Abstract
Environmental pollution is an issue of particular concern, specifically when industrial waste products are not subjected to appropriate treatment. Among various industries in the agri-food sector, the brewing industry holds a significant position in this context, given that beer stands as the predominant choice of consumers. Brewery waste generates significant quantities of organic substances, along with ammonium nitrogen and phosphorus. Among the various methods for their treatment, adsorption has received substantial attention due to its cost-effectiveness and operational simplicity. The present study investigates the adsorption capacity of two materials, zeolite and palygorskite, for the removal of ammonium nitrogen and brewery waste, using columns and batches. Simultaneously, desorption and regeneration experiments were conducted, and the effect of pH on their effectiveness was also examined. To understand the adsorption mechanisms, isotherm and kinetic models have been estimated. The results of the experiments have demonstrated a marked adsorption efficiency of the adsorbent materials, surpassing 90%. In comparison, zeolite has exhibited a better adsorption capacity in the removal of ammonium nitrogen, while palygorskite has shown greater aptitude for phosphorus removal. The purpose of these experiments was to investigate the adsorption capacity of these two materials as a potential medium for brewery wastewater treatment (e.g., as part of adsorption filter, trickling filters, and constructed wetlands).
1. Introduction
One of the current concerns of humanity concerns environmental pollution, particularly in the context of industrial waste disposal into aquatic ecosystems without prior treatment [1,2]. Many industries in the agro-food sector generate substantial quantities of waste and by-products, which exert a profound influence on environmental degradation, climate change, and economic disparities [3].
Among the various types of industrial waste, those emanating from the brewing industry hold notable economic significance. This significance is attributed to the global preeminence of beer consumption [1], given that it accounts for approximately 40% of total alcohol consumption [4]. It is estimated that the production of 1 liter of beer results in the generation of 3–10 liters of wastewater, depending on the production process and the specific water usage [5,6,7], with global beer production calculated to be over 134 billion liters [8]. The volume of beer produced in Greece amounted to four million hectoliters in 2021, an increase compared to the previous year [9].
The brewing industry generates large quantities of organic waste, mainly consisting of brewery wastewater (BWW) and spent grains or barley malt residues (BSG). BWW is characterized by a high organic load that is easily biodegradable, while BSG is a product of difficult biological decomposition due to its lignocellulosic compounds’ content [3]. Furthermore, these waste products exhibit high concentrations of nutrients, which are mainly linked to the brewing process [10]. In Table 1, the main characteristics of wastewater from the brewery industry are presented, as sourced from the available literature [10,11,12,13]. As observed in Table 1, there is a wide range of values for each parameter, which demonstrates that there is no standardization for this category of waste, as its specific characteristics may vary depending on production methodologies, ingredients used, and industry-specific management practices. As a result, the brewing industry discharges large amounts of highly polluting wastewater throughout the year, thereby posing a significant threat to both human health and the environment [11], as pollutant concentrations are much higher than those in municipal wastewater.

Table 1.
Characteristics of brewery wastewater.
In order to address these issues, environmental authorities are exerting pressure on breweries to ensure that their wastewater abides with environmental standards [8]. Numerous wastewater treatment methods have been adopted for the treatment of brewery wastewater, including membrane filtration [13], anaerobic [3] and aerobic processes [14], constructed wetlands [8], and combinations of these [10]. Among these methods, biological wastewater treatment has become a more attractive alternative solution, finding application in both developed and developing nations. Notably, within the spectrum of wastewater treatment options, constructed wetlands (CWs) have gained favor as an eco-friendly technology, while offering numerous benefits through the treatment of variable wastewater volumes. CW systems have been tested for treating different types of industrial wastewater [15], including those from brewery operations [8]. In the CWs, wastewater purification is achieved through a comprehensive array of physical, chemical, and biological processes that occur within their components [16]. However, the effectiveness of CWs in removing pollutants is predominantly subject to hydraulic variables [17,18]. For instance, a lower hydraulic loading rate leads to higher pollutant contact time within the CW, favoring the settling of Total Suspended Solids (TSS) and the degradation of Chemical Oxygen Demand (COD), including the high absorption and retention of nutrients [19], improving the quality of the wastewater. It should be noted that the performance of CWs is contingent upon the local climatic conditions, the vegetation, and the specific substrates employed, all of which contribute to their efficiency.
Regarding nutrient removal, the removal of nitrogen (N) is associated with processes such as ammonification, nitrification, plant uptake, and ammonia adsorption [20]. Conversely, the removal of phosphorus (P) is accomplished through a synergistic approach involving substrates, vegetation, and microorganisms [21]. The substrate plays a vital role in mitigating N and P. Commonly used substrates are generally categorized into three types—natural materials, industrial by-products, and industrial products. Various substrates have been utilized in constructed wetlands, including gravel, clay, bentonite, zeolite, charcoal, palygorskite, and others [22].
In the selection of substrates, the primary considerations are the cost and the availability of raw materials [23]. Zeolite is a natural aluminosilicate mineral with various commercial applications due to its low cost, stable crystal structure, and low density. The primary physical characteristics of zeolites that are relevant for ion exchange applications [24] include porosity (%), thermal stability, ion exchange capacity (mmolM+/g), specific weight (g/cm3), and apparent density (g/cm3) [25]. Zeolite, a natural mineral, exhibits a high absorption rate for both nitrogen (N) and phosphorus (P) due to its internal composition and spatial structure [26]. Specifically, zeolite filters can significantly enhance the removal capacity of CW, with removal rates for organic matter, N, and P reaching 95%, 80%, and 70%, respectively [27].
On the other hand, palygorskite is a hydrated alumino-magnesium phyllosilicate mineral with an ideal chemical formula (Mg, Al)5(Si, Al)8 O20(OH)2 8H2O [28]. Its layer charge and high surface area give palygorskite intermediate cation exchange capacity while also providing high adsorption capacity. This, combined with the mineral’s elongated quality, makes it particularly useful in various industrial applications (such as drilling fluids, suspension fertilizers, environmental absorbents, etc.) [29]. Similarly, palygorskite is a significant industrial mineral that is closely associated with absorption processes [30]. This mineral exhibits removal rates of 90% for N and 85% for P [31].
The use of ion exchange materials, such as zeolite and palygorskite, enhances the efficiency of CWs, including their capacity to handle shock loads and operate effectively over a wider range of temperatures [32]. In general, however, the adsorption capacity of adsorbent substances depends on multiple characteristics, such as their texture properties, including porosity, surface area, pore size, and surface chemistry, all of which play a critical role in adsorption performance. Furthermore, the adsorption capacity is subject to modulation by an array of factors, including the pH of the solution, ionic concentration, temperature, initial concentration of adsorbates, as well as the duration of contact [2].
In this study, the adsorption capacity of zeolite and palygorskite for treatment of brewery wastewater was investigated, as substrates media of a constructed wetland system. Specifically, research was conducted using columns and batch tests to remove ammonia ions from artificial wastewater. An equivalent set of experiments was conducted for wastewater from the Macedonian Thrace Brewery, known as “Vergina”. In tandem with these experiments, desorption and regeneration tests were carried out, and the impact of altering conditions, such as pH, was studied. Furthermore, in order to better understand the adsorption mechanisms, calculations of adsorption isotherm and kinetic models were conducted, including the Elovich model, the intraparticle diffusion (ID) model, the double constant (DC) model, the pseudo-first (PFO) model, and the pseudo-second (PSO) model.
2. Materials and Methods
In order to investigate the ability of zeolite and palygorskite to adsorb ammonium and treat brewery wastewater, a series of experiments was conducted. As a first step, batch experiments using ammonium aqueous solutions were performed in order to define adsorption kinetics and isotherms and to examine the effect on adsorption of ammonium feeding concentration, material size and pH. The next step was to conduct batch column experiments, based on the batch experiments results, using firstly ammonium aqueous solutions and then brewery wastewater. During batch column experiments, adsorption kinetics and isotherms were defined for ammonium, ortho-phosphate and COD. All batch and column experiments were followed by desorption experiments, to investigate the ability of the materials to release the adsorbed ammonium, phosphorus and organic matter.
2.1. Artificial Wastewater and Brewery Wastewater
Ammonium chloride (NH4Cl) was used in the experiments in order to produce artificial wastewater (AWW). After NH4Cl was dried at 104 °C, it was diluted in deionized water leading to several concentrations of NH4+-N, so as to conduct the adsorption and desorption studies in batch and column experiments. In addition, apart from the experiments with artificial wastewater, column experiments were also conducted with brewery wastewater (BWW). For the needs of this experiment, 10 L of brewery wastewater were obtained after the fermentation stage from a Macedonia Trace Brewery called “Vergina” with a capacity of approximately 7000 t/year, which is located in Rodopi. Then, the wastewater was transported to the laboratory in plastic bottles and was used immediately to prevent any change in its composition. The brewery’s wastewater characteristics are presented in Table 2.

Table 2.
Characteristics of brewery wastewater for “Vergina”.
