The Effect of Soluble Sugar Degradation on the Evaporation of Compacted Clay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- (1)
- The representative soluble sugar of domestic source contaminations
- (2)
- Clay
2.2. Sample Preparation
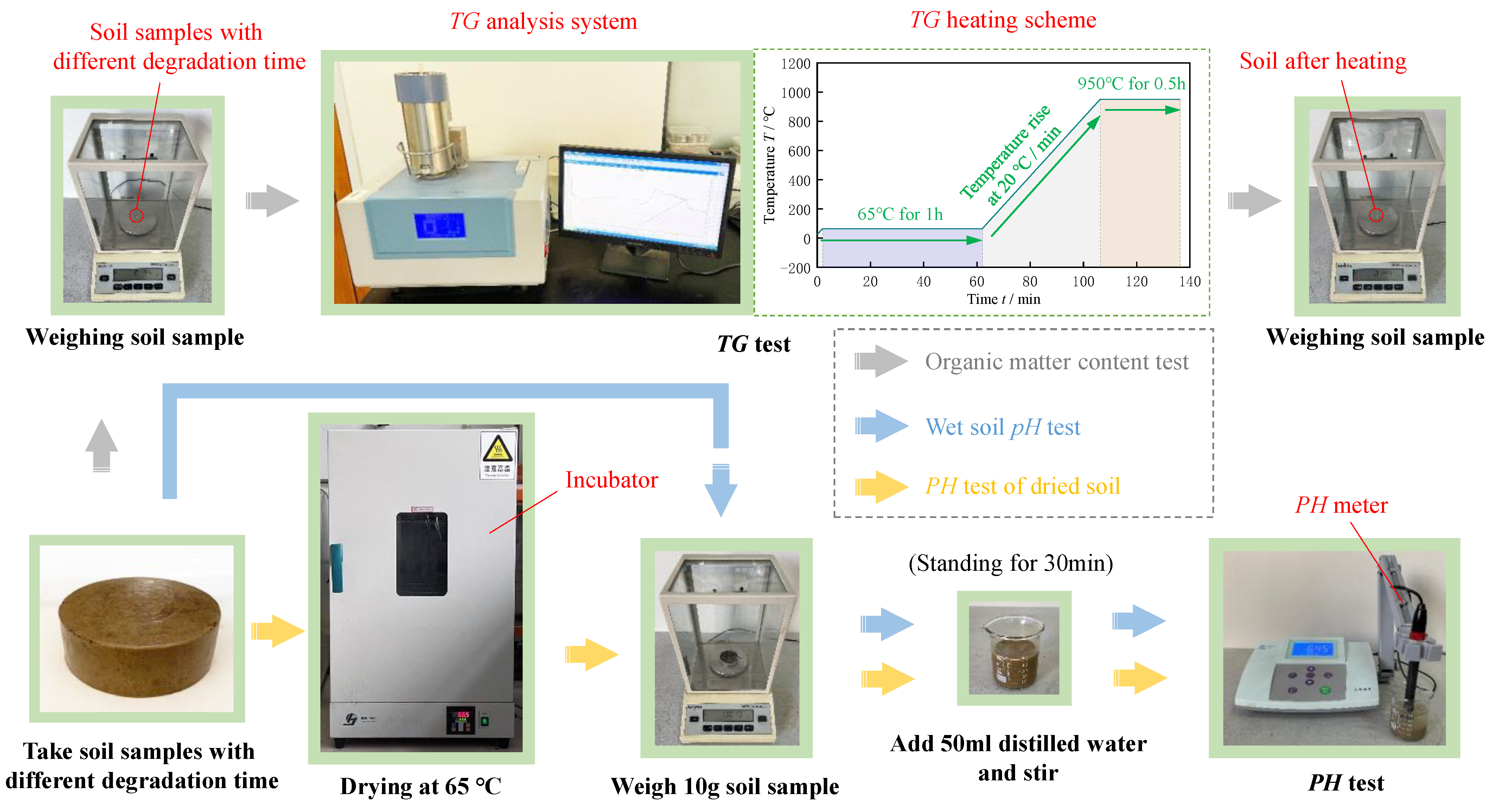
2.3. Experimental Methods
- (1)
- Evaporation test method
- (2)
- Image processing method for evaporation cracks
- (3)
- Experiment Design of the Glucose Anaerobic Degradation Process
3. Results
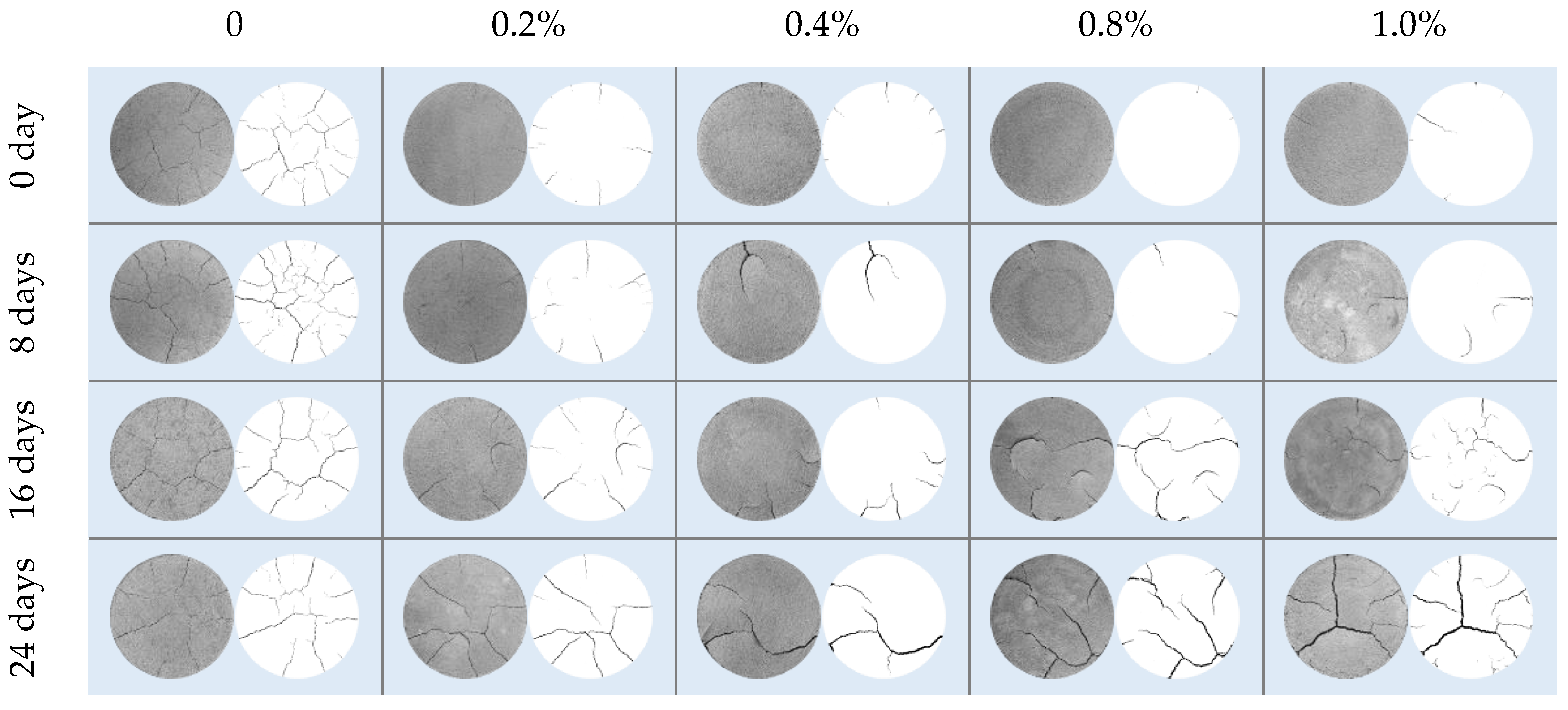
