Limnological Response of Las Curias Reservoir, San Juan, Puerto Rico: Successful Management of the Invasive Aquatic Fern, Salvinia molesta
Abstract
1. Introduction
2. Materials and Methods
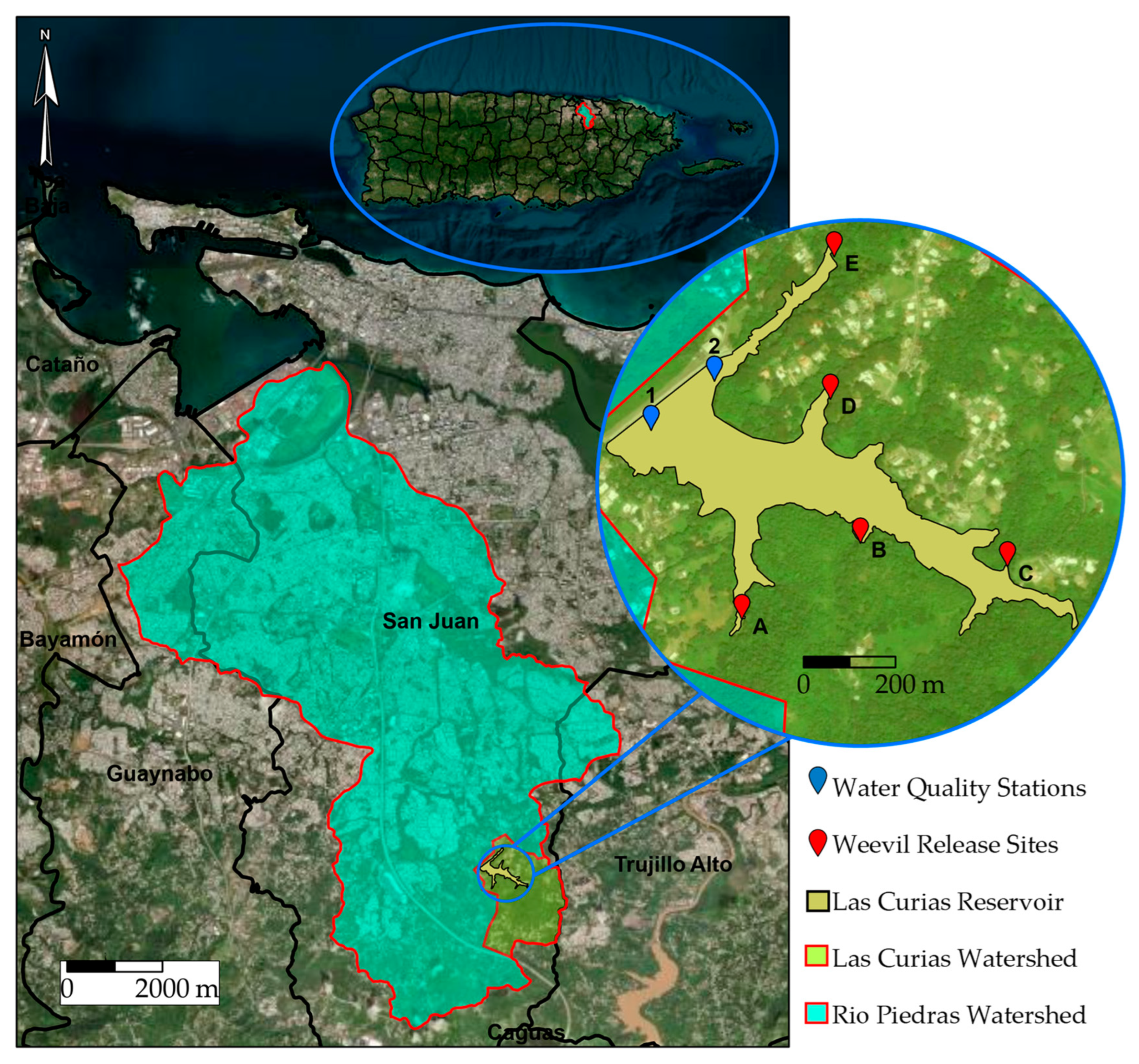
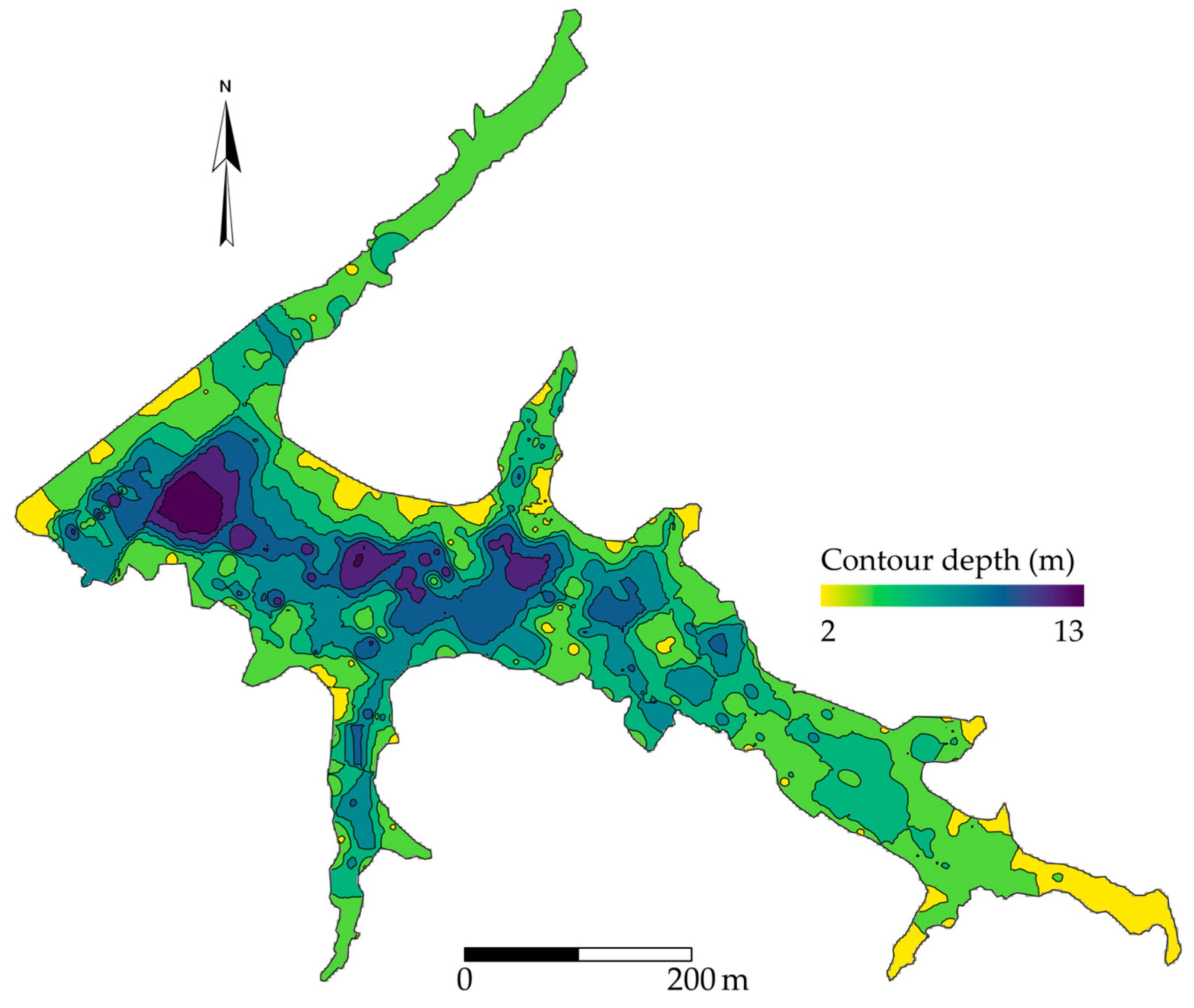
2.1. Site Description
2.2. Salvinia Performance
2.3. Weevil Introduction and Densities
2.4. Water Quality
2.5. Statistical Analysis
3. Results
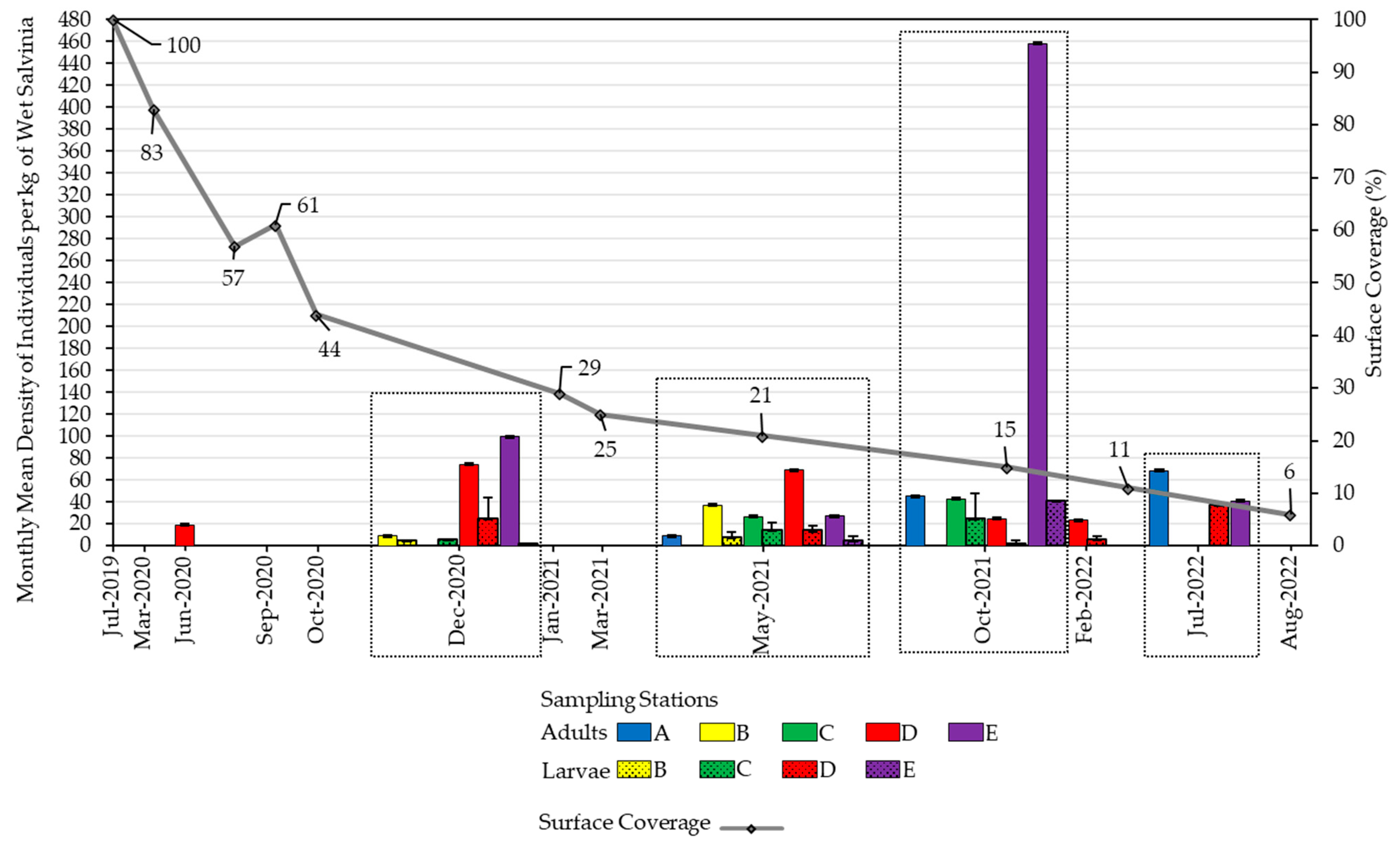
3.1. Salvinia Performance
3.2. Weevil Performance
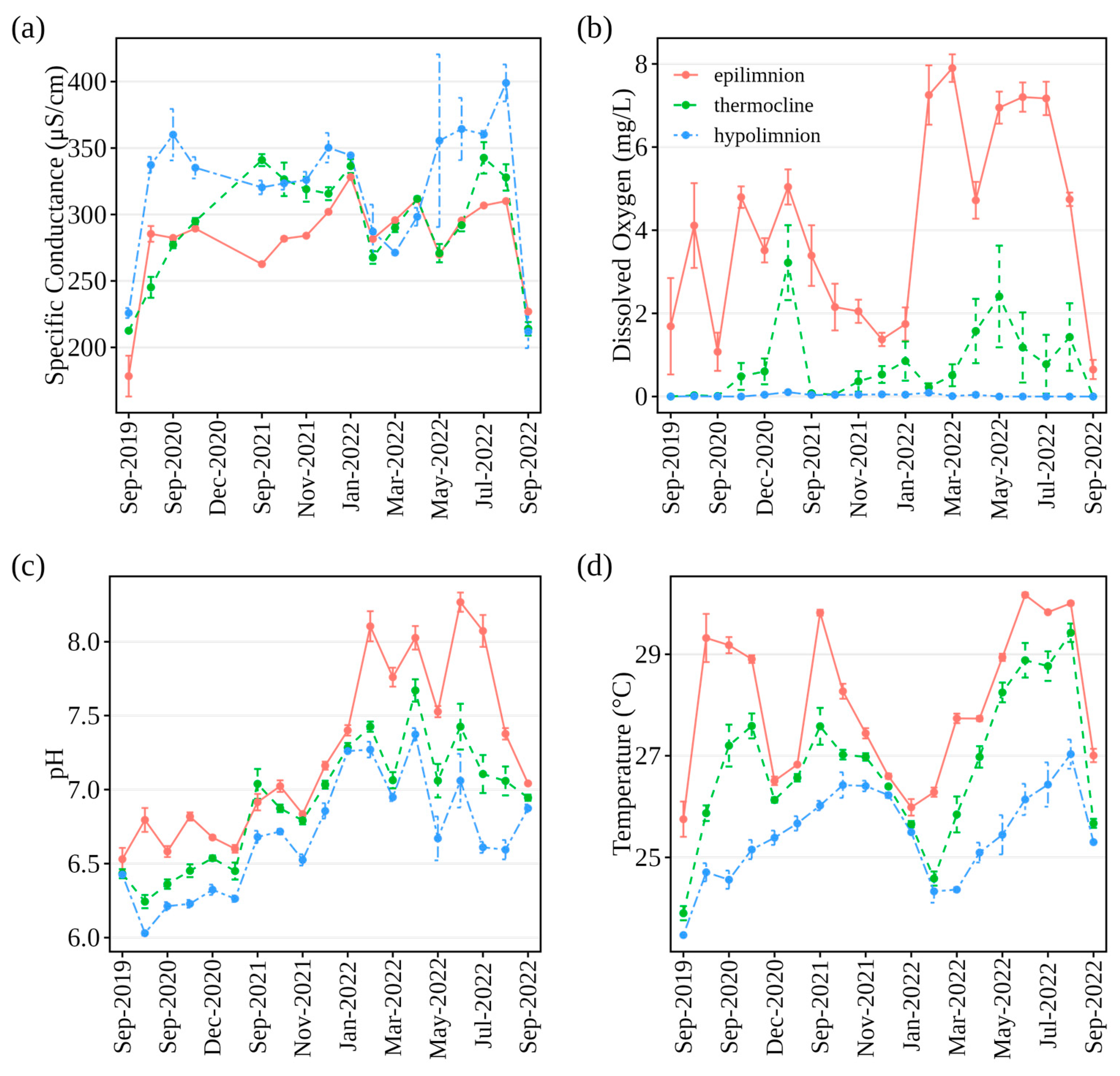
3.3. Water Quality

4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, P.T.J.; Olden, J.D.; Vander Zanden, M.J. Dam Invaders: Impoundments Facilitate Biological Invasions into Freshwaters. Front. Ecol. Environ. 2008, 6, 357–363. [Google Scholar] [CrossRef]
- Havel, J.E.; Lee, C.E.; Vander Zanden, M.J. Do Reservoirs Facilitate Invasions into Landscapes? BioScience 2005, 55, 518–525. [Google Scholar] [CrossRef]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic Invasive Species: Challenges for the Future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Fitzgerald, D.G.; Mayer, C.M.; Rudstam, L.G.; Mills, E.L. Alteration of Ecosystem Function by Zebra Mussels in Oneida Lake: Impacts on Submerged Macrophytes. Ecosystems 2006, 9, 1017–1028. [Google Scholar] [CrossRef]