2.2. Adsorbents: Zeolite and Palygorskite Characterization
The natural zeolite employed in this study originated from Bulgaria (Imerys Minerals Bulgaria AD, Kardzhali, Bulgaria) and subsequently imported into Greece by ZeolifeTM Company, Tessaloniki, Greece. Specifically, this material can be described as a hydrated aluminosilicate mineral of volcanic origin, with a clinoptilolite content of approximately 85%. It contains a moisture content ranging from 10 to 12%, while the residual 3 to 5% is comprised of impurities, including feldspar, micas, and clays that are devoid of fibers and quartz. The chemical composition of the dry clinoptilolite is detailed in Table 3. According to the technical data sheet provided, this particular zeolite exhibits a minimum cation exchange capacity of 150 meq/100 g. In preparation for all experimental procedures, the zeolite was sieved to obtain different granulometries (i.e., 1–2, 2–4 and >4 mm), washed to remove dust, and finally dried.

Table 3.
Chemical composition of the zeolite used, as analyzed by Imerys Minerals Bulgaria AD and reported in the technical data sheet provided online by ZeolifeTM Company. (https://zeolife.gr/products/zeolithos-apo-2-5-eos-5-chiliosta/, accessed on 20 September 2023), and chemical composition of the palygorskite.
Fibrous palygorskite of natural origin, extracted from the Ventzia Basin (Grevena, Greece), was provided by Geohellas S.A. (Athens, Greece) in granular form, albeit with non-uniform sizing. Geohellas S.A. conducted a chemical composition analysis of the palygorskite, and the results are presented in Table 3. The palygorskite possessed a cation exchange capacity (CEC) of 30 meq/100 g [33]. The palygorskite was sieved to obtain different granulometries (i.e., 1–2, 2–4 and >4 mm), washed to remove dust, and finally dried at 105 °C for 24 h before use.
2.3. Batch Studies
For both adsorbents (zeolite and palygorskite), batch tests were carried out in 250 mL borosilicate bottles filled with 100 mL of AWW at several concentrations (5, 10, 50, 100 and 200 ppm) and 5 g of adsorbent of different particle sizes (i.e., 1–2, 2–4, >4 mm). Bottles were continuously stirred at 150 rpm and samples were taken at regular intervals until equilibrium was achieved. The volume sampled at each time interval during desorption was minimal (~1.5 mL) in order to avoid any change in the solid/liquid ratio. The maximum amount of ammonium adsorbed per mass of adsorbent at equilibrium (qe) was determined. In addition, after evaluating the impact of granulometry in the NH4+-N removal, adsorbents were used as purchased without any sieving. Again, the batch test procedure was the same but only one concentration of NH4+-N was applied (50 ppm). In addition, the influence of pH was examined for both adsorbents at 5 ppm NH4+-N initial concentration.
2.4. Column Studies
Based upon batch experiments, further adsorption experiments were also carried out in laboratory-scale columns. The adsorption experiments on fixed-bed reactors were conducted on Plexiglas tubes (columns), 50.0 cm high and of 4.0 cm internal diameter with approximately 450 mL operational volume, equipped with sample valves placed at the bottom of the columns (Figure 1). The columns were firstly filled with approximately 400–450 g of zeolite and fed with 400 mL of AWW at concentrations of 200 and 5000 mg NH4+-N/L. Then the columns operated with palygorskite (~240–340 g) and were filled with AWW (~400 mL) at various NH4+-N concentrations (200, 1000, 1500, 3000 and 5000 mg/L). Samples were collected at regular intervals from the lower sample valve. Generally, in order to achieve complete adsorbent saturation, the columns were fed with an aqueous solution of ammonium chloride of concentration 5000 mg/L. Finally, column studies were conducted with brewery wastewater (BWW) testing both adsorbents (zeolite and palygorskite), following the same procedure as in the experiments with AWW. Specifically, one column was filled with approximately 400 g of zeolite and another with approximately 200 g of palygorskite.

Figure 1.
Column setup.
Then 440–470 mL of brewery wastewater was added to the columns. Time intervals between samplings were the same as in the column studies with AWW. Apart from NH4+-N, other pollutants were also analyzed, including total soluble COD (SCODt) and orthophosphates (OP). The determination of the concentrations of the above pollutants was carried out according to the Standard Methods [34]. To be more precise, the determination of NH4+-N was executed following the Salicylate Method (4500-NH3 B-E), while the measurement of OP was carried out in accordance with the Cassiterite Method (4500-P D). Furthermore, the physico-chemical parameters were assessed using portable instruments, specifically the EcoSense pH100A (YSI Incorporated, Yellow Springs, OH, USA) and EcoSense EC300A (YSI Incorporated, Yellow Springs, OH, USA).
2.5. Adsorption Kinetics
Adsorption experiments were conducted in order to determine the time and to which extent NH4+-N, SCODt and OP are adsorbed on the substrate material (equilibrium), and from which point saturation of binding sites occurs. The experimental data collected from the adsorption tests were fitted to several kinetic models, such as the pseudo-first order, pseudo-second order, Elovich, intraparticle diffusion, and the double-constant model. Details regarding the applied adsorption kinetic models are included in the Appendix A (Appendix A.1).
2.6. Isothermal Adsorption
Adsorption isotherms are used to describe the interactive behavior between solutes and adsorbents, which express the surface properties and affinity of the adsorbent. The experimental data were fitted to four isotherm models: Langmuir, Freundlich, Temkin and Henry models, in order to evaluate which better describes them. Information regarding the isotherm models is shown in the Appendix A (Appendix A.2).
2.7. Desorption and Regeneration Experiments
Zeolite and palygorskite can be used in agriculture for soil fertilization, since they provide slow release of nutrients, which enhance plant growth and, eventually, high crop yield [35]. Therefore, after the conduction of batch and column tests, desorption and regeneration tests were conducted on two consecutive days using deionized water (desorption test) and 0.1 N HCl (regeneration test), respectively, in order to evaluate the adsorbent’s desorption capacity and possible use as a soil additive. The choice of solvents for desorption and regeneration tests was guided by the existing literature [34,35]. The aim of desorption with deionized water is to evaluate the ability of the adsorbent particles to release ions with the flow of a natural solvent, thereby preventing the introduction of a new element and consequently avoiding the contamination of the area with a new chemical product [36]. On the other hand, research has indicated that the use of HCl 0.1 N is the most effective eluent for regenerating these materials [37]. The saturated adsorbent was washed with distilled water and then dried at 45 °C for use in the desorption tests. In batch tests, following the same procedure as the adsorption tests, 100 mL of solvent (deionized water or 0.1 N HCl) were used for the desorption and regeneration experiments with AWW. These experiments were performed for all granulometries and initial NH4+-N concentrations. In column experiments, 350–500 mL of solvent were added to each column for both experiments, with AWW and BWW. Again, experiments were performed for all the applied initial concentrations of NH4+-N (AWW and BWW) and sCODt and PO4−3-P (BWW). Samples were collected at regular intervals for the same period of time applied in the batch and column studies. The desorption and regeneration studies lasted 1440 min until constant values of pollutant concentrations would be observed.
3. Results & Discussion
3.1. Adsorption Batch Experiments
3.1.1. Effect of Particle Size and Initial Concentration
Firstly, the effect of particle size was examined for AWW of several initial concentrations (5, 10, 50, 100 and 200 mg/L NH4+-N). These initial concentrations were chosen to be comparable with previous related studies [35]. Table 4 illustrates the removal efficiencies of both adsorbents at the several initial concentrations. It can be concluded that there are no great differences between the granulometries (1–2 mm, 2–4 mm and >4 mm) (Figure 2). This was also stated by other researchers in previous studies [35]. On the other hand, Muscarella et al. [38] found that grain adsorption ability of zeolite increases by decreasing the particles’ diameter. In addition, for each grain size tested, ammonium removal occurred rapidly within the first 60 min of contact time, with removal efficiencies ranging from 91.57 to 100%. Similar results were reported in another study [39], where the zeolite’s finest particle size presented the fastest and highest ammonium adsorption during the first hour, while past that time the removal rate reduced. However, after 60 min, higher particle sizes demonstrated higher removal capacity, indicating that in smaller particle sizes the available active sites are taken more quickly and therefore saturation is reached in less time. This was explained by the fact that the concentration of available sites is constant; therefore, the availability of active sites is nearly the same between smaller and larger particles. Since the diffusion paths of ions are shorter in smaller particles, the available sites are more accessible to ions. Therefore, smaller particle size is expected to favor kinetics mostly in the early stages of adsorption [39,40]. In the current study, after the first hour, the removal rate gradually decreased and finally reached equilibrium at approximately 120 min. In higher concentrations, the removal efficiency reduced slightly, probably due to the adsorbents’ ammonium capacities.

Table 4.
NH4+-N removal efficiencies (%) for both adsorbents (palygorskite and zeolite) at 400 min.
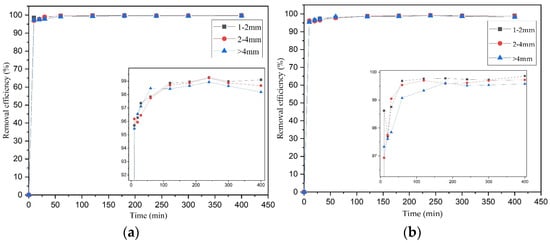
Figure 2.
Effect of particle size on ammonium removal efficiency (10 mg/L NH4+-N) for (a) palygorskite and (b) zeolite at time 400 min.