3.1. Characteristics of Evaporation in Compacted Clay during the Anaerobic Degradation of Glucose
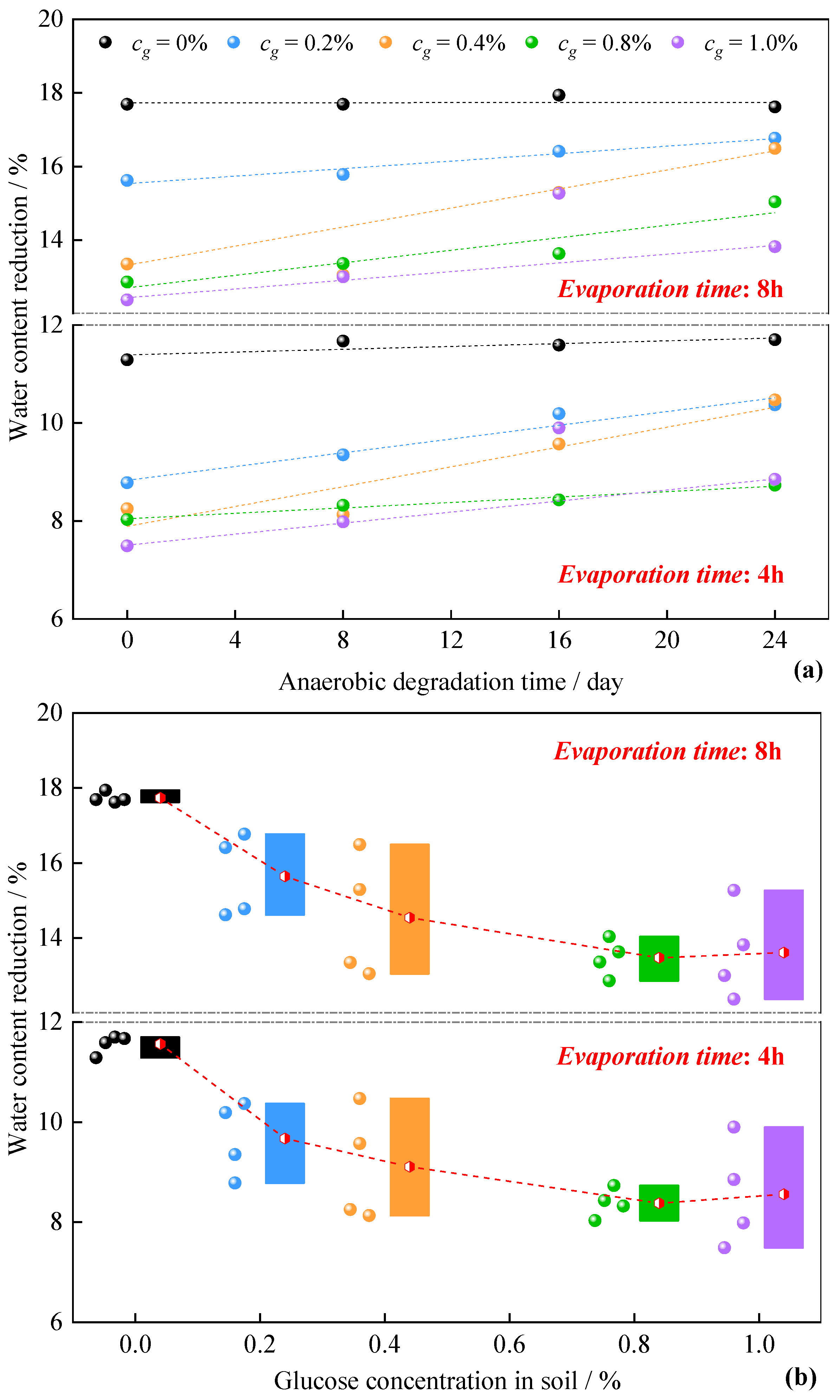
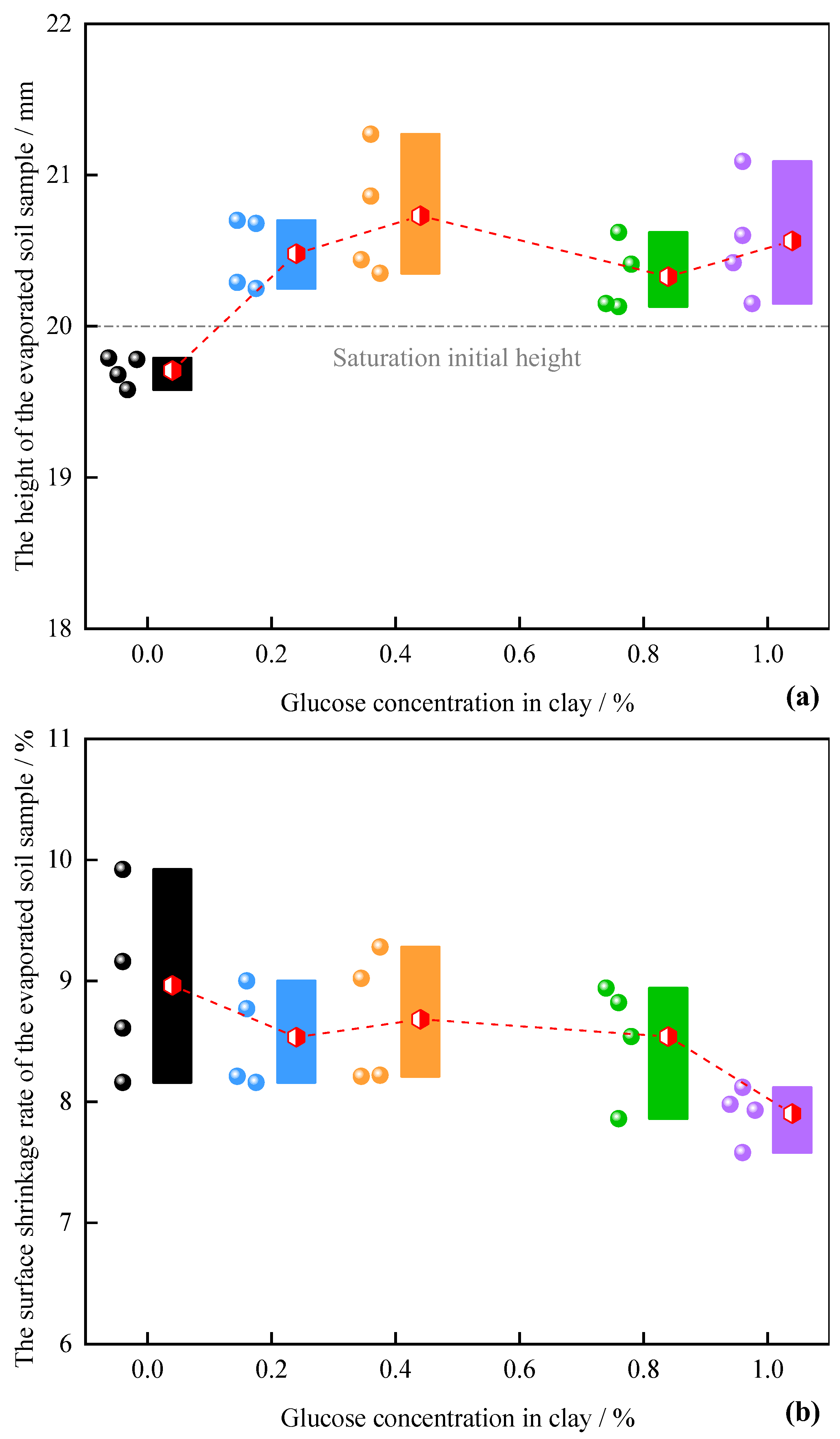
3.2. Characteristics of Evaporation Volume Change in Compacted Clay during the Anaerobic Degradation of Glucose
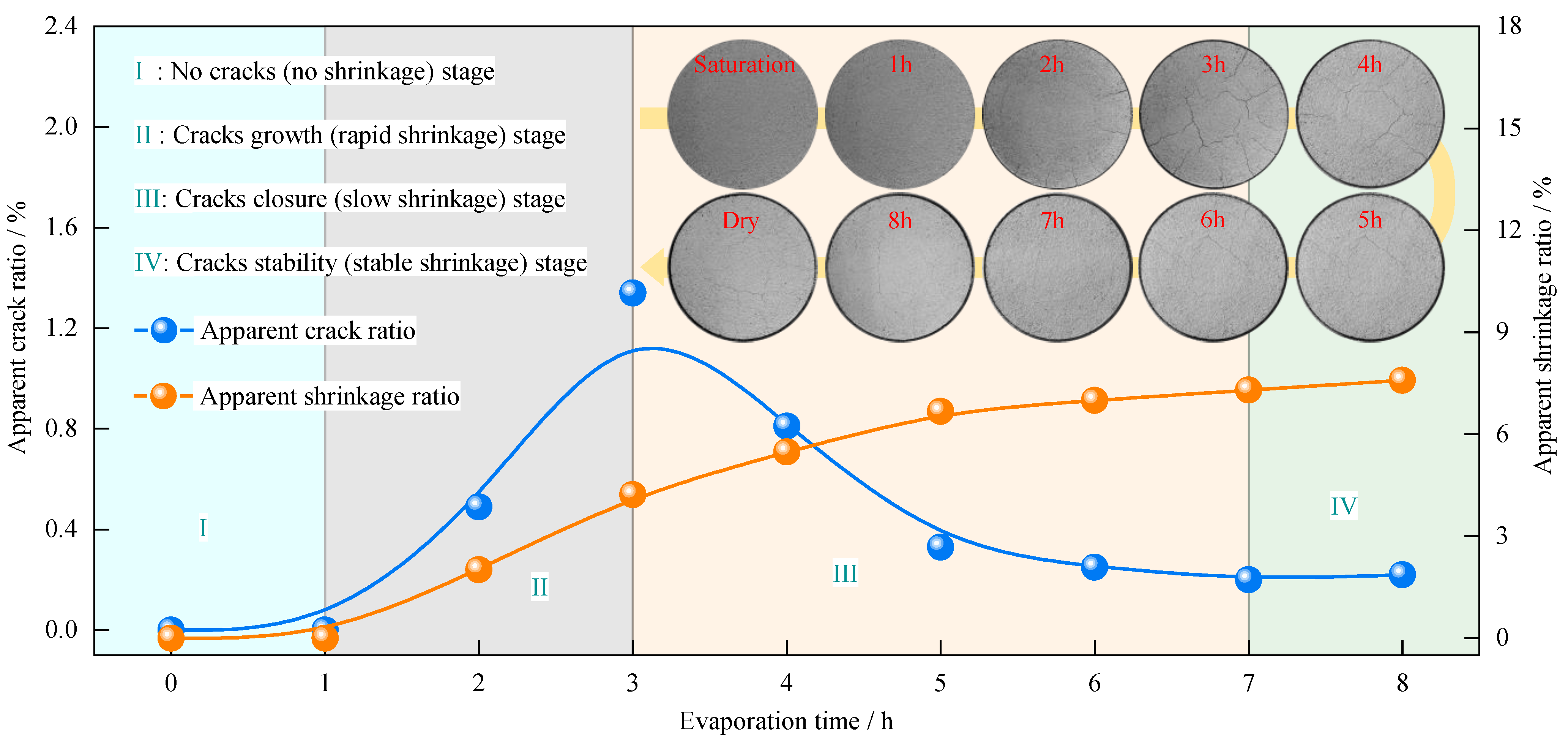
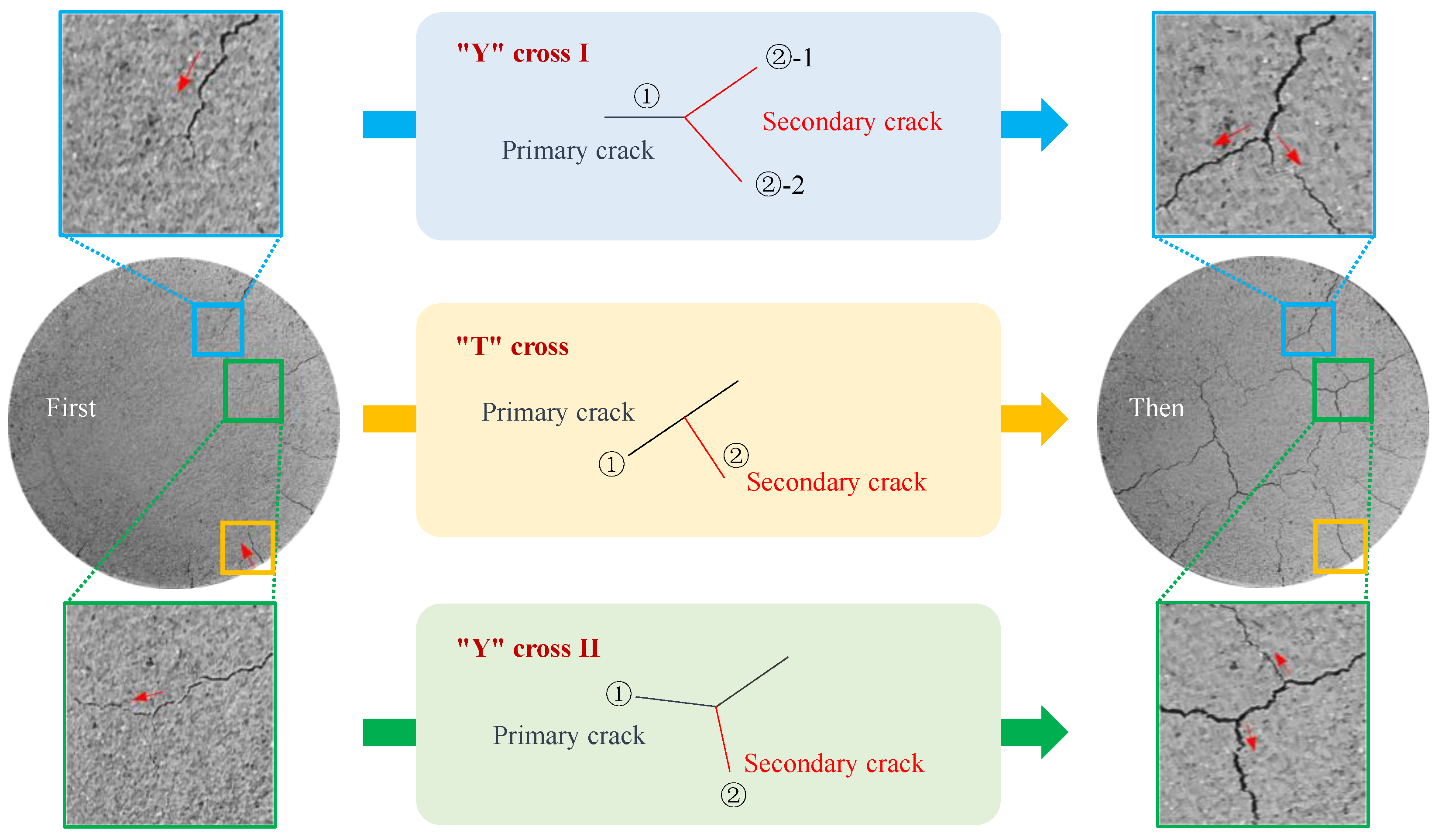
3.3. Characteristics of Evaporation Crack Development in Compacted Clay during the Anaerobic Degradation of Glucose
