Abstract
In the current world, the increasingly developed industries of mankind have caused huge pollution to the earth on which we live. And the water resources, which are the source of human life, are also being seriously polluted and destroyed. Water pollution has become an urgent need to deal with in today’s world. In order to achieve sustainable development, people are constantly using new materials in the process of water treatment. Biochar material is one of them. In the thermochemical process, biomass produces a common by-product coke, which is also called biochar as a result of biomass decomposition. Due to the low price and large specific surface area which can reach over 1000 m2·g−1, it has many applications and advantages in catalysis, adsorption, fuel cell, soil improvement, etc., and has a wide range of application prospects. Therefore, effectively prepared and used biochar in water treatment has become a method to improve the efficiency and economic benefits of thermochemical processes. In this overview, we first introduced the preparation methods of different new types of biomass materials, we then classified and discussed the various modification strategies, and finally discussed the application potential of biochar material for wastewater treatment.
1. Introduction
Nowadays, the increasingly developed industries of mankind have caused huge pollution to the earth in which we live. And the water resources are also being seriously polluted and destroyed. Water pollution has become an urgent need to deal with in today’s world. As a part of sustainable development, people also put forward higher demands and requirements for the material in water treatment in order to reduce carbon emissions, secondary contamination, and consumption. Under such a circumstance, biochar material, as a new type of low-cost and low-pollution material, has been valued by numerous scientists.
The main source of biochar material is biomass, which can be transferred into biofuels and bioproducts through thermochemical processes like pyrolysis and gasification. The net CO2 emissions from the use of biofuels are generally considered to be zero or negative, which we call Negative Emission Technologies (NET), because the released CO2 is recovered from the atmosphere captured in photosynthesis [1]. Besides, the combustion of biofuels reduces the emission of hazardous gases like nitrous oxide (NOx) and sulfur dioxide (SO2) compared to most fossil fuels due to the low sulfur and nitrogen content in biomass [2]. Because of these advantages, biomass has become a potential renewable energy source. Syngas, bio-oil, biochar, and tar are the primary byproducts of the thermochemical processes of biomass; their yields depend on the parameters of the process. The two primary intermediate products that can be used to create alternative fuels to conventional fuels are syngas and bio-oil. Numerous studies have been done on improving and using syngas and bio-oil for different applications [3,4,5,6].
Biochar, as mentioned in this review, is a byproduct resulting from the thermochemical conversion of biomass that is receiving increasing attention in a variety of applications. As an environmentally friendly material, biochar has many advantages compared to other materials like MOFs, such as low-cost, easy-to-obtain, stable, etc. Common applications of biochar in the ecosphere include soil amelioration to reduce greenhouse gas emissions, reduce soil pollution [7], improve soil fertility [8], wastewater treatment [9], etc. Because of the NET concept, biochar has become a promising sustainable material for the ecosystem. The use of biochar as a precursor for the creation of catalysts and pollutant adsorbents is one of its further uses. These novel high-value applications are still in their infancy and need to be developed and researched before they can be commercialized. Although charcoal has been used as a biochar-like carbon material for many centuries, research on the use of biochar as a material for these aspects of catalysts, precursors for pollutant sorbents, and soil amendments has only made some progress in the last few years [10]. The properties of biochar determine its potential for use in various applications. For example, the high electrical conductivity, porosity, and stability at lower temperatures of biochar materials are naturally preferred as electrode materials in microbial fuel cells [11]. In the development of supercapacitors, biochar with high porosity and structural nitrogen groups is preferred [12]. Furthermore, the high surface area of biochar with low ash content may be more suitable as a soil supplement [13]. There are many reviews in the literature on the properties of biochar and its specific applications as a soil amendment, such as biochar effects on water holding capacity [14], soil hydrological indices, and growth characteristics of corn [15], but the review of biochar as an application in water treatment is relatively limited, especially studies contrasted the different feedstocks-based biochar in wastewater treatment. In recent years, due to the pursuit of sustainable development, biochar materials with stable and inexpensive sources, large specific surface area, and various treatment capabilities have been favored as a new star in water treatment, therefore, this paper outlines the recent progress in the application of biochar materials in water treatment and contrasted the different feedstocks-based biochar for wastewater treatment.
2. Preparation of Biochar Materials
Biochar is a charred organic material. The International Biochar Initiative defines biochar as “a solid material obtained from the thermochemical transformation of biomass under oxygen-limited conditions” [16]. Biochar is produced in solid form by dry carbonization, pyrolysis, or gasification of biomass, and in slurry form.
Biomass is formed by hydrothermal carbonization under pressure [17]. Typical operations of different thermochemical processes are listed in Table 1 and Figure 1. It shows that the yield of biochar is relatively temperature dependent. The higher the processing temperature, the lower the yield of biochar [18].

Table 1.
Different thermochemical processes for biomass treatment [19].
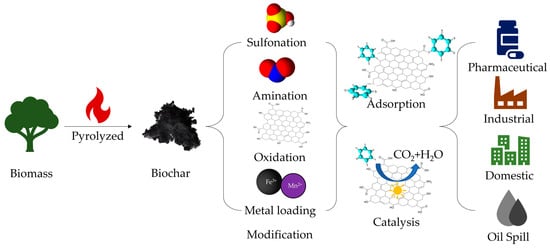
Figure 1.
Biochar materials for a wide range of applications in cleaning sewage.
2.1. Pyrolysis
Pyrolysis is the most common and easy way to obtain biochar. Depending on the heating rate and residence time, pyrolysis can be classified as slow pyrolysis, fast pyrolysis, and flush pyrolysis. By heating biomass at a lower heating rate for a longer residence time, a process known as slow pyrolysis, often referred to as traditional carbonization, creates biochar (up to several days). For ages, this process has been used to make charcoal. On the other hand, rapid pyrolysis creates biochar in a short amount of time (less than 10 s) and at high heating rates (over 200 °C·min−1). On top of this, there is a flush pyrolysis method with a particularly high heating rate and a residence time of fewer than 2 s. The main difference between the three pyrolysis methods is the yield of biochar, bio-oil, and biogas: fast pyrolysis favors a high yield of bio-oil, while slow pyrolysis favors a high yield of biochar. Although slow pyrolysis remains the traditional way of biochar production and is widely used for biochar production, it might be impossible to be the future choice for biochar production due to its inefficient and time-consuming use of energy, so a more cost-effective and environmentally sustainable method is clearly more in line with the expectations of future choices [19].
2.2. Gasification
By adding a tiny quantity of oxidant at high temperatures (>750 °C), gasification transforms biomass mostly into gaseous mixtures (syngas including CO, H2, CO2, CH4, and trace amounts of higher hydrocarbons). Typical biochar yields from gasification average about 10% of the total biomass mass fraction [20,21]. Gasification can use oxygen, air, steam, or a combination of these gases as an oxidant. Syngas produced by air gasification has a calorific value of 4–7 MJ·N−1·m−3, while steam gasification produces syngas with a high calorific value of 10–14 MJ·N−1·m−3 [5].
