Biochemical and Pathophysiological Responses in Capoeta capoeta under Lethal and Sub-Lethal Exposures of Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Fish Maintenance
2.2. Test Chemical
2.3. Experimental Setup
2.3.1. Acute Toxicity Assay
2.3.2. Blood Collection and Assay of Hematological Endpoints
2.3.3. Histopathological Analysis
2.3.4. Semi-Quantitative Scoring
2.4. Statistical Analyses
3. Results and Discussion
3.1. Acute Toxicity
3.2. Hematological and Biochemical Endpoints
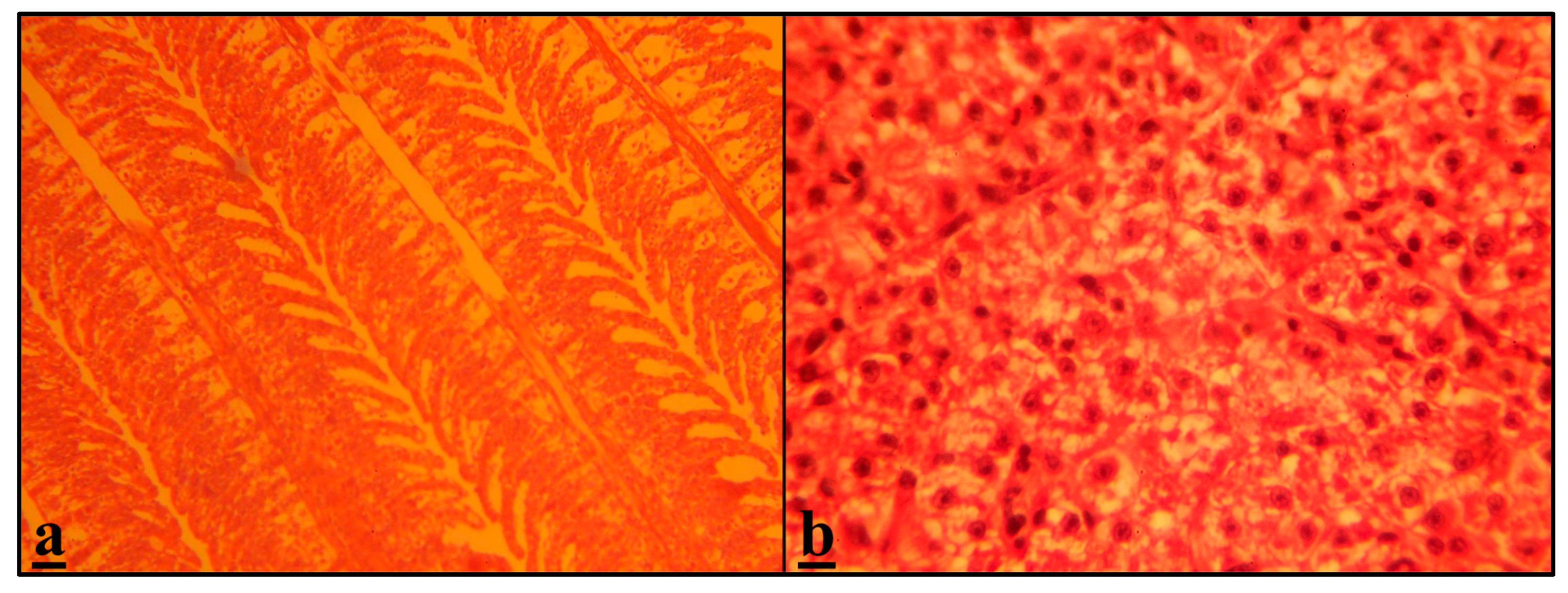
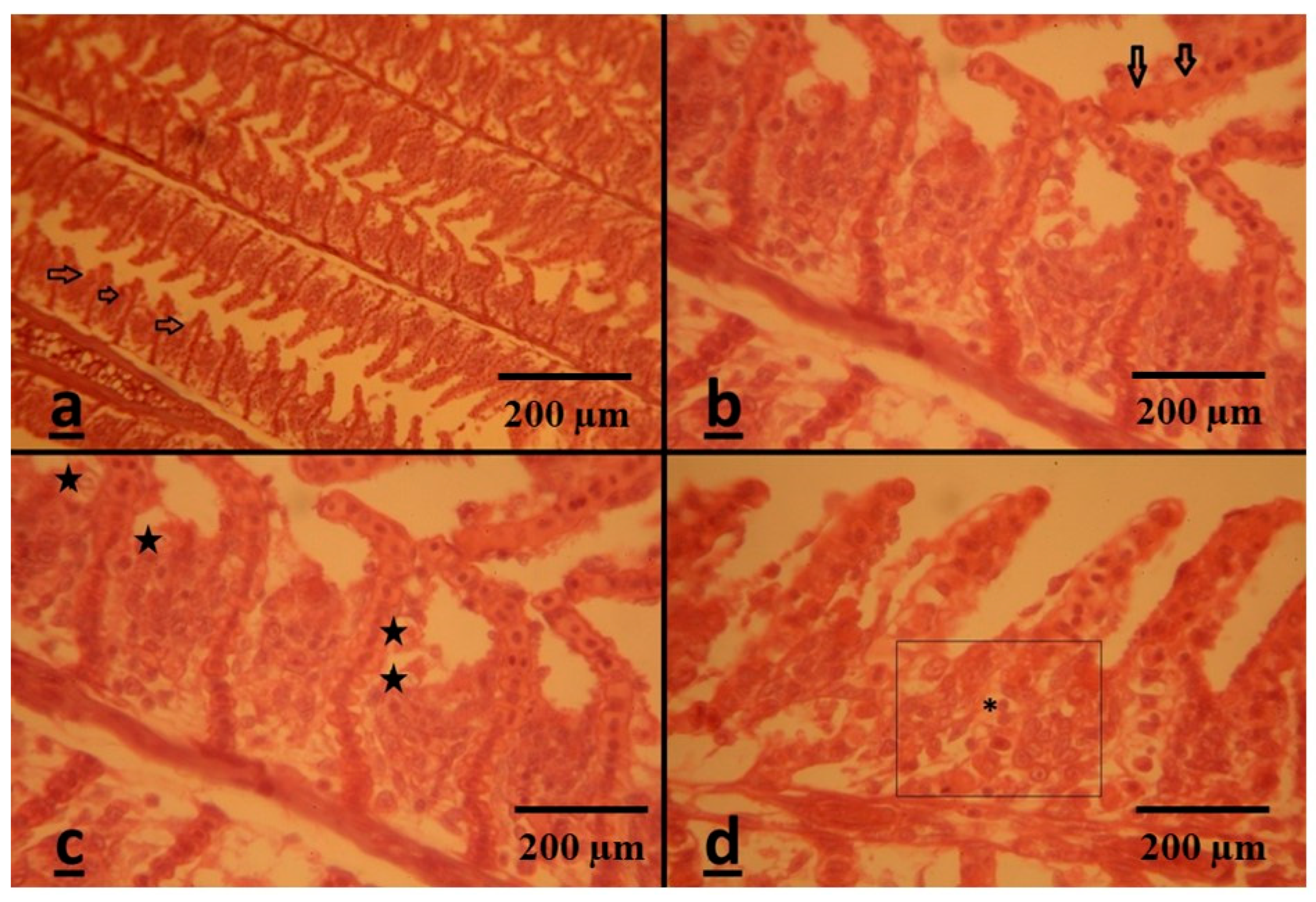
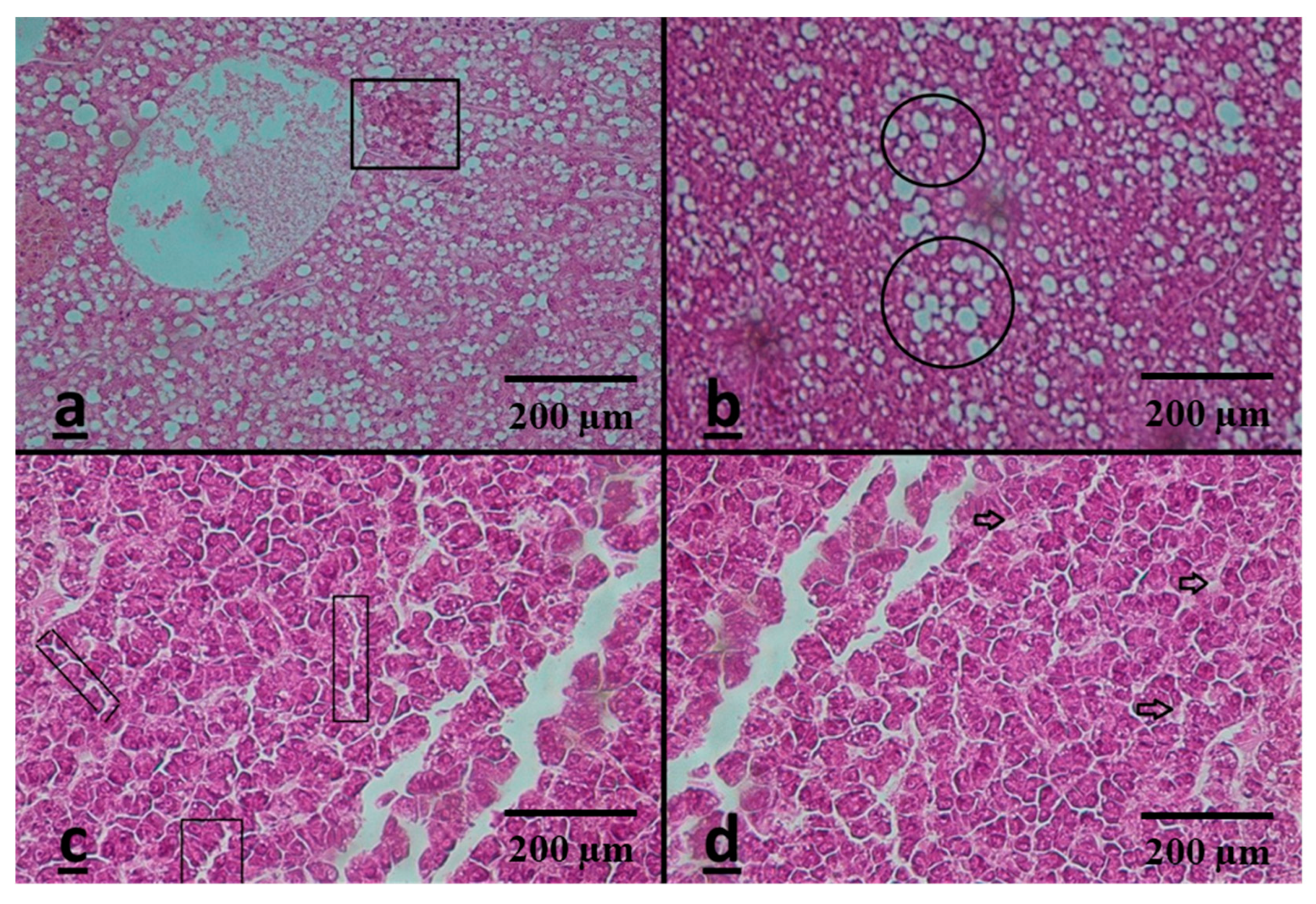
3.3. Histopathological Assessment
Semi-Quantitative Analysis
Gills
Liver
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajkumar, K.; Kanipandian, N.; Thirumurugan, R. Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl. Nanosci. 2016, 6, 19–29. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Durán, N.; Diez, M.; Martínez, M.; Parada, J.; Seabra, A. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chukwuka, A.V.; Mukherjee, D.; Dhara, K.; Saha, N.C.; Faggio, C. Behavioral and physiological toxicity thresholds of a freshwater vertebrate (Heteropneustes fossilis) and invertebrate (Branchiura sowerbyi), exposed to zinc oxide nanoparticles (nZnO): A General Unified Threshold model of Survival (GUTS). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 2022, 109450. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Mahboub, H.H.; Ganapathi, U.; Chandran, S.K.; Al-Onazi, W.; Al-Mohaimeed, A.M.; Chen, T.-W.; Faggio, C.; Paulraj, B. Enhanced photodegradation of methylene blue from aqueous solution using Al-doped ZnS nanoparticles. Environ. Sci. Pollut. Res. 2022, 29, 73528–73541. [Google Scholar] [CrossRef] [PubMed]
- Vali, S.; Majidiyan, N.; Yalsuyi, A.M.; Vajargah, M.F.; Prokić, M.D.; Faggio, C. Ecotoxicological Effects of Silver Nanoparticles (Ag-NPs) on Parturition Time, Survival Rate, Reproductive Success and Blood Parameters of Adult Common Molly (Poecilia sphenops) and Their Larvae. Water 2022, 14, 144. [Google Scholar] [CrossRef]
- Yue, Y.; Behra, R.; Sigg, L.; Fernandez Freire, P.; Pillai, S.; Schirmer, K. Toxicity of silver nanoparticles to a fish gill cell line: Role of medium composition. Nanotoxicology 2015, 9, 54–63. [Google Scholar] [CrossRef]
- Jovanović, B.; Anastasova, L.; Rowe, E.W.; Zhang, Y.; Clapp, A.R.; Palić, D. Effects of nanosized titanium dioxide on innate immune system of fathead minnow (Pimephales promelas Rafinesque, 1820). Ecotoxicol. Environ. Saf. 2011, 74, 675–683. [Google Scholar] [CrossRef]
- Khan, M.S.; Qureshi, N.A.; Jabeen, F.; Asghar, M.S.; Shakeel, M.; Fakhar-e-Alam, M. Eco-friendly synthesis of silver nanoparticles through economical methods and assessment of toxicity through oxidative stress analysis in the Labeo rohita. Biol. Trace Elem. Res. 2017, 176, 416–428. [Google Scholar] [CrossRef]
- Khan, M.S.; Qureshi, N.A.; Jabeen, F.; Shakeel, M.; Asghar, M.S. Assessment of waterborne amine-coated silver nanoparticle (Ag-NP)-induced toxicity in Labeo rohita by histological and hematological profiles. Biol. Trace Elem. Res. 2018, 182, 130–139. [Google Scholar] [CrossRef]
- Ale, A.; Rossi, A.S.; Bacchetta, C.; Gervasio, S.; de la Torre, F.R.; Cazenave, J. Integrative assessment of silver nanoparticles toxicity in Prochilodus lineatus fish. Ecol. Indic. 2018, 93, 1190–1198. [Google Scholar] [CrossRef]
- Paulpandian, P.; Beevi, I.S.; Somanath, B.; Kamatchi, R.K.; Paulraj, B.; Faggio, C. Impact of Camellia sinensis Iron Oxide Nanoparticle on Growth, Hemato-biochemical and Antioxidant Capacity of Blue Gourami (Trichogaster trichopterus) Fingerlings. Biol. Trace Elem. Res. 2022, 201, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, G.; Lazado, C.C.; Mahboub, H.H.; Mohammadi-Aloucheh, R.; Prokić, M.D.; Nada, H.S.; Faggio, C. Chemically and green synthesized ZnO nanoparticles alter key immunological molecules in common carp (Cyprinus carpio) skin mucus. Int. J. Mol. Sci. 2021, 22, 3270. [Google Scholar] [CrossRef]
- Forouhar Vajargah, M.; Mohamadi Yalsuyi, A.; Sattari, M.; Prokić, M.D.; Faggio, C. Effects of copper oxide nanoparticles (CuO-NPs) on parturition time, survival rate and reproductive success of guppy fish, Poecilia reticulata. J. Clust. Sci. 2020, 31, 499–506. [Google Scholar] [CrossRef]