- (1)
- The growth and development laws of cracks
- (2)
- The distribution law of cracks
4. Discussion
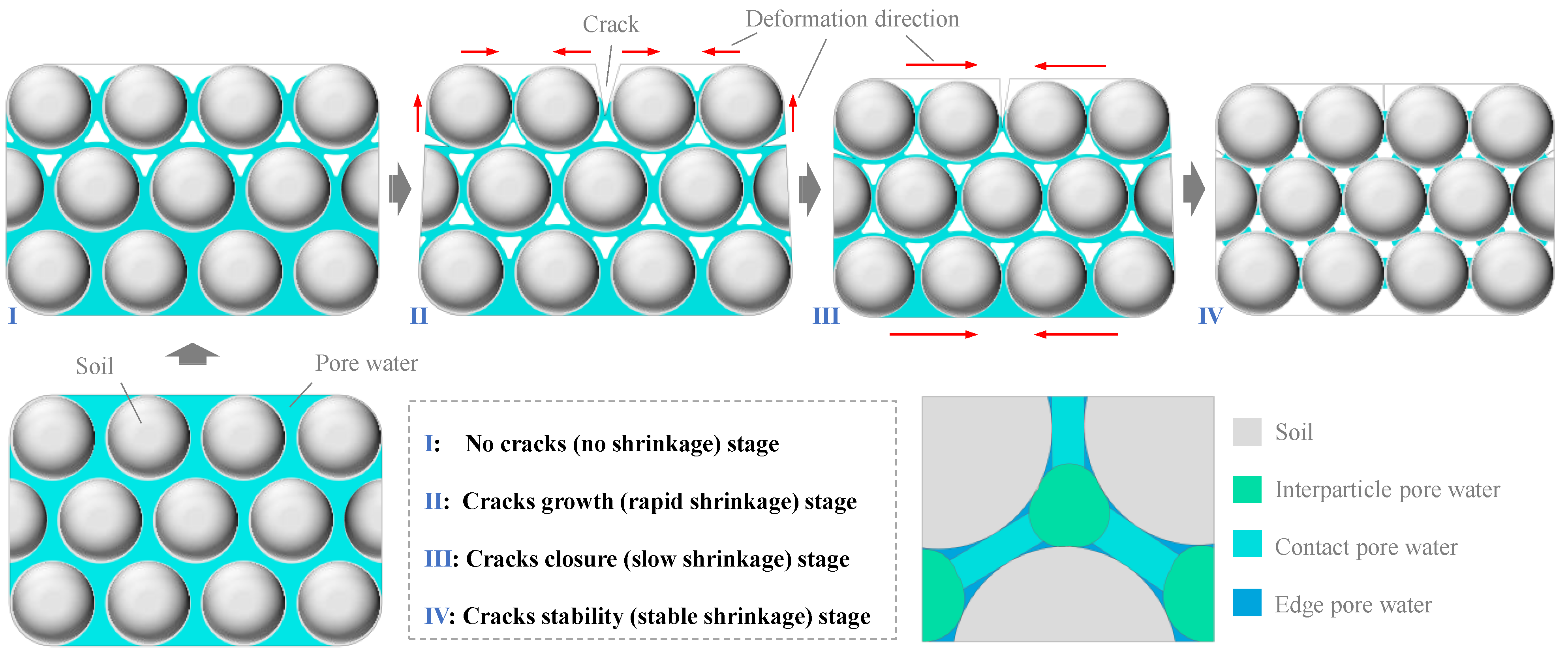
4.1. Evolution Mechanism of Evaporation and Water Loss Deformation in Dense Clay
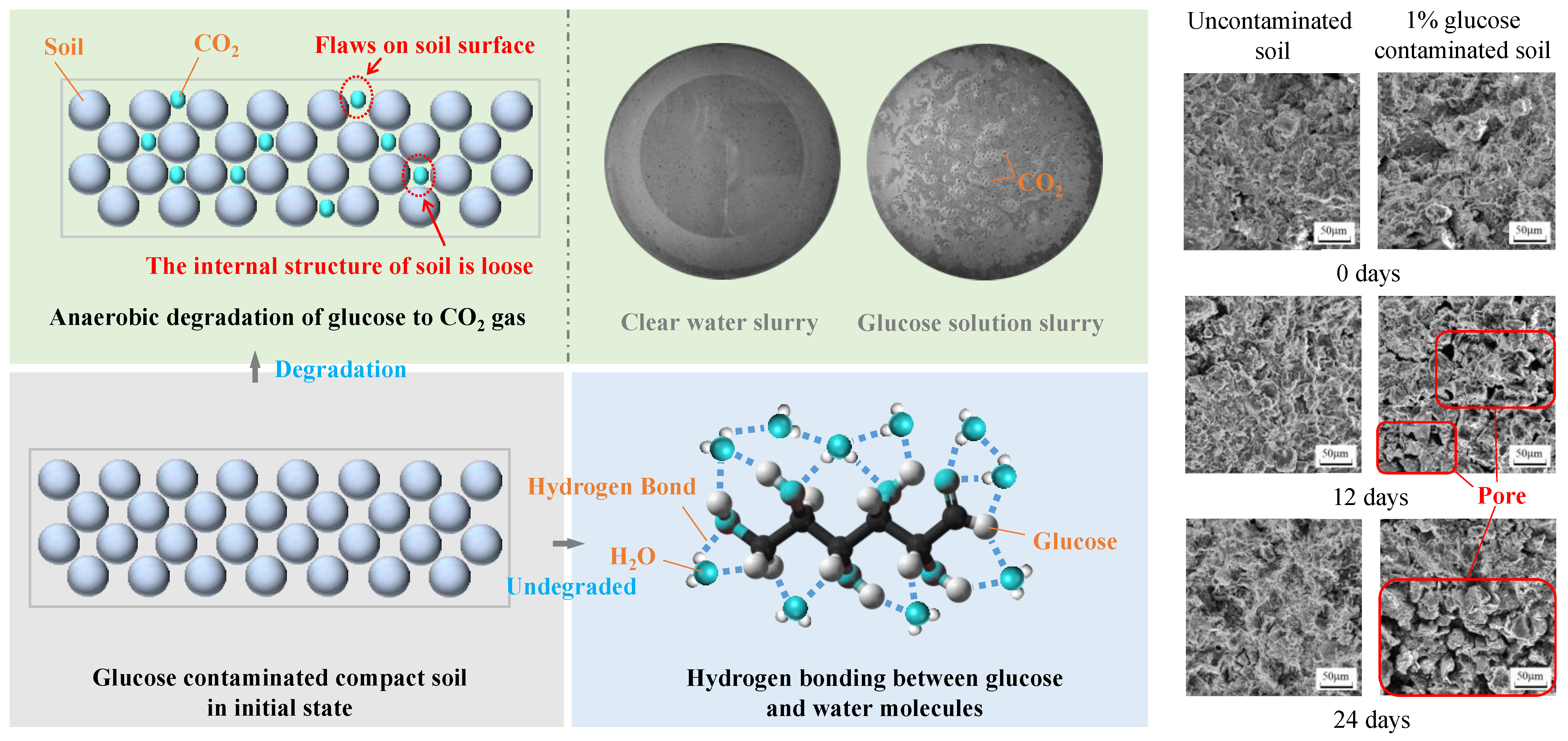
4.2. Anaerobic Degradation Process of Glucose in Soil
4.3. Influence Mechanism of Glucose Anaerobic Degradation on Evaporative Cracks in Dense Clay