Similarly, Gianni et al. [41] found that removal efficiency reached almost 100% for the lowest initial concentration, while it decreased when higher initial concentrations were applied. On the whole, removal efficiencies for both palygorskite and zeolite were higher than 90% for all applied initial concentrations. In addition, among both adsorbents, zeolite was the most efficient, with slightly higher removal efficiencies in comparison to palygorskite regarding ammonium removal (Table 4). This can be attributed to the fact that zeolite illustrates higher selectivity for NH4+-N cations due to the presence of alkaline earth metal cations on its negatively charged surface, exchanged readily with NH4+-N cations in the AWW [42].
Figure 3 illustrates the effect of initial concentration on the adsorbents’ performance. It can be concluded that increased concentrations of initial ammonium lead also to higher adsorbed amounts on the adsorbent. This was also observed by Widiastuti et al. [43], who reported that higher initial concentrations of ammonium provided greater driving force. Probably, the ammonium ion could migrate to the internal micro-pores from the external surface and therefore could exchange with cations on both the external and internal surfaces.
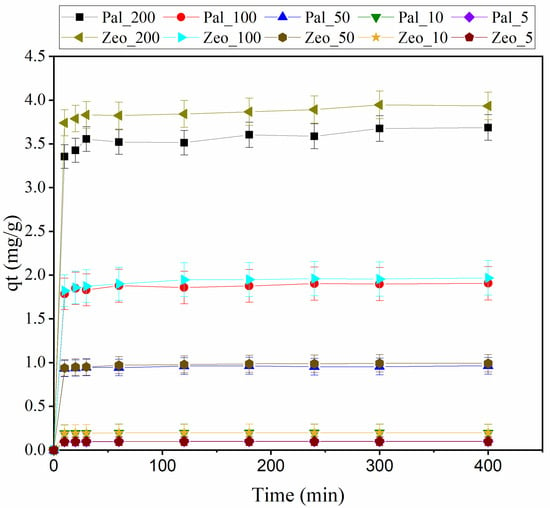
Figure 3.
Amount of NH4+-N adsorbed (mg/g) on the adsorbent (Palygorskite-Pal and Zeolite-Zeo) at time (min) at various initial concentrations (200, 100, 50, 10 and 5 mg/L) for particle size >4 mm.
Consequently, since there wasn’t any great difference in ammonium removal for various granulometries, in the pH batch tests and in the column studies both adsorbents were used with their commercial granulometry (<5 mm and D50 = 150 μm for zeolite and <4 mm and D50 = 75 μm for palygorskite).
3.1.2. Equilibrium Time
In order to investigate the influence of contact time, batch tests were performed with palygorskite and zeolite as purchased with 50 mg/L NH4+-N (initial concentration). The removal efficiency in both adsorbents was very fast initially, with 92.9% and 96.2% achieved in the first 60 min for palygorskite and zeolite, respectively (Figure 4). Then, the rate was lower with increased contact time and remained almost constant until reaching equilibrium at approximately 180 min. After 400 min, ammonium removal reached up to 94.5% and 99.1% in palygorskite and zeolite, respectively.
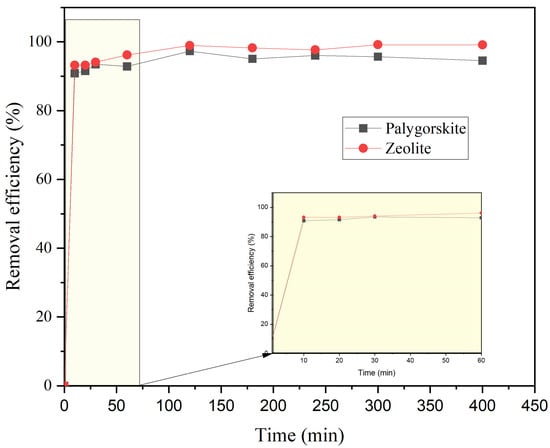
Figure 4.
Effect of contact time for palygorskite and zeolite on ammonium removal (NH4+-N initial concentration = 50 mg/L).
Similar results were found also in previous studies [35,43,44,45]. According to Martin et al. [45], ammonium removal by zeolite occurred within the first 180 min, and the removal rate decreased with increased contact time. Alshameri et al. [44] stated that ammonium removal surpassed 78% within 300 min, contact time higher than that observed in the current study, but then removal rate became constant. In addition, Kotoulas et al. [35] found that ammonium removal reached 85% within the first hour and eventually the removal rate became constant. However, removal rate reached the value of 99% at 2880 min, while in the current study 99% removal was achieved at 400 min. The high removal rate within the first hour (Figure 4) can be explained by the fact that available adsorbing sites are rapidly utilized followed by fast diffusion into its pores and channels, and finally equilibrium is reached [35]. Regarding palygorskite, Alshameri et al. [44] found that removal efficiency of NH4+-N increased rapidly and reached equilibrium very quickly at 30 min. This may also be attributed to the utilization of available sites and eventually equilibrium attainment. Fast adsorption kinetics makes these adsorbents very popular in terms of efficiency and field deployment [44]. However, Alshameri et al. [44] reported lower removal efficiencies (~50%) in comparison to those in this study. Gianni et al. [41] investigated ammonium removal with palygorskite and found that optimal contact time was 15 min. After 15 min they found that removal decreased, probably due to potential NH4+-N desorption, since the available active sites were taken and the agitation may break the bonding between ammonium and the active sites. Once again, removal efficiencies were lower (≤30%) in comparison to the current study. Similar results were found by Widiastuti et al. [43], where ammonium removal by zeolite was fast within the first 15 min but then the removal rate begun decreasing till equilibrium was reached.
3.1.3. Effect of pH
The pH value of the solution is a key parameter for the removal of several pollutants, including ammonium [41,43]. Electrostatic interactions between adsorbents and adsorbate ions are strongly pH dependent [42,46]. The influence of pH on adsorption was evaluated though batch experiments for both adsorbents. Batch tests were conducted at three values of pH, 3 (acid), 7 (neutral) and 9 (alkaline), while NH4+-N initial concentration was constant at 5 mg/L. Figure 5 and Figure 6 illustrate the results pH effect on palygorskite (pal) and zeolite (zeo) performance both in terms of contact time (Figure 6) and pH value at equilibrium (Figure 5). Rozic [47] stated that, besides the adsorption mechanism, ion exchange is also a main mechanism of ammonium removal by zeolite. Ammonium in aqueous solution ca be found in two forms, i.e., as a dissociated form NH4+ or as a non-dissociated form NH3 (ammonia) [43]. In addition, according to Layva-Ramos et al. [48] and Genethliou et al. [39], the ammonium ion NH4+ is the predominant species at pH lower than 7, while at greater pH (alkaline) the NH4+-NH3 equilibria shifts towards NH3. Sundhararasu et al. [46] also avoided alkaline pH in their adsorption experiments with zeolite, since NH4+ ions tend to evaporate as ammonia in high pH values. In addition, at pH values above 8, NH4+ is neutralized by hydroxyl groups, while below 6, H+ and NH4+ compete against each other for adsorption on available active sites of the adsorbent [41,49]. Regarding palygorskite, optimum results were obtained at the highest pH 9 and decreased at lower pH values, with lowest performance at pH 3 (Figure 6). Similar results were published by Gianni et al. [41], who reported that palygorskite under acidic conditions is protonated. Low removal capacity of palygorskite at low pH values can be attributed to the fact that ion exchange between the H+ and NH4+ is not favored (there is potential competition between them for the exchange sites on the adsorbent). On the other hand, higher pH values (above 7) result in a negative charge of the adsorbent’s surface, conditions that favor the adsorption of cationic NH4+ through electrostatic attraction [42,44]. These findings are in absolute agreement with the results of the current study (Figure 6).
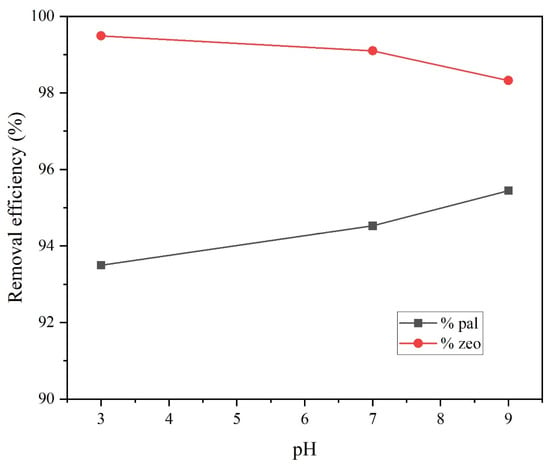
Figure 5.
Adsorbent performance at various pH values applied (3, 7 and 9) for palygorskite (pal) and zeolite (zeo).
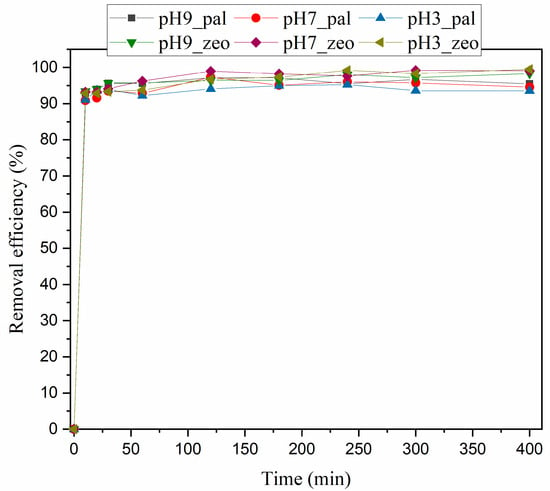
Figure 6.