- Murthy, M.K.; Mohanty, C.S.; Swain, P.; Pattanayak, R. Assessment of toxicity in the freshwater tadpole Polypedates maculatus exposed to silver and zinc oxide nanoparticles: A multi-biomarker approach. Chemosphere 2022, 2022, 133511. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Lademann, J.; Ribaud, C.; Roberts, M.S. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit. Rev. Toxicol. 2007, 37, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Vaseeharan, B.; Sudhakaran, R.; Jeyakandan, J.; Ramasamy, P.; Sonawane, A.; Padhi, A.; Velusamy, P.; Anbu, P.; Faggio, C. Bioinspired zinc oxide nanoparticles using Lycopersicon esculentum for antimicrobial and anticancer applications. J. Clust. Sci. 2019, 30, 1465–1479. [Google Scholar] [CrossRef]
- Mohsenpour, R.; Mousavi-Sabet, H.; Hedayati, A.; Rezaei, A.; Yalsuyi, A.M.; Faggio, C. In vitro effects of silver nanoparticles on gills morphology of female Guppy (Poecilia reticulate) after a short-term exposure. Microsc. Res. Tech. 2020, 83, 1552–1557. [Google Scholar] [CrossRef]
- Forouhar Vajargah, M.; Imanpoor, M.R.; Shabani, A.; Hedayati, A.; Faggio, C. Effect of long-term exposure of silver nanoparticles on growth indices, hematological and biochemical parameters and gonad histology of male goldfish (Carassius auratus gibelio). Microsc. Res. Tech. 2019, 82, 1224–1230. [Google Scholar] [CrossRef]
- Taju, G.; Majeed, S.A.; Nambi, K.; Hameed, A.S. In vitro assay for the toxicity of silver nanoparticles using heart and gill cell lines of Catla catla and gill cell line of Labeo rohita. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 161, 41–52. [Google Scholar] [CrossRef]
- Marin, S.; Mihail Vlasceanu, G.; Elena Tiplea, R.; Raluca Bucur, I.; Lemnaru, M.; Minodora Marin, M.; Mihai Grumezescu, A. Applications and toxicity of silver nanoparticles: A recent review. Curr. Top. Med. Chem. 2015, 15, 1596–1604. [Google Scholar] [CrossRef]
- Cross, S.E.; Innes, B.; Roberts, M.S.; Tsuzuki, T.; Robertson, T.A.; McCormick, P. Human skin penetration of sunscreen nanoparticles: In-vitro assessment of a novel micronized zinc oxide formulation. Ski. Pharmacol. Physiol. 2007, 20, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Galdames, A.; Mendoza, A.; Orueta, M.; de Soto García, I.; Sánchez, M.; Virto, I.; Vilas, J. Development of new remediation technologies for contaminated soils based on the application of zero-valent iron nanoparticles and bioremediation with compost. Resour. -Effic. Technol. 2017, 3, 166–176. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, E.; Zhu, S.; Blickley, T.M.; McClellan-Green, P.; Haasch, M.L. Ecotoxicology of carbon-based engineered nanoparticles: Effects of fullerene (C60) on aquatic organisms. Carbon 2006, 44, 1112–1120. [Google Scholar] [CrossRef]
- Shaw, B.J.; Al-Bairuty, G.; Handy, R.D. Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout,(Oncorhynchus mykiss): Physiology and accumulation. Aquat. Toxicol. 2012, 116, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Al-Bairuty, G.A.; Shaw, B.J.; Handy, R.D.; Henry, T.B. Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2013, 126, 104–115. [Google Scholar] [CrossRef]
- Linhua, H.; Zhenyu, W.; Baoshan, X. Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio). J. Environ. Sci. 2009, 21, 1459–1466. [Google Scholar]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.; Ingle, A.; Gade, A. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Ji, J.H.; Jung, J.H.; Kim, S.S.; Yoon, J.-U.; Park, J.D.; Choi, B.S.; Chung, Y.H.; Kwon, I.H.; Jeong, J.; Han, B.S. Twenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2007, 19, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Chae, Y.; An, Y.-J. Dimension-dependent toxicity of silver nanomaterials on the cladocerans Daphnia magna and Daphnia galeata. Chemosphere 2017, 185, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garraus, A.; Azqueta, A.; Vettorazzi, A.; Lopez de Cerain, A. Genotoxicity of silver nanoparticles. Nanomaterials 2020, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, D.Y. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J. Appl. Toxicol. 2013, 33, 78–89. [Google Scholar] [CrossRef]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef]
- Kaewamatawong, T.; Shimada, A.; Okajima, M.; Inoue, H.; Morita, T.; Inoue, K.; Takano, H. Acute and subacute pulmonary toxicity of low dose of ultrafine colloidal silica particles in mice after intratracheal instillation. Toxicol. Pathol. 2006, 34, 958–965. [Google Scholar] [CrossRef]
