Hydrogeochemical Characterization of Groundwater at the Boundaries of Three Aquifers in Central México
Abstract
1. Introduction
2. Materials and Methods
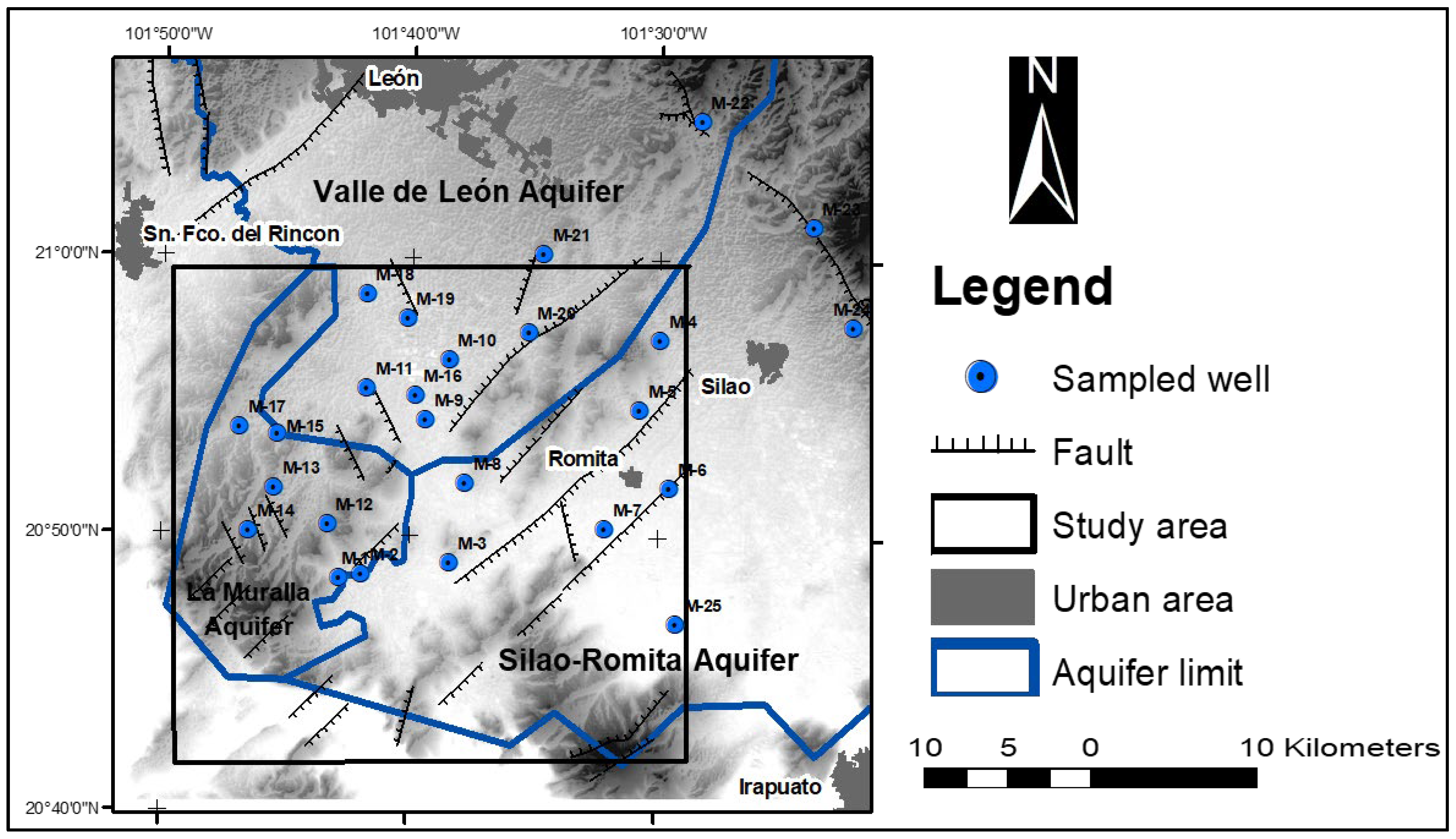
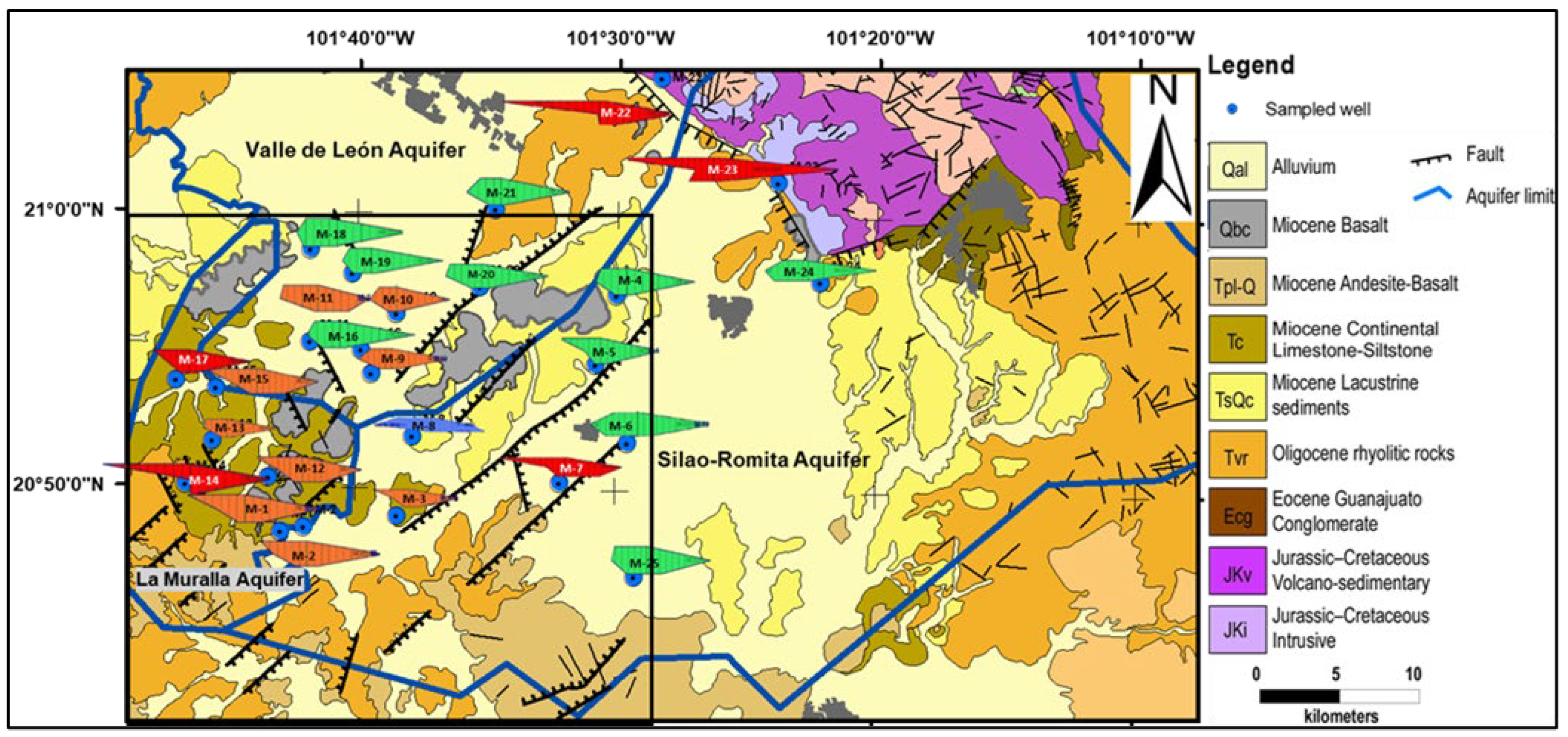
