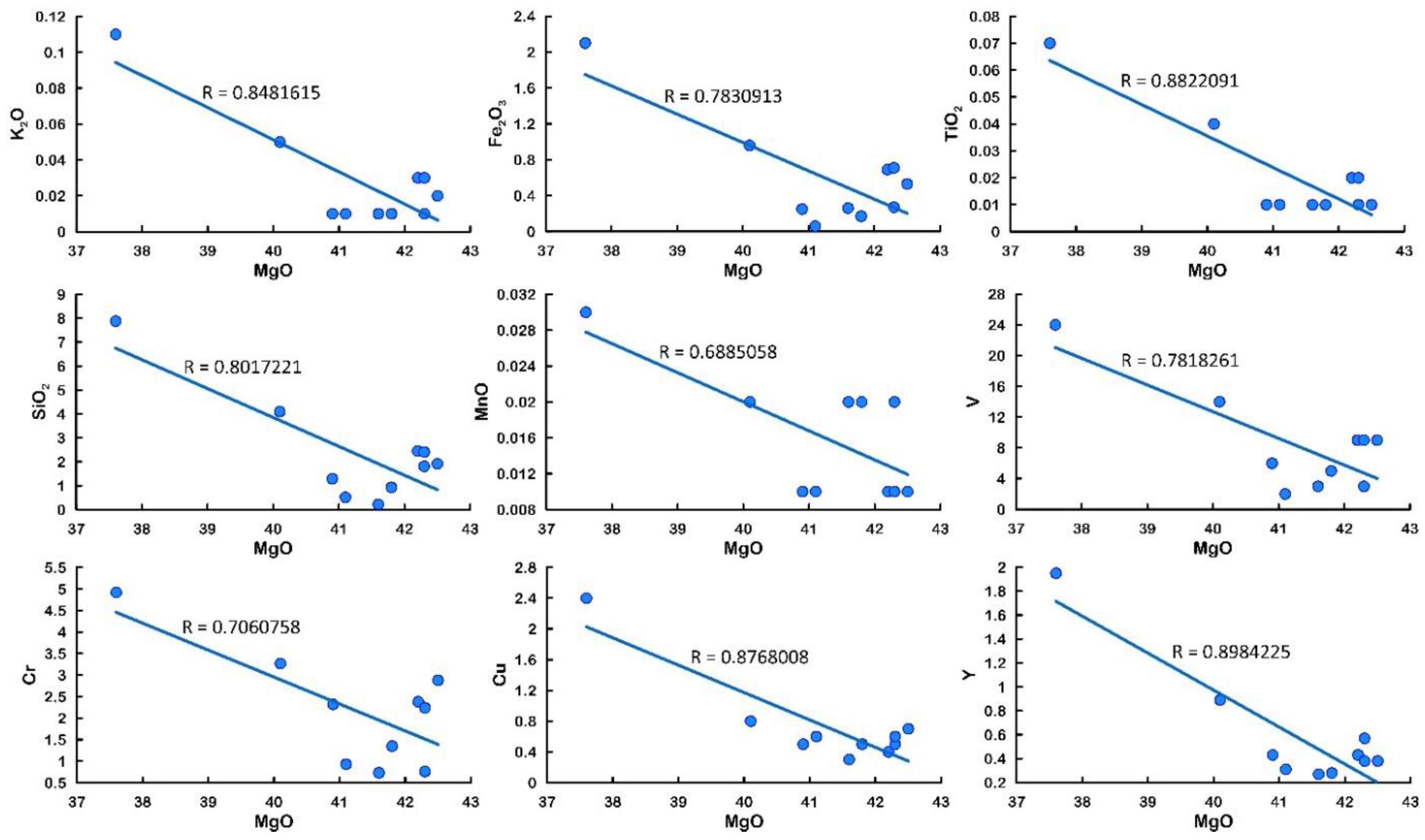
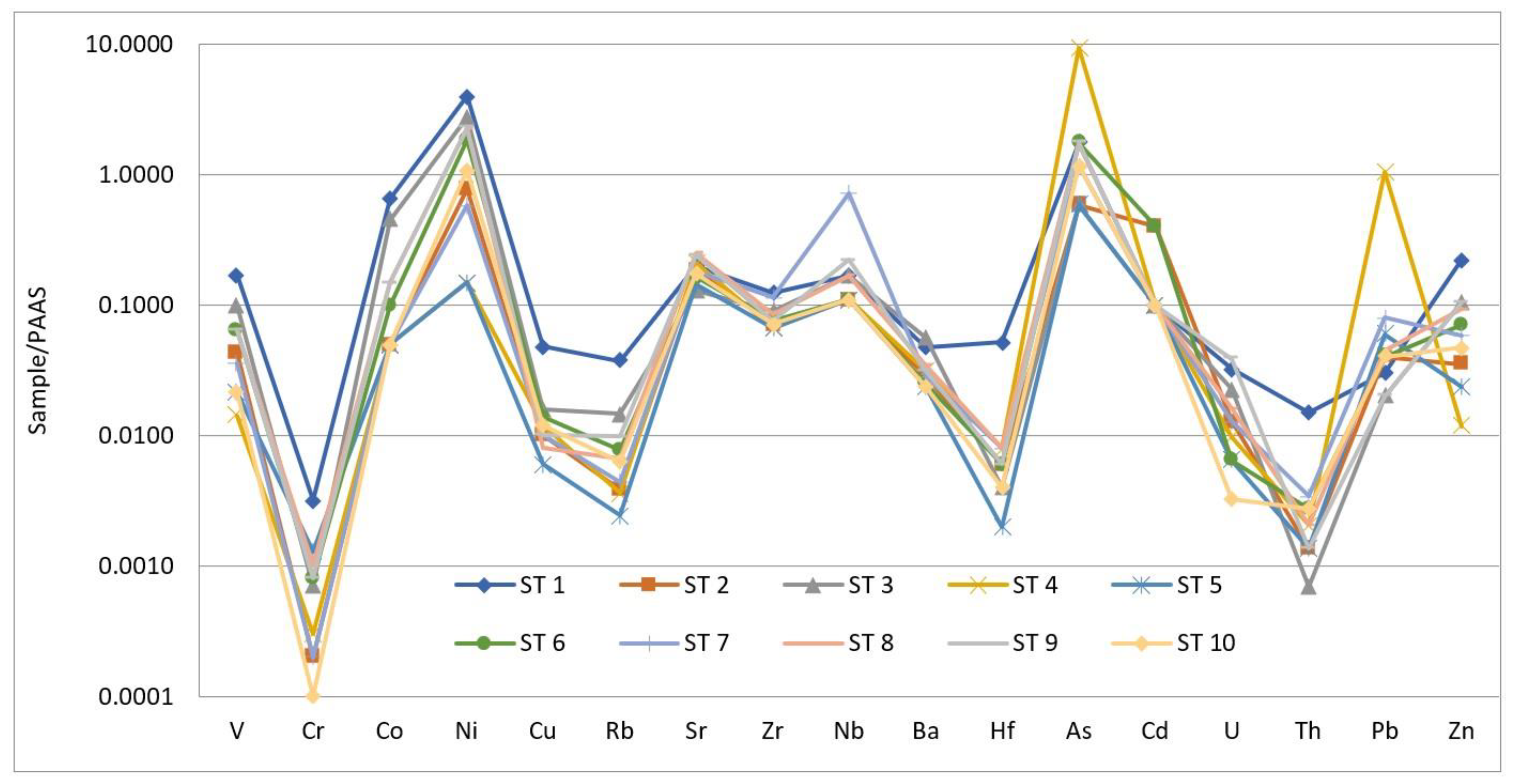
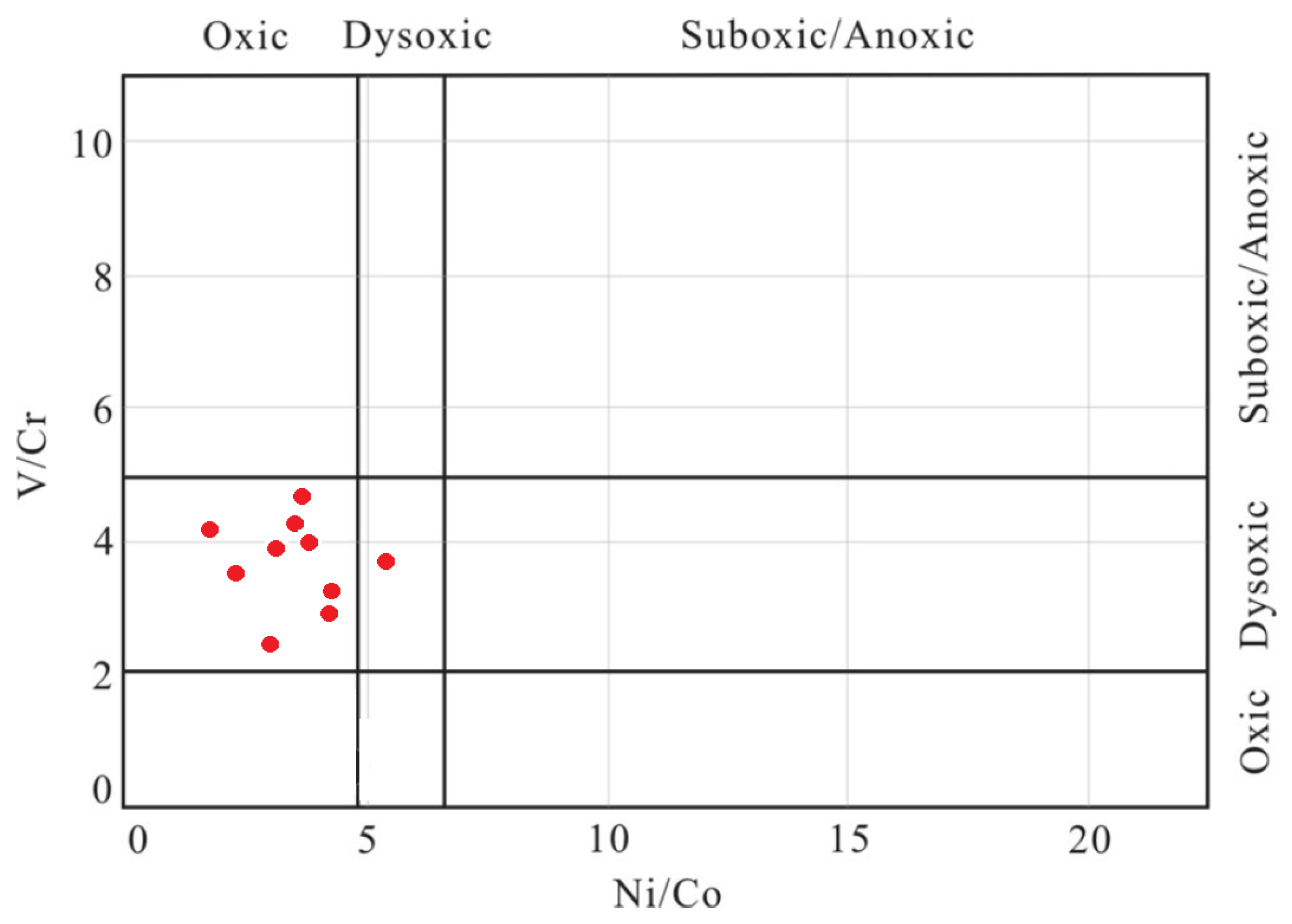
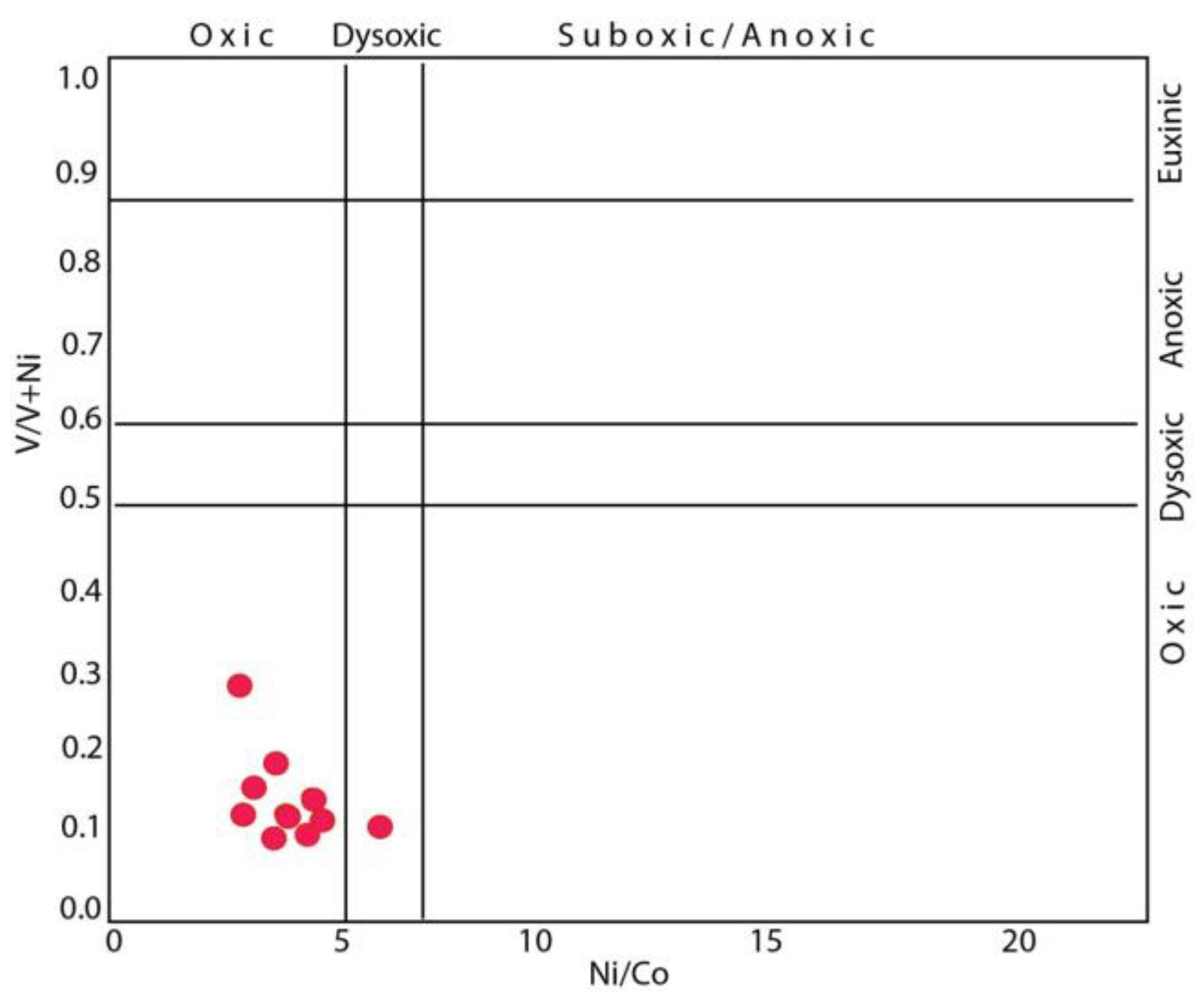
| pH | EC | Eh | Al | Si | B | Br | Cl | NO2 | SO4 | HCO3 | Ca | K | Fe | Mg | Na | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mS/cm | mV | μg/L | μg/L | μg/L | μg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | ||
| SL-01 | 9.49 | 2.62 | −166 | 52 | 400 | 104 | 929 | 192 | 2.06 | 15.6 | 685 | 4.34 | 27.8 | 44 | 281 | 225 |
| SL-02 | 9.33 | 2.18 | −172 | 39 | 376 | 88 | 858 | 196 | 1.88 | 16.8 | 702 | 4.31 | 27.8 | 56 | 289 | 225 |
| SL-03 | 9.27 | 2.36 | −158 | 39 | 366 | 79 | 820 | 193 | 2.24 | 16.2 | 694 | 4.22 | 23.5 | 59 | 285 | 222 |
| SL-04 | 9.52 | 2.55 | −183 | 49 | 386 | 85 | 829 | 194 | 1.96 | 15.4 | 715 | 4.39 | 24.5 | 64 | 286 | 225 |
| SL-05 | 9.26 | 2.22 | −174 | 61 | 543 | 91 | 844 | 204 | 1.78 | 14.8 | 712 | 4.08 | 25.6 | 90 | 284 | 228 |
| SL-06 | 9.33 | 2.38 | −178 | 41 | 415 | 74 | 769 | 178 | 2.08 | 17.3 | 758 | 4.17 | 22.2 | 70 | 275 | 197 |
| SL-07 | 9.44 | 2.12 | −161 | 20 | 510 | 91 | 887 | 208 | 2.01 | 15.6 | 766 | 4.14 | 25.1 | 69 | 281 | 225 |
| SL-08 | 9.38 | 2.44 | −158 | 36 | 701 | 66 | 592 | 139 | 1.92 | 14.6 | 688 | 4.14 | 17.0 | 71 | 234 | 150 |