- Tresnakova, N.; Famulari, S.; Zicarelli, G.; Impellitteri, F.; Pagano, M.; Presti, G.; Filice, M.; Caferro, A.; Gulotta, E.; Salvatore, G. Multi-characteristic toxicity of enantioselective chiral fungicide tebuconazole to a model organism Mediterranean mussel Mytilus galloprovincialis Lamarck, 1819 (Bivalve: Mytilidae). Sci. Total Environ. 2022, 862, 160874. [Google Scholar] [CrossRef]
- Hussain, S.; Hess, K.; Gearhart, J.; Geiss, K.; Schlager, J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Miao, A.-J.; Schwehr, K.A.; Xu, C.; Zhang, S.-J.; Luo, Z.; Quigg, A.; Santschi, P.H. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ. Pollut. 2009, 157, 3034–3041. [Google Scholar] [CrossRef]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef] [PubMed]
- Kakakhel, M.A.; Wu, F.; Sajjad, W.; Zhang, Q.; Khan, I.; Ullah, K.; Wang, W. Long-term exposure to high-concentration silver nanoparticles induced toxicity, fatality, bioaccumulation, and histological alteration in fish (Cyprinus carpio). Environ. Sci. Eur. 2021, 33, 44. [Google Scholar] [CrossRef]
- Mukherjee, D.; Saha, S.; Chukwuka, A.V.; Ghosh, B.; Dhara, K.; Saha, N.C.; Pal, P.; Faggio, C. Antioxidant enzyme activity and pathophysiological responses in the freshwater walking catfish, Clarias batrachus Linn under sub-chronic and chronic exposures to the neonicotinoid, Thiamethoxam®. Sci. Total Environ. 2022, 836, 155716. [Google Scholar] [CrossRef] [PubMed]
- Vali, S.; Majidiyan, N.; Azadikhah, D.; Varcheh, M.; Tresnakova, N.; Faggio, C. Effects of Diazinon on the Survival, Blood Parameters, Gills, and Liver of Grass Carp (Ctenopharyngodon idella Valenciennes, 1844; Teleostei: Cyprinidae). Water 2022, 14, 1357. [Google Scholar] [CrossRef]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of heavy metals on fish physiology–A review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef] [PubMed]
- Lekamge, S.; Miranda, A.F.; Abraham, A.; Li, V.; Shukla, R.; Bansal, V.; Nugegoda, D. The toxicity of silver nanoparticles (AgNPs) to three freshwater invertebrates with different life strategies: Hydra vulgaris, Daphnia carinata, and Paratya australiensis. Front. Environ. Sci. 2018, 6, 152. [Google Scholar] [CrossRef]
- Saha, S.; Dhara, K.; Chukwuka, A.V.; Pal, P.; Saha, N.C.; Faggio, C. Sub-lethal acute effects of environmental concentrations of inorganic mercury on hematological and biochemical parameters in walking catfish, Clarias batrachus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 264, 109511. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Daima, H.K.; Selvakannan, P.; Kandjani, A.E.; Shukla, R.; Bhargava, S.K.; Bansal, V. Synergistic influence of polyoxometalate surface corona towards enhancing the antibacterial performance of tyrosine-capped Ag nanoparticles. Nanoscale 2014, 6, 758–765. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati, S.; Hadibarata, T.; Kueh, A.B.H.; Salim, M.R.; Zaini, M.A.A. Silver nanoparticles in the water environment in Malaysia: Inspection, characterization, removal, modeling, and future perspective. Sci. Rep. 2018, 8, 986. [Google Scholar] [CrossRef]
- Butler, J.D.; Varghese, L.; Deb, N.; Thornhill, B. Extending international toxicity testing guidance to middle eastern test species. Sci. Total Environ. 2020, 716, 136343. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Lin, K.-C.; Yang, D.-T. Comparison of the relative toxicity relationships based on batch and continuous algal toxicity tests. Chemosphere 1997, 35, 1959–1965. [Google Scholar] [CrossRef]
- Schipper, C.A.; Dubbeldam, M.; Feist, S.W.; Rietjens, I.M.; Murk, A.T. Cultivation of the heart urchin Echinocardium cordatum and validation of its use in marine toxicity testing for environmental risk assessment. J. Exp. Mar. Biol. Ecol. 2008, 364, 11–18. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, S.; Huang, Y.; Zhang, T.; Liu, X.; Hu, Y.; Zhang, Z.; Tang, M. Acute toxic effects and gender-related biokinetics of silver nanoparticles following an intravenous injection in mice. J. Appl. Toxicol. 2012, 32, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.S.; De Matos, A.P.A.; Lourenço, J.; Castro, L.; Peres, I.; Mendonça, E.; Picado, A. Liver alterations in two freshwater fish species (Carassius auratus and Danio rerio) following exposure to different TiO2 nanoparticle concentrations. Microsc. Microanal. 2013, 19, 1131–1140. [Google Scholar] [CrossRef]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef]
