Interactions between Aquatic Plants and Cyanobacterial Blooms in Freshwater Reservoir Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
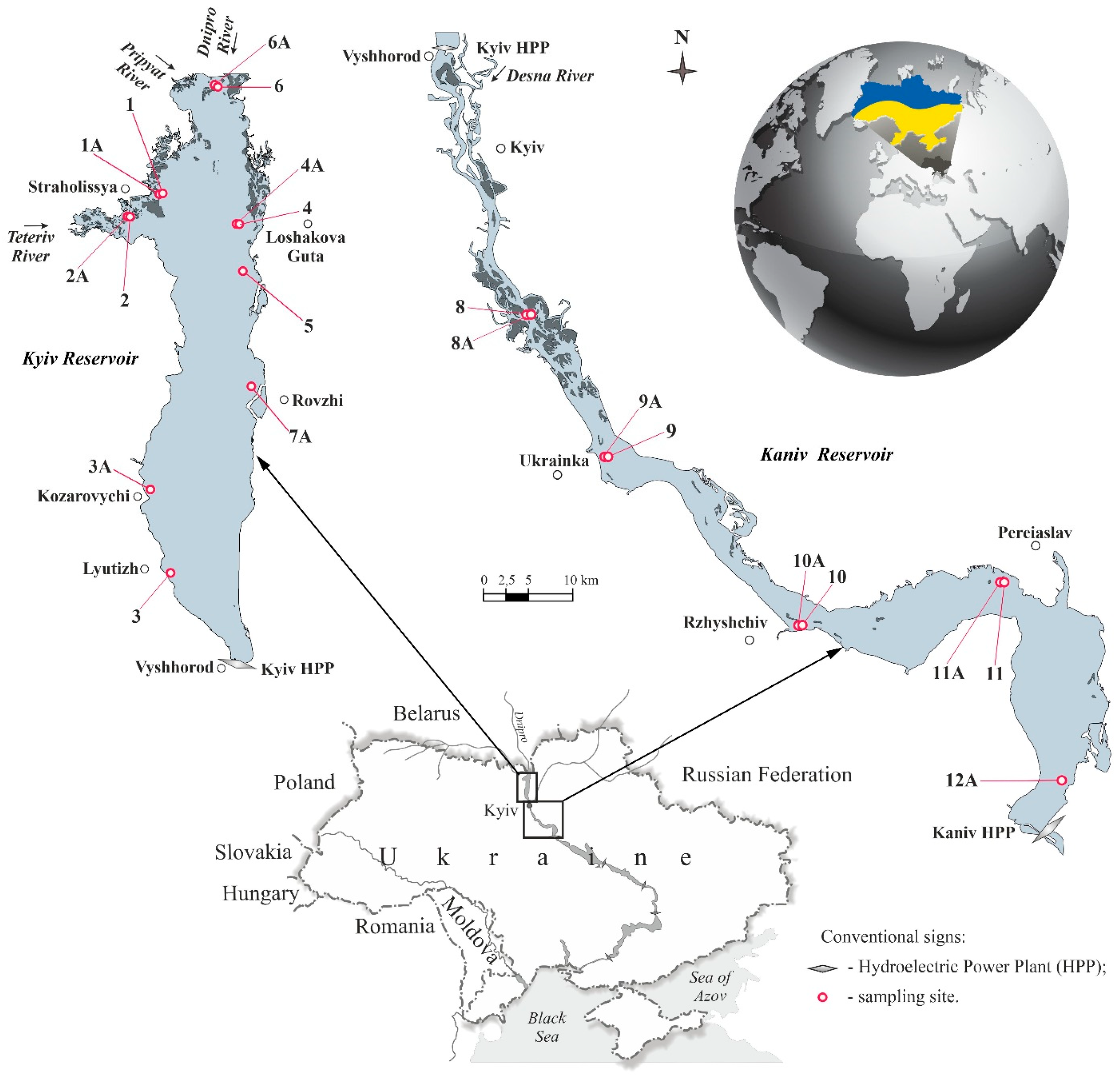
2.1. Study Area
2.2. Sampling Strategy
2.3. Hydrochemical Investigations in the Studied Reservoirs
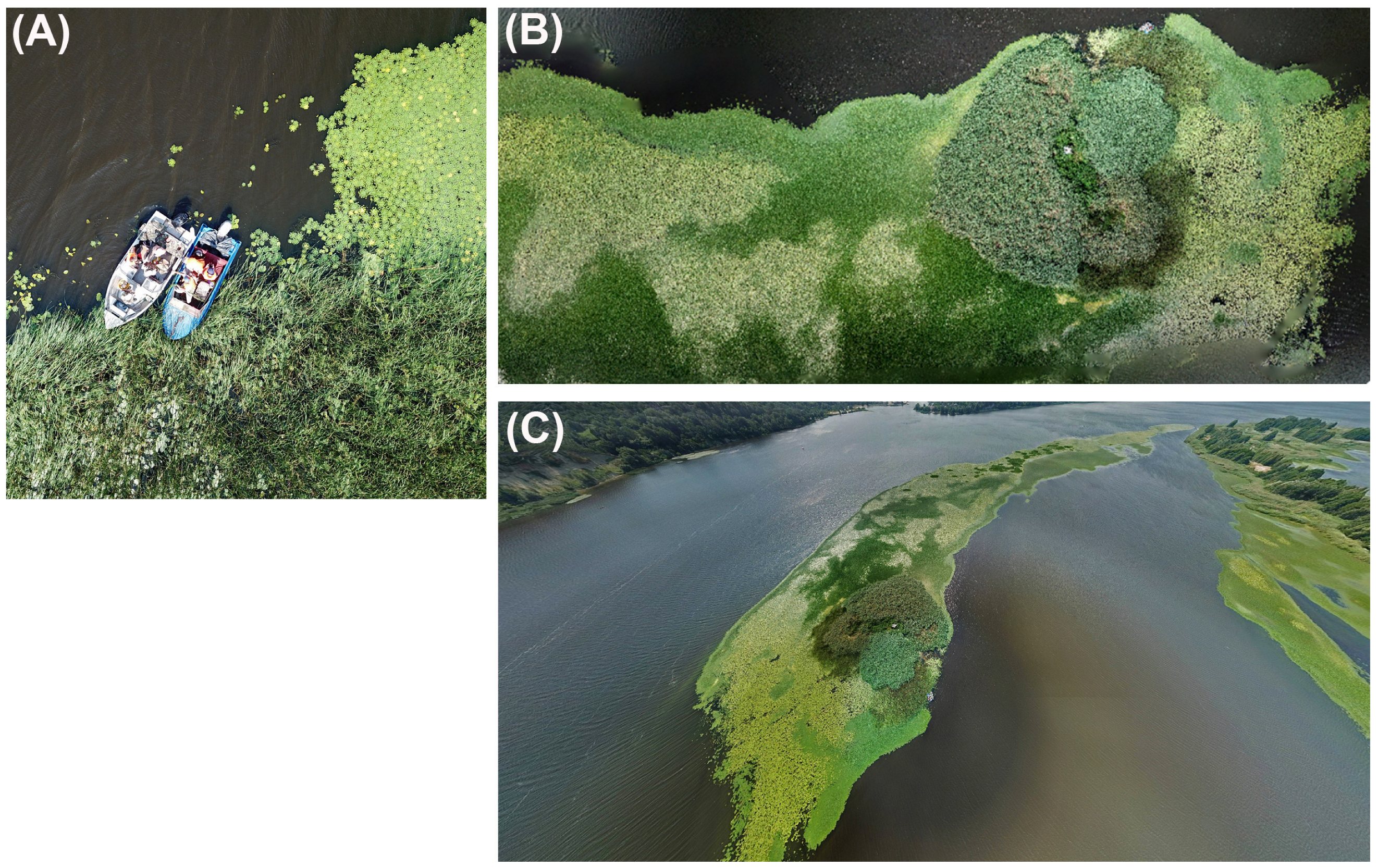
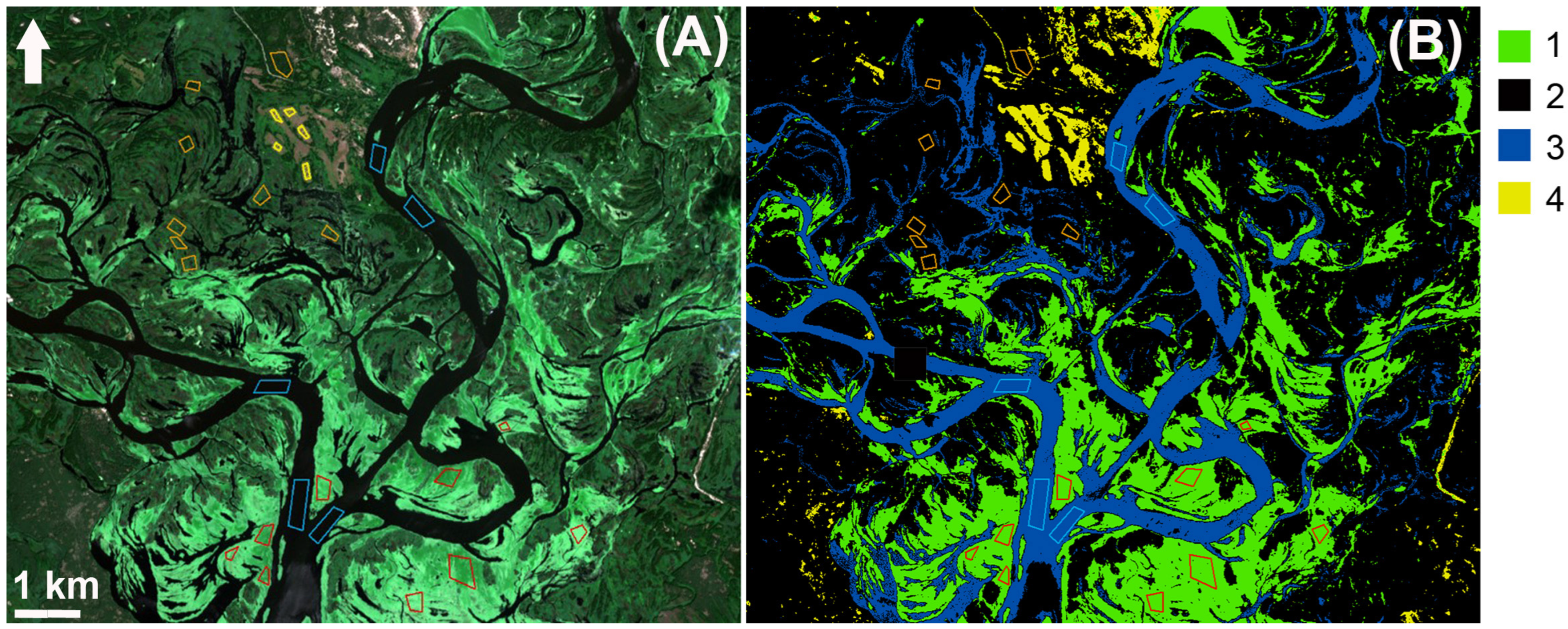
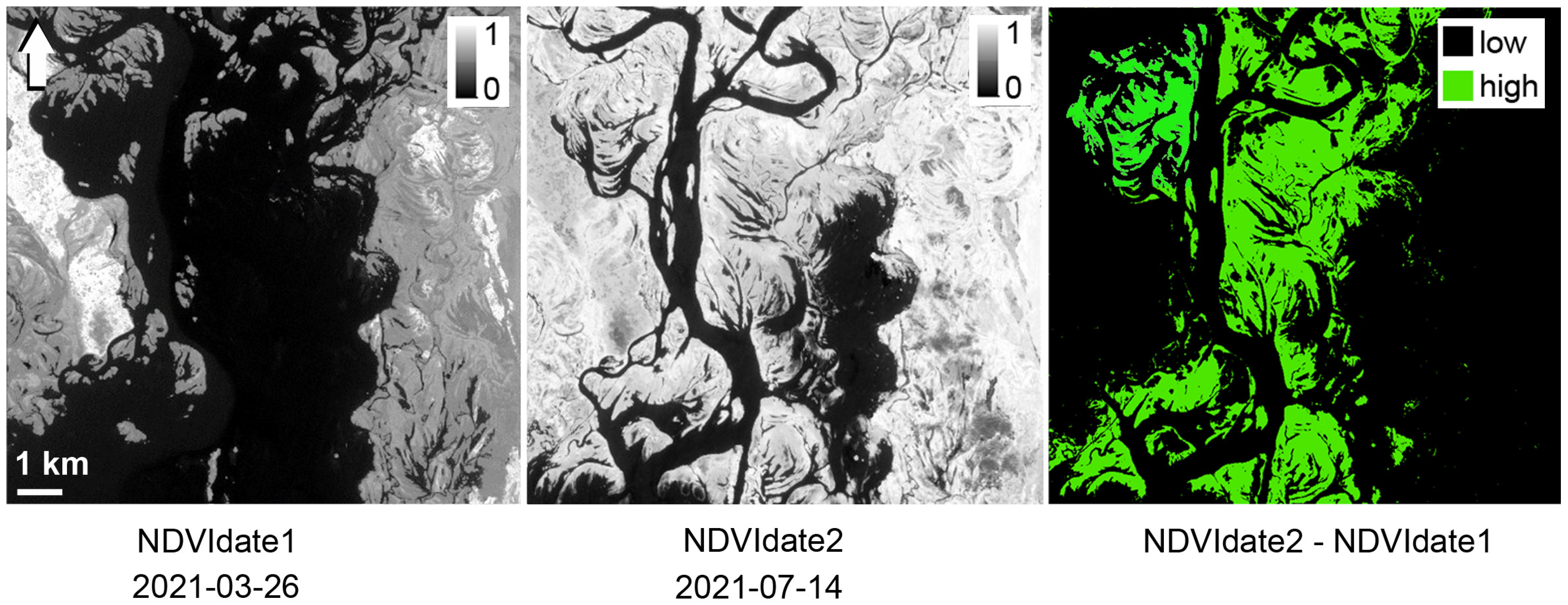
2.4. Mapping of Hydrophytes and Algae Distribution with UAV and Satellite Data
2.5. Sampling Phytoplankton and Further Determination of Algae
2.6. Determination of the Concentration of Chlorophyll a in Phytoplankton
2.7. Statistical Data Analysis
3. Results
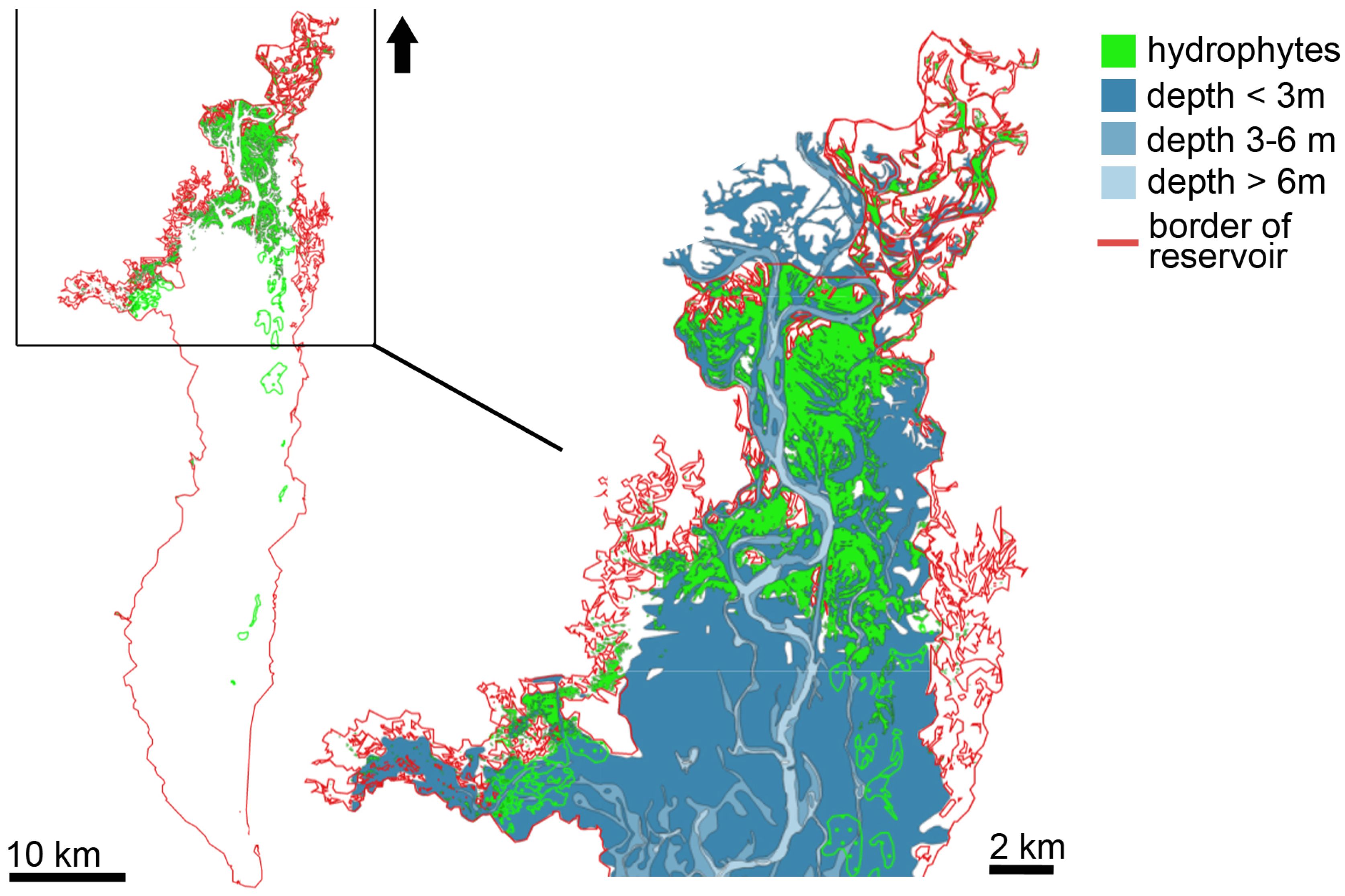
3.1. Distribution of Hydrophytes within the Kyiv and Kaniv Reservoirs
3.1.1. Kyiv Reservoir’s Hydrophytes
3.1.2. Hydrophytes’ Distribution in the Kaniv Reservoir
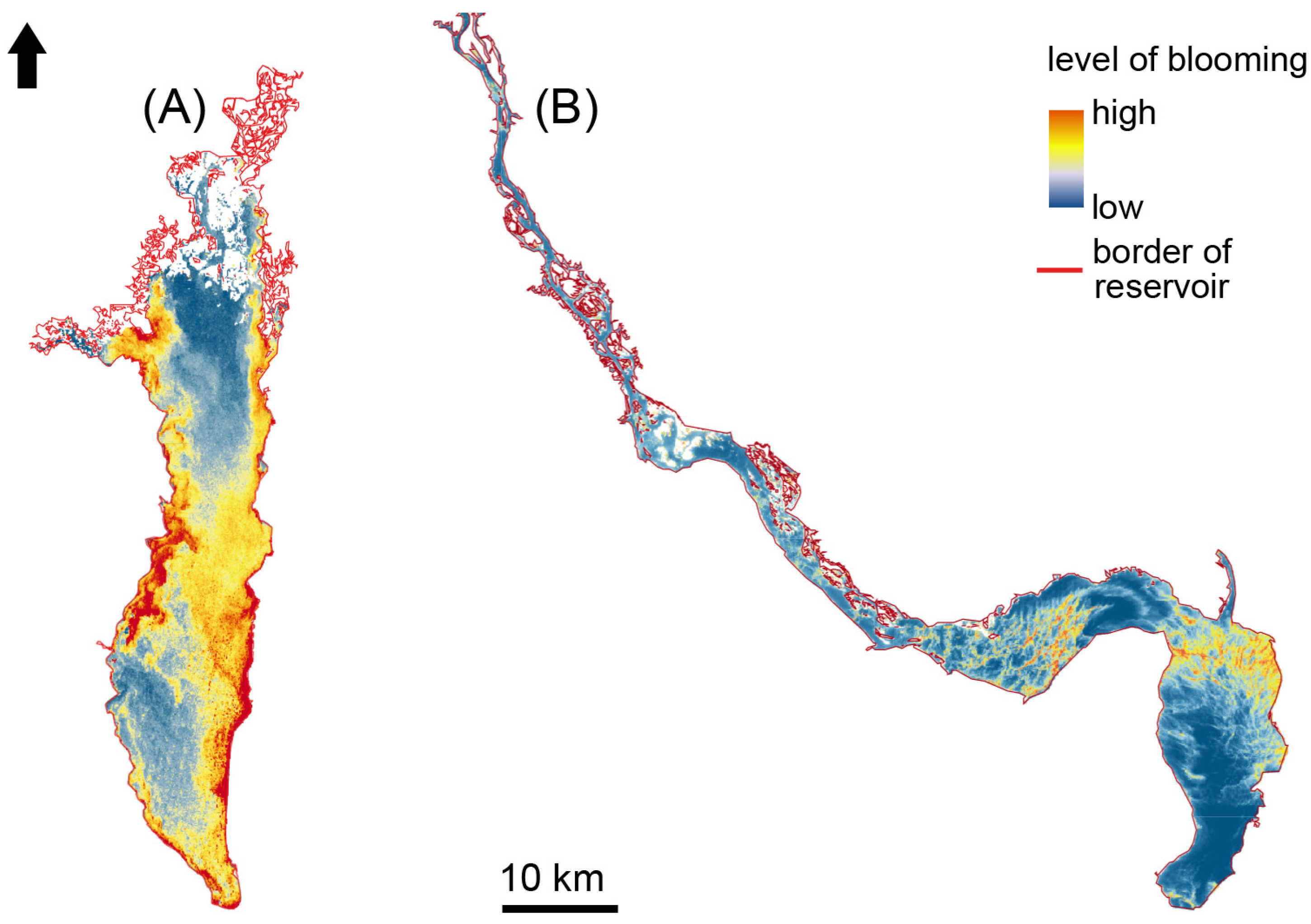
3.2. Distribution of Algal Blooms within the Kyiv and Kaniv Reservoirs
3.3. Hydrochemical Characteristics of the Reservoirs Studied
3.4. Phytoplankton Taxonomic Composition and Quantitative Values in the Kyiv and Kaniv Reservoirs
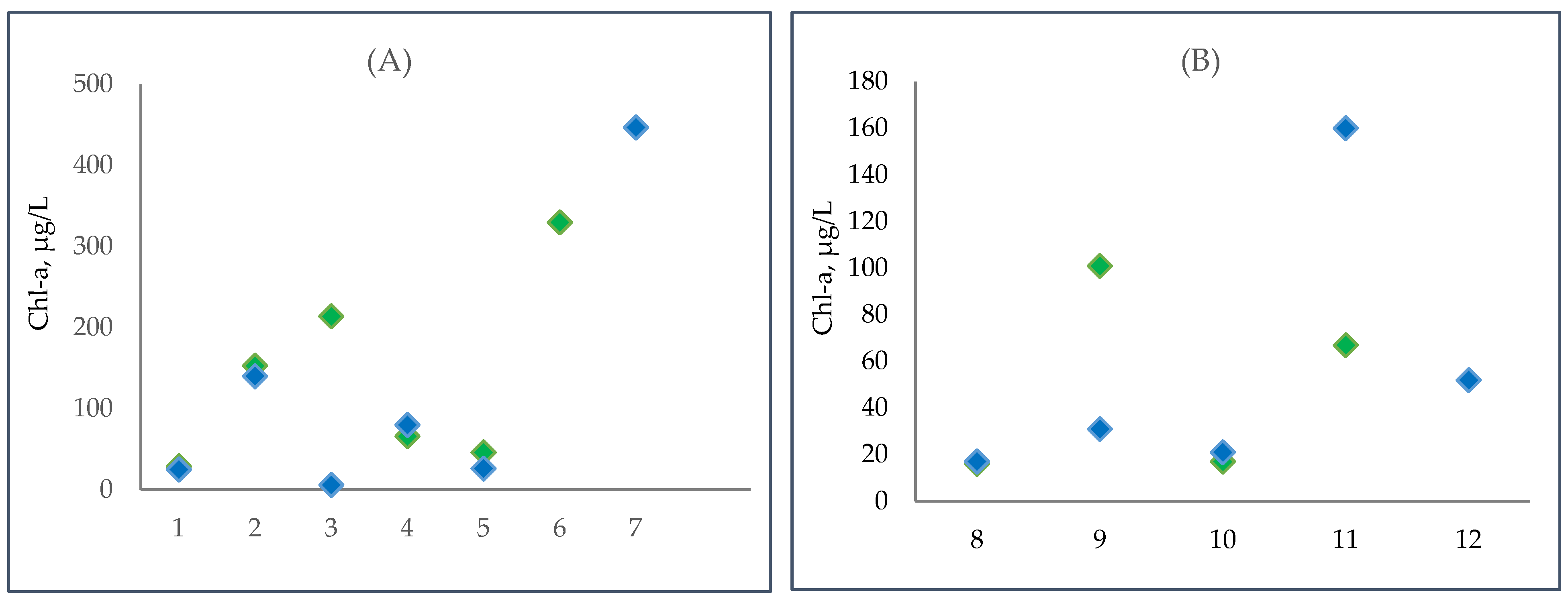
3.5. Changes in Phytoplanktic Chlorophyll a Concentration in the Kyiv and Kaniv Reservoirs
3.6. Statistical Analysis of the Interactions between Aquatic Plants and Cyanobacterial Blooms
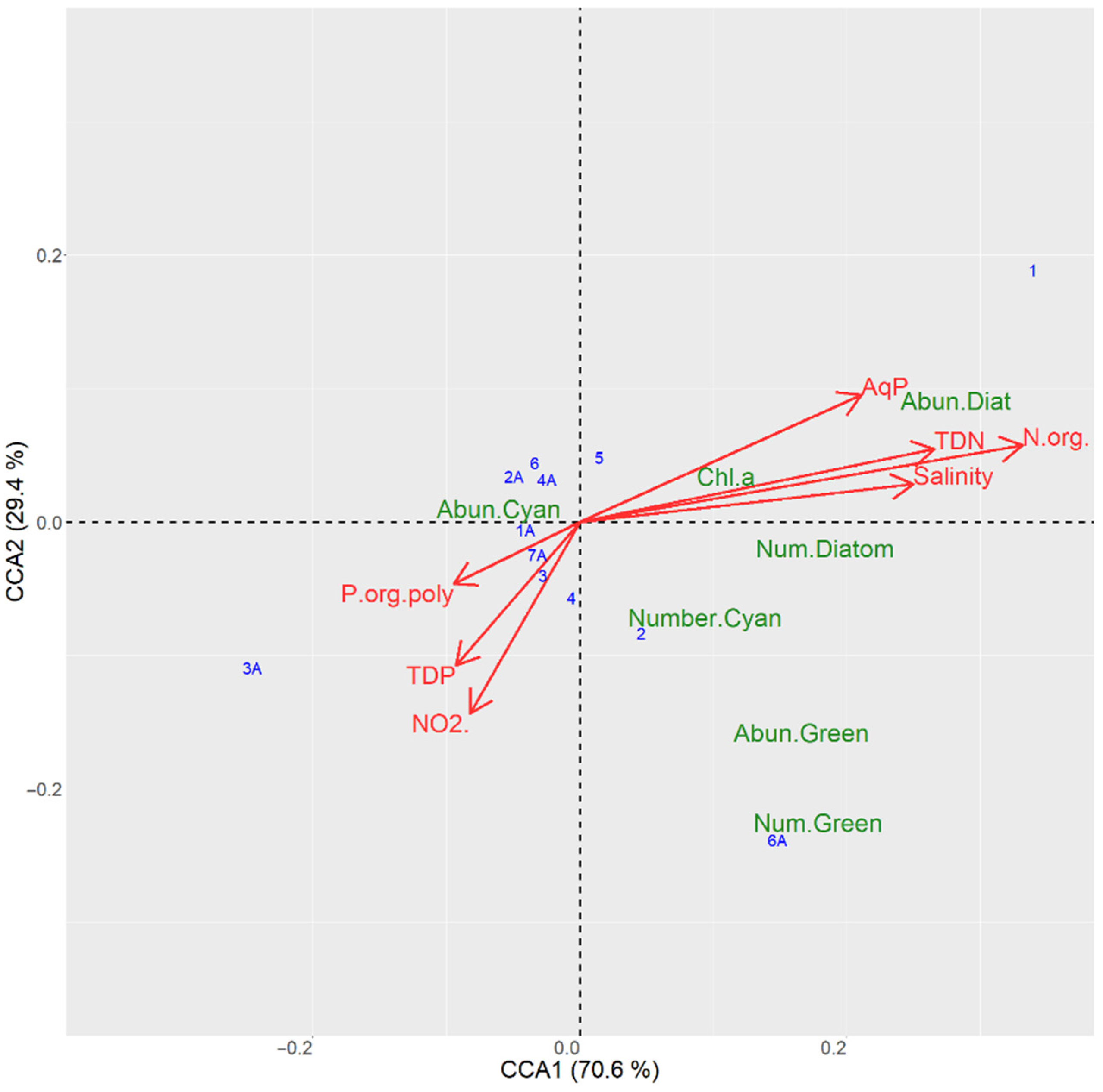
3.6.1. CCA Ordination for the Sampling Sites in the Kyiv Reservoir
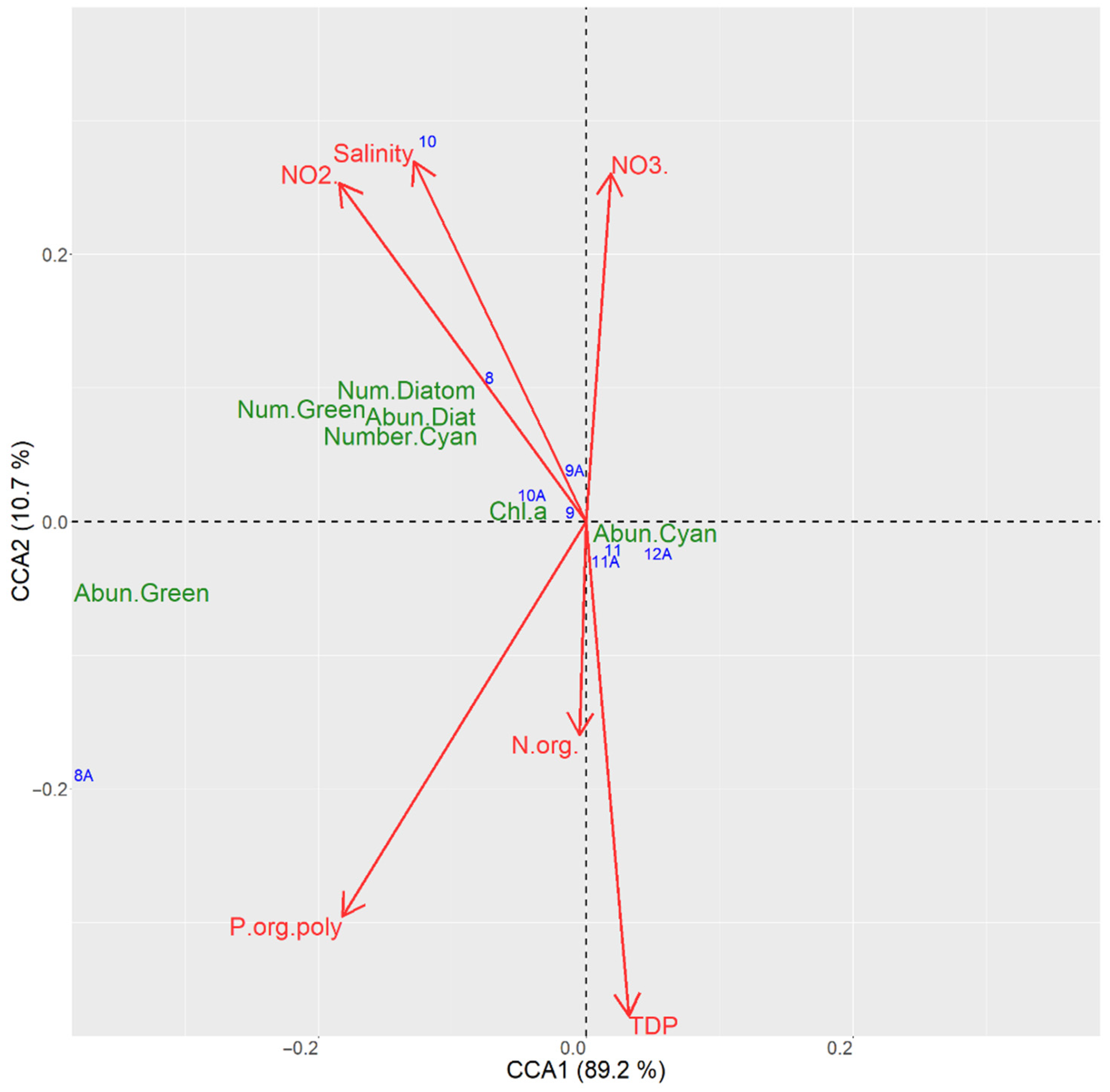
3.6.2. CCA Ordination for the Sampling Sites in the Kaniv Reservoir