2.3. Hydrothermal Carbonization
At high temperatures (160–800 °C), biomass undergoes hydrothermal carbonation (HTC). Since the water temperature is higher than 100 °C, a larger reaction pressure (more than 1 atm) is also necessary to maintain the liquid condition of the water. Hydrothermal carbonation can be classified as high-temperature HTC (between 300 and 800 °C) or low-temperature HTC (below 300 °C) depending on the reaction temperature [22]. The primary reaction of high-temperature HTC is hydrothermal gasification, and the main products are gases like methane and hydrogen since the reaction conditions of high-temperature HTC (over 300 °C) surpass the stability conditions of most organic molecules [23]. Gasification is limited and biomass carbonization to char is more common below 300 °C. Low-temperature HTC can imitate the gradual, centuries-long natural coalescence of biomass, but it can do it with faster reaction times and higher reaction rates. Depending on the characteristics of the feedstock, reaction temperature, and pressure, coke outputs from low-temperature biomass HTC range from 30% to 60%. Given that HTC requires water, it might be an affordable way to produce biochar from feedstocks with a high moisture content [12].
Because of the oxidation of carbon and other functional groups with increasing temperature, the production of biochar often declines. On the other hand, biochar’s fixed carbon content often rises with temperature. The primary cause of this phenomenon is the sporadic emission of volatiles (such as H2O, CO2, CO, NH3, HCN, and CxHyOz) during the pyrolysis stage, which leads to a minor reduction in the concentration of biochar [24]. As the temperature rises further, less carbon-rich compounds, such as CxHyOz, are released from the biochar, while lower carbon-content molecules, such as CO, CO2, and NOx, are constantly released. The fixed carbon content of the residual biochar rises as a result. The pyrolysis temperature has a significant impact on the biochar features, such as surface area and pore size distribution, in addition to the yield and fixed carbon content. The surface area of the biochar increases dramatically as a result of the increased volatiles that are released from the biomass surface as the temperature rises, creating more porous char. Regarding the composition of the biomass feedstock, it has been noted that biochar synthesis at high pressures is increased by biomass with high moisture content (between 42% and 62%) [13]. According to this research, some biomasses with a greater moisture content may be especially suitable for the formation of biochar. In addition to moisture content, the inherent composition of the biomass, which includes cellulose, hemicellulose, lignin, and inorganic materials, should be given consideration. For example, substantial yields of biochar with a fixed carbon content can be produced by pyrolyzing high-lignin biomass (such as pine and spruce wood) [25]. On the other hand, inorganic substances (e.g., alkali metal or alkaline earth metal compounds) have inherent catalytic effects [26].
3. Modification of Biochar Materials
Since biochar materials obtained directly from biomass pyrolysis generally have poor surface functionality, limited active groups, and low porosity (within the range of 0.016–0.083 cm3·g−1) and surface area (usually <150 m2·g−1) [27], these drawbacks limit their wide application as functional materials. However, these surface functions and properties such as porosity can be easily adjusted by modification, which provides a large scope for synthesizing biochar into various functional materials, such as adsorbent materials or catalysts. For example, when it is needed to be used as an adsorbent or catalyst, we can enrich the type and number of surface groups and increase the porosity and surface area through different processes to improve the adsorption capacity or provide more catalytic sites [19]. Research showed that the most efficient method was acidic oxidation, which can increase biochar’s capacity for cation exchange, micro-pores, specific surface area, and oxygen-content functional groups [28]. Some methods can achieve 1000 m2·g−1 specific surface area or higher [29]. Therefore, the modification of biochar materials is essential for enhancing their functionality. There are two main types of modifications, one is a surface modification, and the other is a pore structure change. For reasons of cost and process complexity, surface modification is a very good method because of its efficiency and lower cost. The mainstream surface modification methods for biochar are surface oxidation, surface amination, surface sulfonation, metal nanoparticle loading, and surface binding of nanostructures. Table 2 shows some common modification methods and their features.

Table 2.
Different modification processes of biochar.
3.1. Surface Oxidation
Oxidation functional groups (e.g., C=O, OH, and COOH) are important for improving the performance of biochar in various applications. For example, when biochar is used as an adsorbent for the removal of heavy metals, surface hydroxyl (OH) and carboxyl (COOH) groups can greatly increase the adsorption capacity. This is due to the fact that these groups have hydrogen bonds and thus have a different electronegativity from other groups, which allows them to complex better with metal ions and thus increases their adsorption capacity [30,31]. Additionally, biochar’s hydrophilicity can be significantly increased by the addition of oxidation functional groups to the surface [32], improving its functionality in aqueous systems. The most popular technique for producing oxidation functional groups on the surface of biochar is surface oxidation. The most often utilized surface oxidation reagents are hydrogen peroxide (H2O2), ozone (O3), potassium permanganate (KMnO4), and nitric acid (HNO3) [33,34,35,36]. Surface oxidation processes can produce oxidation functional groups such as carboxyl groups, phenolic hydroxyl groups, lactones, and peroxides. Additionally, oxidizing KMnO4 or HNO3 and treating biochar with an alkali such as NaOH might make it more hydrophilic. Furthermore, HNO3 has been shown to be more efficient than KMnO4 in producing acidic oxidation functional groups, such as COOH and phenolic hydroxyl groups, which can be introduced through surface oxidation onto the surface of biochar [19].
3.2. Surface Amination
Besides surface oxidation to introduce oxidation functional groups, basic amines on the surface of biochar can significantly enhance its performance in applications like CO2 uptake and pollutant adsorption [37,38]. one of the most popular techniques for adding amino groups to organic carbon is surface amination, as shown in Figure 2. ammonia (NH3) treatment at high temperatures is a conventional surface amination technique that has been widely used for decades [39,40,41]. This method is usually combined with a pre-oxidation process because the surface of biochar is structurally graphite-like in structure and its reactivity is not high. Although NH3 treatment can effectively introduce amino groups to the carbon surface, it still does not avoid consuming large amounts of energy and releasing NH3 into the environment, thus causing serious air pollution; therefore, the process is not in line with green chemistry as well as sustainability principles. Therefore, chemical modification with certain amine-containing reagents is often used in experiments, which is considered an environmentally friendly modification method and is therefore commonly used for the surface amination of biochar. Through some experiments and studies [42,43,44], it was discovered that biochar materials with a lot of surface amines function well when it comes to removing heavy metals from wastewater. Organic monomers with various hydrophilic or hydrophobic properties can be added to the surface in addition to amino groups. To accomplish this, amination reagents with organic monomers that have hydrophilic or hydrophobic properties are used. Zhou Y’s team [45] used sodium hydroxide and nitric acid to modify the surface of sludge biochar materials to achieve a better adsorption capacity for tetracycline.
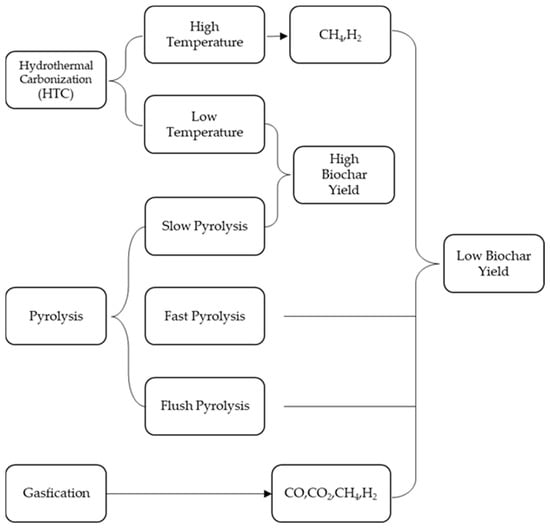
Figure 2.
Typical biochar treatments and their products.
