1. Introduction
Freshwater ecosystems play a major role in the maintenance of sustainability of the planet through the provision of several ecosystem services [
1]. Although freshwater habitats comprise about 0.0091% of the hydrosphere, some 15,200 strictly freshwater species of fish live there [
2]. High levels of endemism are typical for freshwater fish due to the fragmentation or complete isolation of their habitats. Many endemic species are heavily threatened due to ongoing climate change [
3] and human-made disturbances [
4]. The Caucasus region is one of the biodiversity hotspots on the planet [
5]. Sevan trout (
Salmo ischchan Kessler, 1877) is an endemic species native for only Lake Sevan (Armenia) [
6,
7]. Considering its commercial value, Sevan trout were regularly introduced to other water bodies throughout the territory of the former Union of Soviet Socialist Republics (USSR) for commercial trout fishery. But only the stocking of Lake Issyk Kul (Kyrgyzstan) was successful in the long term [
8,
9].
State of Sevan trout. Since the morphology of Lake Sevan was established in the Pleistocene and Holocene [
10], the lake and its endemic species are relatively young. However, human intervention into natural processes of the lake during the 20th century led to the degradation of its ecosystem and increased the vulnerability of native species [
11,
12]. According to the most recent estimation, Sevan trout are a critically endangered species (corresponds to International Union for Conservation of Nature (IUCN) category CR A2cd) [
13]. Until the 1970s, it was represented by four subspecies (morphs) differing in terms of breeding times and places, as well as growth rates [
14,
15]. Particularly, winter bakhtak (
S. ischchan ischchan Kessler, 1877) (growing up to 90 cm and reaching 15 kg) and bodjak (
S. ischchan danilewskii Iakowlev, 1988) (dwarf, slowly growing lacustrine fish, not exceeding 33 cm and 250 g) bred in the lake while summer bakhtak (
S. ischchan aestivalis Fortunatov, 1926) bred both in the tributaries or near the river mouth; gegharkuni (
S. ischchan gegarkuni Kessler, 1877) bred exclusively in the tributaries [
15,
16,
17,
18]. While increased water diversion for irrigation and energy generation has led to extinction of winter bakhtak and bojak [
19] due to loss of spawning ground in the littoral zone, poaching in river mouth parts has significantly reduced the populations of summer bakhtak and gegharkuni. Thus, only these two subspecies have remained in Lake Sevan today [
20].
Theoretical background of the experimental validation. Several human-induced changes in the spawning rivers like the establishment of migration barriers [
21] and increased level of pollution [
22] make the possibilities for a natural reproduction of Sevan trout very limited. Thus, all the relevant authorities and academia in Armenia have been looking for effective solutions to protect Sevan trout since the 1980s. However, conservation measures had small success due to socio-economic problems in the country after the collapse of USSR. The main strategy of restocking wild population by farm-raised smolts also has little success as the natural reproduction of stocked fish in the rivers hardly occurs [
23]. In addition to all the artificial barriers, there is a risk that stocked fish may not return to the spawning rivers [
24]. According to the information from the Foundation for restoration of Sevan trout stocks and development for aquaculture (STF) (personal communication with T. Vardanyan) about 3.818 million specimens of gegharkuni and 2.136 million specimens of summer bakhtak were released in total into the drainage basin of Lake Sevan by the Ministry of the Environment during 2005–2015 and by the STF during 2015–2020. Possible restocking by the population from Lake Issyk Kul was also disputed as the recent findings based on molecular and morphometric analyses are rather controversial. Particularly, molecular analysis shows significant difference between the populations from various farms in Armenia and from Lake Issyk Kul [
17] while morphologically Kyrgyz and wild populations from Lake Sevan are closer than the wild populations and trout released from fish farms in Armenia [
25]. Considering also the risk of domestication when restocking with farm-grown fish, it was supposed that incubation of eggs directly in the river would be an alternative and rather efficient solution for restocking Sevan trout [
24] taking into account the homing reflex of fish.
Thus, pilot projects have been launching since 2015 for the development of methodologies to assess the recent state of Lake Sevan tributaries as spawning grounds for Sevan trout (hereafter theoretical assessment) [
22,
26]. Assessments rely on the analysis of core hydro-physical, hydro-chemical and hydro-morphological parameters that support natural reproduction of Sevan trout. In particular, Sevan trout prefer the gravel, cobble and pebble to settle the nest where depth is about 15–50 cm; velocity is 0.15–0.55 m/s; dissolved oxygen concentration is high (8–15 mg/L) and pH is between 6 and 9. However, gegharquni spawn in cold periods when water temperature drops below +13 °C and summer bakhtak spawn in a warmer period when temperature increases to more than +10 °C [
15,
26].