| SL-09 | 9.42 | 2.46 | −152 | 35 | 359 | 80 | 785 | 195 | 1.69 | 15.8 | 723 | 4.19 | 23.7 | 65 | 283 | 222 |
| SL-10 | 9.36 | 2.28 | −162 | 42 | 366 | 83 | 816 | 202 | 1.94 | 16.1 | 744 | 4.22 | 24.1 | 68 | 285 | 223 |
| Avrg | 9.38 | 2.36 | −166 | 41 | 442 | 84.1 | 813 | 190 | 1.96 | 15.8 | 719 | 4.22 | 24.1 | 66 | 278 | 214 |
| Sample No | ST 1 | ST 2 | ST 3 | ST 4 | ST 5 | ST 6 | ST 7 | ST 8 | ST 9 | ST 10 | Average |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 7.88 | 1.29 | 4.10 | 0.52 | 0.22 | 1.92 | 0.93 | 2.45 | 2.41 | 1.81 | 2.35 |
| Al2O3 | 0.92 | 0.13 | 0.46 | 0.05 | 0.02 | 0.12 | 0.04 | 0.16 | 0.20 | 0.10 | 0.22 |
| MgO | 37.60 | 40.90 | 40.10 | 41.10 | 41.60 | 42.50 | 41.80 | 42.20 | 42.30 | 42.30 | 41.24 |
| CaO | 1.44 | 1.19 | 0.96 | 1.85 | 1.08 | 1.21 | 0.99 | 1.50 | 1.28 | 1.30 | 1.28 |
| Na2O | 0.13 | 0.02 | 0.13 | 0.03 | 0.08 | 0.06 | 0.04 | 0.12 | 0.02 | 0.03 | 0.07 |
| K2O | 0.11 | 0.01 | 0.05 | 0.01 | 0.01 | 0.02 | 0.01 | 0.03 | 0.03 | 0.01 | 0.03 |
| MnO | 0.03 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 |
| Fe2O3 | 2.10 | 0.25 | 0.96 | 0.06 | 0.26 | 0.53 | 0.17 | 0.69 | 0.71 | 0.27 | 0.60 |