Effect of pH over time (total 400 min) on the removal efficiencies for palygorskite (pal) and zeolite (zeo).
Regarding zeolite, the performance changed when pH varied from 3 to 9. Overall, at the lowest pH, the ammonium removal was highest and further increase of pH lowered the removal efficiency (Figure 5). This implies that lower pH intensifies the adsorption and ion exchange, while at higher pH values NH4+ ions are transformed to aqueous NH3, which demonstrates low ion exchange ability. In addition, it can be observed that within the first 150 min the removal efficiency at pH 7 is higher in comparison to that at pH 3, while past 200 min the zeolite’s performance is better at pH 3, reaching equilibrium. The performance of zeolite at pH 9 was the lowest, a fact attributed to the low ion exchange ability of NH3 as the predominant species in an alkaline environment. Partially similar results were reported by previous studies [39,43]. Specifically, Widiastuti et al. [43] reported that optimum adsorption takes place in a pH range between 4 and 8, which is consistent with the results of the current study. Genethliou et al. [39] found that pH had little effect on ammonium removal at pH values in the range from 6 to 8, while zeolite’s performance dropped significantly at pH values above 8. On the other hand, Saltalı et al. [50] found an optimal pH 8, which is in disagreement with previous studies but also with the current study.
3.1.4. Batch Kinetic Models
The knowledge of adsorption kinetics is of critical importance to the design of practical treatment systems and the field deployment of adsorbents in general [43,44]. Kinetics of NH4+-N adsorption on both adsorbents were analyzed using several linear and nonlinear modes, i.e., the Elovich model, the intraparticle diffusion (ID) model, the double constant (DC) model, the pseudo-first (PFO) model and the pseudo-second (PSO) model. The use of these kinetic models may help identify the mechanism of adsorption, which depends on the physico-chemical characteristics of the adsorbents and the mass transport process. The values of adsorption capacity at equilibrium (qe), measured experimentally and calculated from the applied models were compared. The fitting results of the five applied models and the relative kinetic parameters are summarized in Table 5 and illustrated in Figure 7.

Table 5.
Fitting parameters of kinetic models to NH4+-N adsorption (initial ammonium concentration = 5 mg/L) in batch studies.
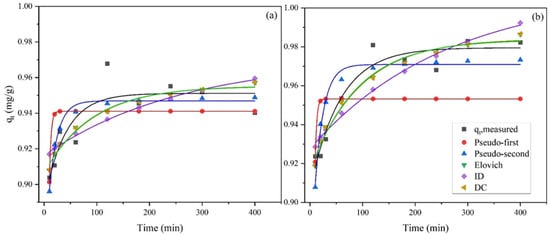
Figure 7.
Kinetic adsorption models of NH4+-N on (a) Palygorskite and (b) Zeolite (NH4-N initial concentration = 5 mg/L) in batch studies.
According to Table 5, the classification of all kinetic modes used to fit the data is PSO > DC > Elovich > ID > PFO for palygorskite and DC > Elovich > ID > PSO > PFO for zeolite. That means that the adsorption mechanism might be different when comparing these adsorbents. The experimental adsorbed amount at equilibrium (qe,exp) was 0.952 mg/g and 0.983 mg/g in palygorskite and zeolite, respectively. Regarding palygorskite, the results indicate that the experimental data fitted best the pseudo-second order kinetic model (R2 = 0.686). In addition, qe, which is a significant parameter for adsorption mechanism, was overestimated in almost all kinetic models, apart from PFO, where it was underestimated (Table 5). The qe from the PSO plot is correlated with the experimental qe, expressing the adsorbents’ removal capacity adequately. Although the correlation coefficient was generally low at all models applied (0.359 < R2 < 0.686) for palygorskite, they confirm the findings presented by Alshameri et al. [44] and Gianni et al. [41], whose results had the best fit to the PSO kinetic model, suggesting that NH4+-N uptake on the palygorskite was controlled mostly by chemisorption process.
Regarding zeolite, the experimental data fitted best to the DC and Elovich model (R2 = 0.902 and 0.901, respectively). This result is also highlighted by the fact that the calculated adsorption capacity of both kinetic models is very close to the experimental adsorption capacity (Table 5). The good agreement with the Elovich model indicates that the activation energy varies greatly during the sorption process [51]. In addition, the conformity of the ammonium adsorption to the Elovich model suggests that the rates of NH4+-N exchange were governed by a heterogeneous diffusion process. However, these results are not in agreement with previous studies [39,43,52], where the PSO kinetic model fitted best to the experimental data. However, it should be noted that previous researchers applied less kinetic models (PFO and PSO) in comparison to the current study (four kinetic models applied). Consequently, applying more kinetic models may give different and more trustworthy results. Overall, it can be assumed that three steps are involved in the adsorption kinetics [44,53,54,55]. Based on the adsorption models’ fitting, it is confirmed that the adsorption mainly uses readily available adsorption sites, which results in a rapid diffusion and quick equilibrium of the adsorbent, while after saturation the adsorbed ions move into the adsorbent places of the adsorbent [44]. These include the diffusion of ammonium ions from liquid-phase to liquid–solid interface, the movement of ions from liquid–solid interface to solid surfaces and finally the diffusion of ions into the particle pores.
3.1.5. Batch Adsorption Isotherms
An adsorption isotherm is very important for designing an adsorption system (in terms of adsorbents’ performance and optimization) and includes several constants related to the surface characteristics, affinity of the adsorbent and adsorption capacity [39,43]. The models were applied for the adsorbents’ removal efficiency for 5, 10, 50, 100 and 200 mg/L NH4+-N and for several granulometries (1–2 mm, 2–4 mm and >4 mm). The fitting curves with the experimental data are presented in Figure 8 and the respective model parameters are listed in Table 6. Since there were no significant differences between the granulometries, Figure 8 illustrates the applied isotherm models only for granulometry >4 mm.
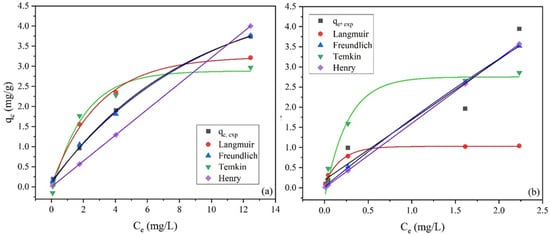
Figure 8.
Adsorption isotherms of ammonium for (a) palygorskite and (b) zeolite (granulometry >4 mm) in batch studies. The qe is the adsorption capacity and Ce is the equilibrium concentration of NH4+-N.

Table 6.
Isotherm parameters for ammonium adsorption onto the adsorbents tested in batch studies.
As illustrated in Table 6, the classification of all isotherm modes used to fit the data is Freundlich > Henry > Langmuir > Temkin for palygorskite and Henry > Langmuir > Freundlich > Temkin for zeolite. It is obvious that the mechanism again might be different when comparing palygorskite and zeolite. Freundlich estimated values demonstrated a better fit for palygorskite (Figure 8a), while Henrys’ model fitted best to the zeolites’ results. Worth noticing is also the fact that Henry’s isotherm model gave satisfactory results for both adsorbents (R2 > 0.943, Table 6), indicating that a very simple linear form can describe the adsorption for both. Regarding palygorskite, the results do not agree with Alshameri et al. [44], who found that Langmuir isotherm gave a better fit than Freundlich, indicating a monolayer adsorption. However, it should be noted that previous researchers focused mainly on only two isotherm models, the Langmuir and Freundlich models [41,44,50,52]. The application of more isotherms cast more light on the isotherm adsorption mechanism. On the other hand, Gianni et al. [41] published similar results, where the Freundlich isotherm fitted best the results highlighting the heterogeneous nature of the procedure. Concerning zeolite, the Langmuir model may have had the second-best fit with the experimental data; however, the estimated equilibrium concentrations (Qe) were lower than the experimental (underestimation, Figure 8b, red line). Then Freundlich gave a better fitting curve (Figure 8b), meaning that the adsorption may be explained by both Henrys’ and Freundlich’s equation, with the second one prevailing. Previous studies are not in complete agreement with the current study, where the results were best described by the Freundlich model [35,43] or both Langmuir and Freundlich models [50,52]. Again, it should be noted that in many previous studies only Langmuir and Freundlich equations were applied. Finally, Genethliou et al. [39] found a better fit to the Temkin model, indicating an uneven surface of the particles.
3.2. Column Studies
3.2.1. AWW Experiments
Based on the batch sorption experiments, palygorskite (pal) and zeolite (zeo) (as purchased) were tested for further sorption studies using columns. Firstly, two concentrations of ammonium were tested, 200 and 5000 mg/L. The results did not indicate any significant difference for zeolite, which demonstrated high removal capacity in both initial concentrations, and the performance of zeolite did not seem to decrease (Table 7). However, this was not the case for palygorskite, where removal efficiencies were lower. Therefore, more initial concentrations were tested with palygorskite in order to evaluate its performance. Zeolite demonstrated higher removal efficiency and lower equilibrium concentration when high ammonium concentration was applied (Table 7), while palygorskite demonstrated better performance at the lowest concentration (200 mg/L). In addition, palygorskites’ performance decreased with the increase of ammonium initial concentration, while zeolite gave opposite results. Furthermore, for palygorskite it appears that, for initial concentrations above 1000, it reaches its maximum adsorption capacity in the first 100 min and after 100 min the material undergoes sorption and desorption cycles (Figure 9). However, according to Figure 9, similar adsorption rates were found for both adsorbents. In addition, a higher amount of adsorbent was used in the zeolite column study, indicating that saturation was reached and a further amount of adsorbent did not necessarily lead to higher removal capacity and adsorbents’ potential.