- Tasker, A.V. USDA Demonstration Project: Giant Salvinia (Toledo Bend Reservoir and Surrounding Areas in Louisiana and Eastern Texas. Environmental Assessment, March 2001); U.S. Department of Agriculture: Riverdale, MD, USA, 2001; pp. 1–30. [Google Scholar]
- Jayan, P.R.; Sathyanathan, N. Aquatic Weed Classification, Environmental Effects and the Management Technologies for Its Effective Control in Kerala, India. Int. J. Agric. Biol. Eng. 2012, 5, 76–91. [Google Scholar]
- Mitchell, D.S. The Kariba Weed: Salvinia Molesta. Brittonia 1972, 24, 228–231. [Google Scholar]
- Luque, G.M.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Genovesi, P.; Simberloff, D.; Courchamp, F. The 100th of the World’s Worst Invasive Alien Species. Biol. Invasions 2014, 16, 981–985. [Google Scholar] [CrossRef]
- Russell, A.; Johnson, S.J.; Mudge, C.; Díaz, R. The Biology and Ecology of the Salvinia Weevil: A Biological Control Agent for the Management of Giant Salvinia.; Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2015; pp. 1–4. [Google Scholar]
- Forno, I.W.; Harley, K.L.S. The Occurrence of Salvinia Molesta in Brazil. Aquat. Bot. 1979, 6, 185–187. [Google Scholar] [CrossRef]