4. Discussion
4.1. Spatial Distribution of Patches of Floating-Leaf and Free-Floating Macrophytes in the Kyiv and Kaniv Reservoirs
4.2. Spatial Distribution of Cyanobacterial Blooms in the Kyiv and Kaniv Reservoirs
4.3. Interactions between Aquatic Plants and Cyanobacterial Blooms
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.-Y.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Zanchett, G.; Oliveira-Filho, E.C. Cyanobacteria and cyanotoxins: From impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins 2013, 5, 1896–1917. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.S.; Zimba, P.V. Cyanobacterial bioactive metabolites-A review of their chemistry and biology. Harmful Algae 2019, 83, 42–94. [Google Scholar] [CrossRef] [PubMed]
- Blinkova, M.; Zdraveski, N.; Krstić, S.S. Blue-Green Algae, the Janus of Nature. In Proceedings of the 5th International Scientific Conference BALWOIS, Ohrid, Republic of Macedonia, 28 May–2 June 2012; pp. 1–12. [Google Scholar]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Burford, M.A.; Beardall, J.; Willis, A.; Orr, P.T.; Magalhaes, V.F.; Rangel, L.M.; Azevedo, S.M.F.O.E.; Neilan, B.A. Understanding the winning strategies used by the bloom-forming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae 2016, 54, 44–53. [Google Scholar] [CrossRef]
- Kurmayer, R.; Deng, L.; Entfellner, E. Role of toxic and bioactive secondary metabolites in colonization and bloom formation by filamentous cyanobacteria Planktothrix. Harmful Algae 2016, 54, 69–86. [Google Scholar] [CrossRef]