2.1. Study Area
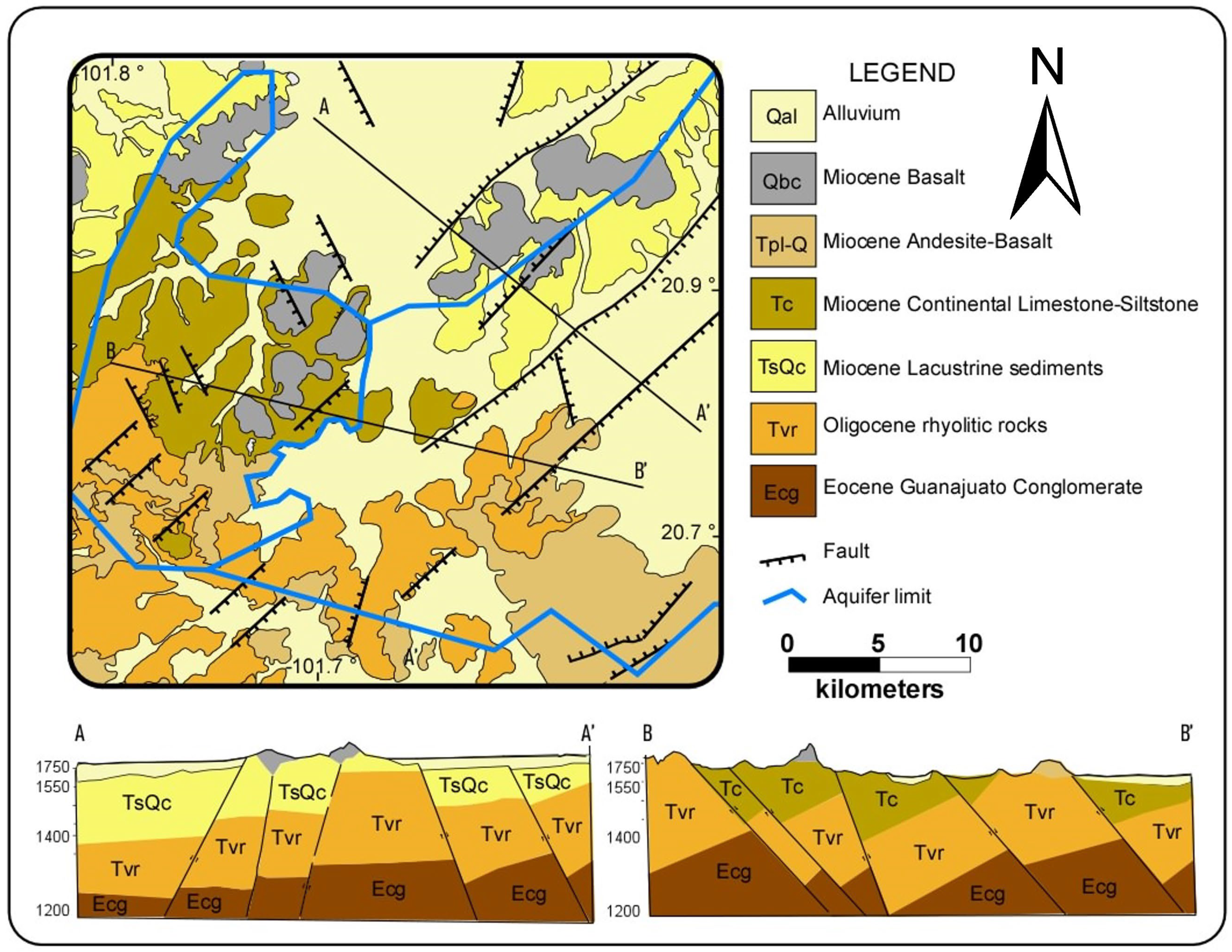
2.2. Geology
2.3. Hydrogeology