- McFarland, D.G.; Nelson, L.S.; Grodowitz, M.J.; Smart, R.M.; Owens, C.S. Salvinia Molesta D. S. Mitchell (Giant Salvinia) in the United States: A Review of Species Ecology and Approaches to Management; APCRP Technical Notes Collection; US Army Engineer Research and Development Center: Vicksburg, MS, USA, 2004; pp. 1–33. [Google Scholar]
- Julien, M.H.; Hill, M.P.; Tipping, P.W. Salvinia Molesta DS Mitchell (Salviniaceae). In Weed Biological Control with Arthropods in the Tropics; Muniappan, R., Ed.; Cambridge University Press: Cambridge, UK, 2009; pp. 378–407. [Google Scholar]
- Finlayson, C.M. Growth Rates of Salvinia Molesta in Lake Moondarra, Mount Isa, Australia. Aquat. Bot. 1984, 18, 257–262. [Google Scholar] [CrossRef]
- Room, P.M.; Thomas, P.A. Nitrogen, Phosphorus and Potassium in Salvinia Molesta Mitchell in the Field: Effects of Weather, Insect Damage, Fertilizers and Age. Aquat. Bot. 1986, 1, 213–232. [Google Scholar] [CrossRef]
- Motitsoe, S.N.; Coetzee, J.A.; Hill, J.M.; Hill, M.P. Biological Control of Salvinia Molesta (D.S. Mitchell) Drives Aquatic Ecosystem Recovery. Diversity 2020, 12, 204. [Google Scholar] [CrossRef]
- Wahl, C.F.; Kaller, M.; Díaz, R. Invasion of Floating Fern Alters Freshwater Macroinvertebrate Community Structure with Implications for Bottom-up Processes. Hydrobiologia 2021, 848, 2523–2537. [Google Scholar] [CrossRef]