- Panacek, A.; Prucek, R.; Safarova, D.; Dittrich, M.; Richtrova, J.; Benickova, K.; Zboril, R.; Kvitek, L. Acute and chronic toxicity effects of silver nanoparticles (NPs) on Drosophila melanogaster. Environ. Sci. Technol. 2011, 45, 4974–4979. [Google Scholar] [CrossRef]
- García, A.; Espinosa, R.; Delgado, L.; Casals, E.; González, E.; Puntes, V.; Barata, C.; Font, X.; Sánchez, A. Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests. Desalination 2011, 269, 136–141. [Google Scholar] [CrossRef]
- Kumar, N.; Krishnani, K.K.; Singh, N.P. Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ. Sci. Pollut. Res. 2018, 25, 8914–8927. [Google Scholar] [CrossRef]
- Saha, S.; Chukwuka, A.V.; Mukherjee, D.; Patnaik, L.; Nayak, S.; Dhara, K.; Saha, N.C.; Faggio, C. Chronic Effects of Diazinon® Exposures Using Integrated Biomarker Responses in Freshwater Walking Catfish, Clarias batrachus. Appl. Sci. 2021, 11, 10902. [Google Scholar] [CrossRef]
- Saha, S.; Chukwuka, A.V.; Mukherjee, D.; Dhara, K.; Pal, P.; Saha, N.C. Physiological (haematological, growth and endocrine) and biochemical biomarker responses in air-breathing catfish, Clarias batrachus under long-term Captan® pesticide exposures. Environ. Toxicol. Pharmacol. 2022, 90, 103815. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Iqbal Dar, O.; Andotra, M.; Sharma, S.; Kaur, A.; Faggio, C. Environmentally relevant concentrations of Triclosan induce cyto-genotoxicity and biochemical alterations in the hatchlings of Labeo rohita. Appl. Sci. 2021, 11, 10478. [Google Scholar] [CrossRef]
- Sharma, S.; Dar, O.I.; Singh, K.; Kaur, A.; Faggio, C. Triclosan elicited biochemical and transcriptomic alterations in Labeo rohita larvae. Environ. Toxicol. Pharmacol. 2021, 88, 103748. [Google Scholar] [CrossRef] [PubMed]
- Gopi, N.; Rekha, R.; Vijayakumar, S.; Liu, G.; Monserrat, J.M.; Faggio, C.; Nor, S.A.M.; Vaseeharan, B. Interactive effects of freshwater acidification and selenium pollution on biochemical changes and neurotoxicity in Oreochromis mossambicus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 250, 109161. [Google Scholar] [CrossRef]
- Sharma, R.; Jindal, R.; Faggio, C. Cassia fistula ameliorates chronic toxicity of cypermethrin in Catla catla. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109113. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Aceves, M.A.; Lionetti, L.; Faggio, C. Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci. Total Environ. 2019, 670, 1170–1183. [Google Scholar] [CrossRef]
- Saha, S.; Chukwuka, A.V.; Mukherjee, D.; Dhara, K.; Adeogun, A.O.; Saha, N.C. Effects of short-term sub-lethal diazinon® exposure on behavioural patterns and respiratory function in Clarias batrachus: Inferences for adaptive capacity in the wild. Chem. Ecol. 2022, 38, 180–194. [Google Scholar] [CrossRef]
- Faggio, C.; Piccione, G.; Marafioti, S.; Arfuso, F.; Fortino, G.; Fazio, F. Metabolic response to monthly variations of Sparus aurata reared in Mediterranean on-shore tanks. Turk. J. Fish. Aquat. Sci. 2014, 14, 567–574. [Google Scholar]
- Hodkovicova, N.; Hollerova, A.; Svobodova, Z.; Faldyna, M.; Faggio, C. Effects of Plastic Particles on Aquatic Invertebrates and Fish—A Review. Environ. Toxicol. Pharmacol. 2022, 96, 104013. [Google Scholar] [CrossRef]
- Banaee, M.; Impellitteri, F.; Evaz-Zadeh Samani, H.; Piccione, G.; Faggio, C. Dietary Arthrospira platensis in Rainbow Trout (Oncorhynchus mykiss): A Means to Reduce Threats Caused by CdCl2 Exposure? Toxics 2022, 10, 731. [Google Scholar] [CrossRef]
- Impellitteri, F.; Curpăn, A.-S.; Plăvan, G.; Ciobica, A.; Faggio, C. Hemocytes: A Useful Tool for Assessing the Toxicity of Microplastics, Heavy Metals, and Pesticides on Aquatic Invertebrates. Int. J. Environ. Res. Public Health 2022, 19, 16830. [Google Scholar] [CrossRef]
- Ahmed, I.; Reshi, Q.M.; Fazio, F. The influence of the endogenous and exogenous factors on hematological parameters in different fish species: A review. Aquac. Int. 2020, 28, 869–899. [Google Scholar] [CrossRef]