- Li, X.; Dreher, T.W.; Li, R. An overview of diversity, occurrence, genetics and toxin production of bloom-forming Dolichospermum (Anabaena) species. Harmful Algae 2016, 54, 54–68. [Google Scholar] [CrossRef]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Lürling, M.; Van Oosterhout, F.; Faassen, E. Eutrophication and Warming Boost Cyanobacterial Biomass and Microcystins. Toxins 2017, 9, 64. [Google Scholar] [CrossRef]
- Reynolds, C.S. Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006; p. 552. [Google Scholar]
- Tarczyńska, M.; Romanowska-Duda, Z.; Jurczak, T.; Zalewski, M. Toxic cyanobacterial blooms in a drinking water reservoir—Causes, consequences and management strategy. Water Sci. Technol. 2001, 1, 237–246. [Google Scholar] [CrossRef]
- Kuo, J.-T.; Lung, W.-S.; Yang, C.-P.; Liu, W.-C.; Yang, M.-D.; Tang, T.-S. Eutrophication modelling of reservoirs in Taiwan. Environ. Model. Softw. 2006, 21, 829–844. [Google Scholar] [CrossRef]
- Khilchevskyi, V.; Grebin, V.; Dubniak, S.; Zabokrytska, M.; Bolbot, H. Large and small reservoirs of Ukraine. J. Water Land Dev. 2022, 52, 101–107. [Google Scholar] [CrossRef]
- Nezbrytska, I.; Usenko, O.; Konovets, I.; Leontieva, T.; Abramiuk, I.; Goncharova, M.; Bilous, O. Potential use of aquatic vascular plants to control cyanobacterial blooms: A review. Water 2022, 14, 1727. [Google Scholar] [CrossRef]
- Tan, K.; Huang, Z.; Ji, R.; Qiu, Y.; Wang, Z.; Liu, J. A review of allelopathy on microalgae. Microbiology 2019, 165, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Stroom, J.M.; Kardinaal, W.E.A. How to combat cyanobacterial blooms: Strategy toward preventive lake restoration and reactive control measures. Aquat. Ecol. 2016, 50, 541–576. [Google Scholar] [CrossRef]