3.3. Surface Sulfonation
The most prevalent functional groups in solid acidic materials are sulfonic acid groups (SO3H). These are frequently employed to catalyze various chemical reactions and serve as replacements for liquid acids [46]. A typical type of solid Bronsted acids is composed of amorphous carbon-containing SO3H groups; these acids typically have a high density of SO3H groups that are easily separable from the reaction [47]. Most of the organic matter that hasn’t fully carbonized is directly sulfonated to produce amorphous carbon compounds with SO3H groups. Biochar is a viable feedstock for the synthesis of amorphous carbon-based solid Bronsted acids since it is a byproduct of incomplete carbonization (pyrolysis) of biomass. The most popular technique for creating biochar-based solid acids is surface sulfonation of biochar using concentrated sulfuric acid or its derivatives (for example, fuming sulfuric acid and chlorosulfonic acid) [19,48,49,50,51,52,53]. Since the strong oxidizing power of concentrated sulfuric acid, surface oxidation is usually always accompanied by sulfonation. This produces oxidized functional groups such as COOH, C=O and OH [54], which can promote further functionalization of biochar-based solid acids with other properties, resulting in synthesized materials with high catalytic efficiency and excellent stability [55].
3.4. Metal Nanoparticle Loading
As was already mentioned above, the pyrolysis reaction typically takes place in an anaerobic environment where the biomass is broken down into charcoal and a few volatile low-molecular-weight chemicals, both of which have significant reducing abilities. Therefore, if certain loaded high-valent metal compounds are pyrolyzed alongside biomass, the reducing capabilities of the low-molecular-weight compounds can convert the high-valent metal compounds to zero-valent metal nanoparticles. In this way, biochar materials loaded with metal nanoparticles can be synthesized by in situ loading on biochar materials. The synthesis of biochar nanocomposites has several advantages: (1) the formation of biochar and the growth of metal nanoparticles occur simultaneously, thus avoiding the introduction of redundant reactions and impurities by using other reducing agents; (2) the metal compounds have a catalytic effect on the pyrolysis of biomass, thus increasing the yield of bio-oil and making the structure of biochar more porous after the reaction; for example, FeCl3 can then act as a catalyst during biomass pyrolysis and help improve the porous structure of the biochar obtained [56]. Richardson’s team [57,58] reported that many of the oxidation groups present in the biochar material can act as active sites for Ni2+ adsorption during the aqueous impregnation step. This produced uniformly dispersed metal precursors in the biomass matrix. Subsequently, these precursors were reduced in situ to metallic Ni nanoparticles by a carbothermal reduction reaction at a temperature of about 500 °C. The metal solution impregnation with loaded metal ions was utilized by Lai C’s team [59], which dried and ground the biochar material and pyrolyzed it, followed by impregnation in a mixture of ferric chloride and manganese chloride and dispersed into ultrasound. After that, ammonia was added drop by drop and stirred, and finally centrifuged to obtain a new biochar catalytic adsorption material capable of adsorption and degradation treatment of tetracycline, and its catalytic decomposition efficiency of tetracycline was able to reach nearly 92%.
3.5. Surface Binding of Nanostructures
The abundance of surface functional groups in biochar above typical activated carbon is a significant benefit. It appears that these functional groups can respond favorably to different nanostructures. For example, surface “modification” of biochar has been reported for hickory chips and sugarcane bagasse by pyrolysis with carbon nanotubes (CNT) [60]. To CNTs with carboxylic acid functionalization, the functional groups on biochar are extremely reactive. The physicochemical characteristics of biochar can be dramatically changed by the hybridization of carbon nanotubes, which also greatly enhances its efficacy in removing pollutants. Therefore, another intriguing area of exploration is the incorporation of naturally occurring inorganic chemicals in biochar for the formation of useful nanostructured materials [61]. Since silicon (Si) is a plentiful element in plant biomass (such as rice husk), the resulting rice husk biochar can be used as a low-cost and economically viable raw material for the manufacturing of silicate and silica products [62].
3.6. Other Modification Methods
Besides the main modification methods mentioned above, there are some uncommon modification methods, such as microwave activation and freeze-thaw processing cycles. Microwave activation is now a very promising technique for biomass and biochar treatment due to its fast, selective, uniform, and volumetric heating [63], the energy-saving performance also meets the current sustainable development requirements. In recent years, the microwave-assisted method is mainly used to improve the specific surface area for biochar, and the research is mainly focused on using biochar for soil remediation and soil amendment [64,65]. As an aspect of wastewater treatment, Qu’s team [66] used a microwave-assisted hydrothermal method to synthesize biochar and achieved an amazingly high specific surface area of 2097.50 m2·g−1.
The freeze-thaw cycle is a traditional process for soil remediation. This method can rapidly age biochar materials in a relatively short period. It was found that the biochar hydrophilicity and polarity increased, whereas its aromaticity decreased [67]. The BET area of processed biochar has become greater than ordinary biochar. It is quite an efficient way to increase the adsorption capacity of biochar.
Although there are many methods of surface modification, in order to search for more efficient and new catalyst materials, there are generally more studies using a combination of modification methods, such as Gholami’s team [68], which combined both loaded metal nanoparticles and surface amination, and their studied catalysts have some degradation effect on some hard-to-treat organic substances.
Table 3 summarized recent research on biochar in the water treatment field. The table shows us that surface oxidation, such as pyrolysis and metal loading, is now very widely used in biochar modification. and nowadays adsorption is still a common way of treating wastewater by using biochar, especially when used for the treatment of metal ions, and that adsorption properties are often the most important property of biochar materials. On the other hand, for the treatment of printing and dyeing wastewater or organic matter, biochar materials are always used in an integrated process combined with adsorption, catalytic, and degradation.

Table 3.
Biochar utilization for water treatment.
4. Application of Biochar Material for Water Treatment
Due to the ease of modification and the porous characteristics, biochar materials have a wide range of applications in the field of wastewater treatment. Since there are various types of water treatment, here is a classification to sort out the uses of different biochar materials in different wastewater treatment situations.
4.1. Treatment of Domestic Sewage
Modern domestic wastewater treatment consists of a series of physical and chemical processes aimed at reducing nitrogen, phosphorus, and potassium in domestic wastewater for discharge back into the environment [88]. Nowadays, China has the second-largest wastewater treatment capacity in the world after the United States [89]. In this context, environmentally friendly wastewater treatment methods are better in order to achieve the goal of sustainable development. As a NET material [1], biochar not only has the advantage of being green and low emission but also has a cost advantage in terms of price compared to activated carbon [9]. For example, in primary treatment, biochar can be used to precipitate and remove some of the suspended matter in wastewater, and mixing biochar with a coagulant for precipitation can enhance the effect of precipitation [90], while in the traditional aerobic activated sludge process, the addition of biochar can also improve the dewatering of sludge and increase the settling [91]. In anaerobic digestion, biochar can accelerate the process of anaerobic digestion and improve the efficiency of anaerobic digestion because of its redox activity [92]. In addition, biochar is often used to absorb malodorous gases (e.g., NH3, CH4, H2S, etc.) from wastewater due to its loose and porous nature [93]. Finally, biochar materials that are rich in nitrogen and phosphorus elements are also good for increasing soil fertility when dehydrated digested sludge containing biochar is discharged into the environment [94]. It was found that sewage sludge biochar was quite an efficient soil supplement [95]. However, due to the complex composition of wastewater, the toxicity of the treated biochar material should be evaluated to avoid secondary contamination due to the treatment of wastewater.