2.4. Inventory of Groundwater Exploitations
2.5. Groundwater Quality Analysis and Sampling Program
2.6. Isotopic Analysis
2.7. Statistical Data Analysis
3. Results and Discussion
3.1. Hydrogeochemistry
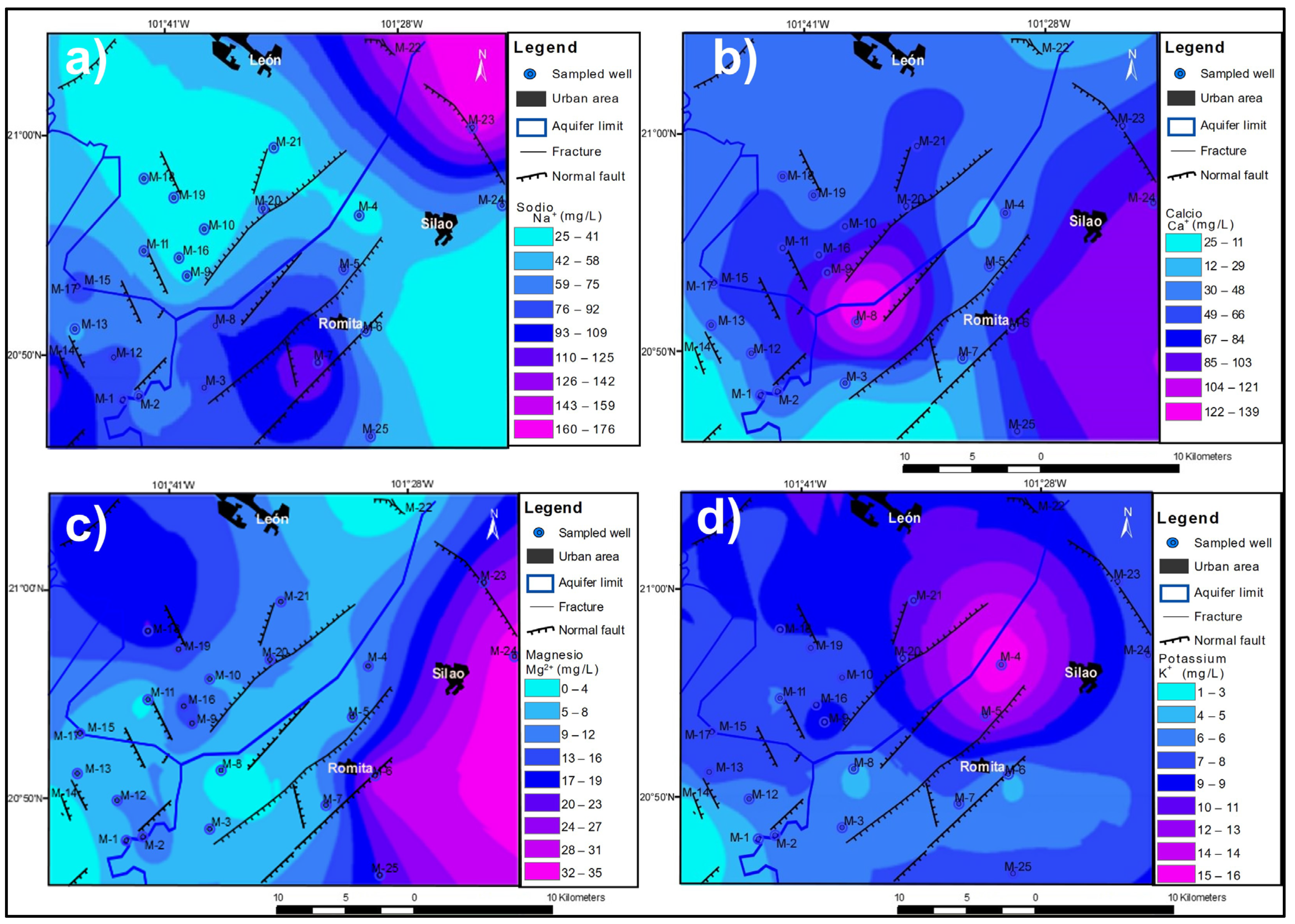