Table 7.
NH4+-N removal efficiencies and equilibrium concentrations for palygorskite and zeolite in column studies.
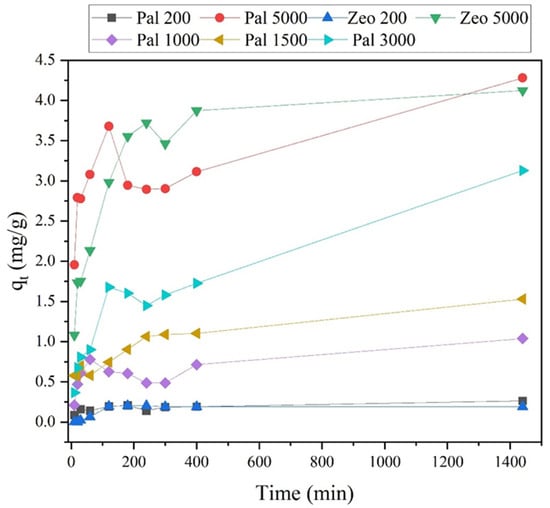
Figure 9.
Amount of NH4+-N adsorbed (mg/g) on the adsorbent (Palygorskite-Pal and Zeolite-Zeo) at time (min) at various initial concentrations (200, 1000, 1500, 3000 and 5000 mg/L).
It can be concluded that in smaller ammonium concentrations equilibrium was reached sooner (~400 min for 200 mg/L), while in higher concentrations the system took more time to reach saturation (1440 min) (Figure 9).
3.2.2. Column Kinetic Studies
Kinetics of ammonium adsorption on both adsorbents were analyzed again as in the batch studies using the same kinetic models (Elovich, ID, DC, PFO and PSO kinetic models) in order to shed light on the adsorption mechanism. Table 8 illustrates all the fitting parameters along with the qe measured experimentally and estimated with the use of kinetic models. The applied concentrations for palygorskite were 200, 1000, 1500, 3000 and 5000, while for zeolite only 200 and 5000 were applied, since no decrease in performance was observed (Table 8). It is obvious from the curves in Figure 10a,b that the adsorption mechanism is different between the two tested adsorbents. This is highlighted also by the correlation coefficients (R2) shown in Table 8. Specifically, regarding palygorskite, it was observed that at low ammonium concentrations the governing adsorption model was the Elovich (R2 = 0.726), while at concentrations higher than 1000 mg/L, the adsorption was governed by the ID model where the highest correlation coefficients were calculated (Table 8). When intraparticle diffusion occurs, an adsorbate hops from one available adsorption site to another through an adsorption–desorption reaction [56]. This can be produced by pore volume diffusion, surface diffusion or their combination.

Table 8.
Fitting parameters of kinetic models to NH4+-N adsorption in column studies at various initial concentrations.
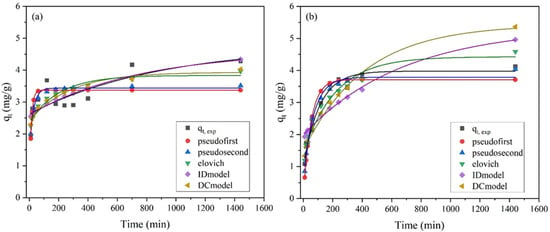
Figure 10.
Kinetic adsorption models of NH4+-N on (a) Palygorskite and (b) Zeolite (NH4-N initial concentration = 5000 mg/L) in column studies.
The Elovich kinetic model is often used to describe second-order kinetics assuming that the surface is energetically heterogeneous [57]. In palygorskite, initially, at low concentrations, the main mechanism follows the Elovich model, while with high concentrations (≥1000 mg/L) the mechanism follows the ID model pattern. On the other hand, zeolite demonstrated different behavior, with better fit to the PFO kinetic model at low concentration (R2 = 0.895, Ci = 200 mg/L) and with the PSO model at higher concentration (R2 = 0.958, Ci = 5000 mg/L). In the PFO kinetic model, the reaction rate depends only on the concentration of the adsorbate and the process is controlled by physisorption. However, in the PSO kinetic model the rate-determining step is considered as a chemical reaction between the adsorbent (zeolite) and adsorbate (ammonium solution), described as chemisorption [43]. This means that in zeolite, according to the above results, at lower adsorbate concentrations (200 mg/L) adsorption was governed mainly by physisorption, but when the concentration increased significantly (5000 mg/L) the mechanism changed to chemisorption, which involves the sharing of electrons or their transfer between adsorbate and adsorbent.
On the whole, it can be concluded that the removal mechanism changes with the increase of the initial concentration of ammonium for both adsorbents. This result is very crucial for the selection, design and operation (conditions) of an effective adsorption system.
3.3. Brewery Wastewater Experiment
Apart from the experiments with artificial wastewater, column experiments were also conducted with brewery wastewater (fixed bed column experiments). In comparison to AWW, which contained only NH4+-N as a pollutant, natural brewery wastewater contains several other components (e.g., pollutants, physico-chemical parameters, etc.) that may interact with each other, enhancing or inhibiting the columns’ overall performance in terms of adsorption. Therefore, apart from NH4+-N, sCODt and OP were also analyzed, and physico-chemical parameters (pH, Electrical Conductivity, EC) were measured. According to previous studies, fixed bed columns were more efficient than fluidized bed columns [35,58]. The columns operated as fixed bed reactors and were fed with brewery wastewater. Samples were taken at specific time intervals until equilibrium to determine the kinetics of NH4+-N, sCODt and PO4−3-P removal (Section 2.4). Table 9 illustrates the initial concentrations of pollutants in the brewery wastewater and the results obtained at equilibrium state.

Table 9.
Concentrations and removal efficiencies at equilibrium for both adsorbents at equilibrium.
Figure 11 illustrates the variation in pH and Electrical Conductivity (EC) measurements over time. It is noteworthy that a significant initial decline is marked within the first few minutes of the process for both adsorbents and parameters, followed by a stabilization of the values. Figure 12 presents the results of NH4+-N, sCODt and OP adsorption obtained in column kinetic experiments (under batch operation) with brewery wastewater for palygorskite (pal) and zeolite (zeo). It can be observed that both palygorskite and zeolite were able to adsorb most of the available NH4+-N within 400 min (>85%, Table 9, Figure 12b1), while equilibrium was reached at 1440 min. In a previous study [35], zeolite demonstrated very high NH4+-N removal efficiencies (>99%) within a smaller period of time (120 min).
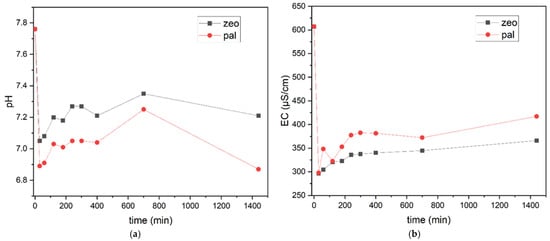
Figure 11.
(a) pH and (b) EC values in effluent brewery wastewater in column studies for palygorskite (pal) and zeolite (zeo).
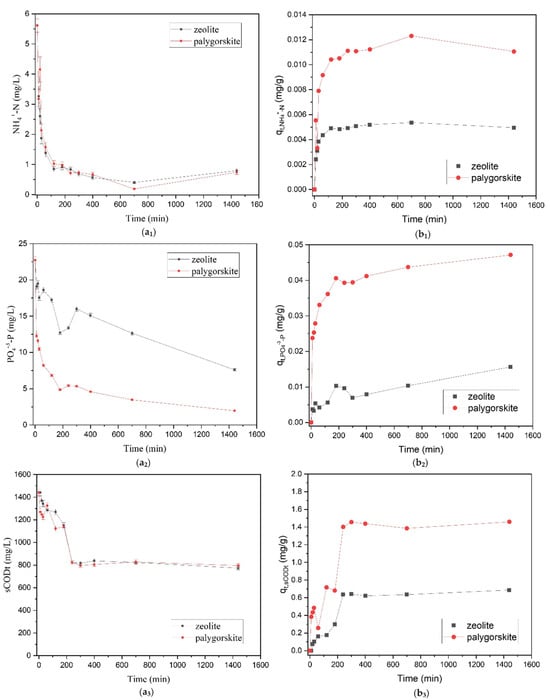
Figure 12.
Effluent concentrations and adsorbed amount of (a1,b1) NH4+-N, (a2,b2) sCODt and (a3,b3) PO4−3-P through time (min).