- Woodley, S.E.; Wahl, C.F.; Tryforos, A.; Díaz, R. Biological Control of Invasive Floating Fern Leads to Rapid Recovery of Ecological Functions in Coastal Freshwater Wetlands in Louisiana. J. Aquat. Plant Manag. 2023, 61, 42–54. [Google Scholar] [CrossRef]
- Hilt, S.; Gross, E.M.; Hupfer, M.; Morscheid, H.; Mählmann, J.; Melzer, A.; Poltz, J.; Sandrock, S.; Scharf, E.M.; Schneider, S.; et al. Restoration of Submerged Vegetation in Shallow Eutrophic Lakes–A Guideline and State of the Art in Germany. Limnologica 2006, 36, 155–171. [Google Scholar] [CrossRef]
- Wahl, C.F.; Díaz, R.; Kaller, M. Nutrients Enhance the Negative Impact of an Invasive Floating Plant on Water Quality and a Submerged Macrophyte. J. Aquat. Plant Manag. 2021, 59, 57–65. [Google Scholar]
- van Donk, E.; van de Bund, W.J. Impact of Submerged Macrophytes Including Charophytes on Phyto- and Zooplankton Communities: Allelopathy versus Other Mechanisms. Aquat. Bot. 2002, 72, 261–274. [Google Scholar] [CrossRef]
- Thayer, D.D.; Pfingsten, I.A.; Jacono, C.C.; Richerson, M.M.; Howard, V. Salvinia Molesta Mitchell. Available online: https://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=298 (accessed on 12 December 2020).