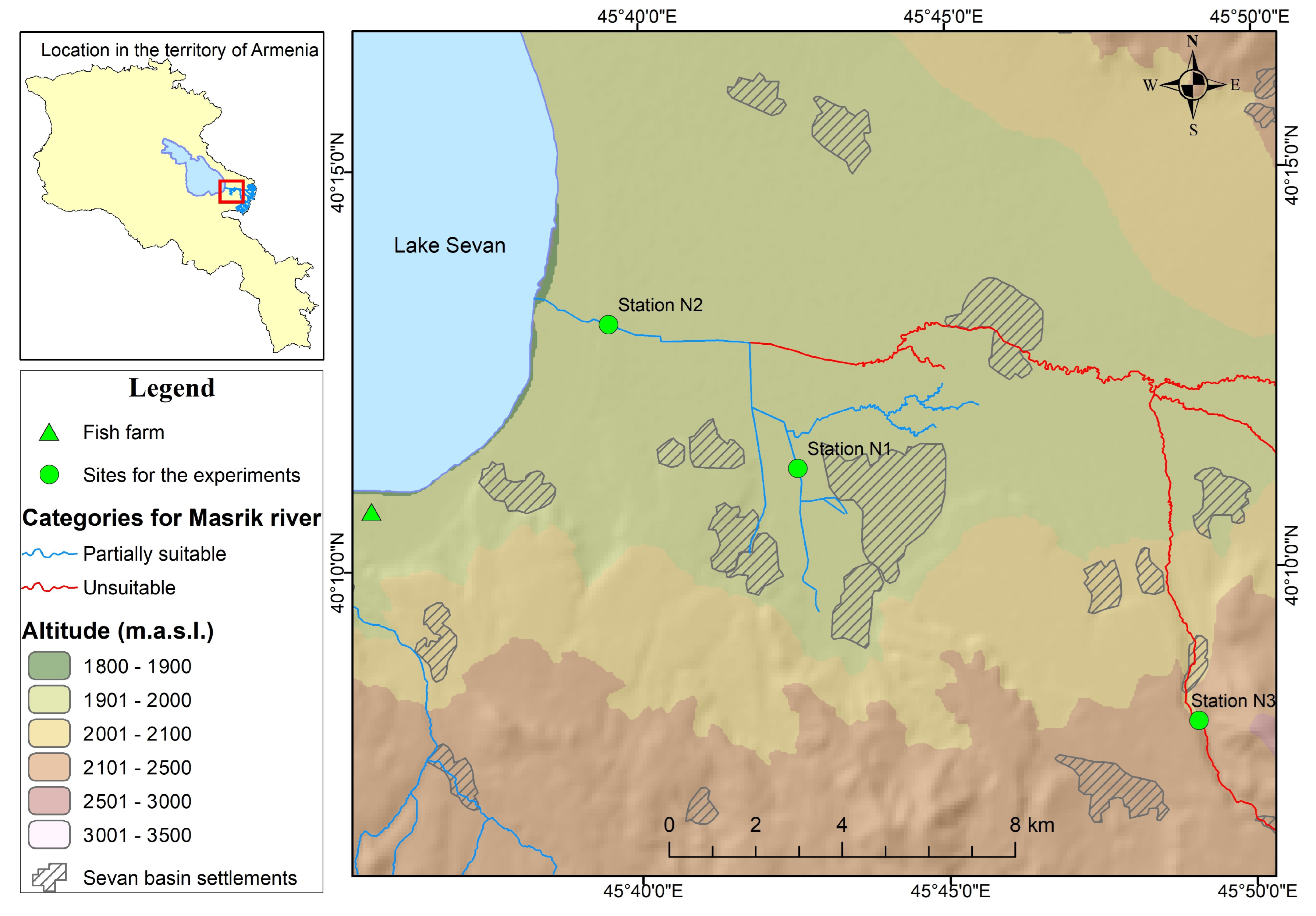
The recent experimental validation of the results of theoretical assessments were conducted in the Masrik River system. According to the results of the theoretical assessment [
26], only two categories of river stretches could be distinguished in the Masrik River (
Figure 1): 1. partly suitable for natural reproduction and 2. unsuitable. “Suitable” spawning grounds are missing in the system of the Masrik River because of two major reasons: (1) artificial barriers for the spawning migrations; and (2) anthropogenic pressures on aquatic ecosystems in terms of wastewater discharge, solid waste, etc.