- Basu, B.K.; Kalff, J.; Pinel-Alloul, B. The influence of macrophyte beds on plankton communities and their export from fluvial lakes in the St Lawrence River. Freshw. Biol. 2000, 45, 373–382. [Google Scholar] [CrossRef]
- Peretyatko, A.; Triest, L.; Symoens, J.J. Impact of macrophytes on phytoplankton in eutrophic peri-urban ponds, implications for pond management and restoration. Belg. J. Bot. 2007, 140, 83–99. [Google Scholar] [CrossRef]
- Usenko, O.M.; Sakevich, A.I. Allelopathic influence of higher aquatic plants on the functional activity of plankton algae. Hydrobiol. J. 2005, 41, 54–66. [Google Scholar] [CrossRef]
- De Tezanos Pinto, P.; Allende, L.; O’Farrell, I. Influence of free-floating plants on the structure of a natural phytoplankton assemblage: An experimental approach. J. Plankton Res. 2006, 29, 47–56. [Google Scholar] [CrossRef]
- Timchenko, V.M.; Linnyk, P.M.; Kholodko, O.P. Abiotic Components of the Ecosystem of the Kyiv Reservoir: A Monograph; LOGOS: Kyiv, Ukraine, 2013; p. 60. (In Ukrainian) [Google Scholar]
- Denisova, A.I.; Timchenko, V.M.; Nakshina, E.P.; Novikov, B.Y.; Ryabov, A.K.; Bass, Y.I. Hydrology and Hydrochemistry of the Dnieper River and Its Basin; Naukova Dumka: Kyiv, Ukraine, 1989; p. 215. (In Russian) [Google Scholar]
- Timchenko, V.M. Ecological Hydrology of Reservoirs of Ukraine: Monograph; Naukova Dumka: Kyiv, Ukraine, 2006; p. 383. (In Ukrainian) [Google Scholar]