| TiO2 | 0.07 | 0.01 | 0.04 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 |
| P2O5 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.03 | 0.01 |
| SO3 | 0.32 | 0.13 | 0.25 | 0.03 | 0.02 | 0.06 | 0.15 | 0.15 | 0.14 | 0.05 | 0.13 |
| Cl | 0.09 | 0.02 | 0.09 | 0.03 | 0.02 | 0.05 | 0.03 | 0.10 | 0.05 | 0.01 | 0.05 |
| LOI | 48.9 | 55.9 | 52.7 | 56.4 | 56.6 | 53.5 | 55.9 | 52.4 | 52.7 | 54.1 | 53.9 |
| Total | 99.6 | 100.0 | 99.9 | 100.0 | 99.9 | 100.0 | 100.0 | 99.9 | 99.9 | 100.0 | 99.9 |
| MgO/CaO | 26.11 | 34.37 | 41.77 | 22.22 | 38.52 | 35.12 | 42.22 | 28.13 | 33.05 | 32.54 | 33.41 |
| Sample No | ST 1 | ST 2 | ST 3 | ST 4 | ST 5 | ST 6 | ST 7 | ST 8 | ST 9 | ST 10 | Average |
|---|---|---|---|---|---|---|---|---|---|---|---|
| V | 24.00 | 6.00 | 14.00 | 2.00 | 3.00 | 9.00 | 5.00 | 9.00 | 9.00 | 3.00 | 8.40 |
| Cr | 4.92 | 2.32 | 3.27 | 0.93 | 0.73 | 2.88 | 1.35 | 2.38 | 2.24 | 0.76 | 2.18 |
| Co | 23.00 | 10.00 | 24.00 | 7.00 | 4.00 | 19.00 | 13.00 | 25.00 | 22.00 | 14.00 | 16.10 |
| Ni | 140.00 | 47.00 | 165.00 | 24.00 | 9.00 | 111.00 | 35.00 | 137.00 | 138.00 | 65.00 | 87.10 |
| Cu | 2.40 | 0.50 | 0.80 | 0.60 | 0.30 | 0.70 | 0.50 | 0.40 | 0.50 | 0.60 | 0.73 |
| Rb | 5.99 | 0.62 | 2.35 | 0.58 | 0.39 | 1.25 | 0.70 | 1.06 | 1.58 | 1.00 | 1.55 |