Considering the lack of recent opportunities to observe the natural reproduction of Sevan trout in the rivers, we launched an experiment with semi-natural reproduction via nesting in a simple box-incubator in the wilderness and regularly maintained it until the possible emergence of sac-fry. Such method is also common for increasing salmonid densities in different countries [
27]. Thus, the aim of the current study was to validate the results of theoretical assessment of the suitability of rivers as spawning grounds for Sevan trout through incubation of eggs in the wild. One more objective was to estimate the difference of the egg survival until the sac-fry stage in the wild and microcosm conditions created in the farm for restocking Sevan trout. Because there are only two categories of stretches, the main hypothesis tested was that sac-fry should emerge from the eggs of gegharkuni in the zones assessed as “partly suitable”, while there should be no emergence in the “unsuitable” zones. At the same time the mortality rate of eggs in the wild should be higher than in the microcosm conditions. Also, we have hypothesized that the success rate of the experiments should be higher in case of planting eyed eggs (a stage when black spot of the eyes of embryo are already visible) in the wild compared with planting green eggs (just fertilized egg stage).
2. Materials and Methods
Experiment sites. Due to the conservation status of Sevan trout and a very limited availability of their eggs, we have strictly limited many aspects of wild experiments. For instance, they were conducted only in the system of the Masrik River which provides a wide range of abiotic conditions and is recently better studied than other spawning rivers [
22]. Two sites (Station N1—40°11′15″ N; 45°42′30″ E; Station N2—40°13′4″ N; 45°39′26″ E) were chosen at so-called “partly suitable” zones with slightly different abiotic conditions [
28] and one at an “unsuitable” zone for comparison (Station N3—40°8′3″ N; 45°49′3″ E) (
Figure 1). Microcosm experiments were conducted in parallel in the Karchaghbyur fish farm located nearby the Masrik River which specialized in hatching Lake Sevan trout.
Conditions of experiments. For all experiments, eggs and milt were collected from the same parents simultaneously to avoid any bias on a quality of eggs or success of fertilization and all the experiments were conducted by the same order of steps. The fertilization was strictly performed in frames of annual fertilization works in STF by their specialists. However, considering some differences in temperature conditions between Station N3 and the two other stations (
Supplementary Table S1), we launched the experiment for Station N3 on 23 October 2018 and for Stations N1 and N2 on 30 October 2018.
In the case of the experiment in Station N3, about one third and, in the case of the experiment in Stations N1 and N2, about half of the green eggs were directly transported to the experimental sites using special box-refrigerators. The remaining part was transported to the farm for the incubation. The time between fertilization and planting in the experimental sites fluctuated from 1 to 2 h. Because we set two stages for the experiments, when the eggs emerge to the eyed stage in the farm, we transported almost half of the eggs to the wild experiment sites using the same box-refrigerators. In all cases, semi-natural planting of eggs was performed by putting the eggs into the incubator boxes and burying them into the artificially established nests made by the cobble, gravel and pebble taken in situ.
In order to set the best possible conditions for the wild experiments, the spawning habits of gegharkuni were followed as much as possible. In particular, the experiments were launched when optimum temperatures for breeding were established in the chosen stretches. Nests were established at optimal depths following the observations of Dadikyan [
29] and, in optimal water velocity, following the observations of Savvaitova et al. [
30].
Microcosm conditions. Planting was conducted in two special incubators of 42 cm in width, 360 cm in length and 17 cm in depth. Sieves had 39 × 39 cm size. Water was supplied from a nearby source that also fed the Karchaghbyur River. During the experiment Dissolved oxygen (DO) fluctuated from 8.5 to 9 mg/L, water temperature 8.3–9.5 °C and pH was 7 (
Supplementary Table S1).