- Romanenko, V.D. The Dnieper Reservoirs, Their Significance and Problems. Hydrobiol. J. 2018, 54, 3–9. [Google Scholar] [CrossRef]
- Tseeba, Y.Y.; Maystrenko, Y.G. Kiev Reservoir: Hydrochemistry, Biology, Productivity; Naukova Dumka: Kyiv, Ukraine, 1972; p. 460. (In Ukrainian) [Google Scholar]
- Almazov, A.M.; Denisova, A.I.; Majstrenko, Y.G.; Nakhshina, E.P. Hydrochemistry of the Dnieper, Its Reservoirs and Tributaries; Naukova Dumka: Kyiv, Ukraine, 1967; p. 316. (In Ukrainian) [Google Scholar]
- Nabyvanets, B.Y.; Osadchiy, V.I.; Osadcha, N.M.; Nabyvanets, Y.B. Analytical Chemistry of Surface Waters; Naukova Dumka: Kyiv, Ukraine, 2007; p. 456. (In Ukrainian) [Google Scholar]
- DSTU 4078-2001; Water Quality. Determination of Nitrate. Part 3: Spectrometric Method Using Sulfosalicylic Acid (ISO 7890 3:1998, MOD); State Standard of Ukraine: Kyiv, Ukraine, 2002; p. 7. (In Ukrainian)
- Sentinel Online. Level-2A Algorithm Overview. Available online: https://sentinels.copernicus.eu/web/sentinel/technical-guides/sentinel-2-msi/level-2a/algorithm (accessed on 14 December 2022).
- Hawryło, P.; Wężyk, P. Predicting growing stock volume of scots pine stands using Sentinel-2 satellite imagery and airborne image-derived point clouds. Forests 2018, 9, 274. [Google Scholar] [CrossRef]
- Image Classification Using the ArcGIS Spatial Analyst Extension. Available online: https://desktop.arcgis.com/en/arcmap/latest/extensions/spatial-analyst/image-classification/image-classification-using-spatial-analyst.htm (accessed on 15 December 2022).