| Sr | 40.00 | 37.00 | 26.00 | 42.00 | 29.00 | 33.00 | 36.00 | 50.00 | 48.00 | 35.00 | 37.60 |
| Zr | 26.00 | 15.00 | 19.00 | 14.00 | 14.00 | 16.00 | 24.00 | 18.00 | 16.00 | 15.00 | 17.70 |
| Nb | 3.00 | 2.00 | 3.00 | 2.00 | 2.00 | 2.00 | 13.00 | 3.00 | 4.00 | 2.00 | 3.60 |
| Ba | 30.00 | 18.00 | 36.00 | 20.00 | 15.00 | 16.00 | 18.00 | 22.00 | 20.00 | 15.00 | 21.00 |
| Hf | 0.26 | 0.03 | 0.02 | 0.04 | 0.01 | 0.03 | 0.04 | 0.04 | 0.03 | 0.02 | 0.05 |
| As | 3.00 | 1.00 | 1.00 | 16.00 | 1.00 | 3.00 | 2.00 | 3.00 | 3.00 | 2.00 | 3.50 |
| Cd | 0.01 | 0.04 | 0.01 | 0.01 | 0.01 | 0.04 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 |
| U | 0.10 | 0.04 | 0.04 | 0.03 | 0.02 | 0.04 | 0.05 | 0.03 | 0.04 | 0.03 | 0.04 |
| Th | 0.12 | 0.07 | 0.06 | 0.04 | 0.05 | 0.07 | 0.08 | 0.06 | 0.07 | 0.05 | 0.07 |
| Pb | 0.60 | 0.80 | 0.40 | 21.00 | 1.20 | 0.80 | 1.60 | 0.90 | 0.40 | 0.80 | 2.85 |
| Zn | 19.0 | 3.0 | 9.0 | 1.0 | 2.0 | 6.0 | 5.0 | 8.0 | 9.0 | 4.0 | 6.6 |
| Zr/Hf | 100 | 500 | 950 | 350 | 1400 | 533 | 600 | 450 | 533 | 750 | 617 |
| V/Cr | 4.88 | 2.59 | 4.28 | 2.15 | 4.11 | 3.13 | 3.70 | 3.78 | 4.02 | 3.95 | 3.66 |
| Ni/Co | 6.09 | 4.70 | 5.89 | 3.43 | 2.25 | 5.84 | 2.69 | 5.48 | 6.27 | 4.64 | 4.73 |