All experiments in the wild were conducted using Whitlock-Vibert Boxes (WVB’s from the Fly Fishers International) as their effectiveness for incubation of gegharkuni eggs in the tributaries of Lake Sevan was already proven [
24]. During the experiments, some basic abiotic parameters as well as survival rates were monitored on a weekly basis from 23 October to 11 November at Station N3 and from 30 October to 15 February at Stations N1 and N2. In particular, temperature, pH and dissolved oxygen conditions were measured by handheld Hanna HI9813-5N pH/EC/TDS and HI9147-10 DO meters twice per day in the early morning and in late afternoon, while the fluctuations in water level near nests were measured by a meter stick once per visit. All the measurements were strictly conducted near the places where the WVB’s were installed. Then, the mean values were calculated for each visit. Success of hatching in all sites was estimated through empirical observation of discolored eggs’ proportion in each WVB, which shows the mortality rate.
Experiments with green eggs. The number of eggs planted in each WVB was derived by dividing the total mass of planted eggs by the average mass of gegharkuni egg (0.07 g). Thus, in total, six WVB’s (two per station) with 2657 green eggs were installed in three chosen wild stretches and 2374 eggs remained in the farm.
Experiments with eyed eggs. A second set of experiments was launched on 6 December 2018 when the green eggs from the first set of experiments reached the eyed stage in the farm. As the average weight of such eggs was 0.09 g, it was calculated that we have installed two WVBs with a total number of 744 eyed eggs only in the “partly suitable” zones (one per site). Considering the loss until the eyed stage, about 890 eggs remained in the farm after the launch of the second stage of the experiments. Observations of abiotic conditions in Station N3 (unsuitable zone) have shown no chance for egg survival as the water was mostly frozen. Thus, it was preferable to interrupt the experiment there to avoid loss of eyed eggs.
Statistical processing of data. All statistical calculations were conducted using the IBM SPSS 17 software. Series of one-way ANOVA tests were performed to compare means for the results of different experiments to check the results of theoretical assessment and to reveal the part of the Masrik River where further implementation of artificial incubation of eggs could be more effective. In all tests, the survival rates were set as a dependent parameter. Basically, the hypothesis of homogeneity of variance was checked through Levene’s test and the equality of means was checked through the Welch and Brown–Forsythe tests. In the first set of analyses, we separately check the hypotheses of significance of differences for mean values of survival rates of (1) green and (2) eyed eggs between two stations and also the fish farm. The experiment sites were set as an independent factor here. As a post hoc test for the results of survival rates between the stations, the Tukey test was used. Because in the first set of experiments the eggs were planted using two WVB’s in each station, the mortality rates were derived by calculating the average numbers of discolored eggs per station for each visit. In the second set of analyses, the means for green and eyed egg survival rates were compared for the same stations in the wild. Here, we assumed that if the variance between the groups is significantly higher than the variance within the groups (F factor), then there are proven differences in the effectiveness of hatching green and eyed eggs. The F value was derived through univariate analysis of variance.
As abiotic parameters and their fluctuations were considered decisive for the spawning success in theoretical assessment, we performed General Linear Model analysis to estimate a significance of effects of measured basic parameters on hatching success. The main assumption made was that if the mean difference of the parameter is significantly different among two stations, then it could lead to differences in success rates of the experiments. For that, stations were set as an independent factor and the measured abiotic parameters–dependent. Then, nonparametric correlation analysis (Spearman) was conducted between the values of abiotic parameters and the recorded mortality rates.
Ethics statement. All eggs for the experiments were supplied by the STF in frames of their restocking and breeding action plans; all the experiments were approved by the STF and Scientific Center of Zoology and Hydroecology of National Academy of Sciences (NAS) of Armenia in frames of the contract with the Ministry of Environment of RA on annual assessment of the stocks of fish and crayfish in Lake Sevan basin.