3.2. Spatial Distribution of Major Ions
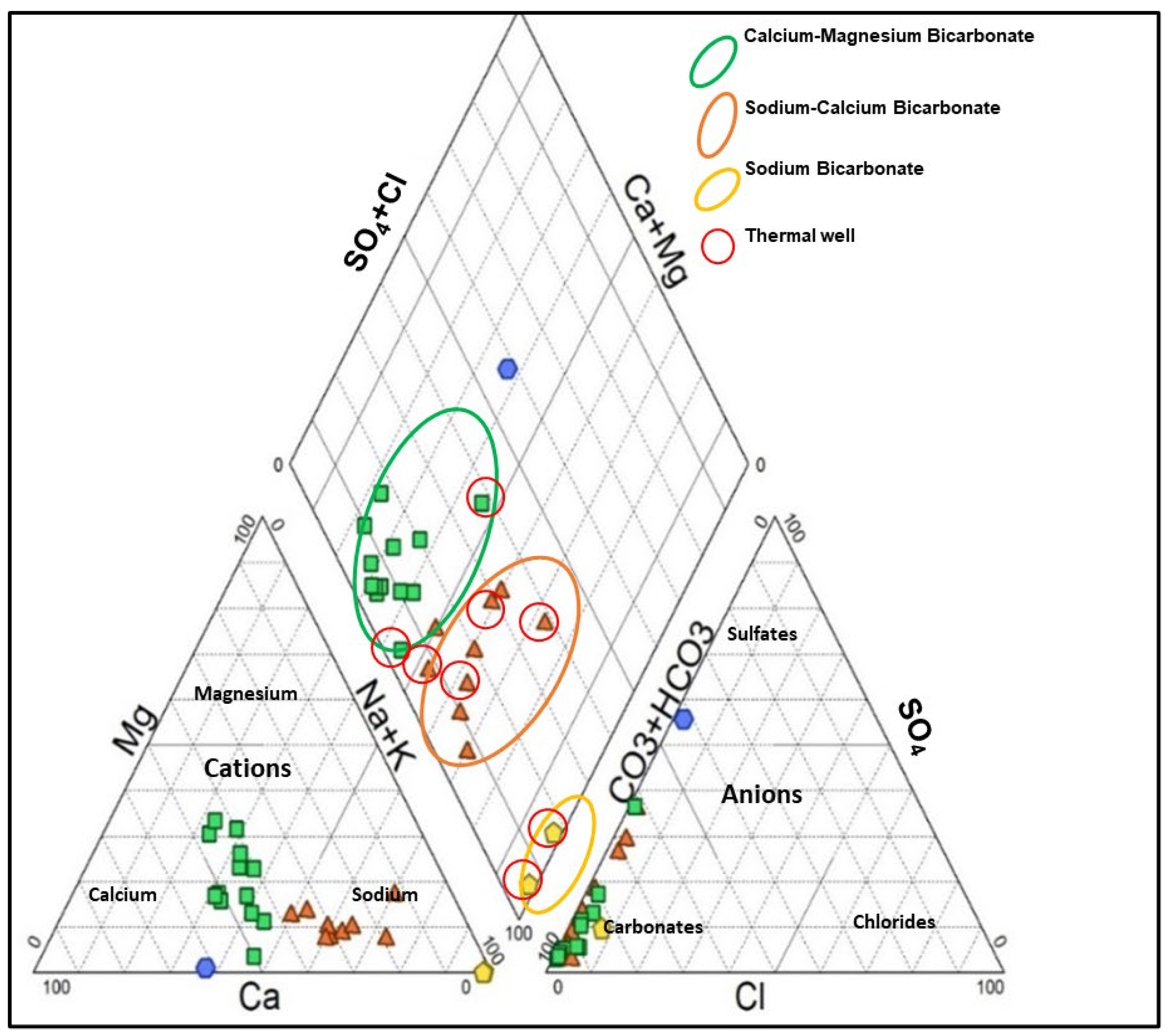
3.3. Groundwater Types