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, D.; Gong, J.; Ni, X.; Ren, J. Experimental and numerical analysis of soil cracking characteristics under evaporation. Math. Probl. Eng. 2021, 2021, 3790345. [Google Scholar] [CrossRef]
- Izzo, M.Z.; Mileti, M. Sustainable improvement of the crack resistance of cohesive soils. Sustainability 2019, 11, 5806. [Google Scholar] [CrossRef]
- Cheng, W.Q.; Bian, H.B.; Gao, Q.F.; Hattab, M. Experimental and Numerical Study of the Drying Process of a Consolidated Clay Soil. Adv. Civ. Eng. 2022, 2022, 1607963. [Google Scholar] [CrossRef]
- Hillel, D. Introduction to Environmental Soil Physics; Elsevier Academic Press: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Yan, J.P.; Chen, X.Y.; Cai, Y.; Cheng, F.K.; Fan, T.Y. A review of genetic classification and characteristics of soil cracks. Open Geosci. 2021, 13, 1509–1522. [Google Scholar] [CrossRef]
- Tang, C.S.; Zhu, C.; Cheng, Q.; Zeng, H.; Xu, J.J.; Tian, B.G.; Shi, B. Desiccation cracking of soils: A review of investigation approaches, underlying mechanisms, and influencing factors. Earth-Sci. Rev. 2021, 216, 103586. [Google Scholar] [CrossRef]
- Morris, P.H.; Graham, J.; Wiliams, D.J. Cracking in drying soils. Can. Geotech. J. 1992, 29, 263–267. [Google Scholar] [CrossRef]
- Yin, Z.Z.; Yuan, J.P.; Wei, J.; Cao, X.S.; Liu, H.Q.; Xu, B. Influence of fissures on slope stability of expansive soil. Chin. J. Geotech. Eng. 2012, 34, 2155–2161. Available online: http://www.cgejournal.com/cn/article/id/14958 (accessed on 1 October 2023). (In Chinese).