- Pasch, R.J.; Penny, A.B.; Berg, R. National Hurricane Center Tropical Cyclone Report: Hurricane Maria (AL152017), 16–30 September 2017; National Oceanic And Atmospheric Administration and the National Weather Service: Washington, DC, USA, 2023; pp. 1–48. [Google Scholar]
- Wahl, C.F.; Díaz, R.; Ortiz-Zayas, J. Assessing Salvinia Molesta Impact on Environmental Conditions in an Urban Lake: Case Study of Lago Las Curias, Puerto Rico. Aquat. Invasions 2020, 15, 562–577. [Google Scholar] [CrossRef]
- Calder, A.A.; Sands, D.P.A. A New Brazilian Cyrtobagous Hustache (Coleoptera: Curculionidae) Introduced into Australia to Control Salvinia. J. Aust. Entomol. Soc. 1985, 24, 57–64. [Google Scholar] [CrossRef]
- Coetzee, J.A.; Hill, M.P. Salvinia Molesta D. Mitch. (Salviniaceae): Impact and Control. CAB Rev. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- U.S. Geological Survey Water-Year Summary for Site USGS 50048680. Available online: https://waterdata.usgs.gov/pr/nwis/wys_rpt/?site_no=50048680&agency_cd=USGS (accessed on 23 October 2023).
- García-Martinó, A. The Construction of Dams in the Face of Economic Development: Puerto Rico in the 20th Century. Dimens. CIAPR 2000, 4, 7–14. [Google Scholar]
- Tetra Tech. Las Curias Reservoir Lake Watershed Characterization.; Tetra Tech, Inc.: Fairfax, VA, USA, 2018; pp. 1–22. [Google Scholar]
- U.S. Census Bureau American Community Survey 5-Year Estimates. Available online: https://censusreporter.org/profiles/06000US7212722847-cupey-barrio-san-juan-municipio-pr/ (accessed on 3 October 2023).
- Puerto Rico 2022- 305(b) and 303(d) Integrated Report; Plans and Special Projects Division, Water Quality Area: San Juan, PR, USA, 2023; pp. 1–259.
- Lugo, A.E.; Ramos, O.; Rodriguez, C. The Río Piedras Watershed and Its Surrounding Environment.; US Department of Agriculture, Forest Service, International Institute of Tropical Forestry: Washington, DC, USA, 2011; pp. 1–52. [Google Scholar]
- Soler-López, L.R.; Webb, R.M. Sedimentation Survey of Lago Dos Bocas, Puerto Rico, June 1997; U.S. Geological Survey (USGS): Reston, VA, USA, 1998; pp. 1–21. [Google Scholar]
- Soler-López, L.R. Sedimentation Survey of Lago Caonillas, Puerto Rico, February 2000; U.S. Geological Survey (USGS): Reston, VA, USA, 2001; pp. 1–32. [Google Scholar]
- Ireland, P.; Knutson, A.; Gregory, L. Rearing the Salvinia Weevil in Outdoor Tanks at Caddo Lake, Texas. In A guide to mass rearing the salvinia weevil for biological control of giant salvinia; Knutson, A., Nachtrieb, J., Eds.; Texas A&M AgriLife Extension Service, Special Publication ESP-475: College Station, TX, USA, 2012; pp. 25–36. [Google Scholar]
- Wahl, C.F.; Díaz, R. Winter and Spring Conditions Determine the Production of the Salvinia Weevil Mass Rearing Programme. Biocontrol Sci. Technol. 2020, 30, 569–580. [Google Scholar] [CrossRef]
- Nachtrieb, J.G. Rearing the Salvinia Weevil for Biological Control of Giant Salvinia at the U.S. Army Corps of Engineers Lewisville Aquatic Ecosystem Research Facility. In A Guide to Mass Rearing the Salvinia Weevil for Biological Control of Giant Salvinia.; Knutson, A., Nachtrieb, J., Eds.; Texas A&M AgriLife Extension Service, Special Publication: College Station, TX, USA, 2012; pp. 13–24. [Google Scholar]
- Latto, A.; Hagen, A.; Berg, R. National Hurricane Center Tropical Cyclone Report: Hurricane Isaias (AL092020).; National Oceanic And Atmospheric Administration and the National Weather Service: Washington, DC, USA, 2021; pp. 1–84. [Google Scholar]
- Pasch, R.J.; Reinhart, B.J.; Alaka, L. National Hurricane Center Tropical Cyclone Report: Hurricane Fiona (AL072022), 14–23 September 2022; National Oceanic And Atmospheric Administration and the National Weather Service: Washington, DC, USA, 2022; pp. 1–60. [Google Scholar]
- NWS Internet Services Team Advanced Hydrologic Prediction Service. Available online: https://water.weather.gov/precip/index.php (accessed on 20 October 2023).