4.2. Treatment of Industrial Wastewater
Among the industrial wastewater produced by modern industries, the three main sources of wastewater are dyeing wastewater, pharmaceutical wastewater, and mining wastewater [90] (showed in Figure 3), and different biochar modification and treatment strategies are used to cope with different wastewater, depending on their active ingredients (e.g., Figure 4). Pharmaceutical and dyeing wastewaters contain a high content of organic matter, and a better method for the removal of this organic matter is to generate free radicals through chemical catalysis to promote the oxidation of polluting organic matter in the wastewater by catalytic oxidants.
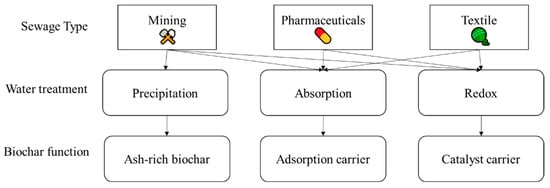
Figure 3.
Industrial wastewater from different sources and their treatment methods.
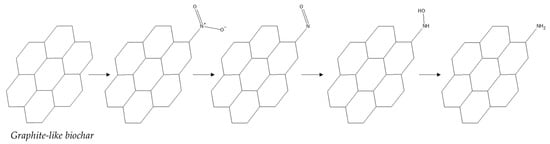
Figure 4.
Different stages of graphite-like biochar amination process.
In mining wastewater, due to its richness in metal ions, precipitation is generally used [96], together with subsequent redox reactions to remove metal ions from polluted water. The precipitation capacity of biochar comes mainly from its ability to form insoluble phosphates, hydroxides, or carbonates with metals [97]. In order to increase its precipitation capacity, materials with higher ash content possess a greater advantage [98,99], from which it can be reflected that biomass with low lignin content is more suitable as biochar material for precipitation compared to biomass with high lignin content [25,26].
In terms of catalysis, different modification methods and preparations of biochar materials are basically aimed at increasing the specific surface area and introducing active substances to improve the efficiency of catalysis. Biochar materials with graphite-like structures possess greater advantages in this regard [100]. Zheng Fan’s team used Modified Caulis spatholobi Residue Biochar to improve the adsorption of tetracycline in water by using potassium modification. It is a special way to modify by using alkali to oxidize the surface of biochar [101]. Gholami’s team [68] used a mixture of sulfuric acid and nitric acid to treat the biochar materials and added Cu(NO3)2.3H2O, Fe(NO3)3.6H2O, NH4F and CO(NH2)2 to the modified biochar powder by dispersing it in deionized water and performing a hydrothermal reaction. Hydrothermal reactions were carried out and the materials thus obtained were subjected to degradation experiments on cefazolin sodium. Both Mao Q’s team and Rubeena’s team [102,103] modified the biochar material by loading nanoparticles, with the difference that Mao Q’s team used a reducing agent, firstly adjusting the FeSO4–7H2O solution to pH 5.0, followed by adding NaBH4 solution to the mixture and stirring vigorously at 200 rpm for 30 min to reduce the iron ions in the solution to zero-valent iron for the degradation of ciprofloxacin solution, while the Rubeena team only impregnated the biochar material and later used it for the degradation of acidic Big Red dye. Rong X’s team and Li S’s team [104,105], on the other hand, took advantage of the properties of Fe3O4 and dispersed them in biomass materials to make catalysts containing magnetic properties for easy recovery and reuse. The latter achieved 95.5% degradation of carbamazepine. Shengquan Zeng’s team [106] used ferric chloride to activate the biochar material by dipping bermudagrass into ferric chloride solution and then drying and pyrolyzing it in a tube furnace, and the obtained product was able to achieve 99.94% removal of sulfamethoxazole in the Fenton reaction. Lingamdinne’s team [107] even used watermelon rind to modify it to make biochar materials that can purify uranium-containing wastewater, using a similar method to the one previously listed, where soaking followed by pyrolysis was used to complete the modification of the biochar material using metal ion loading. It can be found that in the direction of catalysis, the use of iron salt-based materials for activation and modification, and the selection of various biochar materials have become a consensus in the academic community.
4.3. Biochar in Oil Spills
Due to industrialization, the dependence on oil and its derivatives is increasing, and the globalized market inevitably leads to a large-scale transnational oil trade. In this context, environmental pollution caused by oil and its derivatives is on the rise. Such as well blowouts during oil field development, various accidents that tend to lead to pipeline leaks and storage tank leaks, tankers and their leaks, oil well drilling, refineries, and production equipment maintenance are common causes of oil and its derivatives leaks and discharges during oil development process [108,109]. Such discharges, in turn, can easily cause pollution in water bodies and terrestrial ecosystems. Oil refineries, gas stations, and petrochemical and pharmaceutical industries are the most important sources of these pollutants in the soil circle and water bodies. Many pollutants such as hydrocarbons are non-biodegradable due to their high molecular weight, complex structure, and hydrophobicity and can persist in the environment for a long time, while they contain toxic and carcinogenic properties [110], so such compounds in the ecosystem pose a threat to the health of animals and humans. To deal with these pollutants, biochar, which is inexpensive and environmentally friendly and at the same time a good adsorbent and photocatalytic carrier [111], came into the spotlight. Zeng’s team [112] used bamboo powder to synthesize magnetic porous biochar for cleaning up high-viscosity oil spills. Bazargan’s team [113] completed experiments on the direction of oil-water separation using cellulose-rich biomass, which was pyrolyzed on rice husk under the nitrogen atmosphere, followed by treatment of biomass materials using alkali, and obtained better oil-water separation results.
4.4. Different Biochar in Water Treatment
Rhodamine B, one of the most main hazardous xanthene dyes in wastewater [114], is a common water-soluble organic dye that has been the subject of a very large number of experiments. There are much data on the adsorption capacity of different biochar materials on rhodamine B, which can reflect the treatment capacity of different biochar materials on organic wastewater or printing and dyeing wastewater. Table 4 shows different biochar materials’ degradation abilities on rhodamine B.

Table 4.
Different biochar material effect on rhodamine B.
From the table, we can find that the absorption effect of rhodamine B varies greatly among different biochar materials, while the effect of catalysis basically achieves a very high degradation rate. Besides, it shows that porous materials are easier to gain more adsorption capacity and efficiency. In addition, there are multiple modifiers or methods for modifying adsorption biochar, while the methods for modifying catalytic biochar materials are basically related to iron or manganese metal salts.
5. Conclusions
In recent years, new biochar materials have made great developments in water treatment, which have improved the efficiency of environmental wastewater treatment and reduced the cost of treating wastewater. The adsorption and catalysis efficiency has reached a very high level. It seems that a similar method of biochar processing can be widely used in different ways in water treatment. This will be the key to reducing costs and increasing yields, and we can also see the effects of biochar materials in different areas. This is consistent with the sustainable development concept of low cost and low emissions. However, on the other hand, in the process of wastewater treatment, the toxicity of biochar for adsorption needs to be assessed, otherwise, there will be a risk of secondary pollution, and the environmental friendliness of the chemical materials added or influenced by the preparation of biochar materials is still worth studying and debating, if more chemical materials are consumed in the preparation process and more difficult to treat industrial effluents are produced, it will be more than worth the loss. In addition, today’s biochar modification methods are similar and not very distinguishable, with most of the preparation methods revolving around immersion in metal solutions and alkaline or acidic solutions. While immersion in metal solutions is undeniably the simplest and most efficient method, new preparation methods are equally important in the research of novel materials. Microwave activation as well as hydrothermal carbonization is a new direction for modification. Microwave-assisted biochar has various applications but is rare in wastewater treatment. This might be a new direction to study.