- Albrecht, B.A.; Benson, C.H. Effect of desiccation on compacted natural clays. J. Geotech. Geoenvironmental Eng. 2001, 127, 67–75. [Google Scholar] [CrossRef]
- Ye, W.; Ma, F.H.; Hu, J.; Li, Z.Y. Seepage Behavior of an Inclined Wall Earth Dam under Fluctuating Drought and Flood Conditions. Geofluids 2018, 2018, 4734138. [Google Scholar] [CrossRef]
- Keller, A.A.; Roberts, P.V.; Blunt, M.J. Effect of fracture aperture variations on the dispersion of contaminants. Water Resour. Res. 1999, 35, 55–63. [Google Scholar] [CrossRef]
- Konrad, J.M.; Ayad, R. A idealized framework for the analysis of cohesive soils undergoing desiccation. Can. Geotech. J. 1997, 34, 477–488. [Google Scholar] [CrossRef]
- Zhang, S.H.; Yue, J.W.; Huang, X.J.; Zhao, L.M.; Wang, Z.F. Study on Cracking Law of Earthen Soil under Dry Shrinkage Condition. Materials 2022, 15, 8281. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.D.; Najm, M.R.A. Field measurements of soil cracks. Soil Sci. Soc. Am. J. 2020, 84, 1462–1476. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Tang, C.S.; Zeng, H.; Wang, Y.S.; Cheng, Q.; Shi, B. Laboratory characterization and discrete element modeling of desiccation cracking behavior of soils under different boundary conditions. Chin. J. Geotech. Eng. 2020, 42, 372–380. (In Chinese) [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, J.M.; Zhou, Z.; Chikhotkin, V.; Mi, M.; Shen, J. Evolution law of critical moisture content of soil cracking under rainfall-evaporation conditions. Rock Soil Mech. 2020, 41, 2592–2600. [Google Scholar] [CrossRef]
- Zeng, H.; Tang, C.S.; Cheng, Q.; Inyang, H.I.; Rong, D.Z.; Lin, L.; Shi, B. Coupling effects of interfacial friction and layer thickness on soil desiccation cracking behavior. Eng. Geol. 2019, 260, 105220. [Google Scholar] [CrossRef]
- Cordero, J.A.; Useche, G.; Prat, P.C.; Ledesma, A.; Santamarina, J.C. Soil desiccation cracks as a suction–contraction process. Géotechnique Lett. 2017, 7, 272–278. [Google Scholar] [CrossRef]
- Yesiller, N.; Miller, C.J.; Inci, G.; Yaldo, K. Desiccation and cracking behavior of three compacted landfill liner soils. Eng. Geol. 2000, 57, 105–121. [Google Scholar] [CrossRef]
- Lakshmikantha, M.R.; Prat, P.C.; Ledesma, A. Experimental evidence of size effect in soil cracking. Can. Geotech. J. 2012, 49, 264–284. [Google Scholar] [CrossRef]
- An, N.; Tang, C.S.; Xu, S.K.; Gong, X.P.; Shi, B.; Inyang, H.I. Effects of soil characteristics on moisture evaporation. Eng. Geol. 2018, 239, 126–135. [Google Scholar] [CrossRef]
- Cheng, Q.; Tang, C.S.; Chen, Z.G.; EI-Maarry, M.R.; Zeng, H.; Shi, B. Tensile behavior of clayey soils during desiccation cracking process. Eng. Geol. 2020, 279, 105909. [Google Scholar] [CrossRef]
- Tang, C.S.; Cui, Y.J.; Tang, A.M.; Shi, B. Experiment evidence on the temperature dependence of desiccation cracking behavior of clayey soils. Eng. Geol. 2010, 114, 261–266. [Google Scholar] [CrossRef]
- Omar, H.; Rohani, S. Treatment of landfill waste, leachate and landfill gas: A review. Front. Chem. Sci. Eng. 2015, 9, 15–32. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Asakura, H. Treatment of landfill leachate with different techniques: An overview. Water Reuse 2021, 11, 66–96. [Google Scholar] [CrossRef]
- Gülser, C.; Minkina, T.; Sushkova, S.; Kızılkaya, R. Changes of soil hydraulic properties during the decomposition of organic waste in a coarse textured soil. J. Geochem. Explor. 2017, 174, 66–69. [Google Scholar] [CrossRef]
- Matthews, A.M.; Portwood, A.M.; Armstrong, A.C.; Leeds-Harrison, P.B.; Harris, G.L.; Catt, J.A.; Addiscott, T.M. CRACK-NP, development of a model for predicting pollutant transport in cracking clay soils. Soil Use Manag. 2006, 16, 279–284. [Google Scholar] [CrossRef]
- Hafid, H.S.; Rahman, N.A.A.; Mokhtar, M.N.; Talib, A.T.; Baharuddin, A.S.; Shah, U.K.M. Over production of fermentable sugar for bioethanol production from carbohydrate-rich Malaysian food waste via sequential acid-enzymatic hydrolysis pretreatment. Waste Manag. 2017, 67, 95–105. [Google Scholar] [CrossRef]
- Jeckelmann, J.M.; Erni, B. Transporters of glucose and other carbohydrates in bacteria. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1129–1153. [Google Scholar] [CrossRef]