| U/Th | 0.83 | 0.57 | 0.67 | 0.75 | 0.40 | 0.29 | 0.50 | 0.50 | 0.86 | 0.60 | 0.60 |
| V/V+Ni | 0.15 | 0.11 | 0.08 | 0.08 | 0.25 | 0.08 | 0.13 | 0.06 | 0.06 | 0.04 | 0.10 |
| ∑TE | 324.4 | 143.9 | 304.8 | 151.5 | 82.0 | 221.2 | 155.6 | 280.3 | 274.4 | 158.7 | 209.7 |
| V | Cr | Co | Ni | Cu | Rb | Sr | Y | Zr | Nb | Ba | Hf | As | Cd | U | Th | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V | 1 | |||||||||||||||||
| Cr | 0.96 | 1 | ||||||||||||||||
| Co | 0.72 | 0.73 | 1 | |||||||||||||||
| Ni | 0.75 | 0.77 | 0.97 | 1 | ||||||||||||||
| Cu | 0.87 | 0.79 | 0.40 | 0.42 | 1 | |||||||||||||
| Rb | 0.95 | 0.86 | 0.57 | 0.60 | 0.97 | 1 | ||||||||||||
| Sr | 0.05 | 0.06 | 0.31 | 0.21 | 0.02 | 0.05 | 1 | |||||||||||
| Y | 0.95 | 0.86 | 0.53 | 0.57 | 0.96 | 0.99 | 0.03 | 1 | ||||||||||
| Zr | 0.72 | 0.63 | 0.48 | 0.37 | 0.70 | 0.71 | 0.04 | 0.69 | 1 | |||||||||
| Nb | −0.07 | −0.12 | 0.01 | −0.17 | −0.09 | −0.09 | 0.03 | −0.12 | 0.60 | 1 | ||||||||
| Ba | 0.76 | 0.72 | 0.62 | 0.70 | 0.56 | 0.68 | −0.12 | 0.71 | 0.53 | −0.05 | 1 | |||||||
| Hf | 0.82 | 0.74 | 0.35 | 0.33 | 0.96 | 0.92 | 0.20 | 0.92 | 0.73 | −0.01 | 0.45 | 1 | ||||||
| As | −0.26 | −0.26 | −0.30 | −0.29 | −0.01 | −0.13 | 0.33 | −0.13 | −0.25 | −0.15 | −0.06 | 0.03 | 1 | |||||