- Yousefi, M.; Shabunin, S.V.; Vatnikov, Y.A.; Kulikov, E.V.; Adineh, H.; Hamidi, M.K.; Hoseini, S.M. Effects of lavender (Lavandula angustifolia) extract inclusion in diet on growth performance, innate immunity, immune-related gene expression, and stress response of common carp, Cyprinus carpio. Aquaculture 2020, 515, 734588. [Google Scholar] [CrossRef]
- Barathinivas, A.; Ramya, S.; Neethirajan, K.; Jayakumararaj, R.; Pothiraj, C.; Balaji, P.; Faggio, C. Ecotoxicological effects of pesticides on hematological parameters and oxidative enzymes in freshwater Catfish, Mystus keletius. Sustainability 2022, 14, 9529. [Google Scholar] [CrossRef]
- Clark, N.J.; Shaw, B.J.; Handy, R.D. Low hazard of silver nanoparticles and silver nitrate to the haematopoietic system of rainbow trout. Ecotoxicol. Environ. Saf. 2018, 152, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Bayir, A.; Sirkecioğlu, A.N.; Polat, H.; Aras, N.M. Biochemical profile of blood serum of siraz Capoeta capoeta umbla. Comp. Clin. Pathol. 2007, 16, 119–126. [Google Scholar] [CrossRef]
- Yousefzadeh, F.; Khara, H. Changes in blood chemistry and hematological indices of Capoeta capoeta gracilis in relation to age, sex, and geographic location. Comp. Clin. Pathol. 2015, 24, 791–795. [Google Scholar] [CrossRef]
- Imani, M.; Halimi, M.; Khara, H. Effects of silver nanoparticles (AgNPs) on hematological parameters of rainbow trout, Oncorhynchus mykiss. Comp. Clin. Pathol. 2015, 24, 491–495. [Google Scholar] [CrossRef]
- Ololade, I.; Oginni, O. Toxic stress and hematological effects of nickel on African catfish, Clarias gariepinus, fingerlings. J. Environ. Chem. Ecotoxicol. 2010, 2, 014–019. [Google Scholar]
- Barboza, L.G.A.; Vieira, L.R.; Guilhermino, L. Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchus labrax): Changes in behavioural responses and reduction of swimming velocity and resistance time. Environ. Pollut. 2018, 236, 1014–1019. [Google Scholar] [CrossRef]
- Bojarski, B.; Witeska, M.J.E.S.; Research, P. Blood biomarkers of herbicide, insecticide, and fungicide toxicity to fish—A review. Environ. Sci. Pollut. Res. 2020, 27, 19236–19250. [Google Scholar] [CrossRef] [PubMed]
- Lakra, K.C.; Mistri, A.; Banerjee, T.K.; Lal, B. Analyses of the health status, risk assessment and recovery response of the nutritionally important catfish Clarias batrachus reared in coal mine effluent-fed pond water: A biochemical, haematological and histopathological investigation. Environ. Sci. Pollut. Res. 2022, 29, 47462–47487. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Bose, M.; Harish, D. Haematological changes in the blood of Clarias batrachus exposed to mercuric chloride. J. Ecotoxicol. Environ. Monit. 2002, 12, 119–122. [Google Scholar]
- Dhara, K.; Saha, S.; Mukherjee, D.; Saha, N.C. Comparative acute toxicity of mercury to air breathing fish, Channa gachua (Ham.) and non-air breathing fish Cyprinus carpio (Linn.): Ethological and Haematological Consideration. Indian J. Ecol. 2021, 48, 1243–1253. [Google Scholar]
- Mohamed, F.; Gad, N. Environmental pollution-induced biochemical changes in tissues of Tilapia zillii, Solea vulgaris and Mugil capito from Lake Qarun, Egypt. Glob. Vet. 2008, 2, 327–336. [Google Scholar]
- Allen, T.; Singhal, R.; Rana, S. Resistance to oxidative stress in a freshwater fish Channa punctatus after exposure to inorganic arsenic. Biol. Trace Elem. Res. 2004, 98, 63–72. [Google Scholar] [CrossRef]
- Jaheed, E. Study of Blood Serum Biochemical Profile and Pathological Changes in Haemonchosis Experimentally Induced in Goats. Am. J. BioScience 2021, 9, 95. [Google Scholar] [CrossRef]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Batalova, A.A.; Goncharov, N.V. Serum Albumin. Encyclopedia 2020, 1, 65–75. [Google Scholar] [CrossRef]
- Nematdoost Haghi, B.; Banaee, M. Effects of micro-plastic particles on paraquat toxicity to common carp (Cyprinus carpio): Biochemical changes. Int. J. Environ. Sci. Technol. 2017, 14, 521–530. [Google Scholar] [CrossRef]
- Mahmoud, U.M.; Mekkawy, I.A.; Naguib, M.; Sayed, A.E.-D.H. Silver nanoparticle–induced nephrotoxicity in Clarias gariepinus: Physio-histological biomarkers. Fish Physiol. Biochem. 2019, 45, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, B.; Maleki, A.; Davari, B.; Johari, S.A.; Shahmoradi, B.; Mohammadi, E.; Shahsavari, S. Histopathological effects following short-term coexposure of Cyprinus carpio to nanoparticles of TiO2 and CuO. Environ. Monit. Assess. 2016, 188, 575. [Google Scholar] [CrossRef] [PubMed]