3. Results
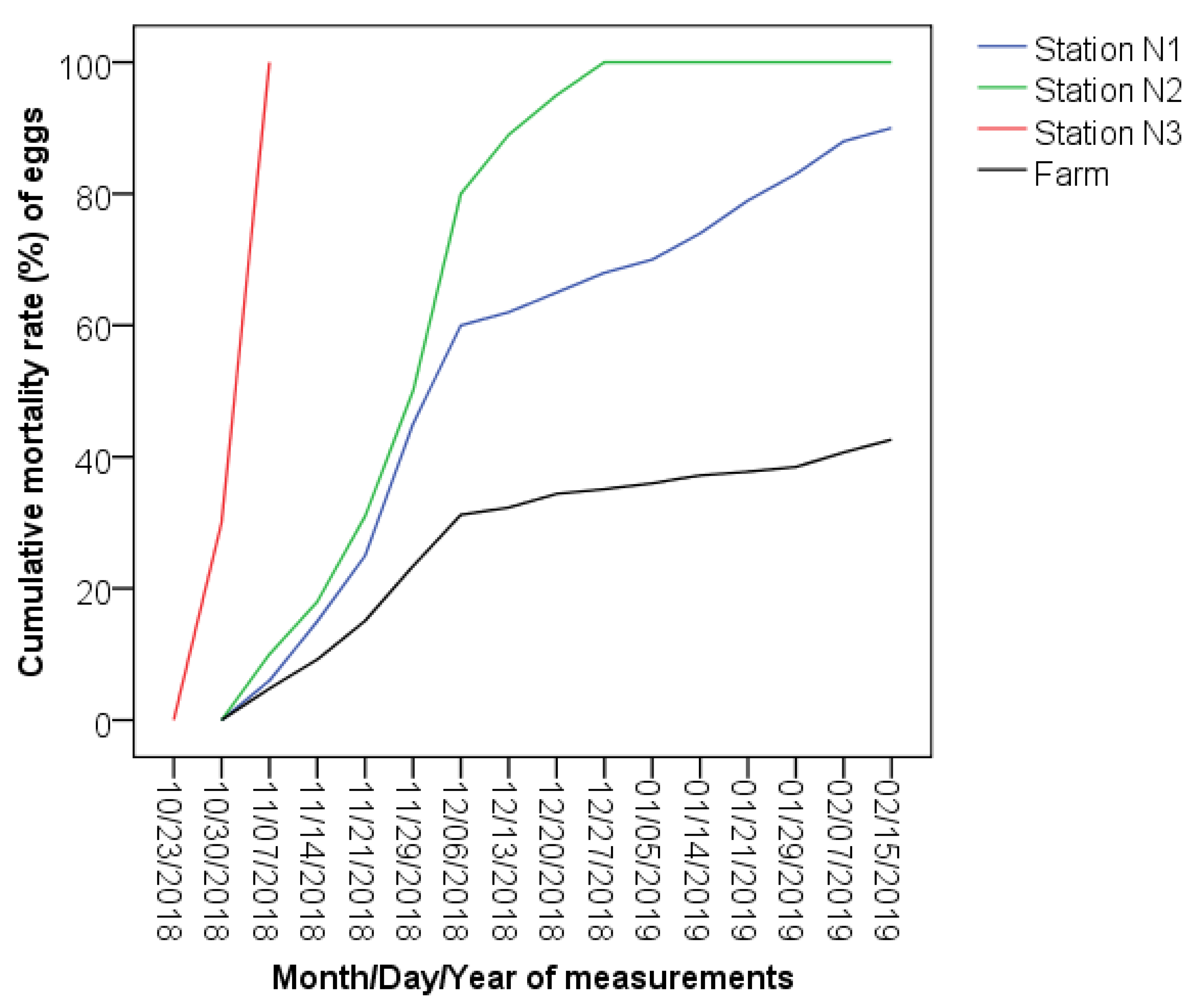
Hatching of green eggs. The success rate of the experiments varies in a wide range between the stations (
Figure 2). Null success of hatching was recorded in Station N3. Moreover, the main experiment was interrupted after two weeks due to frozen water in the station. The results of ANOVA test for hatching success claim the significance of mean differences between the fish farm and both Stations N1 and N2 (
Table 1). In particular, there was no survival of green eggs in Station N2 while the success rate in Station N1 was 10%. Also, it should be considered that while there was a yield from Station N1, the success rate of the hatching in the farm was 57.38% in the same time period. Thus, environmental conditions in Station N1 significantly affected survival. This also proves the results of theoretical assessment and the assumption that incubation in the wild will have a lower success rate than in the farm.
Hatching of eyed eggs. The experiments with the eyed eggs were launched after about five weeks from fertilization. Considering the emergence of green eggs at the farm was 68.78%, only a 7.25% loss was recorded during the eyed stage (
Figure 3). Unlike the green eggs, the yield from the eyed eggs in Station N2 was 5%, while, in Station N1, it was 80%. However, compared with the 92.75% yield in the farm, it is also quite low. The results of ANOVA claim the significance of difference of eyed egg survival between Station N2 and the remaining two sites of the experiments (
Table 2). However, a null hypothesis was rejected for the pair Station1-Farm. Considering the results of theoretical assessment, such result raises some questions on its precision as both stations were previously concluded “partly suitable”.