To meet the requirements of the sustainable development concept, the development of new biochar can be studied in depth mainly from the following aspects: (1) developing new methods for material synthesis, optimizing preparation processes, and using multiple composite modifications to add more functionality to biochar materials; (2) exploring in depth catalysts with different structures and functions to achieve controllable preparation of high-performance catalysts; (3) exploring new catalysts that are recyclable, low-cost and environment-friendly to be used in environmental pollution control projects; (4) opening up new uses and functions of biochar materials to reduce costs and emission.
Author Contributions
Conceptualization, R.L. and L.Q.; methodology, J.C. and L.Q.; formal analysis, Y.W. and H.L.; writing-original draft preparation, R.L., X.L. and L.Q.; and writing—review and editing, B.S. and L.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 22278373), Zhejiang Provincial Natural Science Foundation of China (Grant No. TGS23B070004), Open Research Fund of Key Laboratory of Drug Monitoring and Control of Zhejiang Province (Grant number 2022KLDM006), Open Research Fund of State Environmental Protection Key Laboratory of Estuarine and Coastal Environment (Grant number HKHA2022003) and the Fundamental Research Funds for the Central Public-Interest Scientific Institution (Grant number 2022YSKY-03), and Zhejiang Provincial Postdoctoral Science Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Reprinted (adapted) with permission from {Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285.}. Copyright {2023} American Chemical Society.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Selvarajoo, A.; Lee, C.W.; Oochit, D.; Almashjary, K.H.O. Bio-pellets from empty fruit bunch and durian rinds with cornstarch adhesive for potential renewable energy. Mater. Sci. Energy Technol. 2021, 4, 242–248. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Swain, P.K.; Das, L.M.; Naik, S.N. Biomass to liquid: A prospective challenge to research and development in 21st century. Renew. Sustain. Energy Rev. 2011, 15, 4917–4933. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.; Hanna, M. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Petrus, L.; Noordermeer, M.A. Biomass to biofuels, a chemical perspective. Green Chemistry 2006, 8, 861. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Kopecky, M.; Amirahmadi, E.; Moudry, J., Jr.; Mensik, L. Preliminary Findings on Cadmium Bioaccumulation and Photosynthesis in Rice (Oryza sativa L.) and Maize (Zea mays L.) Using Biochar Made from C3- and C4-Originated Straw. Plants 2022, 11, 1424. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Kopecký, M.; Amirahmadi, E.; Bucur, D.; Walkiewicz, A. Interaction of Biochar with Chemical, Green and Biological Nitrogen Fertilizers on Nitrogen Use Efficiency Indices. Agronomy 2022, 12, 2106. [Google Scholar] [CrossRef]
- Thompson, K.A.; Shimabuku, K.K.; Kearns, J.P.; Knappe, D.R.U.; Summers, R.S.; Cook, S.M. Environmental Comparison of Biochar and Activated Carbon for Tertiary Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11253–11262. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Huggins, T.; Wang, H.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol. 2014, 157, 114–119. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796. [Google Scholar] [CrossRef]
- Manyà, J.J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef]
- Ghorbani, M.; Neugschwandtner, R.W.; Konvalina, P.; Asadi, H.; Kopecký, M.; Amirahmadi, E. Comparative effects of biochar and compost applications on water holding capacity and crop yield of rice under evaporation stress: A two-years field study. Paddy Water Environ. 2023, 21, 47–58. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Konvalina, P.; Moudrý, J.; Bárta, J.; Kopecký, M.; Teodorescu, R.I.; Bucur, R.D. Comparative Influence of Biochar and Zeolite on Soil Hydrological Indices and Growth Characteristics of Corn (Zea mays L.). Water 2022, 14, 3506. [Google Scholar] [CrossRef]
- IBI. Standardized Product Definition and Product Testing Guidelines for Biochar that Is Used in Soil (Version 1.1), International Biochar Initiative. 2012. Available online: http://www.biochar-international.org/characterizationstandard (accessed on 12 January 2023).
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Neugschwandtner, R.W.; Konvalina, P.; Kopecký, M.; Moudrý, J.; Perná, K.; Murindangabo, Y.T. The Impact of Pyrolysis Temperature on Biochar Properties and Its Effects on Soil Hydrological Properties. Sustainability 2022, 14, 14722. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, Economical, and Climate-Related Aspects of Biochar Production Technologies: A Literature Review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Patil, K.; Bellmer, D.; Wang, D.; Yuan, W.; Huhnke, R. Effects of Biomass Feedstocks and Gasification Conditions on the Physiochemical Properties of Char. Energies 2013, 6, 3972–3986. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.-H.; Antonietti, M.; Titirici, M.-M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Becidan, M.; Skreiberg, Ø.; Hustad, J.E. NOx and N2O precursors (NH3 and HCN) in pyrolysis of biomass residues. Energy Fuels 2007, 21, 1173–1180. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Kopecký, M.; Kolář, L. A meta-analysis on the impacts of different oxidation methods on the surface area properties of biochar. Land Degrad. Dev. 2022, 1–14. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen, D.T.; Nguyen, T.T.; Nguyen, H.P.T.; Khuat, H.B.; Nguyen, T.H.; Tran, V.K.; Woong Chang, S.; Nguyen-Tri, P.; Nguyen, D.D.; et al. Activated carbon with ultrahigh surface area derived from sawdust biowaste for the removal of rhodamine B in water. Environ. Technol. Innov. 2021, 24, 101811. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Guo, L.; Zhang, Y.; Lou, Z.; Wang, Y.; Qian, G. Cu(II) removal from aqueous solution by Spartina alterniflora derived biochar. Bioresour. Technol. 2013, 141, 83–88. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, Y.; Sun, K.; Gao, B.; Wang, Z.; Jin, J.; Zhang, Z.; Wang, S.; Yan, Y.; Liu, X.; et al. Cadmium adsorption on plant- and manure-derived biochar and biochar-amended sandy soils: Impact of bulk and surface properties. Chemosphere 2014, 111, 320–326. [Google Scholar] [CrossRef]
- He, H.; Qian, T.-T.; Liu, W.-J.; Jiang, H.; Yu, H.-Q. Biological and chemical phosphorus solubilization from pyrolytical biochar in aqueous solution. Chemosphere 2014, 113, 175–181. [Google Scholar] [CrossRef]
- Anfruns, A.; García-Suárez, E.J.; Montes-Morán, M.A.; Gonzalez-Olmos, R.; Martin, M.J. New insights into the influence of activated carbon surface oxygen groups on H2O2 decomposition and oxidation of pre-adsorbed volatile organic compounds. Carbon 2014, 77, 89–98. [Google Scholar] [CrossRef]