- Murali, M.; Suganthi, P.; Athif, P.; Bukhari, A.S.; Mohamed, H.S.; Basu, H.; Singhal, R. Histological alterations in the hepatic tissues of Al2O3 nanoparticles exposed freshwater fish Oreochromis mossambicus. J. Trace Elem. Med. Biol. 2017, 44, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Merola, C.; Fabrello, J.; Matozzo, V.; Faggio, C.; Iannetta, A.; Tinelli, A.; Crescenzo, G.; Amorena, M.; Perugini, M. Dinitroaniline herbicide pendimethalin affects development and induces biochemical and histological alterations in zebrafish early-life stages. Sci. Total Environ. 2022, 828, 154414. [Google Scholar] [CrossRef] [PubMed]
- Vajargah, M.; Mohamadi Yalsuyi, A.; Hedayati, A.; Faggio, C. Histopathological lesions and toxicity in common carp (Cyprinus carpio L. 1758) induced by copper nanoparticles. Microsc. Res. Tech. 2018, 81, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Ravi, R.; Athisuyambulingam, M.; Kanagaraj, S.; Tresnakova, N.; Impellitteri, F.; Viswambaran, G.; Faggio, C. Impact of Chlorpyrifos on Cytopathological Indices in Mangrove Crab, Episesarma tetragonum (Fabricius). Vet. Sci. 2023, 10, 53. [Google Scholar] [CrossRef]
- Jeyasree, J.; Bupesh, G.; Vasanth, S.; Beulah, J.P.J.; Pandian, K.; Anand, A.V.; Vijayakumar, T.S.; Narayanan, L. In-vivo toxicological (acute) characterization of bio-synthesized silver nanoparticles in labeo rohita. Nano Biomed. Eng. 2020, 12, 115–123. [Google Scholar] [CrossRef]
- Govindasamy, R.; Rahuman, A.A. Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus). J. Environ. Sci. 2012, 24, 1091–1098. [Google Scholar] [CrossRef]
- El Euony, O.I.; Elblehi, S.S.; Abdel-Latif, H.M.; Abdel-Daim, M.M.; El-Sayed, Y.S. Modulatory role of dietary Thymus vulgaris essential oil and Bacillus subtilis against thiamethoxam-induced hepatorenal damage, oxidative stress, and immunotoxicity in African catfish (Clarias garipenus). Environ. Sci. Pollut. Res. 2020, 27, 23108–23128. [Google Scholar] [CrossRef]
- Sula, E.; Aliko, V.; Marku, E.; Nuro, A.; Faggio, C. Evaluation of kidney histopathological alterations in Crucian Carp, Carassius carassius, from a pesticide and PCB-contaminated freshwater ecosystem, using light microscopy and organ index mathematical model. Int. J. Aquat. Biol. 2020, 8, 154–165. [Google Scholar]
- Sula, E.; Aliko, V.; Pagano, M.; Faggio, C. Digital light microscopy as a tool in toxicological evaluation of fish erythrocyte morphological abnormalities. Microsc. Res. Tech. 2020, 83, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Vajargah, M.F.; Namin, J.I.; Mohsenpour, R.; Yalsuyi, A.M.; Prokić, M.D.; Faggio, C. Histological effects of sublethal concentrations of insecticide Lindane on intestinal tissue of grass carp (Ctenopharyngodon idella). Vet. Res. Commun. 2021, 45, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Plhalova, L.; Sehonova, P.; Blahova, J.; Doubkova, V.; Tichy, F.; Faggio, C.; Berankova, P.; Svobodova, Z. Evaluation of tramadol hydrochloride toxicity to juvenile zebrafish—Morphological, antioxidant and histological responses. Appl. Sci. 2020, 10, 2349. [Google Scholar] [CrossRef]
- Carraschi, S.; Florêncio, T.; Ignácio, N.; Ikefuti, C.; Cruz, C.; Ranzani-Paiva, M.J.T. Hematological and histopathological assessment of pacu (Piaractus mesopotamicus) after treatment of pathogens with veterinary medicinal products. Comp. Clin. Pathol. 2017, 26, 105–114. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Seben, D.; Sippert, L.R.; Salbego, J.; Marchesan, E.; Zanella, R.; Baldisserotto, B.; Golombieski, J. Gill bioenergetics dysfunction and oxidative damage induced by thiamethoxam exposure as relevant toxicological mechanisms in freshwater silver catfish Rhamdia quelen. Sci. Total Environ. 2018, 636, 420–426. [Google Scholar] [CrossRef]
- Sales, C.F.; Dos Santos, K.P.E.; Rizzo, E.; de Azambuja Ribeiro, R.I.M.; Dos Santos, H.B.; Thomé, R.G. Proliferation, survival and cell death in fish gills remodeling: From injury to recovery. Fish Shellfish Immunol. 2017, 68, 10–18. [Google Scholar] [CrossRef]