- Mclnerneg, M.J.; Bryant, M.P. Biomass Conversion Processes for Energy and Fuels; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Guo, Y.L.; Cao, L.W.; Sang, S.X.; Zhang, R. An experimental investigation on the effect of soluble sugar degradation on the of domestic-source-contaminated soil sites formation and evolution. Environ. Pollut. 2023, 316, 120613. [Google Scholar] [CrossRef]
- Luo, Y.Z.; Zhang, W.W.; Li, Y.Y.; Wang, Y.J.; Yao, H.Y.; Han, J.G. Sanitary landfill improved CNPS microbial functional gene abundance compared to non-sanitary landfill. J. Soils Sediments 2020, 20, 99–108. [Google Scholar] [CrossRef]
- Xiao, G.Y.; Ye, Z.M.; Xu, G.L.; Zeng, J.; Zhang, L. Temperature Effect on Crack Evolution of Red Clay in Guilin. Water 2021, 13, 3025. [Google Scholar] [CrossRef]
- Tang, C.S.; Shi, B.; Liu, C.; Zhao, L.Z.; Wang, B.J. Influencing factors of geometrical structure of surface shrinkage cracks in clayey soils. Eng. Geol. 2008, 101, 204–217. [Google Scholar] [CrossRef]
- Warnaka, K.; Pochop, L. Analyses of equations for free water evaporation estimates. Water Resour. Res. 1988, 24, 979–984. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Li, D.D.; Zhang, S.W. The Influences of Sand Content and Particle Size on the Desiccation Cracks of Compacted Expansive Soil. Adv. Mater. Sci. Eng. 2021, 2021, 7752352. [Google Scholar] [CrossRef]
- Mu, Q.Y.; Meng, L.L.; Shen, Y.Q.; Zhou, C.; Gu, Z.L. Effects of clay content on the desiccation cracking behavior of low-plasticity soils. Bull. Eng. Geol. Environ. 2023, 82, 317. [Google Scholar] [CrossRef]
- Li, D.D.; Yang, B.B.; Gao, Z.L.; Sun, L.X. The effects of biomass ash on soil evaporation and cracking. Arab. J. Geosci. 2021, 14, 837. [Google Scholar] [CrossRef]
- Song, W.K.; Cui, Y.J.; Tang, A.M.; Ding, W.Q.; Wang, Q. Experimental study on water evaporation from compacted clay using environmental chamber. Can. Geotech. J. 2016, 53, 1293–1304. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, Z.Y.; Wang, C. Desiccation and cracking behaviour of clay loam subjected to different irrigation methods during wetting–drying cycles. Eur. J. Soil Sci. 2020, 72, 793–809. [Google Scholar] [CrossRef]
- Wu, J.H.; Yuan, J.P.; Ng, C.W.W. Theoretical and experimental study of initial cracking mechanism of an expansive soil due to moisture-change. J. Cent. South Univ. 2012, 19, 1437–1446. [Google Scholar] [CrossRef]
- Kim, M.; Cho, A.E. The role of water molecules in stereoselectivity of glucose/galactose-binding protein. Sci. Rep. 2016, 6, 36807. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.S.; Shi, B.; Cui, Y.J. Behaviors and mechanisms of desiccation cracking of soils. Chin. J. Geotech. Eng. 2018, 40, 1415–1423. [Google Scholar] [CrossRef]
- Tang, C.S.; Zhu, C.; Leng, T.; Shi, B.; Cheng, Q.; Zeng, H. Three-dimensional characterization of desiccation cracking behavior of compacted clayey soil using X-ray computed tomography. Eng. Geol. 2019, 255, 1–10. [Google Scholar] [CrossRef]
- Xu, X.H.; Xin, P.; Zhou, T.Z.; Yu, X.Y. Effects of Large Macropores on Saline Water Evaporation from Marsh Soil. Water Resour. Res. 2023, 59, e2022WR033276. [Google Scholar] [CrossRef]
- Chertkov, V.V. Modeling the pore structure and shrinkage curve of soil clay matrix. Geoderma 2000, 95, 215–246. [Google Scholar] [CrossRef]






| Type of Aqueous Solution | Glucose | Pyruvic Acid | Ethanol | Carbonic Acid | Lactic Acid |
|---|---|---|---|---|---|
| Acidity and basicity | Acidity | Acidity | Neutral | Acidity | Acidity |
| Stability | Stable | Easy to decompose | Volatile | Instable | It can volatilize with superheated water vapor |
| Melting point/°C | 146 | 11.8 | −114.1 | — | 16.8 |
| Boiling point/°C | 527.1 | 165 | 78.3 | — | 122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Cao, L.; Sang, Z.; Zhang, R. The Effect of Soluble Sugar Degradation on the Evaporation of Compacted Clay. Water 2023, 15, 4180. https://doi.org/10.3390/w15234180
Guo Y, Cao L, Sang Z, Zhang R. The Effect of Soluble Sugar Degradation on the Evaporation of Compacted Clay. Water. 2023; 15(23):4180. https://doi.org/10.3390/w15234180
Chicago/Turabian StyleGuo, Yuliang, Liwen Cao, Zihaohan Sang, and Rui Zhang. 2023. "The Effect of Soluble Sugar Degradation on the Evaporation of Compacted Clay" Water 15, no. 23: 4180. https://doi.org/10.3390/w15234180
APA StyleGuo, Y., Cao, L., Sang, Z., & Zhang, R. (2023). The Effect of Soluble Sugar Degradation on the Evaporation of Compacted Clay. Water, 15(23), 4180. https://doi.org/10.3390/w15234180