- Wu, L.; Sitamraju, S.; Xiao, J.; Liu, B.; Li, Z.; Janik, M.J.; Song, C. Effect of liquid-phase O3 oxidation of activated carbon on the adsorption of thiophene. Chem. Eng. J. 2014, 242, 211–219. [Google Scholar] [CrossRef]
- Sun, C.; Snape, C.E.; Liu, H. Development of Low-Cost Functional Adsorbents for Control of Mercury (Hg) Emissions from Coal Combustion. Energy Fuels 2013, 27, 3875–3882. [Google Scholar] [CrossRef]
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. [Google Scholar] [CrossRef]
- Yang, G.; Chen, H.; Qin, H.; Feng, Y. Amination of activated carbon for enhancing phenol adsorption: Effect of nitrogen-containing functional groups. Appl. Surf. Sci. 2014, 293, 299–305. [Google Scholar] [CrossRef]
- Adelodun, A.A.; Lim, Y.H.; Jo, Y.M. Stabilization of potassium-doped activated carbon by amination for improved CO2 selective capture. J. Anal. Appl. Pyrolysis 2014, 108, 151–159. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Wan Daud, W.M.A.; Houshmand, A.; Arami-Niya, A. The application of response surface methodology to optimize the amination of activated carbon for the preparation of carbon dioxide adsorbents. Fuel 2012, 94, 465–472. [Google Scholar] [CrossRef]
- Jansen, R.J.J.; Van Bekkum, H. Amination and ammoxidation of activated carbons. Carbon 1994, 32, 1507–1516. [Google Scholar] [CrossRef]
- Stöhr, B.; Boehm, H.P.; Schlögl, R. Enhancement of the catalytic activity of activated carbons in oxidation reactions by thermal treatment with ammonia or hydrogen cyanide and observation of a superoxide species as a possible intermediate. Carbon 1991, 29, 707–720. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Thomas, A.; Antonietti, M. Aminated hydrophilic ordered mesoporous carbons. J. Mater. Chem. 2007, 17, 3412. [Google Scholar] [CrossRef]
- Zhao, L.; Bacsik, Z.; Hedin, N.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.-M. Carbon Dioxide Capture on Amine-Rich Carbonaceous Materials Derived from Glucose. ChemSusChem 2010, 3, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, W.-J.; Zhang, N.; Li, Y.-S.; Jiang, H.; Sheng, G.-P. Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresour. Technol. 2014, 169, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, Y.; He, Y.; Liu, X.; Xu, B.; Yu, J.; Dai, C.; Huang, A.; Pang, Y.; Luo, L. Analyses of tetracycline adsorption on alkali-acid modified magnetic biochar: Site energy distribution consideration. Sci. Total Environ. 2019, 650, 2260–2266. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Tagusagawa, C.; Hayashi, S.; Hara, M.; Domen, K. Nanosheets as highly active solid acid catalysts for green chemical syntheses. Energy Environ. Sci. 2010, 3, 82–93. [Google Scholar] [CrossRef]
- Nakajima, K.; Hara, M. Amorphous Carbon with SO3H groups as a Solid Brønsted Acid Catalyst. ACS Catal. 2012, 2, 1296–1304. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Ellis, N. Biochar-based catalyst for simultaneous reactions of esterification and transesterification. Catal. Today 2013, 207, 86–92. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Kastner, J.R.; Miller, J.; Geller, D.P.; Locklin, J.; Keith, L.H.; Johnson, T. Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catal. Today 2012, 190, 122–132. [Google Scholar] [CrossRef]
- Ormsby, R.; Kastner, J.R.; Miller, J. Hemicellulose hydrolysis using solid acid catalysts generated from biochar. Catal. Today 2012, 190, 89–97. [Google Scholar] [CrossRef]
- Li, S.; Gu, Z.; Bjornson, B.E.; Muthukumarappan, A. Biochar based solid acid catalyst hydrolyze biomass. J. Environ. Chem. Eng. 2013, 1, 1174–1181. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, Z.; Yin, D.; Xu, Q.; Liu, F.; Lu, C.; Mao, L. Microwave-assisted hydrolysis of crystalline cellulose catalyzed by biomass char sulfonic acids. Green Chem. 2010, 12, 696. [Google Scholar] [CrossRef]
- Hara, M. Biomass conversion by a solid acid catalyst. Energy Environ. Sci. 2010, 3, 601. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Z.; Liu, Y.C.; Dai, B.; Zou, Y.; Gong, X.; Wang, Y.; Deng, X.; Wu, H.; Xu, Q.; et al. Ionic liquid-functionalized biochar sulfonic acid as a biomimetic catalyst for hydrolysis of cellulose and bamboo under microwave irradiation. Green Chem. 2012, 14, 1928. [Google Scholar] [CrossRef]
- Liu, W.-J.; Tian, K.; Jiang, H.; Yu, H.-Q. Facile synthesis of highly efficient and recyclable magnetic solid acid from biomass waste. Sci. Rep. 2013, 3, 2419. [Google Scholar] [CrossRef]
- Richardson, Y.; Blin, J.; Volle, G.; Motuzas, J.; Julbe, A. In situ generation of Ni metal nanoparticles as catalyst for H2-rich syngas production from biomass gasification. Appl. Catal. A Gen. 2010, 382, 220–230. [Google Scholar] [CrossRef]
- Richardson, Y.; Motuzas, J.; Julbe, A.; Volle, G.; Blin, J. Catalytic Investigation of in Situ Generated Ni Metal Nanoparticles for Tar Conversion during Biomass Pyrolysis. J. Phys. Chem. C 2013, 117, 23812–23831. [Google Scholar] [CrossRef]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S.; et al. Fabrication of novel magnetic MnFe2O4/bio-char composite and heterogeneous photo-Fenton degradation of tetracycline in near neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Zimmerman, A.; Zhang, M.; Chen, H. Synthesis, characterization, and dye sorption ability of carbon nanotube–biochar nanocomposites. Chem. Eng. J. 2014, 236, 39–46. [Google Scholar] [CrossRef]
- Su, D.S.; Chen, X.-W. Natural Lavas as Catalysts for Efficient Production of Carbon Nanotubes and Nanofibers. Angew. Chem. 2007, 119, 1855–1856. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q. Porous silica and carbon derived materials from rice husk pyrolysis char. Microporous Mesoporous Mater. 2014, 188, 46–76. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Liu, T.; Fu, W.; Li, B. A review of biochar prepared by microwave-assisted pyrolysis of organic wastes. Sustain. Energy Technol. Assess. 2022, 50, 101873. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, I.K.M.; Tsang, D.C.W.; Fan, J.; Clark, J.H.; Luo, G.; Zhang, S.; Khan, E.; Graham, N.J.D. Tailored design of graphitic biochar for high-efficiency and chemical-free microwave-assisted removal of refractory organic contaminants. Chem. Eng. J. 2020, 398, 125505. [Google Scholar] [CrossRef]
- Qu, J.; Wang, S.; Jin, L.; Liu, Y.; Yin, R.; Jiang, Z.; Tao, Y.; Huang, J.; Zhang, Y. Magnetic porous biochar with high specific surface area derived from microwave-assisted hydrothermal and pyrolysis treatments of water hyacinth for Cr(Ⅵ) and tetracycline adsorption from water. Bioresour. Technol. 2021, 340, 125692. [Google Scholar] [CrossRef]