- Crooks, J.A. Characterizing Ecosystem-Level Consequences of Biological Invasions: The Role of Ecosystem Engineers. Oikos 2002, 97, 153–166. [Google Scholar] [CrossRef]
- Schirmel, J.; Bundschuh, M.; Entling, M.; Kowarik, I.; Buchholz, S. Impacts of Invasive Plants on Resident Animals across Ecosystems, Taxa, and Feeding Types: A Global Assessment. Glob. Change Biol. 2016, 22, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Linders, T.E.W.; Schaffner, U.; Eschen, R.; Abebe, A.; Choge, S.K.; Nigatu, L.; Mbaabu, P.R.; Shiferaw, H.; Allan, E. Direct and Indirect Effects of Invasive Species: Biodiversity Loss Is a Major Mechanism by Which an Invasive Tree Affects Ecosystem Functioning. J. Ecol. 2019, 107, 2660–2672. [Google Scholar] [CrossRef]
- Jones, H.P.; Schmitz, O.J. Rapid Recovery of Damaged Ecosystems. PLOS ONE 2009, 4, e5653. [Google Scholar] [CrossRef] [PubMed]
- Tanentzap, A.J.; Burrows, L.E.; Lee, W.G.; Nugent, G.; Maxwell, J.M.; Coomes, D.A. Landscape-Level Vegetation Recovery from Herbivory: Progress after Four Decades of Invasive Red Deer Control. J. Appl. Ecol. 2009, 46, 1064–1072. [Google Scholar] [CrossRef]
- Prior, K.M.; Adams, D.C.; Klepzig, K.D.; Hulcr, J. When Does Invasive Species Removal Lead to Ecological Recovery? Implications for Management Success. Biol. Invasions 2018, 20, 267–283. [Google Scholar] [CrossRef]
- Martin, G.D.; Coetzee, J.A.; Weyl, P.S.; Parkinson, M.C.; Hill, M.P. Biological Control of Salvinia Molesta in South Africa Revisited. Biol. Control 2018, 125, 74–80. [Google Scholar] [CrossRef]
- Pieterse, A.H.; Kettunen, M.; Diouf, S.; Ndao, I.; Sarr, K.; Tarvainen, A.; Hellsten, S. Effective Biological Control of Salvinia Molesta in the Senegal River by Means of the Weevil Cyrtobagous Salviniae. AMBIO J. Hum. Environ. 2003, 32, 458–462. [Google Scholar] [CrossRef]
- Sullivan, P.R.; Postle, L.A.; Julien, M. Biological Control of Salvinia Molesta by Cyrtobagous Salviniae in Temperate Australia. Biol. Control 2011, 57, 222–228. [Google Scholar] [CrossRef]
- Room, P.M.; Harley, K.L.S.; Forno, T.W.; Sands, D.P.A. Successful Biological Control of the Floating Weed Salvinia. Nature 1981, 294, 78–80. [Google Scholar] [CrossRef]
- Tipping, P.W.; Martin, M.R.; Center, T.D.; Davern, T.M. Suppression of Salvinia Molesta Mitchell in Texas and Louisiana by Cyrtobagous Salviniae Calder and Sands. Aquat. Bot. 2008, 88, 196–202. [Google Scholar] [CrossRef]
- Forno, I.W.; Sands, D.P.A.; Sexton, W. Distribution, Biology and Host Specificity of Cyrtobagous Singularis Hustache (Coleoptera: Curculionidae) for the Biological Control of Salvinia Molesta. Bull. Entomol. Res. 1983, 73, 85–95. [Google Scholar] [CrossRef]
- Micinski, S.; Fitzpatrick, B.J.; Ferro, M.L.; Johnson, S.J.; Johnson, B.; Williams, S. Flight Activity of Cyrtobagous Salvinia Calder and Sands in Louisiana. Southwest. Entomol. 2016, 41, 313–320. [Google Scholar] [CrossRef]
- Sands, D.P.A.; Schotz, M.; Bourne, A.S. The Feeding Characteristics and Development of Larvae of a Salvinia Weevil Cyrtobagous Sp. Entomol. Exp. Appl. 1983, 34, 291–296. [Google Scholar] [CrossRef]
- Wahl, C.F.; Díaz, R.; Kaller, M. Optimising Berlese Funnel Extraction for Population Estimates of Adult Cyrtobagous Salviniae from Salvinia Molesta. Biocontrol Sci. Technol. 2022, 32, 1232–1247. [Google Scholar] [CrossRef]