- Vieira, L.; Saldanha, A.A.; Moraes, A.M.; de Oliveira, F.M.; Lopes, D.O.; de Oliveira Barbosa, L.A.; de Azambuja Ribeiro, R.I.M.; Thomé, R.G.; Dos Santos, H.B.; Villar, J.A.F.P. 21-Benzylidene digoxin, a novel digoxin hemi-synthetic derivative, presents an anti-inflammatory activity through inhibition of edema, tumour necrosis factor alpha production, inducible nitric oxide synthase expression and leucocyte migration. Int. Immunopharmacol. 2018, 65, 174–181. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Hyndman, K.; Denslow, N.D.; Barber, D.S. Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol. Sci. 2009, 107, 404–415. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. 2008, 27, 1972–1978. [Google Scholar] [CrossRef]
- Kumar, N.; Jeena, N.; Gangola, S.; Singh, H. Phytoremediation facilitating enzymes: An enzymatic approach for enhancing remediation process. In Smart Bioremediation Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 289–306. [Google Scholar]
- Brusle, J.; Anadon, G.G. The structure and function of fish liver. In Fish Morphology; Routledge: Abingdon, UK, 2017; pp. 77–93. [Google Scholar]
- Paulino, M.G.; Tavares, D.; Terezan, A.P.; Sakuragui, M.M.; Pesenti, E.; Giani, A.; Cestari, M.M.; Fernandes, J.B.; Fernandes, M.N. Biotransformations, antioxidant system responses, and histopathological indexes in the liver of fish exposed to cyanobacterial extract. Environ. Toxicol. Chem. 2020, 39, 1041–1051. [Google Scholar] [CrossRef]
- Saud Alarifi, D.A.; Al-Doaiss, A.A.; Ali, B.A.; Ahmed, M.; Al-Khedhairy, A.A. Histologic and apoptotic changes induced by titanium dioxide nanoparticles in the livers of rats. Int. J. Nanomed. 2013, 8, 3937. [Google Scholar]
- Paunovic, J.; Vucevic, D.; Radosavljevic, T.; Pantic, S.; Nikolovski, D.; Dugalic, S.; Pantic, I. Effects of metallic nanoparticles on physiological liver functions. Rev. Adv. Mater. Sci. 2017, 49, 123–128. [Google Scholar]
- Baldissera, M.D.; Souza, C.F.; Descovi, S.N.; Zanella, R.; Prestes, O.D.; da Silva, A.S.; Baldisserotto, B. Organophosphate pesticide trichlorfon induced neurotoxic effects in freshwater silver catfish Rhamdia quelen via disruption of blood-brain barrier: Implications on oxidative status, cell viability and brain neurotransmitters. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2019, 218, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Serafini, S.; de Freitas Souza, C.; Baldissera, M.D.; Baldisserotto, B.; Segat, J.C.; Baretta, D.; Zanella, R.; da Silva, A.S. Fish exposed to water contaminated with eprinomectin show inhibition of the activities of AChE and Na+/K+-ATPase in the brain, and changes in natural behavior. Chemosphere 2019, 223, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiong, L.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Yuan, F.; Xi, T. Distribution, translocation and accumulation of silver nanoparticles in rats. J. Nanosci. Nanotechnol. 2009, 9, 4924–4932. [Google Scholar] [CrossRef]
- Faedmaleki, F.; Shirazi, F.H.; Salarian, A.-A.; Ashtiani, H.A.; Rastegar, H. Toxicity effect of silver nanoparticles on mice liver primary cell culture and HepG2 cell line. Iran. J. Pharm. Res. 2014, 13, 235. [Google Scholar]
- Aghamirkarimi, S.; Mashinchian Moradi, A.; Sharifpour, I.; Jamili, S.; Ghavam Mostafavi, P. Sublethal effects of copper nanoparticles on the histology of gill, liver and kidney of the Caspian roach, Rutilus rutilus caspicus. Global J. Environ. Sci. Manag. 2017, 3, 323–332. [Google Scholar] [CrossRef]
- Yazdanparast, T.; Sharifpour, I.; Soltani, M.; Esfahani, H.K. Evaluation of silver retention in different organs of zebrafish (Danio rerio) fed diet supplemented with silver nanoparticles. 2016, 5, 269–274. Int. J. Eng. Res. 2016, 5, 269–274. [Google Scholar]
- Naguib, M.; Mahmoud, U.M.; Mekkawy, I.A.; Sayed, A.E.-D.H. Hepatotoxic effects of silver nanoparticles on Clarias gariepinus; Biochemical, histopathological, and histochemical studies. Toxicol. Rep. 2020, 7, 133–141. [Google Scholar] [CrossRef]