| Cd | −0.07 | 0.17 | −0.11 | −0.07 | −0.11 | −0.20 | −0.18 | −0.19 | −0.28 | −0.25 | −0.31 | −0.16 | −0.18 | 1 | ||||
| U | 0.86 | 0.80 | 0.43 | 0.40 | 0.94 | 0.91 | 0.10 | 0.90 | 0.84 | 0.19 | 0.51 | 0.94 | −0.11 | −0.05 | 1 | |||
| Th | 0.83 | 0.80 | 0.47 | 0.42 | 0.82 | 0.83 | 0.12 | 0.81 | 0.84 | 0.28 | 0.39 | 0.85 | −0.34 | 0.07 | 0.95 | 1 | ||
| Pb | −0.37 | −0.36 | −0.45 | −0.43 | −0.09 | −0.23 | 0.19 | −0.22 | −0.30 | −0.13 | −0.08 | −0.07 | 0.98 | −0.17 | −0.20 | −0.43 | 1 | |
| Zn | 0.96 | 0.89 | 0.76 | 0.76 | 0.86 | 0.95 | 0.21 | 0.92 | 0.75 | 0.02 | 0.67 | 0.84 | −0.27 | −0.21 | 0.86 | 0.85 | −0.40 | 1 |
| ST 1 | ST 2 | ST 3 | ST 4 | ST 5 | ST 6 | ST 7 | ST 8 | ST 9 | ST 10 | Average | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| La | 1.38 | 0.88 | 0.62 | 0.36 | 0.32 | 0.64 | 0.56 | 0.42 | 0.78 | 0.75 | 0.67 |
| Ce | 1.45 | 0.37 | 0.56 | 0.26 | 0.28 | 0.42 | 0.28 | 0.35 | 0.75 | 0.38 | 0.51 |
| Pr | 0.26 | 0.03 | 0.09 | 0.04 | 0.04 | 0.05 | 0.04 | 0.06 | 0.08 | 0.03 | 0.07 |
| Nd | 0.92 | 0.02 | 0.28 | 0.06 | 0.04 | 0.16 | 0.08 | 0.19 | 0.21 | 0.12 | 0.21 |
| Sm | 0.37 | 0.05 | 0.07 | 0.06 | 0.02 | 0.06 | 0.05 | 0.05 | 0.08 | 0.07 | 0.09 |