In terms of overall performance, palygorskite demonstrated better results in comparison to zeolite, since removal efficiencies were either similar or higher (Table 9, Figure 12). Worth noticing also is the high adsorption of phosphorus to the palygorskite substrate (>90%). The distinctive structural and adsorptive characteristics of palygorskite for the purpose of phosphorus removal are widely recognized and have found extensive application [33,59,60]. In addition, as illustrated Figure 12a3, phosphorus concentration continued to decrease even after 1400 min, meaning that there are available adsorption sites even after this period of time, while adsorption of other pollutants (such as sCODt and NH4+-N) reached equilibrium earlier. In zeolite, adsorption of PO4−3-P was less than that of NH4+-N, indicating that zeolite’s ability to adsorb PO4−3-P is limited compared to its ability to adsorb NH4+-N [35,61]. PO4−3-P is mainly adsorbed in zeolite through an inner-sphere complexation [35]. Zeolite’s law adsorption capacity for PO4−3-P was also reported by previous studies [35,62]. Concerning sCODt removal, the columns presented the same pattern, with maximum removal reaching 44.67% and 46.30% in palygorskite and zeolite, respectively. Due to the relative short experimental time period, sCODt removal is mainly attributed to adsorption.
Worth noticing is the fact that adsorbed amounts of all pollutants (NH4+-N, sCODt, PO4−3-P) per gram of the adsorbent were higher in the column filled with palygorskite in comparison to the one filled with zeolite (Figure 12(b1,b2,b3)). This highlights the better performance of palygorskite as an adsorbent material in the treatment of brewery wastewater.
Table 10 illustrates all the fitting parameters along with the qe measured experimentally and estimated with the use of kinetic models for the brewery wastewater test. Kinetic models were applied for all three pollutants (NH4+-N, sCODt, PO4−3-P). It is obvious that the adsorption mechanism regarding NH4+-N and sCODt follows the same pattern in both adsorbent materials (palygorskite and zeolite), with governing kinetic models PSO and PFO for NH4+-N and sCODt, respectively (correlation coefficients R2, Table 10). Ammonium’s adsorption mechanism is mainly described by chemisorption, including a chemical reaction between the adsorbent (zeolite) and adsorbate (ammonium solution). These results agree with those reported by Genethliou et al. [42], who stated that the formation of relatively strong bonds between the palygorskite particles and adsorbed NH4+-N and thus the uptake of NH4+-N on palygorskite were dominated by chemisorption.

Table 10.
Fitting parameters of kinetic models to NH4+-N, sCODt and PO4−3 adsorption in column studies with brewery wastewater.
However, these results do not agree with those produced in the column studies testing various concentrations of ammonium (synthetic wastewater, Table 8). This may be attributed to the fact that brewery wastewater contained lower ammonium concentrations (5.61 mg/L, Table 9) in comparison to those applied in the column studies (range 200–5000 mg/L). This means that the adsorption mechanism changes with the increase of initial concentrations. Regarding sCODt, the adsorption mechanism was described best by physisorption, depending only on the concentration of the adsorbent. The same was reported by Genethliou et al. [42], who suggested that the predominant adsorbent-adsorbate forces are physical. On the other hand, phosphorus adsorption did not show similar behavior between the two tested adsorbents. Specifically, the experimental data in palygorskite fitted best to the Elovich model, while in zeolite to the ID model (R2 = 0.955 and R2 = 0.841 for palygorskite and zeolite respectively). The results are also highlighted by the fact that the calculated adsorption capacities of kinetic models are very close to the experimental adsorption capacities (Table 10). The good agreement of phosphorus adsorption to palygorskite suggests that the mechanism was governed by a heterogeneous diffusion process.
3.4. Desorption and Regeneration Experiments
The investigation of the desorption process is very significant, since zeolite and palygorskite are useful for agricultural soil fertilization as they provide slow release of nutrients [35,39], which enhance plant growth and, eventually, high crop yield [35]. The saturated adsorbents were tested for desorption in deionized water. Regeneration of the adsorbents is important since it may affect the substrate selection and process cost [39,40]. The regeneration of the adsorbents was performed with 0.1 N HCl. Results of all desorption experiments are illustrated in Table 11, Table 12 and Table 13.

Table 11.
Batch Desorption results with AWW.

Table 12.
Column desorption results with AWW.

Table 13.
Column desorption results with BWW.
In the batch desorption tests, the results showed a remarkably slow release even after equilibrium, where the NH4+-N recoveries were below 6% for all granulometries and both adsorbents (Table 11), with slightly higher recoveries found at the finer granulometry (1–2 mm) and zeolite. The results of Kotoulas et al. [35] agree with the results of the current study, reporting zeolites’ recovery varying from 2.77 to 4.39%, with higher values found at the lowest granulometry. In addition, Genethliou et al. [42], who found similar results, stated that NH4+-N uptake by palygorskite was practically irreversible (9.1 ± 0.1% and 7.8 ± 0.1% recoveries for distilled and tap water). On the contrary, Genethliou et al. [39] found that for zeolite the regeneration efficiency of NH4+-N was satisfactory, reaching 63.75% of the adsorbed quantity. Regarding column desorption studies, slightly higher values were observed in palygorskite (recoveries 6.96–17.77%), while in zeolite these values were again very low (recoveries 3.45 and 2.07%, Table 12). These results can be explained by the fact that the aqueous solution lacked any ion exchange capacity to release and replace ammonium anions. In order to generate used adsorbents, a strong ion exchange solution is needed [63]. However, as is shown in Table 11 and Table 12, although the regeneration of NH4+-N was higher in batch studies than in desorption studies, the recoveries were again insignificant, following a similar pattern to the desorption with deionized water. On the other hand, in column studies regarding palygorskite, the recoveries were in all cases higher than those found in batch studies (Table 12), indicating that a column system is more effective in desorption and regeneration activities. Regarding zeolite, the pattern was similar to that found in batch studies, with low NH4+-N recoveries.
Regarding desorption column studies with BWW, recoveries of NH4+-N were low, 5.36 and 5.72% for palygorskite and zeolite, respectively (Table 13). In regeneration tests, NH4+-N recovery was significantly higher (36.02%) in palygorskite, while in zeolite it slightly increased but again remained low (15%). sCODt recoveries were generally low, with values of 21.13 and 19.99% in palygorskite and zeolite, respectively. Genethliou et al. [42] found desorption efficiencies of sCODt for tap and distilled water of 61.7 ± 0.4% and 54.0 ± 0.1%, respectively, suggesting that sCODt uptake was reversible and that the predominant forces between adsorbent-adsorbate are mainly physical. Finally, PO4−3-P recoveries were also generally low (Table 13) indicating that PO4−3-P uptake by both adsorbents was practically irreversible.
4. Conclusions
In this study, a sequence of experiments was undertaken to evaluate the processes taking place within the substrate of a constructed wetland system. More specifically, the investigation primarily centered on the utilization of ion exchange materials, namely zeolite and palygorskite, for the removal of ammonium from artificial wastewater and the treatment of brewery wastewater. Experimental assessments were conducted both in batch and column configurations. In batch experiments, both of the aforementioned adsorbent materials exhibited remarkable efficiency, surpassing the 90% threshold, for the removal of ammonium. In comparison, zeolite displayed higher performance in the removal of ammonium. This can be attributed to zeolite’s higher selectivity for cations due to the presence of alkaline earth metal cations on its negatively charged surface, which can easily exchange with NH4+-N ions in the aqueous wastewater. In column experiments, the zeolite material showcased higher efficiency in NH4+-N removal, coupled with lower equilibrium concentrations when subjected to higher ammonium concentrations. Conversely, palygorskite demonstrated better performance when the ammonium concentrations were lower. A noteworthy observation is that both palygorskite and zeolite demonstrated the capacity to adsorb a substantial portion of the available NH4+-N in brewery wastewater, exceeding 85% removal rate. It is worth mentioning that palygorskite exhibited higher phosphorus removal efficiency, which can be attributed to its unique structure. To identify the adsorption mechanisms in both experiments, kinetic models, including the pseudo-first order, pseudo-second order, Elovich, intraparticle diffusion, and double-constant model, were employed. These mechanisms depend on the physico-chemical characteristics of the adsorbents and the mass transfer process, indicating different adsorption mechanisms for these materials. Isotherm models were also calculated, which are essential for designing an adsorption system and include several constants related to surface characteristics, adsorbent affinity, and adsorption capacity. Finally, desorption and recovery experiments were conducted, as these materials find applicability in agriculture due to their ability to facilitate the controlled release of nutrients.