Considering the faster emergence in the farm, the observations continued on yolk sacs after the 13th week. During the last two weeks of the experiments, some 4.15% of sac-fry had also died in the farm. However, it was impossible to measure the semi-natural mortality of yolk sac in the river to compare as they were attacked by the crustaceans of the genus Gammarus (Fabricius, 1775). Thus, we conclude it inappropriate to involve such comparisons in the further discussion.
The effectiveness of incubation in the wild. Comparison of green and eyed egg incubation success. It was initially assumed that the survival rate of green and eyed eggs at the same stations will be different because of the sensitivity criterion. The results of Univariate Analysis of Variance partially prove the assumption (
Table 3). In particular, differences between the groups were significantly higher than within the groups in Station N1 but not in Station N2. This means that the environmental conditions almost equally influence the hatching of eggs in both stages in Station N2. Considering the low success rates of hatching there, it could be concluded that the area is not well suited for the proposed method in general.
Fluctuations in abiotic parameters. In general, Station N1 has the most stable conditions for all the measured parameters except oxygen saturation, while Station N3 has the most unstable conditions (
Supplementary Table S1). Moreover, all monitored parameters were at the margins of optimum during the experiments besides temperature (
Table 4).
For instance, during 15 visits to the experimental sites, the optimum temperatures were never exceeded in Station N1, were exceeded seven times in Station N2 and were always exceeded in Station N3 (
Supplementary Table S1). The results of the general linear mixed model analysis show that the null hypothesis of equality of error variance of the dependent variable across groups is rejected for pH, DO (mg/L) and depth parameters. Thus, the results of Tukey’s HSD are not accurate for them. Therefore, Tukey’s test was performed only for temperature and DO (%) parameters (
Table 5), and revealed significant differences only in terms of the temperature parameter.
However, considering time lag between visits, the expected negative impact on the incubation should be rather small in Station N1 but high in Stations N2 and N3. Also, the gap between optimal and registered average temperatures for Station N1 was only 0.5 °C and from 0.2 to 1.2 °C (SD ± 0.38) in Station N2. Such a situation with the temperature conditions also led us to state that the results of theoretical assessment for the remaining upstream parts of the Masrik River system were also valid. When comparing the results for the wild with the farm conditions, it was obvious that the biggest gap in all parameters was in Station N3 and relatively closer conditions were in Station N1.
Correlations with hatching success. Because it has already been shown that only temperature and DO (%) parameter effects on hatching could be considered confident, the correlation analysis (
Table 6) was also performed for these two parameters only.
The results show the existence of strong links between tested parameters and hatching success. Particularly, it could be derived that temperature changes correlate strongly with the mortality of both green and eyed eggs in Station N1 but only with the mortality of eyed eggs in Station N2. Meanwhile, oxygen saturation correlates well with the mortality of green eggs in Station N1 and with the mortality rate of eyed eggs in Station N2. However, because the DO parameter was always in the optimum range, only the temperature was influential. Such results do not neglect the hypothesis that other environmental conditions could have a strong effect on the natural reproduction success of trout of Lake Sevan in the rivers.
4. Discussion
Environmental factors. The results of abiotic factors prove that the temperature conditions generally remain the main constraining factor for the successful growth of eggs in the Masrik River as was also shown during the theoretical assessment [
26]. In particular, compared with Station 1 where temperatures remained in the optimum range throughout all the experiment, there was less success in hatching in Station 2 for both green and eyed eggs. For the failure of hatching in Station 3, the temperature factor was decisive. The results of analyses also revealed that various environmental parameters or features should be involved in the theoretical assessment. Particularly, solid waste and organic matter were regularly covering nests in Stations N1 and N2. This issue is regularly reported [
22] but no action from the authorities to prevent solid waste dumping directly into the rivers. If not maintained properly, this issue will lead to closure of pores and reduced aeration in the nest or even their destruction.
For the particular case of the Masrik River, it seems meaningful to also use a criterion of a suspension level, because a fast stream current is eroding away significant amount of clay from the floodplain [
31] which blocks the pores of WVB and we assume will do the same with the pores of the nests.
One more feature to consider is the wind chill effect on water temperatures [
32]. Because most of the tributaries of Lake Sevan flow through gorges and V-shape valleys in their upper and middle course parts [
33], obviously wind chill significantly contributes to early drop of temperatures, which makes such areas unsuitable for gegharkuni’s spawning.