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the great plains with ERTS. In Third ERTS-1 Symposium NASA, NASA SP-351; NASA: Washington, DC, USA, 1974; pp. 309–317. [Google Scholar]
- Krammer, K. Bacillariophyceae 1. Naviculaceae. In Süßwasserflora von Mitteleuropa; Gustav Fischer Verlag: Stuttgart, Germany, 1986; pp. 1–876. [Google Scholar]
- Krammer, K. Bacillariophyceae. 4. Achnanthaceae. Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema. In Süßwasserflora von Mitteleuropa; Gustav Fischer Verlag: Stuttgart, Germany, 1991; pp. 1–437. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Centrales, Fragilariaceae, Eunotiaceae. In Süßwasserflora von Mitteleuropa; Gustav Fischer Verlag: Stuttgart, Germany, 1991; pp. 1–600. [Google Scholar]
- Komárek, J. Cyanoprokaryota 3. Teil/3rd Part: Heterocystous genera. In Süsswasserflora von Mitteleuropa; Budel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer Spektrum: Heidelberg, Germany, 2013; pp. 1–1130. [Google Scholar]
- Komarek, J.; Anagnostidis, K. Cyanoprokaryota. 1. Chroococcales. In Susswasserflora von Mirreleuropa; Bd. 19/1; Gustav Vischer Verlag: Jena, Germany, 1998; pp. 1–548. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. Teil 2. Oscillatoriales. In Süßwasserflora von Mitteleuropa; Bd 19/2; Elsevier: München, Germany, 2005; pp. 1–759. [Google Scholar]
- Krammer, K. The Genus Pinnularia. In Diatoms of Europe; A.R.G. Gantner Verlag: Königstein, Germany, 2000; Volume 1, pp. 1–703. [Google Scholar]
- Krammer, K. Cymbella. In Diatoms of Europe; A.R.G. Gantner Verlag, K.-G.: Ruggell, Liechtenstein, 2002; Volume 3, pp. 1–584. [Google Scholar]
- Krammer, K. Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella. In Diatoms of Europe; A.R.G. Gantner Verlag, K.-G.: Ruggell, Liechtenstein, 2003; Volume 4, pp. 1–530. [Google Scholar]
- Vetrova, Z.I. Euglenophyta. In Flora of Algae of the Continental Water Bodies of the Ukrainian SSR. 1; Naukova Dumka: Kiev, Ukraine, 1986; pp. 1–348. (In Russian) [Google Scholar]
- Tsarenko, P.M. Short Identification Book of Chlorococcales of the Ukraine SSR; Naukova Dumka: Kiev, Ukraine, 1990; pp. 1–208. (In Russian) [Google Scholar]
- Vetrova, Z.I. Euglenales, Family 2. Euglenaceae. Genus 1. Trachelomonas. Group II. Genus 2. Strombomonas. Genus 3. Euglena. In Flora of Algae of the Continental Water Bodies of Ukraine. 1; Naukova Dumka: Kiev, Ukraine, 1993; pp. 1–260. (In Russian) [Google Scholar]
- Krammer, K. Die cymbelloiden Diatomeen. Eine Monographie der weltweit bekannten Taxa. 1. Allegmeins und Encyonema Part. In Bibliotheca Diatomologica; J. Cramer: Berlin Germany; Stuttgart, Germany, 1997; pp. 1–382. [Google Scholar]
- Palamar-Mordvintseva, G.M. Desmidiales. Gonatozygaceae, Peniaceae, Closteriaceae, Desmidiaceae. In Flora of Algae of the Continental Water Bodies of Ukraine; Fasc. 1, Part 1; Akademperiodyka Press: Kiev, Ukraine, 2003; pp. 1–355. (In Ukrainian) [Google Scholar]
- Vetrova, Z.I. Euglenofitovye vodorosli. In Flora of Algae of the Continental Water Bodies of Ukraine. 2; Lileia Press: Kiev, Ukraine, 2004; pp. 1–272. (In Russian) [Google Scholar]
- Palamar-Mordvintseva, G.M. Desmidiales. In Flora of Algae of the Continental Water Bodies of Ukraine. Fasc. 1, Pars 2; Akademperiodyka Press: Kyiv, Ukraine, 2005; pp. 1–573. (In Ukrainian) [Google Scholar]
- Kovalenko, O.V. Cyanoprokaryota. Ordo Chroococcales. In Flora of Algae of Ukraine. 1. Fasc. 1; VELES: Kyiv, Ukraine, 2009; pp. 1–397. (In Ukrainian) [Google Scholar]
- Palamar-Mordvintseva, G.M.; Petlovany, O.A. Streptophyta. Familia Mesotaeniaceae. In Flora of Algae of Ukraine. 12. Fasc. 1; VELES: Kyiv, Ukraine, 2009; pp. 1–158. (In Ukrainian) [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; pp. 1–942. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2021. Available online: http://www.algaebase.org (accessed on 21 December 2022).
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- SCOR-UNESCO. Determination of Photosynthetic Pigments in Sea Water; Monographs on Oceanographic Methodology, 1; UNESCO: Paris, France, 1966; p. 69. [Google Scholar]
- Jeffrey, S.; Humphrey, F.H. New spectrophotometric equations for determining chlorophyll a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 171–194. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier Publishing: Amsterdam, The Netherlands, 2012; pp. 1–989. [Google Scholar]
- Oksanen, F.J.; Blanchet, G.; Friendly, U.M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-3. 2017. Available online: https://CRAN.R-project.org/package=vegan (accessed on 14 December 2022).
- Šmilauer, P.; Lepš, J. Using Canoco 5. In Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: Cambridge, UK, 2014; pp. 15–38. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Dodds, W.K.; Jones, J.R.; Welch, E.B. Suggested classification of stream trophic state: Distributions of temperate stream types by chlorophyll, total nitrogen, and phosphorus. Water Res. 1998, 32, 1455–1462. [Google Scholar] [CrossRef]
- Kutser, T. Quantitative Detection of Chlorophyll in Cyanobacterial Blooms by Satellite Remote Sensing. Limnol. Oceanogr. 2004, 49, 2179–2189. [Google Scholar] [CrossRef]
- Aranha, T.R.B.T.; Martinez, J.-M.; Souza, E.P.; Barros, M.U.G.; Martins, E.S.P.R. Remote Analysis of the Chlorophyll-a Concentration Using Sentinel-2 MSI Images in a Semiarid Environment in Northeastern Brazil. Water 2022, 14, 451. [Google Scholar] [CrossRef]
- Timchenko, V.M.; Timchenko, O.V.; Guliyaeva, O.O.; Kholodko, O.P. Ecological and hydrodynamic zoning of cascade valley reservoirs. Hydrol. Hydrochem. Hydroecology 2010, 19, 23–29. [Google Scholar]
- Timchenko, V.M.; Timchenko, O.V.; Lakhai, Y.O.; Ivanova, N.O.; Kushnir, N.I. Environmental Regulation of the operation mode of Dnipro hydroelectric power stations. Sci. Technol. 2009, 2, 1–4. (In Ukrainian) [Google Scholar]
- Timchenko, V.M. Ecological hydrology of Dnieper reservoirs. Hydrobiol. J. 2006, 42, 76–90. [Google Scholar] [CrossRef]
- Tsaplina, K.M.; Linchuk, M.I. Distribution of plant communities in the Kaniv reservoir depending on environmental factors. Sci. Work. UkrNDGMI 2003, 251, 184–189. (In Ukrainian) [Google Scholar]
- Tsaplina, Y.N.; Kholodko, O.P.; Linchuk, M.I. Thickets of higher aquatic plants in the estuarine sections of the rivers flowing into the Kiev reservoir. Hydrobiol. J. 2014, 50, 17–31. [Google Scholar] [CrossRef]
- Kurhanskyi, S.V.; Buzevych, O.A. The influence of the development of the water nut (Trapa natans) on the conditions of grazing of young fish of the Kyiv reservoir. Fish. Sci. Ukr. 2014, 28, 5–13. (In Ukrainian) [Google Scholar]
- Shevchuk, S.A.; Vishnevskyi, V.I.; Shevchenko, I.A. The use of DZD data to establish the ecological state of the Dnipro reservoirs. In Proceedings of the Central Geophysical Observatory; Kosovets, O.O., Ed.; Interpress LTD: Kyiv, Ukraine, 2014; Volume 24, pp. 72–78. (In Ukrainian) [Google Scholar]
- Shevchuk, S.A.; Vyshnevskyi, V.I.; Shevchenko, I.A.; Kozytskyi, O.M. Research of water objects of Ukraine using the data of remote sensing of the Earth. Land Reclam. Water Mngmn. 2019, 2, 146–156. (In Ukrainian) [Google Scholar]
- Soloveychuk, Y.S.; Starodubtsev, V.M. Seasonal dynamics of the Kaniv reservoir overgrow. In Proceedings of the Intellectual potential of the XXI century ‘2015. Biology—Ecology and Biotechnology, Odesa, Ukraine, 10–22 November 2015; (In Ukrainian). Available online: https://sworld.education/konferm2/2.pdf (accessed on 21 December 2022).