- Grigore, M.E.; Biscu, E.R.; Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Chojnacki, M.; Kamaszewski, M.; Sawosz-Chwalibóg, E. Histopathological effects of silver and copper nanoparticles on the epidermis, gills, and liver of Siberian sturgeon. Environ. Sci. Pollut. Res. 2016, 23, 1621–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avci, A.; Kaçmaz, M.; Durak, İ. Peroxidation in muscle and liver tissues from fish in a contaminated river due to a petroleum refinery industry. Ecotoxicol. Environ. Saf. 2005, 60, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Mela, M.; Randi, M.; Ventura, D.; Carvalho, C.; Pelletier, E.; Ribeiro, C.O. Effects of dietary methylmercury on liver and kidney histology in the neotropical fish Hoplias malabaricus. Ecotoxicol. Environ. Saf. 2007, 68, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Velma, V.; Tchounwou, P.B. Chromium-induced biochemical, genotoxic and histopathologic effects in liver and kidney of goldfish, Carassius auratus. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2010, 698, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Jerome, F.C.; Hassan, A.; Omoniyi-Esan, G.O.; Odujoko, O.O.; Chukwuka, A.V. Metal uptake, oxidative stress and histopathological alterations in gills and hepatopancreas of Callinectes amnicola exposed to industrial effluent. Ecotoxicol. Environ. Saf. 2017, 139, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.S.; França, F.M.; Marcantonio, A.S.; Viriato, C.; Fonseca, A.M.C.R.P.; Pedroso, C.B.; Ferreira, C.M. Histopathological changes in the gills of zebrafish (Danio rerio) and bullfrog tadpoles (Lithobates catesbeianus) caused by the use of formaldehyde. Braz. J. Anim. Environ. Res. 2021, 4, 3832–3847. [Google Scholar] [CrossRef]
| Concentration (mgL−1) * | Number | No. of Mortality | |||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | ||
| 0 | 21 | 0 | 0 | 0 | 0 |
| 5 | 21 | 1 | 3 | 4 | 5 |
| 10 | 21 | 3 | 6 | 8 | 10 |
| 15 | 21 | 9 | 11 | 14 | 18 |
| 20 | 21 | 13 | 17 | 20 | 21 |
| Point | Concentration (mgL−1) | |||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | |
| LC10 | 8.161 | 5.049 | 4.164 | 3.609 |
| LC20 | 11.268 | 8.135 | 6.708 | 5.672 |
| LC30 | 13.509 | 10.360 | 8.542 | 7.160 |
| LC40 | 15.424 | 12.261 | 10.109 | 8.431 |
| LC50 | 17.213 | 14.038 | 11.574 | 9.619 |
| LC60 | 19.003 | 15.815 | 13.039 | 10.808 |
| LC70 | 20.918 | 17.716 | 14.606 | 12.079 |
| LC80 | 23.158 | 19.941 | 16.440 | 13.567 |
| LC90 | 26.266 | 23.027 | 18.984 | 15.630 |
| LC95 | 28.832 | 25.575 | 21.084 | 17.334 |
| Hematological and Biochemical Indices | Control (0 mgL−1) | 5 mgL−1 | 10 mgL−1 | 15 mgL−1 |
|---|---|---|---|---|
| RBC (106 μL) | 2.43 ± 0.06 a | 1.97 ± 0.3 b | 1.63 ± 0.12 c | 1.28 ± 0.24 d |
| WBC (104 μL) | 2.35 ± 0.68 a | 1.94 ± 0.5 b | 1.90 ± 0.62 b | 1.51 ± 0.50 c |
| Hematocrit (%) | 46.63 ± 0.3 a | 35.3 ± 0.5 b | 35.3 ± 0.22 b | 29.8 ± 0.38 c |
| Total serum glucose (mg/dL) | 4.24 ± 0.07 a | 4.19 ± 0.11 a | 3.15 ± 0.17 b | 2.02 ± 0.05 c |
| Total serum protein (mg/dL) | 1.92 ± 0.31 d | 2.98 ± 0.25 b | 2.96 ± 0.13 b | 3.78 ± 0.11 a |
| Triglyceride (mg/dL) | 1.90 ± 0.09 d | 2.23 ± 0.08 b | 2.23 ± 0.03 b | 2.98 ± 0.27 a |
| Cholesterol (mg/dL) | 3.97 ± 0.21 c | 3.98 ± 0.30 c | 4.14 ± 0.14 b | 4.12 ± 0.16 b |
| Albumin (mg/dL) | 0.49 ± 0.13 c | 0.63 ± 0.16 b | 0.62 ± 0.11 b | 0.78 ± 0.22 a |
| Tissue Damages | Reaction Pattern | Nominal Concentrations (mgL−1) | |||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | ||
| Epithelial lifting of secondary lamellae | R | − | ++ | ++++ | +++ |
| Epithelial hypertrophy | P | − | +++ | ++++ | +++ |
| Bottom hyperplasia | R | − | ++ | ++++ | ++++ |
| Leukocyte infiltration | R | − | ++ | +++ | ++++ |
| Tissue Damages | Reaction Pattern | Nominal Concentrations (mgL−1) | |||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | ||
| Macrophage aggregates | C | − | +++ | +++ | +++ |
| fatty liver | R | − | +++ | +++ | ++++ |
| Dilation of sinusoid | C | − | ++ | +++ | ++++ |
| necrosis | R | − | + | +++ | ++++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azadikhah, D.; Yalsuyi, A.M.; Saha, S.; Saha, N.C.; Faggio, C. Biochemical and Pathophysiological Responses in Capoeta capoeta under Lethal and Sub-Lethal Exposures of Silver Nanoparticles. Water 2023, 15, 585. https://doi.org/10.3390/w15030585
Azadikhah D, Yalsuyi AM, Saha S, Saha NC, Faggio C. Biochemical and Pathophysiological Responses in Capoeta capoeta under Lethal and Sub-Lethal Exposures of Silver Nanoparticles. Water. 2023; 15(3):585. https://doi.org/10.3390/w15030585
Chicago/Turabian StyleAzadikhah, Dariush, Ahmad Mohamadi Yalsuyi, Shubhajit Saha, Nimai Chandra Saha, and Caterina Faggio. 2023. "Biochemical and Pathophysiological Responses in Capoeta capoeta under Lethal and Sub-Lethal Exposures of Silver Nanoparticles" Water 15, no. 3: 585. https://doi.org/10.3390/w15030585