- Masifwa, W.F.; Okello, W.; Ochieng, H.; Ganda, E. Phosphorus Release from Decomposing Water Hyacinth and Effects of Decomposition on Water Quality. Uganda J. Agric. Sci. 2004, 9, 389–395. [Google Scholar]
- Wu, Y.; Wen, Y.; Zhou, J.; Wu, Y. Phosphorus Release from Lake Sediments: Effects of pH, Temperature and Dissolved Oxygen. J. Civ. Eng. 2014, 18, 323–329. [Google Scholar] [CrossRef]
- Cary, P.R.; Weerts, P.G. Growth of Salvinia Molesta as Affected by Water Temperature and Nutrition. III. Nitrogen-Phosphorus Interactions and Effect of pH. Aquat. Bot. 1984, 19, 171–182. [Google Scholar] [CrossRef]
- Hupfer, M.; Lewandowski, J. Oxygen Controls the Phosphorus Release from Lake Sediments–a Long-lasting Paradigm in Limnology. Int. Rev. Hydrobiol. 2008, 93, 415–432. [Google Scholar] [CrossRef]
- Sondergaard, M.; Jensen, P.J.; Jeppesen, E. Retention and Internal Loading of Phosphorus in Shallow, Eutrophic Lakes. Sci. World J. 2001, 1, 427–442. [Google Scholar] [CrossRef]
- Frodge, J.D.; Thomas, G.L.; Pauley, G.B. Sediment Phosphorus Loading beneath Dense Canopies of Aquatic Macrophytes. Lake Reserv. Manag. 1991, 7, 61–71. [Google Scholar] [CrossRef][Green Version]
- Hutchinson, G.E. A Treatise on Limnology: Geography, Physics and Chemistry; Wiley: Hoboken, NJ, USA, 1957. [Google Scholar]
- Buffle, J.; Stumm, W. General Chemistry of Aquatic Systems. In Chemical and Biological Regulation of Aquatic Systems; Buffle, J., De Vitre, R.R., Eds.; CRC: Boca Raton, FL, USA, 1994; pp. 1–42. [Google Scholar]
- Room, P.M. Ecology of a Simple Plant-Herbivore System: Biological Control of Salvinia. Trends Ecol. Evol. 1990, 5, 74–79. [Google Scholar] [CrossRef]
- Bianchini, I.; da Cunha-Santino, M.B. CH4 and CO2 from Decomposition of Salvinia Auriculata Aublet, a Macrophyte with High Invasive Potential. Wetlands 2016, 36, 557–564. [Google Scholar] [CrossRef]
- O’Sullivan, C.; Rounsefell, B.; Grinham, A.; Clarke, W.; Udy, J. Anaerobic Digestion of Harvested Aquatic Weeds: Water Hyacinth (Eichhornia Crassipes), Cabomba (Cabomba Caroliniana) and Salvinia (Salvinia Molesta). Ecol. Eng. 2010, 36, 1459–1468. [Google Scholar] [CrossRef]
- Grasset, C.; Mendonça, R.; Villamor Saucedo, G.; Bastviken, D.; Roland, F.; Sobek, S. Large but Variable Methane Production in Anoxic Freshwater Sediment upon Addition of Allochthonous and Autochthonous Organic Matter. Limnol. Oceanogr. 2018, 63, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Quiñones-Márquez, F. Limnology of Lago Loiza, Puerto Rico; U.S. Geological Survey (USGS): Reston, VA, USA, 1980; pp. 1–130. [Google Scholar]
- Lewis, W.M., Jr. The Thermal Regime of Lake Lanao (Philippines) and Its Theoretical Implications for Tropical Lakes 1. Limnol. Oceanogr. 1973, 18, 200–217. [Google Scholar] [CrossRef]
- Lewis, W.M., Jr. Temperature, Heat, and Mixing in Lake Valencia, Venezuela. Limnol. Oceanogr. 1983, 28, 273–286. [Google Scholar] [CrossRef]
- MacIntyre, S.; Melack, J.M. Vertical and Horizontal Transport in Lakes: Linking Littoral, Benthic, and Pelagic Habitats. J. N. Am. Benthol. Soc. 1995, 14, 599–615. [Google Scholar] [CrossRef]
- Crowe, S.A.; O’Neill, A.H.; Katsev, S.; Hehanussa, P.; Haffner, G.D.; Sundby, B.; Fowle, D.A. The Biogeochemistry of Tropical Lakes: A Case Study from Lake Matano, Indonesia. Limnol. Oceanogr. 2008, 53, 319–331. [Google Scholar] [CrossRef]