Author Contributions
Conceptualization, V.P. and C.S.A.; Methodology, V.P., K.A.B. and J.T.; Investigation, V.P., K.A.B. and J.T.; Data Curation, V.P. and K.A.B.; Writing—Original Draft Preparation, V.P. and K.A.B.; Writing—Review and Editing, V.P. and C.S.A.; Visualization, K.A.B.; Supervision, V.P. and C.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank Geohellas S.A. for providing palygorskite and ZeolifeTM and Chrysovallantis Chatzigeorgiou for providing zeolites.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Appendix A.1. Adsorption Kinetic Models
In the Elovich model, which is one of the most widely used, chemisorption is dominant for the sorption process and can be calculated by Equation (A1), where C0 and k0 are two empirical constants determined by the experimental data. The intraparticle diffusion (ID) model is used to analyze and elucidate the diffusion mechanism and is calculated by Equation (A2), where C1 is an intercept constant indicating the effect of boundary layer to adsorption and k1 is an intraparticle diffusion rate constant. The double-constant model is suitable for the heterogenous diffusion process and is given by Equation (A3), where C2 is an intercept constant and k2 an adsorption constant. The pseudo-first order (PFO) kinetic model provides a simplified description of the kinetic-sorption mechanism and is calculated by Equation (A4), where qe (mg/g) is the amount adsorbed at equilibrium and k3 is a rate constant of the PFO model. The pseudo-second order (PSO) kinetic model is calculated by Equation (A5), where k4 is the pseudo-second order rate constant for adsorption.
qt = C0 + k0 lnt,
qt = C1 + k1 t1/2,
lnqt = C2 + k2 lnt,
qt = qe (1 − e−k3t),
qt = (qe2 k4 t)/(1 + k4 qe t),
Appendix A.2. Isotherm Models
The Langmuir model is applied to monolayer adsorption onto a homogenous surface, with a finite active number of identical sites which, once occupied by a metal ion, are no longer available for further adsorption. The Langmuir model is described by Equation (A6), where Ce and qe are defined as the adsorbed amount (mg/kg) and the concentration (mg/L), qm is the saturated adsorption (or adsorption capacity) (mg/kg), and KL is a constant (L/kg), which reflects the bonding energy between solution and ions. According to the Freundlich isotherm, due to the heterogeneity of the surface charges, the adsorption may be multilayered (Equation (A7)). Qe is the adsorbed amount (mg kg−1) and Ce is the concentration (mg L−1), KF is a constant (mg/ kg) indicative of efficiency of adsorption of ions and n is a constant indicative of the intensity of adsorption of ions. The Temkin model is often used to describe the uneven surface of a soil particle, which can be expressed by Equation (A8), where A and B are two empirical constants. The Henry model is a simple linear form which describes the adsorption through Equation (A9), where Kp is an adsorption constant (L/kg) and D is the initial amount of adsorbed ions (mg/g) and is considered as 0.
qe = (KL Ce qm)/(1+ KL Ce),
qe = KF Ce1/n,
qe = A lnCe + B,
qe = D + Kp Ce,
References
- Maria, M.P.; Torres, N.H.; Nascimento, V.R.S.; Chagas, T.S.A.; Saratale, G.D.; Mulla, S.I.; Bharagava, R.N.; Cavalcanti, E.B.; Romanholo Ferreira, L.F. Current Advances in the Brewery Wastewater Treatment from Anaerobic Digestion for Biogas Production: A Systematic Review. Environ. Adv. 2023, 13, 100394. [Google Scholar] [CrossRef]
- Othmani, A.; Magdouli, S.; Senthil Kumar, P.; Kapoor, A.; Chellam, P.V.; Gökkuş, Ö. Agricultural Waste Materials for Adsorptive Removal of Phenols, Chromium (VI) and Cadmium (II) from Wastewater: A Review. Environ. Res. 2022, 204, 111916. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Perez, M. Effect of Temperature and Bagasse Addition on Anaerobic Co-Digestion of Brewery Waste by Biochemical Methane Potential Test. Fuel 2024, 357, 129737. [Google Scholar] [CrossRef]
- Organization for Economic Co-operation and Development (OECD). Health Data; Organization for Economic Co-Operation and Development (OECD): Paris, France, 2005.
- Braeken, L.; Van Der Bruggen, B.; Vandecasteele, C. Regeneration of Brewery Waste Water Using Nanofiltration. Water Res. 2004, 38, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
- Fillaudeau, L.; Blanpain-Avet, P.; Daufin, G. Water, Wastewater and Waste Management in Brewing Industries. J. Clean. Prod. 2006, 14, 463–471. [Google Scholar] [CrossRef]
- Kanagachandran, K.; Jayaratne, R. Utilization Potential of Brewery Waste Water Sludge as an Organic Fertilizer. J. Inst. Brew. 2006, 112, 92–96. [Google Scholar] [CrossRef]
- Alayu, E.; Leta, S. Post Treatment of Anaerobically Treated Brewery Effluent Using Pilot Scale Horizontal Subsurface Flow Constructed Wetland System. Bioresour. Bioprocess. 2021, 8, 8. [Google Scholar] [CrossRef]
- Brewers of Europe European Beer Trends Statistics Report|2022 Edition. Available online: https://brewersofeurope.eu/european-beer-trends-2022-edition-and-previous-years/ (accessed on 20 September 2023).
- Simate, G.S.; Cluett, J.; Iyuke, S.E.; Musapatika, E.T.; Ndlovu, S.; Walubita, L.F.; Alvarez, A.E. The Treatment of Brewery Wastewater for Reuse: State of the Art. Desalination 2011, 273, 235–247. [Google Scholar] [CrossRef]
- Ashraf, A.; Ramamurthy, R.; Rene, E.R. Wastewater Treatment and Resource Recovery Technologies in the Brewery Industry: Current Trends and Emerging Practices. Sustain. Energy Technol. Assess. 2021, 47, 101432. [Google Scholar] [CrossRef]
- Zacharof, M.-P. Industrial Symbiosis: Beer Brewery Wastewater-Based Biorefinery. Circ. Econ. Sust. 2021, 1, 593–609. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.-G.; Wei, X.-D.; Liu, Y.-S.; Liu, S.-S.; Hu, L.-X.; He, L.-Y.; Chen, Z.-F.; Chen, F.-R.; Yang, Y.-Q. Removal of Antibiotics and Antibiotic Resistance Genes from Domestic Sewage by Constructed Wetlands: Effect of Flow Configuration and Plant Species. Sci. Total Environ. 2016, 571, 974–982. [Google Scholar] [CrossRef]
- Demeke, M.; Gabbiye, N. Organic Biofertilizer from Brewery Wastewater Sludges via Aerobic Composting Process. In Advances of Science and Technology; Habtu, N.G., Ayele, D.W., Fanta, S.W., Admasu, B.T., Bitew, M.A., Eds.; Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering; Springer International Publishing: Cham, Switzerland, 2020; Volume 308, pp. 3–15. ISBN 978-3-030-43689-6. [Google Scholar]
- Alayu, E.; Leta, S. Evaluation of Irrigation Suitability Potential of Brewery Effluent Post Treated in a Pilot Horizontal Subsurface Flow Constructed Wetland System: Implications for Sustainable Urban Agriculture. Heliyon 2021, 7, e07129. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, D.P.L.; Vanrolleghem, P.A.; De Pauw, N. Model-Based Design of Horizontal Subsurface Flow Constructed Treatment Wetlands: A Review. Water Res. 2004, 38, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Gopal, B. Effect of Hydraulic Retention Time on the Treatment of Secondary Effluent in a Subsurface Flow Constructed Wetland. Ecol. Eng. 2010, 36, 1044–1051. [Google Scholar] [CrossRef]
- Weerakoon, G.; Jinadasa, K.; Herath, G.; Mowjood, M.; Ng, W. Applicability of Constructed Wetlands for Water Quality Improvement in a Tea Estate Catchment: The Pussellawa Case Study. Water 2018, 10, 332. [Google Scholar] [CrossRef]
- Alayu, E.; Leta, S. Performance Efficiency and Water Quality Index of a Two-Stage Horizontal Subsurface Flow Constructed Wetland System Polishing Anaerobically Treated Brewery Effluent. J. Water Process Eng. 2021, 42, 102156. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of Nutrients in Various Types of Constructed Wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Lavrnić, S.; Toscano, A. Potential of Constructed Wetland Treatment Systems for Agricultural Wastewater Reuse under the EU Framework. J. Environ. Manag. 2020, 275, 111219. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, B.; Chen, X.; Li, Z.; Xia, Q.; Wang, H.; Yang, Y.; Zhou, Y.; Yang, H. The Use of Constructed Wetland for Mitigating Nitrogen and Phosphorus from Agricultural Runoff: A Review. Water 2021, 13, 476. [Google Scholar] [CrossRef]
- Dordio, A.V.; Carvalho, A.J.P. Organic Xenobiotics Removal in Constructed Wetlands, with Emphasis on the Importance of the Support Matrix. J. Hazard. Mater. 2013, 252–253, 272–292. [Google Scholar] [CrossRef]
- Zijun, Z.; Effeney, G.; Millar, G.J.; Stephen, M. Synthesis and Cation Exchange Capacity of Zeolite W from Ultra-Fine Natural Zeolite Waste. Environ. Technol. Innov. 2021, 23, 101595. [Google Scholar] [CrossRef]