- Blaženčić, J. The efect on the water chestnut (Trapa L.) survival in associations with the white and yellow water lily [Nymphaea alba L. and Nuphar lutea (L.) Sm. in Sibth. et Sm.]. In 8th Symposium on the Lora of Southeastern Serbia and Neighbouring Regions; Faculty of Natural Sciences and Mathematics: Niš, Serbia, 2005; p. 68. [Google Scholar]
- Schneider, E. Aquatic macrophytes in the Danube Delta—Indicators for water quality and habitat parameters. Stud. Univ. Babeş-Bolyai, Biol. 2009, 54, 21–31. [Google Scholar]
- Džigurski, D.; Ljevnaić-Mašić, B.; Nikolić, L. Trapetum natantis Müller et Görs 1960 in hydromeliorative facilities in Serbia. Acta Soc. Bot. Pol. 2013, 82, 125–133. [Google Scholar] [CrossRef]
- Klochenko, P.D.; Shevchenko, T.F. Phytoepiphyton of macrophytes of various ecological groups of the Kiev Reservoir. Hydrobiol. J. 2016, 52, 3–16. [Google Scholar] [CrossRef]
- Klochenko, P.D.; Shevchenko, T.F.; Tarashchuk, O.S. Phytoepiphyton of the additional net of the Kanev Reservoir. Hydrobiol. J. 2016, 52, 22–37. [Google Scholar] [CrossRef]
- Sirenko, L.A.; Korelyakova, I.L.; Mikhaylenko, L.E.; Kostikova, L.E.; Litvinova, M.A.; Myslovich, V.O.; Skorik, L.V.; Khoroshikh, L.A.; Shcherbak, V.I.; Yakubovsky, A.B.; et al. Vegetation and Bacterial Population of the Dnieper and Its Reservoirs; Naukova Dumka: Kiev, Ukraine, 1989; p. 232. (In Russian) [Google Scholar]
- Shcherbak, V.I. Structural and Functional Characteristics of the Dnieper Phytoplankton. Ph.D. Thesis, Institute of Hydrobiol. NASU, Kyiv, Ukraine, 2000. (In Ukrainian). [Google Scholar]
- Oksiyuk, O.P.; Davydov, O.A.; Melenchuk, G.V. Formation of species diversity of phytoplankton in river sections of the Dnieper reservoirs. Algology 2005, 15, 78–85. (In Russian) [Google Scholar]
- Maistrova, N.V. Phytoplankton diversity of the Kyiv reservoir. Ukr. Bot. J. 2009, 66, 220–233. (In Ukrainian) [Google Scholar]
- Shcherbak, V.I. Phytoplankton succession of the Kyiv water reservoir and assessment of its transboundary section. TNPU Ser. Biol. 2015, 3–4, 757–760. (In Ukrainian) [Google Scholar]
- Kimmel, B.L.; Groeger, A.W. Factors controlling primary production in lakes and reservoirs: A perspective. Lake Reserv. Manag. 1984, 1, 277–281. [Google Scholar] [CrossRef]
- Thornton, K.W.; Kimmel, B.L.; Payne, F.E. Reservoir Limnology: Ecological Perspectives; John Wiley and Sons: Hoboken, NJ, USA, 1990; p. 256. [Google Scholar]
- Calijuri, M.C. Temporal changes in the phytoplankton community structure in a tropical and eutrophic reservoir (Barra Bonita, S.P.—Brazil). J. Plankton Res. 2002, 24, 617–634. [Google Scholar] [CrossRef]
- Brooks, B.W.; Scott, J.T.; Forbes, M.G.; Valenti, T.W.; Stanley, J.K.; Doyle, R.D. Reservoir zonation and water quality. Lake Line 2009, 25, 39–41. [Google Scholar]
- Mac Donagh, M.E.; Casco, M.A.; Claps, M.C. Plankton relationships under small water level fluctuations in a subtropical reservoir. Aquat. Ecol. 2008, 43, 371–381. [Google Scholar] [CrossRef]
- Araújo, F.G.; Costa de Azevedo, M.C.; Lima Ferreira, M.d.N. Seasonal changes and spatial variation in the water quality of a eutrophic tropical reservoir determined by the inflowing river. Lake Reserv. Manag. 2011, 27, 343–354. [Google Scholar] [CrossRef]
- Dai, H.; Mao, J.; Jiang, D.; Wang, L. Longitudinal Hydrodynamic Characteristics in Reservoir Tributary Embayments and Effects on Algal Blooms. PLoS ONE 2013, 8, e68186. [Google Scholar] [CrossRef]
- Gil Guarín, I.C.; Villabona-González, S.L.; Parra-García, E.; Echenique, R.O. Environmental factors driving phytoplankton biomass and diversity in a tropical reservoir. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 2020, 171, 423–436. [Google Scholar] [CrossRef]
- Mhlanga, L.; Madzivanzira, T.; Nhiwatiwa, T.; Tendaupenyu, P.; Barson, M.; Marufu, L.; Songore, N. Physico-chemical limnology during the filling phase of Tugwi-Mukosi, a tropical reservoir in Zimbabwe. Afr. J. Aquat. Sci. 2020, 45, 412–420. [Google Scholar] [CrossRef]
- Ruggiero, A.; Solimini, A.G.; Carchini, G. Nutrient and chlorophyll a temporal patterns in eutrophic mountain ponds with contrasting macrophyte coverage. Hydrobiologia 2003, 506–509, 657–663. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M. Effect of free floating macrophyte, Azolla pinnata R. Brown on water physico-chemistry, primary productivity, and the production of Nile Tilapia, Oreochromis niloticus (L.), and common carp, Cyprinus carpio L., in fertilized earthen ponds. J. Appl. Aquac. 2006, 18, 21–41. [Google Scholar] [CrossRef]
- Cunha, D.G.F.; Bottino, F.; do Carmo Calijuri, M. Can free-floating and emerged macrophytes influence the density and diversity of phytoplankton in subtropical reservoirs? Lake Reserv. Manag. 2012, 28, 255–264. [Google Scholar] [CrossRef]
- De Tezanos Pinto, P.; O’Farrell, I. Regime shifts between free-floating plants and phytoplankton: A review. Hydrobiologia 2014, 740, 13–24. [Google Scholar] [CrossRef]
- Gross, E.M. Allelopathy of aquatic autotrophs. Crit. Rev. Plant Sci. 2003, 22, 313–339. [Google Scholar] [CrossRef]
- Hu, H.; Hong, Y. Algal-bloom control by allelopathy of aquatic macrophytes—A review. Front. Environ. Sci. Eng. China 2008, 2, 421–438. [Google Scholar] [CrossRef]
- Rodriguez, P.; Solange Vera, M.; Pizarro, H. Primary production of phytoplankton and periphyton in two humic lakes of a South American wetland. Limnology 2012, 13, 281–287. [Google Scholar] [CrossRef]
- Shi, P.; Xie, P.; Liu, L.; Cui, J.; Wang, X.; Yang, P. Photoacclimation in freshwater diatoms: Interspecific and inter-habitat comparisons. J. Freshw. Ecol. 2020, 35, 391–407. [Google Scholar] [CrossRef]
- Lukina, L.F.; Smirnova, N.N. Physiology of Higher Aquatic Plants; Naukova Dumka: Kiev, Ukraine, 1988; p. 185. (In Russian) [Google Scholar]
- Akabori, Y.; Takagi, S.; Nishihiro, J.; Kagami, M. Effects of Floating-Leaved Plant, Trapa Natans, on the Water Qualities in lake Inba. Jpn. J. Limnol. 2015, 77, 155–166. [Google Scholar] [CrossRef]
- Kemp, W.M.; Boynton, W.R.; Adolf, J.E.; Boesch, D.F.; Boicourt, W.C.; Brush, G.; Cornwell, J.C.; Fisher, T.R.; Glibert, P.M.; Hagy, J.D.; et al. Eutrophication of Chesapeake Bay: Historical trends and ecological interactions. Mar. Ecol. Prog. Ser. 2005, 303, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Nezbrytska, I.M.; Shamanskyi, S.Y.; Boichenko, S.V.; Kharchenko, G.V. Some Problems of the Use of Microalgae for Nitrogen and Phosphorus Removal from Wastewater (a Review). Hydrobiol. J. 2021, 57, 62–78. [Google Scholar] [CrossRef]