- Fan, J.; Morris, G.L. Reservoir Sedimentation Handbook: Design and Management of Dams, Reservoirs, and Watersheds for Sustainable Use; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Carpenter, S.R.; Lodge, D.M. Effects of Submersed Macrophytes on Ecosystem Processes. Aquat. Bot. 1986, 26, 341–370. [Google Scholar] [CrossRef]
- Scheffer, M.; Szabo, S.; Gragnani, A.; van Nes, E.H.; Rinaldi, S.; Kautsky, N.; Norberg, J.; Roijackers, R.M.M.; Franken, R.J.M. Floating Plant Dominance as a Stable State. Proc. Natl. Acad. Sci. USA 2003, 100, 4040–4045. [Google Scholar] [CrossRef] [PubMed]
- Briese, D.T. Classical Biological Control. In Australian weed management systems; R.G. and F.J. Richardson: Meredith, Australia, 2000; Volume 2, pp. 161–192. [Google Scholar]


| Depth Zone | Year | Specific Conductance (μS/cm) | Dissolved Oxygen (mg L−1) | pH | Water Temperature (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (±SE) | N | Mean (±SE) | N | Mean (±SE) (Range) | N | Mean (±SE) | ||
| Epilimnion | 2019 | 6 | 178.4 (±15.4) a | 6 | 1.7 (±1.2) a | 6 | 6.53 (±0.08) a (6.34–6.8) | 6 | 25.8 (±0.4) a |
| 2020 | 18 | 285.7 (±2.0) b | 24 | 3.4 (±0.4) a | 24 | 6.72 (±0.03) a (6.47–6.96) | 24 | 28.5 (±0.3) b | |
| 2021 | 24 | 282.6 (±2.9) b | 30 | 2.8 (±0.3) a | 30 | 6.91 (±0.04) a (6.51–7.29) | 30 | 27.8 (±0.2) b | |
| 2022 | 54 | 291.8 (±3.9) b | 54 | 5.4 (±0.4) b | 54 | 7.73 (±0.06) b (7.01–8.41) | 54 | 28.2 (±0.2) b | |
| Thermocline | 2019 | 6 | 212.6 (±0.4) b | 6 | 0.0 (±0.0) | 6 | 6.43 (±0.03) a (6.35–6.51) | 6 | 23.9 (±0.1) a |
| 2020 | 18 | 272.4 (±5.7) b | 24 | 0.3 (±0.1) | 24 | 6.40 (±0.03) a (6.09–6.6) | 24 | 26.7 (±0.2) b | |
| 2021 | 24 | 325.5 (±4.5) c | 30 | 0.9 (±0.3) | 30 | 6.84 (±0.05) b (6.27–7.5) | 30 | 26.9 (±0.1) b | |
| 2022 | 53 | 295.3 (±5.7) b | 53 | 1.0 (±0.2) | 53 | 7.23 (±0.04) c (6.76–7.96) | 53 | 27.1 (±0.2) a | |
| Hypolimnion | 2019 | 17 | 225.9 (±3.8) a | 17 | 0.0 (±0.00) | 17 | 6.43 (±0.01) a (6.36–6.49) | 17 | 23.5 (±0.0) a |
| 2020 | 27 | 344.3 (±7.3) b | 36 | 0.0 (±0.00) | 36 | 6.19 (±0.02) a (5.97–6.46) | 36 | 24.9 (±0.1) b | |
| 2021 | 17 | 331.0 (±4.8) b | 24 | 0.1 (±0.01) | 24 | 6.57 (±0.05) ab (6.15–6.95) | 24 | 26.1 (±0.1) c | |
| 2022 | 37 | 322.1 (±11.8) b | 37 | 0.0 (±0.01) | 37 | 6.97 (±0.05) b (6.24–7.63) | 37 | 25.5 (±0.2) bc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-López, X.A.; Ortiz-Zayas, J.R.; Díaz, R.; Castro-Jiménez, A.; Wahl, C.F. Limnological Response of Las Curias Reservoir, San Juan, Puerto Rico: Successful Management of the Invasive Aquatic Fern, Salvinia molesta. Water 2023, 15, 3966. https://doi.org/10.3390/w15223966
García-López XA, Ortiz-Zayas JR, Díaz R, Castro-Jiménez A, Wahl CF. Limnological Response of Las Curias Reservoir, San Juan, Puerto Rico: Successful Management of the Invasive Aquatic Fern, Salvinia molesta. Water. 2023; 15(22):3966. https://doi.org/10.3390/w15223966
Chicago/Turabian StyleGarcía-López, Xavier A., Jorge R. Ortiz-Zayas, Rodrigo Díaz, Aurelio Castro-Jiménez, and Charles F. Wahl. 2023. "Limnological Response of Las Curias Reservoir, San Juan, Puerto Rico: Successful Management of the Invasive Aquatic Fern, Salvinia molesta" Water 15, no. 22: 3966. https://doi.org/10.3390/w15223966
APA StyleGarcía-López, X. A., Ortiz-Zayas, J. R., Díaz, R., Castro-Jiménez, A., & Wahl, C. F. (2023). Limnological Response of Las Curias Reservoir, San Juan, Puerto Rico: Successful Management of the Invasive Aquatic Fern, Salvinia molesta. Water, 15(22), 3966. https://doi.org/10.3390/w15223966