| Eu | 0.08 | 0.03 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.03 | 0.02 | 0.03 |
| Gd | 0.22 | 0.05 | 0.08 | 0.04 | 0.02 | 0.05 | 0.02 | 0.01 | 0.06 | 0.03 | 0.06 |
| Tb | 0.05 | 0.03 | 0.02 | 0.03 | 0.02 | 0.01 | 0.01 | 0.02 | 0.03 | 0.02 | 0.02 |
| Dy | 0.33 | 0.04 | 0.06 | 0.03 | 0.05 | 0.01 | 0.01 | 0.07 | 0.08 | 0.02 | 0.07 |
| Y | 0.07 | 0.02 | 0.03 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 |
| Ho | 0.06 | 0.02 | 0.03 | 0.02 | 0.03 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 |
| Er | 0.09 | 0.05 | 0.06 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.05 | 0.02 | 0.04 |
| Tm | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.01 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 |
| Yb | 0.16 | 0.04 | 0.09 | 0.03 | 0.05 | 0.03 | 0.05 | 0.04 | 0.04 | 0.06 | 0.06 |
| Lu | 0.03 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 |
| ∑REE | 5.5 | 1.67 | 2.04 | 1.03 | 0.96 | 1.51 | 1.19 | 1.31 | 2.28 | 1.57 | 1.9 |
| (Dy/Sm)N | 0.79 | 0.02 | 0.28 | 0.12 | 0.12 | 0.16 | 0.16 | 0.1 | 0.21 | 0.12 | 0.21 |
| Ce/Ce* | 0.56 | 0.35 | 0.53 | 0.47 | 0.55 | 0.47 | 0.37 | 0.49 | 0.64 | 0.41 | 0.48 |
| Eu/Eu* | 1.08 | 2.07 | 1.22 | 1.89 | 2.09 | 1.74 | 2.64 | 1.55 | 2.02 | 1.95 | 1.83 |