| Site | Coordinates | Water Temperature, °C | pH | Dissolved Oxygen (DO), mg/L | DO % | Conductivity, µS/cm | Salinity, g/L (ppt) | Total Dissolved Solids, ppm | |
|---|---|---|---|---|---|---|---|---|---|
| Longitude | Latitude | ||||||||
| Kyiv Reservoir | |||||||||
| 1/1A | 30.420816 | 51.067504 | 26.8/27.3 | 7.89/8.45 | 5.5–5.3 | 61.6/58.9 | 348/340 | 0.20/0.20 | 233/227 |
| 2/2A | 30.366592 | 51.047131 | 26.9/26.7 | 8.02/8.02 | 8.1/8.3 | 99.8/104.4 | 338/343 | 0.20/0.19 | 226/229 |
| 3/3A | 30.377484 | 50.767120 | 26.6/27.6 | 8.59/8.42 | 4.0/6.4 | 50.1/90 | 337/333 | 0.18/0.18 | 225/223 |
| 4/4A | 30.545976 | 51.035895 | 27.9/30.7 | 9.00/8.96 | 13.5/13.7 | 164/182 | 306/314 | – | 205/211 |
| 5 | 30.554469 | 50.984399 | 27.1 | 8.98 | 16.3 | 190 | 288 | – | 192 |
| 6/6A | 30.519634 | 51.175135 | 27.7/27.4 | 8.13/8.07 | 3./4.0 | 38.1/46.1 | 367/337 | 0.21/0.20 | 245/225 |
| 7A | 30.551327 | 50.767121 | 27.4 | 8.86 | 18.0 | 190.0 | 304 | – | 203 |
| Kaniv Reservoir | |||||||||
| 8/8A | 30.638962 | 50.301470 | 25.9/26.1 | 7.88/8.21 | 3.3/4.0 | 41.0/49.1 | 359/346 | 0.19/0.19 | 240/231 |
| 9/9A | 30.761261 | 50.154172 | 27.4/29.1 | 8.16/8.21 | 4.3/3.9 | 53.6/51.8 | 354/351 | 0.19/0.20 | 237/235 |
| 10/10A | 31.073836 | 49.980736 | 27.7/27.3 | 8.07/7.86 | 4.4/4.6 | 52.5/59.7 | 346/342 | 0.19/0.20 | 231/229 |
| 11/11A | 31.402835 | 50.025115 | 25.1/26.5 | 8.77/9.12 | 6.2/10.4 | 74.6/127.0 | 328/315 | 0.18/0.17 | 219/211 |
| 12A | 31.503625 | 49.814495 | 26.2 | 8.68 | 5.6 | 68.1 | 322 | 0.18 | 215 |
| Site | Transpa-rency, m | Mass of Suspended Matter, mg/L | NH4+, mg N/L | NO2−, mg N/L | NO3−, mg N/L | N inorg., mg N/L | N org., mg N/L | Total Dissolved Nitrogen (TDN), mg N/L | P inorg., mg P/L | P org.+ Polyph., mg P/L | Total Dissolved Phosphorus (TDP), mg P/L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kyiv Reservoir | |||||||||||
| 1/1A | 0.65/1.10 | 14.4/8.7 | 0.113/0.047 | 0.007/0.008 | 0.070/0.058 | 0.190/0.113 | 1.723/0.762 | 1.913/0.875 | 0.113/0.138 | 0.141/0.138 | 0.254/0.276 |
| 2/2A | 0.50/0.50 | 37.7/23.2 | 0.079/0.091 | 0.010/0.008 | 0.045/0.047 | 0.134/0.146 | 0.763/0.738 | 0.897/0.884 | 0.164/0.158 | 0.185/0.167 | 0.349/0.325 |
| 3/3A | 2.00/0.40 | 4.8/18.0 | 0.155/0.127 | 0.012/0.012 | 0.075/0.125 | 0.242/0.264 | 0.931/1.027 | 1.173/1.291 | 0.221/0.128 | 0.167/0.347 | 0.388/0.475 |
| 4/4A | 0.90 | 10.7/30.2 | 0.091/0.071 | 0.012/0.007 | 0.027/0.045 | 0.130/0.123 | 0.395/0.769 | 0.525/0.892 | 0.085/0.052 | 0.207/0.265 | 0.292/0.317 |
| 5 | 0.35 | 13.8 | 0.204 | 0.011 | 0.048 | 0.263 | 1.069 | 1.332 | 0.117 | 0.346 | 0.463 |
| 6/6A | 0.85/0.85 | 12.7/22.2 | 0.055/0.053 | 0.007/0.003 | 0.054/0.036 | 0.116/0.092 | 1.118/1.252 | 1.234/1.344 | 0.152/0.148 | 0.242/0.209 | 0.394/0.357 |
| 7A | 0.25 | 7.6 | 0.249 | 0.018 | 0.037 | 0.304 | 1.297 | 1.601 | 0.132 | 0.374 | 0.506 |
| Kaniv Reservoir | |||||||||||
| 8/8A | 0.60/.50 | 7.2/5.0 | 0.040/0.023 | 0.021/0.026 | 0.040/0.031 | 0.101/0.080 | 1.195/1.240 | 1.296/1.320 | 0.193/0.203 | 0.256/0.414 | 0.449/0.617 |
| 9/9A | 0.60/0.60 | 10.9/4.2 | 0.154/0.074 | 0.045/0.047 | 0.109/0.120 | 0.308/0.241 | 1.013/1.013 | 1.321/1.254 | 0.276/0.254 | 0.380/0.197 | 0.656/0.451 |
| 10/10A | 1.00/1.25 | 8.8/6.8 | 0.043/0.042 | 0.032/0.025 | 0.119/0.118 | 0.194/0.185 | 0.886/0.547 | 1.080/0.732 | 0.235/0.229 | 0.187/0.201 | 0.422/0.430 |
| 11/11A | 0.75/0.45 | 5.5/9.5 | 0.101/0.109 | 0.000/0.000 | 0.100/0.077 | 0.201/0.186 | 0.816/1.216 | 1.017/1.402 | 0.292/0.323 | 0.244/0.303 | 0.536/0.626 |
| 12A | 1.40 | 6.8 | 0.215 | 0.000 | 0.048 | 0.263 | 1.266 | 1.529 | 0.331 | 0.250 | 0.581 |
| Band Name | Spatial Resolution, m | Spectral Range, nm | Band Description |
|---|---|---|---|
| B1 | 60 | 433–453 | Coastal aerosol |
| B2 | 10 | 458–523 | Blue |
| B3 | 10 | 543–578 | Green |
| B4 | 10 | 650–680 | Red |
| B5 | 20 | 698–713 | Red edge 1 |
| B6 | 20 | 733–748 | Red edge 2 |
| B7 | 20 | 773–793 | Red edge |
| B8 | 10 | 785–900 | Near-infrared (NIR) |
| B8A | 20 | 855–875 | Near-infrared narrow (NIRn) |
| B9 | 60 | 935–955 | Water vapour |
| B10 | 60 | 1360–1390 | Shortwave infrared/Cirrus |
| B11 | 20 | 1565–1655 | Shortwave infrared 1 (SWIR 1) |
| B12 | 20 | 2100–2280 | Shortwave infrared 2 (SWIR 2) |
| Date No. | Kyiv Reservoir | Kaniv Reservoir |
|---|---|---|
| Date 1 | 26 March 2021 | 28 March 2021 |
| Date 2 | 17 July 2021 | 21 June 2021 |
| Date 3 | 10 August 2021 | 31 July 2021 |
| Environmental Variable | Variability Explained, % | F-Value | p-Value |
|---|---|---|---|
| TP | 32.8 | 124.2 | 0.001 |
| P org poly | 24.4 | 92.3 | 0.000 |
| NO2 | 21.6 | 81.6 | 0.001 |
| Salinity | 8.6 | 32.4 | 0.006 |
| TN | 6.5 | 24.5 | 0.010 |
| N org | 5.9 | 22.4 | 0.009 |
| CCA Axis | Variability Explained, % | F-Value | p-Value |
|---|---|---|---|
| Axis 1 | 70.6 | 534.1 | 0.002 |
| Axis 2 | 29.1 | 220.6 | 0.032 |
| Environmental Variable | Variability Explained, % | F-Value | p-Value |
|---|---|---|---|
| TP | 60.2 | 812.7 | 0.001 |
| N org | 14.3 | 192.3 | 0.000 |
| Salinity | 10.7 | 144.7 | 0.002 |
| NO2 | 6.6 | 89.6 | 0.001 |
| NO3 | 6.3 | 85.4 | 0.002 |
| P org poly | 1.7 | 23.5 | 0.007 |
| CCA Axis | Variability Explained, % | F-Value | p-Value |
|---|---|---|---|
| Axis 1 | 89.2 | 3611.4 | 0.000 |
| Axis 2 | 10.7 | 432.9 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilous, O.P.; Nezbrytska, I.; Zhezherya, V.; Dubniak, S.; Batoh, S.; Kazantsev, T.; Polishchuk, O.; Zhezherya, T.; Leontieva, T.; Cantonati, M. Interactions between Aquatic Plants and Cyanobacterial Blooms in Freshwater Reservoir Ecosystems. Water 2023, 15, 672. https://doi.org/10.3390/w15040672
Bilous OP, Nezbrytska I, Zhezherya V, Dubniak S, Batoh S, Kazantsev T, Polishchuk O, Zhezherya T, Leontieva T, Cantonati M. Interactions between Aquatic Plants and Cyanobacterial Blooms in Freshwater Reservoir Ecosystems. Water. 2023; 15(4):672. https://doi.org/10.3390/w15040672
Chicago/Turabian StyleBilous, Olena P., Inna Nezbrytska, Vladyslav Zhezherya, Serhii Dubniak, Svitlana Batoh, Taras Kazantsev, Oleksandr Polishchuk, Tetyana Zhezherya, Tetyana Leontieva, and Marco Cantonati. 2023. "Interactions between Aquatic Plants and Cyanobacterial Blooms in Freshwater Reservoir Ecosystems" Water 15, no. 4: 672. https://doi.org/10.3390/w15040672