- Wangi, G.M.; Olupot, P.W.; Byaruhanga, J.; Kulabako, R. Characterization of Natural Zeolite and Determination of Its Ion-Exchange Potential for Selected Metal Ions in Water. Environ. Process. 2023, 10, 53. [Google Scholar] [CrossRef]
- Sethia, G.; Somani, R.S.; Chand Bajaj, H. Adsorption of Carbon Monoxide, Methane and Nitrogen on Alkaline Earth Metal Ion Exchanged Zeolite-X: Structure, Cation Position and Adsorption Relationship. RSC Adv. 2015, 5, 12773–12781. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Akratos, C.S.; Gikas, G.D.; Tsihrintzis, V.A. Effluent Quality Improvement of Two Pilot-Scale, Horizontal Subsurface Flow Constructed Wetlands Using Natural Zeolite (Clinoptilolite). Microporous Mesoporous Mater. 2009, 124, 131–143. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Wang, X.; Mackinnon, I.D.R.; Xi, Y. Influence of Palygorskite on In-Situ Thermal Behaviours of Clay Mixtures and Properties of Fired Bricks. Appl. Clay Sci. 2022, 216, 106384. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and New Applications for Kaolin, Smectite, and Palygorskite: A General Overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Krekeler, M.P.S.; Kearns, L.E. A New Locality of Palygorskite-Rich Clay from the Southeastern Yucatán: A Potential Material Source for Environmental Applications. Environ. Geol. 2009, 58, 715–726. [Google Scholar] [CrossRef]
- Zeng, Y.; Xu, W.; Wang, H.; Zhao, D.; Ding, H. Nitrogen and Phosphorus Removal Efficiency and Denitrification Kinetics of Different Substrates in Constructed Wetland. Water 2022, 14, 1757. [Google Scholar] [CrossRef]
- Cyrus, J.S.; Reddy, G.B. Sorption and Desorption of Ammonium by Zeolite: Batch and Column Studies. J. Environ. Sci. Health Part A 2011, 46, 408–414. [Google Scholar] [CrossRef]
- Kong, L.; Tian, Y.; Li, N.; Liu, Y.; Zhang, J.; Zhang, J.; Zuo, W. Highly-Effective Phosphate Removal from Aqueous Solutions by Calcined Nano-Porous Palygorskite Matrix with Embedded Lanthanum Hydroxide. Appl. Clay Sci. 2018, 162, 507–517. [Google Scholar] [CrossRef]
- Baird, R.; Eaton, A.; Rice, E. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.; Tatoulis, T.; Akratos, C.; Tekerlekopoulou, A.; Vayenas, D. Zeolite as a Potential Medium for Ammonium Recovery and Second Cheese Whey Treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef]
- Rocha, L.C.C.; Zuquette, L.V. Evaluation of Zeolite as a Potential Reactive Medium in a Permeable Reactive Barrier (PRB): Batch and Column Studies. Geosciences 2020, 10, 59. [Google Scholar] [CrossRef]
- Patel, H. Review on Solvent Desorption Study from Exhausted Adsorbent. J. Saudi Chem. Soc. 2021, 25, 101302. [Google Scholar] [CrossRef]
- Muscarella, S.M.; Badalucco, L.; Cano, B.; Laudicina, V.A.; Mannina, G. Ammonium Adsorption, Desorption and Recovery by Acid and Alkaline Treated Zeolite. Bioresour. Technol. 2021, 341, 125812. [Google Scholar] [CrossRef] [PubMed]
- Genethliou, C.; Triantaphyllidou, I.E.; Giannakis, D.; Papayianni, M.; Sygellou, L.; Tekerlekopoulou, A.G.; Koutsoukos, P.; Vayenas, D.V. Simultaneous Removal of Ammonium Nitrogen, Dissolved Chemical Oxygen Demand and Color from Sanitary Landfill Leachate Using Natural Zeolite. J. Hazard. Mater. 2021, 406, 124679. [Google Scholar] [CrossRef] [PubMed]
- Malamis, S.; Katsou, E. A Review on Zinc and Nickel Adsorption on Natural and Modified Zeolite, Bentonite and Vermiculite: Examination of Process Parameters, Kinetics and Isotherms. J. Hazard. Mater. 2013, 252–253, 428–461. [Google Scholar] [CrossRef] [PubMed]
- Gianni, E.; Lazaratou, C.V.; Panagopoulos, G.; Sarantari, P.; Martsouka, F.; Papagiannopoulos, K.; Panagiotaras, D.; Papoulis, D. Raw and Modified Palygorskite in Water Treatment Applications for Low-concentration Ammonium Removal. Water Environ. Res. 2021, 93, 1979–1994. [Google Scholar] [CrossRef] [PubMed]
- Genethliou, C.; Lazaratou, C.V.; Triantaphyllidou, I.E.; Xanthaki, E.; Mourgkogiannis, N.; Sygellou, L.; Tekerlekopoulou, A.G.; Koutsoukos, P.; Vayenas, D.V. Adsorption Studies Using Natural Palygorskite for the Treatment of Real Sanitary Landfill Leachate. J. Environ. Chem. Eng. 2022, 10, 108545. [Google Scholar] [CrossRef]
- Widiastuti, N.; Wu, H.; Ang, H.M.; Zhang, D. Removal of Ammonium from Greywater Using Natural Zeolite. Desalination 2011, 277, 15–23. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.; Zhu, J.; Xi, Y.; Zhu, R.; Ma, L.; Tao, Q. Adsorption of Ammonium by Different Natural Clay Minerals: Characterization, Kinetics and Adsorption Isotherms. Appl. Clay Sci. 2018, 159, 83–93. [Google Scholar] [CrossRef]
- Martin, M.J.; Thottathil, S.E.; Newman, T.B. Antibiotics Overuse in Animal Agriculture: A Call to Action for Health Care Providers. Am. J. Public Health 2015, 105, 2409–2410. [Google Scholar] [CrossRef]
- Sundhararasu, E.; Runtti, H.; Kangas, T.; Pesonen, J.; Lassi, U.; Tuomikoski, S. Column Adsorption Studies for the Removal of Ammonium Using Na-Zeolite-Based Geopolymers. Resources 2022, 11, 119. [Google Scholar] [CrossRef]
- Rozic, M. Ammoniacal Nitrogen Removal from Water by Treatment with Clays and Zeolites. Water Res. 2000, 34, 3675–3681. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Aguilar, G.; Gonzalez-Gutierrez, L.V. Ammonia Exchange on Clinoptilolite from Mineral Deposites Located in Mexico. J. Chem. Technol. Biotechnol. 2004, 79, 651–657. [Google Scholar] [CrossRef]
- Aydın Temel, F.; Kuleyin, A. Ammonium Removal from Landfill Leachate Using Natural Zeolite: Kinetic, Equilibrium, and Thermodynamic Studies. Desalin. Water Treat. 2016, 57, 23873–23892. [Google Scholar] [CrossRef]
- Saltalı, K.; Sarı, A.; Aydın, M. Removal of Ammonium Ion from Aqueous Solution by Natural Turkish (Yıldızeli) Zeolite for Environmental Quality. J. Hazard. Mater. 2007, 141, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wen, Z.; Zhan, H.; Jin, M. An Experimental Study on the Adsorption and Desorption of Cu(II) in Silty Clay. Geofluids 2018, 2018, 3610921. [Google Scholar] [CrossRef]
- Tang, H.; Xu, X.; Wang, B.; Lv, C.; Shi, D. Removal of Ammonium from Swine Wastewater Using Synthesized Zeolite from Fly Ash. Sustainability 2020, 12, 3423. [Google Scholar] [CrossRef]
- Liao, P.; Yuan, S.; Xie, W.; Zhang, W.; Tong, M.; Wang, K. Adsorption of Nitrogen-Heterocyclic Compounds on Bamboo Charcoal: Kinetics, Thermodynamics, and Microwave Regeneration. J. Colloid Interface Sci. 2013, 390, 189–195. [Google Scholar] [CrossRef]
- Sheela, T.; Nayaka, Y.A.; Viswanatha, R.; Basavanna, S.; Venkatesha, T.G. Kinetics and Thermodynamics Studies on the Adsorption of Zn(II), Cd(II) and Hg(II) from Aqueous Solution Using Zinc Oxide Nanoparticles. Powder Technol. 2012, 217, 163–170. [Google Scholar] [CrossRef]
- Uğurlu, M.; Karaoğlu, M.H. Adsorption of Ammonium from an Aqueous Solution by Fly Ash and Sepiolite: Isotherm, Kinetic and Thermodynamic Analysis. Microporous Mesoporous Mater 2011, 139, 173–178. [Google Scholar] [CrossRef]
- Saleh, T.A. Chapter 3—Kinetic Models and Thermodynamics of Adsorption Processes: Classification. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 65–97. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Prelot, B. Chapter 7—Adsorption Processes for the Removal of Contaminants from Wastewater: The Perspective Role of Nanomaterials and Nanotechnology. In Micro and Nano Technologies, Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. [Google Scholar] [CrossRef]
- Delkash, M.; Ebrazi Bakhshayesh, B.; Kazemian, H. Using Zeolitic Adsorbents to Cleanup Special Wastewater Streams: A Review. Microporous Mesoporous Mater. 2015, 214, 224–241. [Google Scholar] [CrossRef]
- Carazo, E.; Borrego-Sánchez, A.; García-Villén, F.; Sánchez-Espejo, R.; Viseras, C.; Cerezo, P.; Aguzzi, C. Adsorption and Characterization of Palygorskite-Isoniazid Nanohybrids. Appl. Clay Sci. 2018, 160, 180–185. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Li, A.; Yang, H. Adsorption Properties and Mechanisms of Palygorskite for Removal of Various Ionic Dyes from Water. Appl. Clay Sci. 2018, 151, 20–28. [Google Scholar] [CrossRef]
- Moharami, S.; Jalali, M. Use of Modified Clays for Removal of Phosphorus from Aqueous Solutions. Environ. Monit. Assess. 2015, 187, 639. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, X.Y.; Liu, J.L. Ion-Exchange Capability for Ammonium Removal Using Zeolite Modified by Potassium Permanganate. Chem. Eng. Trans. 2016, 55, 163–168. [Google Scholar] [CrossRef]
- You, X.; Valderrama, C.; Queroland, J.L.C.X. Recovery of Ammonium by Powder Synthetic Zeolites from Wastewater Effluents: Optimization of the Regeneration Step. Water Air Soil Pollut. 2017, 228, 396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).