- Wang, H.; Teng, H.; Wang, X.; Xu, J.; Sheng, L. Physicochemical modification of corn straw biochar to improve performance and its application of constructed wetland substrate to treat city tail water. J. Environ. Manag. 2022, 310, 114758. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Hassani, A.; Bhatnagar, A. Facile hydrothermal synthesis of novel Fe-Cu layered double hydroxide/biochar nanocomposite with enhanced sonocatalytic activity for degradation of cefazolin sodium. J. Hazard. Mater. 2020, 381, 120742. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, J.; Saleem, M.; Yang, Y.; Zhang, Q. Enhanced adsorptive removal of carbendazim from water by FeCl3-modified corn straw biochar as compared with pristine, HCl and NaOH modification. J. Environ. Chem. Eng. 2022, 10, 107024. [Google Scholar] [CrossRef]
- Mao, W.; Zhang, Y.; Luo, J.; Chen, L.; Guan, Y. Novel co-polymerization of polypyrrole/polyaniline on ferrate modified biochar composites for the efficient adsorption of hexavalent chromium in water. Chemosphere 2022, 303, 135254. [Google Scholar] [CrossRef]
- Du, H.; Xi, C.; Tang, B.; Chen, W.; Deng, W.; Cao, S.; Jiang, G. Performance and mechanisms of NaOH and ball-milling co-modified biochar for enhanced the removal of Cd2+ in synthetic water: A combined experimental and DFT study. Arab. J. Chem. 2022, 15, 103817. [Google Scholar] [CrossRef]
- Wang, Q.; Duan, C.-J.; Xu, C.-Y.; Geng, Z.-C. Efficient removal of Cd(II) by phosphate-modified biochars derived from apple tree branches: Processes, mechanisms, and application. Sci. Total Environ. 2022, 819, 152876. [Google Scholar] [CrossRef]
- Khan, B.A.; Ahmad, M.; Iqbal, S.; Bolan, N.; Zubair, S.; Shafique, M.A.; Shah, A. Effectiveness of the engineered pinecone-derived biochar for the removal of fluoride from water. Environ. Res. 2022, 212, 113540. [Google Scholar] [CrossRef]
- Liu, Z.; Zhen, F.; Zhang, Q.; Qian, X.; Li, W.; Sun, Y.; Zhang, L.; Qu, B. Nanoporous biochar with high specific surface area based on rice straw digestion residue for efficient adsorption of mercury ion from water. Bioresour. Technol. 2022, 359, 127471. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L. Removal mechanisms of Cd from water and soil using Fe–Mn oxides modified biochar. Environ. Res. 2022, 212, 113406. [Google Scholar] [CrossRef]
- Iqbal, J.; Mohamed Al Hajeri, B.; Shah, N.S.; Wilson, K.; Xavier, C.; Shaalan, J.; Al-Taani, A.A.; Howari, F.; Nazzal, Y. Preparation of H3PO4 modified Sidr biochar for the enhanced removal of ciprofloxacin from water. Int. J. Phytoremediat. 2022, 24, 1231–1242. [Google Scholar] [CrossRef]
- Li, X.; Shi, J.; Luo, X. Enhanced adsorption of rhodamine B from water by Fe-N co-modified biochar: Preparation, performance, mechanism and reusability. Bioresour. Technol. 2022, 343, 126103. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Y.; Song, T.; Su, R.; Luo, J. Activated peroxymonosulfate with ferric chloride-modified biochar to degrade bisphenol A: Characteristics, influencing factors, reaction mechanism and reuse performance. Sep. Purif. Technol. 2022, 300, 121857. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, S.; Liu, C.; Chen, T.; Tang, Y.; Ma, J.; Yin, K.; Luo, S. Efficient removal of arsenic from groundwater using iron oxide nanoneedle array-decorated biochar fibers with high Fe utilization and fast adsorption kinetics. Water Res. 2019, 167, 115107. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Wang, Z.; Yin, K.; Chen, T.; Liu, C. Enhanced removal of As(III) by heterogeneous catalytic oxidation of As(III) on Fe-biochar fibers with H2O2 and hydroxylamine. Chem. Eng. J. 2022, 428, 131200. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Feng, W.; Wang, Y.; Liu, S.; Dong, Z.; Li, X. Removal of EDTA-Cu(II) from Water Using Synergistic Fenton Reaction-Assisted Adsorption by Nanomanganese Oxide-Modified Biochar: Performance and Mechanistic Analysis. ACS EST Water 2021, 1, 1302–1312. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.; Wang, C.; Cao, Y. Co-carbonization of red mud and waste sawdust for functional application as Fenton catalyst: Evaluation of catalytic activity and mechanism. J. Environ. Chem. Eng. 2021, 9, 105368. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Xie, X.; Wang, Z. Bifunctional MnFe2O4/chitosan modified biochar composite for enhanced methyl orange removal based on adsorption and photo-Fenton process. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126104. [Google Scholar] [CrossRef]
- Sang, F.; Yin, Z.; Wang, W.; Almatrafi, E.; Wang, Y.; Zhao, B.; Gong, J.; Zhou, C.; Zhang, C.; Zeng, G.; et al. Degradation of ciprofloxacin using heterogeneous Fenton catalysts derived from natural pyrite and rice straw biochar. J. Clean. Prod. 2022, 378, 134459. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y. Preparation of hydrogen sulfide adsorbent derived from spent Fenton-like reagent modified biochar and its removal characteristics for hydrogen sulfide. Fuel Process. Technol. 2022, 238, 107495. [Google Scholar] [CrossRef]
- Xin, S.; Liu, G.; Ma, X.; Gong, J.; Ma, B.; Yan, Q.; Chen, Q.; Ma, D.; Zhang, G.; Gao, M.; et al. High efficiency heterogeneous Fenton-like catalyst biochar modified CuFeO2 for the degradation of tetracycline: Economical synthesis, catalytic performance and mechanism. Appl. Catal. B Environ. 2021, 280, 119386. [Google Scholar] [CrossRef]
- Yi, K.; Lei, M.; Peng, L.; Chen, A.; Luo, S. Sunlight-driven degradation of diethyl phthalate via magnetically modified biochar catalysts in water: Internal electron transfer mechanism. Chemosphere 2021, 269, 129366. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH's Water Treatment: Principles and Design; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 34–35. [Google Scholar]
- Jin, L.; Zhang, G.; Tian, H. Current state of sewage treatment in China. Water Res. 2014, 66, 85–98. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, L.; Huang, J.; Mo, Z.; Guan, Z.; Sun, S.; Liang, J.; Huang, S. Enhanced sludge dewaterability by a novel MnFe2O4-Biochar activated peroxymonosulfate process combined with Tannic acid. Chem. Eng. J. 2022, 429, 132280. [Google Scholar] [CrossRef]
- He, M.; Xu, Z.; Hou, D.; Gao, B.; Cao, X.; Ok, Y.S.; Rinklebe, J.; Bolan, N.S.; Tsang, D.C.W. Waste-derived biochar for water pollution control and sustainable development. Nat. Rev. Earth Environ. 2022, 3, 444–460. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Gao, X.; Chen, H.; Xu, X.; Zhu, L. Role of biochar in the granulation of anaerobic sludge and improvement of electron transfer characteristics. Bioresour. Technol. 2018, 268, 28–35. [Google Scholar] [CrossRef]
- Barbusiński, K.; Parzentna-Gabor, A.; Kasperczyk, D. Removal of Odors (Mainly H2S and NH3) Using Biological Treatment Methods. Clean Technol. 2021, 3, 138–155. [Google Scholar] [CrossRef]