| Y/Y* | 0.04 | 0.05 | 0.05 | 0.03 | 0.02 | 0.02 | 0.04 | 0.06 | 0.04 | 0.06 | 0.04 |
| Pr/Pr* | 0.93 | 0.69 | 0.91 | 1.09 | 1.1 | 0.74 | 0.96 | 0.94 | 0.72 | 0.52 | 0.86 |
| Y/Ho | 1.17 | 1 | 1 | 1 | 0.67 | 1 | 0.5 | 2 | 1 | 1 | 1.03 |
| (Yb/Gd)N | 16.67 | 50 | 33.33 | 50 | 33.33 | 100 | 50 | 100 | 50 | 100 | 58.33 |
| (La/Gd)N | 13.14 | 1 | 9.33 | 6 | 4 | 16 | 8 | 9.5 | 10.5 | 12 | 8.95 |
| (La/Sm)N | 18.4 | 0.67 | 14 | 2 | 2 | 16 | 8 | 9.5 | 7 | 6 | 8.36 |
| (Dy/Sm)N | 1.8 | 1.67 | 3 | 0.67 | 1 | 2 | 1 | 1 | 1.67 | 1 | 1.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaya, M.; Yildirim, B.A.; Kumral, M.; Sasmaz, A. Trace and Rare Earth Element (REE) Geochemistry of Recently Formed Stromatolites at Lake Salda, SW Turkey. Water 2023, 15, 733. https://doi.org/10.3390/w15040733
Kaya M, Yildirim BA, Kumral M, Sasmaz A. Trace and Rare Earth Element (REE) Geochemistry of Recently Formed Stromatolites at Lake Salda, SW Turkey. Water. 2023; 15(4):733. https://doi.org/10.3390/w15040733
Chicago/Turabian StyleKaya, Mustafa, Belgin Aydin Yildirim, Mustafa Kumral, and Ahmet Sasmaz. 2023. "Trace and Rare Earth Element (REE) Geochemistry of Recently Formed Stromatolites at Lake Salda, SW Turkey" Water 15, no. 4: 733. https://doi.org/10.3390/w15040733