Benthic macroinvertebrates were considered in the theoretical assessment, but only from a perspective of food base for the fry and the indicators of ecological status. However, as our observations show, gammarids are also predating eggs, which should be considered in the theoretical assessment. Moreover, some zooplankton species are also known as predators of fish eggs [
34]. Thus, the next important step towards the restoration of spawning rivers in Lake Sevan basin should reflect such concerns.
Hatching success. Natural and Semi-Natural mortality. One of the most complicated issues is the precise estimation of eggs’ hatching success in the wild. In general, it is stated that the emergence of smolt of some salmonids (involving Rainbow and Brown trouts) varies between 85 and 95% in hatcheries and between 1 and 5% in the wild [
35]. According to Hoitsy et al. [
36], the fertility rate of eggs of Brown trout—a close relative of Sevan trout—is 95–100% in the farms while the hatching rate from green eggs is 90–100% if incubated at 10 °C. Such results correspond to the experimental results from the Karchaghbyur farm. However, natural mortality of Sevan trout eggs in the wild has not yet been studied sufficiently. Some hyperlocal studies in this issue could be found in the works dated back to the first half of 20th century. Particularly, Vladimirov has found a 7–65% yield from green egg [
37], while Fortunatov has found a 0–50% [
38] yield in the Gavaraget River. Vladimirov has also found a 64–93% yield of green eggs in the Karchaghbyur River [
37]. On average, he estimates the success rate of the natural reproduction of gegharkuni in tributaries of Lake Sevan at a level of 10%. However, considering the changes in the basin of Lake Sevan during 20th century [
39], these data are less relevant today. Moreover, the Masrik River cannot be mechanically compared to the Karchaghbyur or Gavaraget rivers. Thus, there are insufficient data to compare with our results. Moreover, our results cannot be considered as natural mortality because we used some artificial incubation in man-made nests thus we prefer to call the results semi-natural. In general, hatching rate in Station N1 corresponds to the estimations of Vladimirov. The success rate in Station N2 claims that some natural reproduction there is still possible, but with a lower yield compared with Station N1 and average survival rate given by the Vladimirov. At the same time, in the current climatic conditions, the natural reproduction of Sevan trout is impossible in the middle or the upper course parts of the Masrik River since mid-November given the low temperatures that lead to freezing water.
Impact of different conditions. Considering the artificial conditions established for the experiments, there should be some differences from natural hatching success rates in the wild. More probably, natural reproduction would be more successful which means that it still can happen in Station N2 but with a significantly lower success rate than in Station N1. However, taking into account the issue with the high level of suspension, the overall success rate of natural reproduction of Sevan trout in the Masrik River is expected to be lower than that measured for the Gavaraget and Karchaghbyur rivers back in the 1940s [
37]. Suspension in the Masrik River is able to block the pores significantly and lessen the aeration and thus increase mortality [
40]. Considering the results of analyses, we conclude it meaningful to revise the “partly suitable” class of a river for natural reproduction of Sevan trout to address the range of impacts of environmental conditions more precisely.
The effectiveness of proposed method for restocking Sevan trout. The results of hatching rates for the experiments have just proven the general pattern of sensitivity of green eggs towards environmental conditions is higher than in case of eyed eggs, e.g., [
27,
41,
42]. However, the results of experiments for the different stretches of the Masrik River and the results of almost similar experiments conducted in the Lichq River [
24] have shown obvious differences. In particular, the emergence of green eggs to the eyed eggs in the Lichq River was about 34%, while it was 40% in Station N1 and 20% in Station N2 (please, see the reading for the 12 June 2018 on
Figure 2). In the eyed stage, the emergence at the end of the experiment in Station N1 was 2% less and in Station N2 77% less than in the Lichq River. Moreover, the eggs were treated in a different way in the farm and in the wild, which further contributes to the drop of the success level in the wild experiments. Thus, if treated more properly, the success rate can increase in the wild.
It can be concluded that the method of artificial incubation of eyed egg can be quite effective in restocking Sevan trout population and a significantly cheaper solution compared with restocking by smolt or reintroduction from Lake Issyk Kul. However, the effectiveness of this method is low in the Masrik River given the environmental conditions and the current state of water resource management in the area. Thus, further action on river restoration is still necessary to conduct prior to introducing this method in a wider scale in Lake Sevan basin. Considering the importance of conservation of an endemic and native fish species in the world, this work also comes to show that egg incubation in the wild is still an effective method for restocking salmonids in the rivers with limited human-induced modifications in river regime and an ecological status.