- He, M.; Xiong, X.; Wang, L.; Hou, D.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Tsang, D.C.W. A critical review on performance indicators for evaluating soil biota and soil health of biochar-amended soils. J. Hazard. Mater. 2021, 414, 125378. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Walkiewicz, A.; Neugschwandtner, R.W.; Kopecky, M.; Zamanian, K.; Chen, W.H.; Bucur, D. Feasibility of Biochar Derived from Sewage Sludge to Promote Sustainable Agriculture and Mitigate GHG Emissions-A Review. Int. J. Environ. Res. Public Health 2022, 19, 12983. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Wang, J.; Zhang, Y.; Pan, W.-P. A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: Enhanced the ion exchange and precipitation capacity. Sci. Total Environ. 2021, 754, 142150. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2012, 20, 358–368. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, X.; Tsang, D.C.W.; Cao, X. Contrasting impacts of pre- and post-application aging of biochar on the immobilization of Cd in contaminated soils. Environ. Pollut. 2018, 242, 1362–1370. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Sun, T.; Levin, B.D.A.; Guzman, J.J.L.; Enders, A.; Muller, D.A.; Angenent, L.T.; Lehmann, J. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nat. Commun. 2017, 8, 14873. [Google Scholar] [CrossRef]
- Fan, Z.; Fang, J.; Zhang, G.; Qin, L.; Fang, Z.; Jin, L. Improved Adsorption of Tetracycline in Water by a Modified Caulis spatholobi Residue Biochar. ACS Omega 2022, 7, 30543–30553. [Google Scholar] [CrossRef]
- Mao, Q.; Zhou, Y.; Yang, Y.; Zhang, J.; Liang, L.; Wang, H.; Luo, S.; Luo, L.; Jeyakumar, P.; Ok, Y.S.; et al. Experimental and theoretical aspects of biochar-supported nanoscale zero-valent iron activating H2O2 for ciprofloxacin removal from aqueous solution. J. Hazard. Mater. 2019, 380, 120848. [Google Scholar] [CrossRef]
- Rubeena, K.K.; Hari Prasad Reddy, P.; Laiju, A.R.; Nidheesh, P.V. Iron impregnated biochars as heterogeneous Fenton catalyst for the degradation of acid red 1 dye. J. Environ. Manag. 2018, 226, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Xie, M.; Kong, L.; Natarajan, V.; Ma, L.; Zhan, J. The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation. Chem. Eng. J. 2019, 372, 294–303. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Zhao, X.; Yang, X.; Liang, G.; Xie, X. Insight into enhanced carbamazepine photodegradation over biochar-based magnetic photocatalyst Fe3O4/BiOBr/BC under visible LED light irradiation. Chem. Eng. J. 2019, 360, 600–611. [Google Scholar] [CrossRef]
- Zeng, S.; Kan, E. FeCl3-activated biochar catalyst for heterogeneous Fenton oxidation of antibiotic sulfamethoxazole in water. Chemosphere 2022, 306, 135554. [Google Scholar] [CrossRef] [PubMed]
- Lingamdinne, L.P.; Choi, J.-S.; Angaru, G.K.R.; Karri, R.R.; Yang, J.-K.; Chang, Y.-Y.; Koduru, J.R. Magnetic-watermelon rinds biochar for uranium-contaminated water treatment using an electromagnetic semi-batch column with removal mechanistic investigations. Chemosphere 2022, 286, 131776. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, J.; Wang, J.; Gao, S. Combining stable carbon isotope analysis and petroleum-fingerprinting to evaluate petroleum contamination in the Yanchang oilfield located on loess plateau in China. Environ. Sci. Pollut. Res. Int. 2018, 25, 2830–2841. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef]
- Saeed, M.; Ilyas, N.; Jayachandran, K.; Shabir, S.; Akhtar, N.; Shahzad, A.; Sayyed, R.Z.; Bano, A. Advances in Biochar and PGPR engineering system for hydrocarbon degradation: A promising strategy for environmental remediation. Environ. Pollut. 2022, 305, 119282. [Google Scholar] [CrossRef]
- Zeng, G.; Huang, X.; Yue, J.; Fan, B.; Liu, Y.; Tang, X.-Z. Solar-assisted efficient cleanup of high-viscosity oil spills using magnetic porous biochar. J. Alloy. Compd. 2022, 924, 166474. [Google Scholar] [CrossRef]
- Bazargan, A.; Tan, J.; Hui, C.W.; McKay, G. Utilization of rice husks for the production of oil sorbent materials. Cellulose 2014, 21, 1679–1688. [Google Scholar] [CrossRef]
- Jain, R.; Mathur, M.; Sikarwar, S.; Mittal, A. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manag. 2007, 85, 956–964. [Google Scholar] [CrossRef]
- Da Silva, W.L.; Muraro, P.C.L.; Pavoski, G.; Espinosa, D.C.R.; Dos Santos, J.H.Z. Preparation and characterization of biochar from cement waste for removal of rhodamine B dye. J. Mater. Cycles Waste Manag. 2022, 24, 1333–1342. [Google Scholar] [CrossRef]
- Adekola, F.; Ayodele, S.; Inyinbor, A. Efficient Rhodamine B Removal Using Acidand Alkaline-Activated Musa paradisiaca Biochar. Pol. J. Environ. Stud. 2019, 28, 3063–3070. [Google Scholar] [CrossRef]
- Albanio, I.I.; Muraro, P.C.L.; Da Silva, W.L. Rhodamine B Dye Adsorption onto Biochar from Olive Biomass Waste. Water, Air, Soil Pollut. 2021, 232, 1–11. [Google Scholar] [CrossRef]
- Adekola, F.A.; Ayodele, S.B.; Inyinbor, A.A. Activated biochar prepared from plaintain peels: Characterization and Rhodamine B adsorption data set. Chem. Data Collect. 2019, 19, 100170. [Google Scholar] [CrossRef]
- Peng, Z.; Fan, Z.; Chen, X.; Zhou, X.; Gao, Z.F.; Deng, S.; Wan, S.; Lv, X.; Shi, Y.; Han, W. Fabrication of Nano Iron Oxide–Modified Biochar from Co-Hydrothermal Carbonization of Microalgae and Fe(II) Salt for Efficient Removal of Rhodamine B. Nanomaterials 2022, 12, 2271. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, G.; Li, J.; Yang, Q.; Huang, S.; Cai, J. Hydrothermal conversion of bamboo shoot shell to biochar: Preliminary studies of adsorption equilibrium and kinetics for rhodamine B removal. J. Anal. Appl. Pyrolysis 2019, 143, 104694. [Google Scholar] [CrossRef]
- Zhu, B.; Yu, Y.; Ding, Y.; Ge, S. Iron-modified granular sludge biochar-based catalysts for improved Rhodamine B degradation by activating peroxymonosulfate. Biomass Convers. Biorefinery 2022, 1–11. [Google Scholar] [CrossRef]
- Fatimah, I.; Purwiandono, G.; Sahroni, I.; Wijayana, A.; Faraswati, M.; Dwi Putri, A.; Oh, W.-C.; Doong, R.-A. Magnetically-separable photocatalyst of magnetic biochar from snake fruit peel for rhodamine B photooxidation. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100669. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, S.; Nguyen, T.T.; Gao, X.; Luo, S.; Guo, M. Biochar loaded on MnFe2O4 as Fenton catalyst for Rhodamine B removal: Characterizations, catalytic performance, process optimization and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127651. [Google Scholar] [CrossRef]
- Kang, F.; Shi, C.; Li, W.; Eqi, M.; Liu, Z.; Zheng, X.; Huang, Z. Honeycomb like CdS/sulphur-modified biochar composites with enhanced adsorption-photocatalytic capacity for effective removal of rhodamine B. J. Environ. Chem. Eng. 2022, 10, 106942. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).