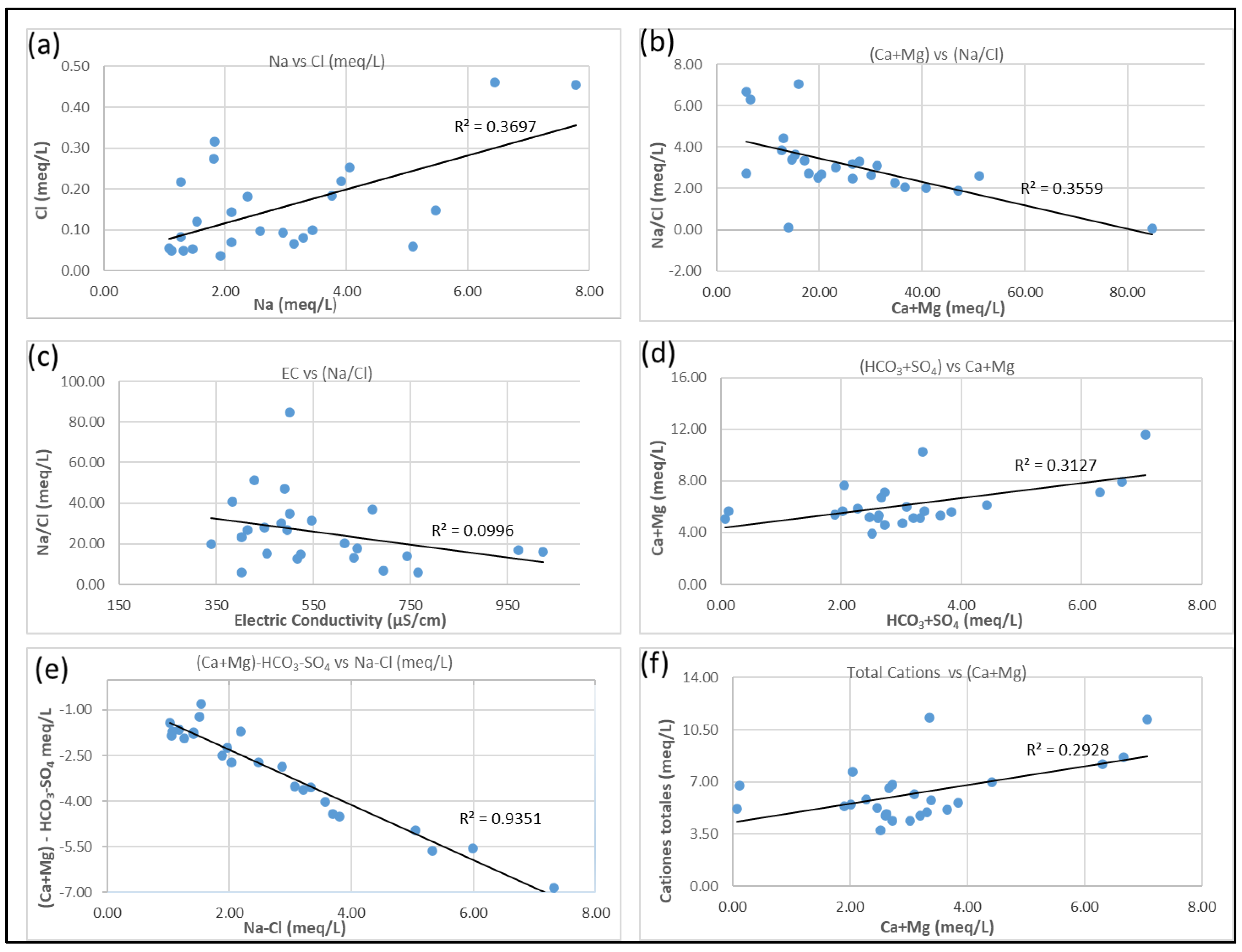
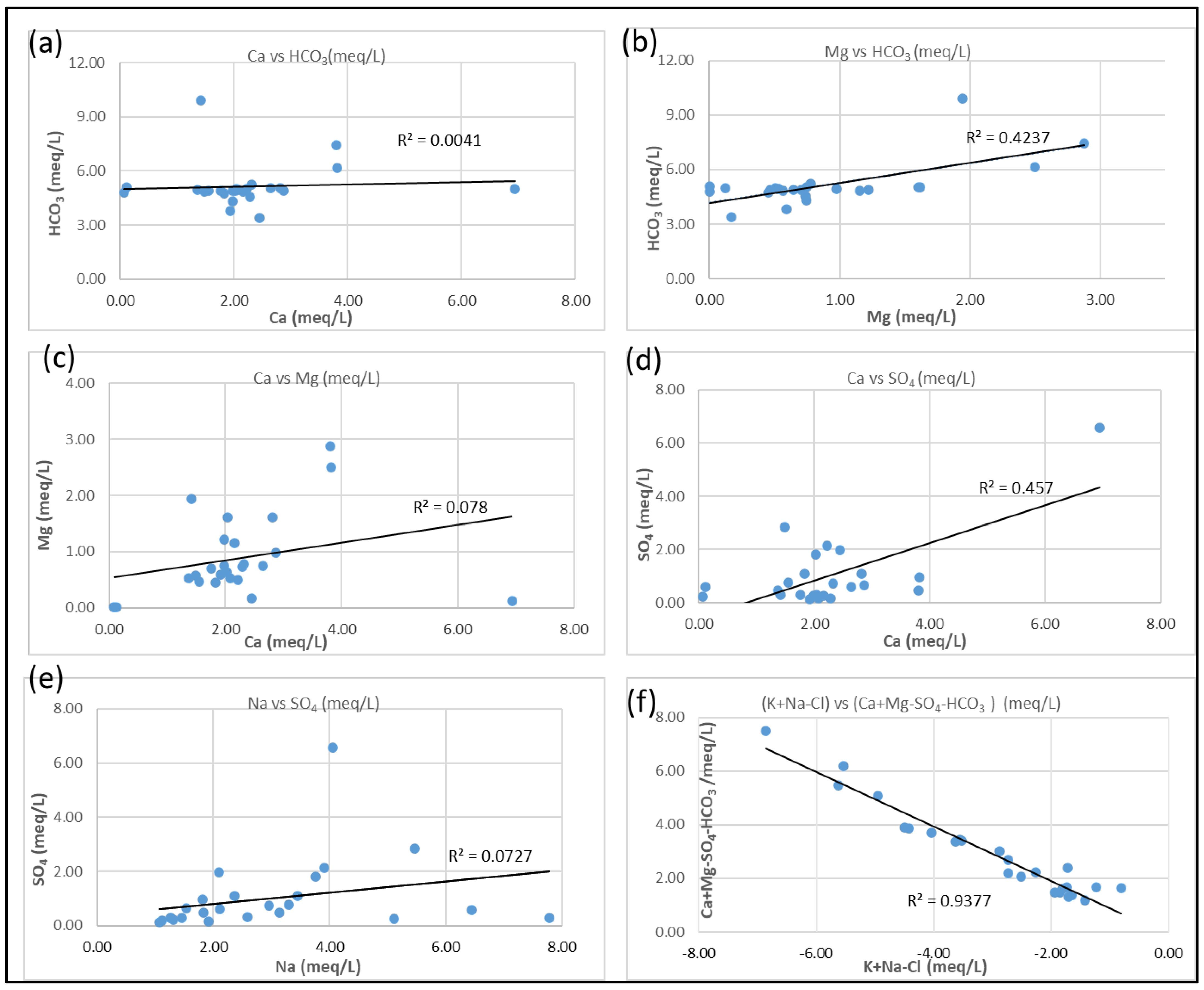
3.4. Hydrogeochemical Processes
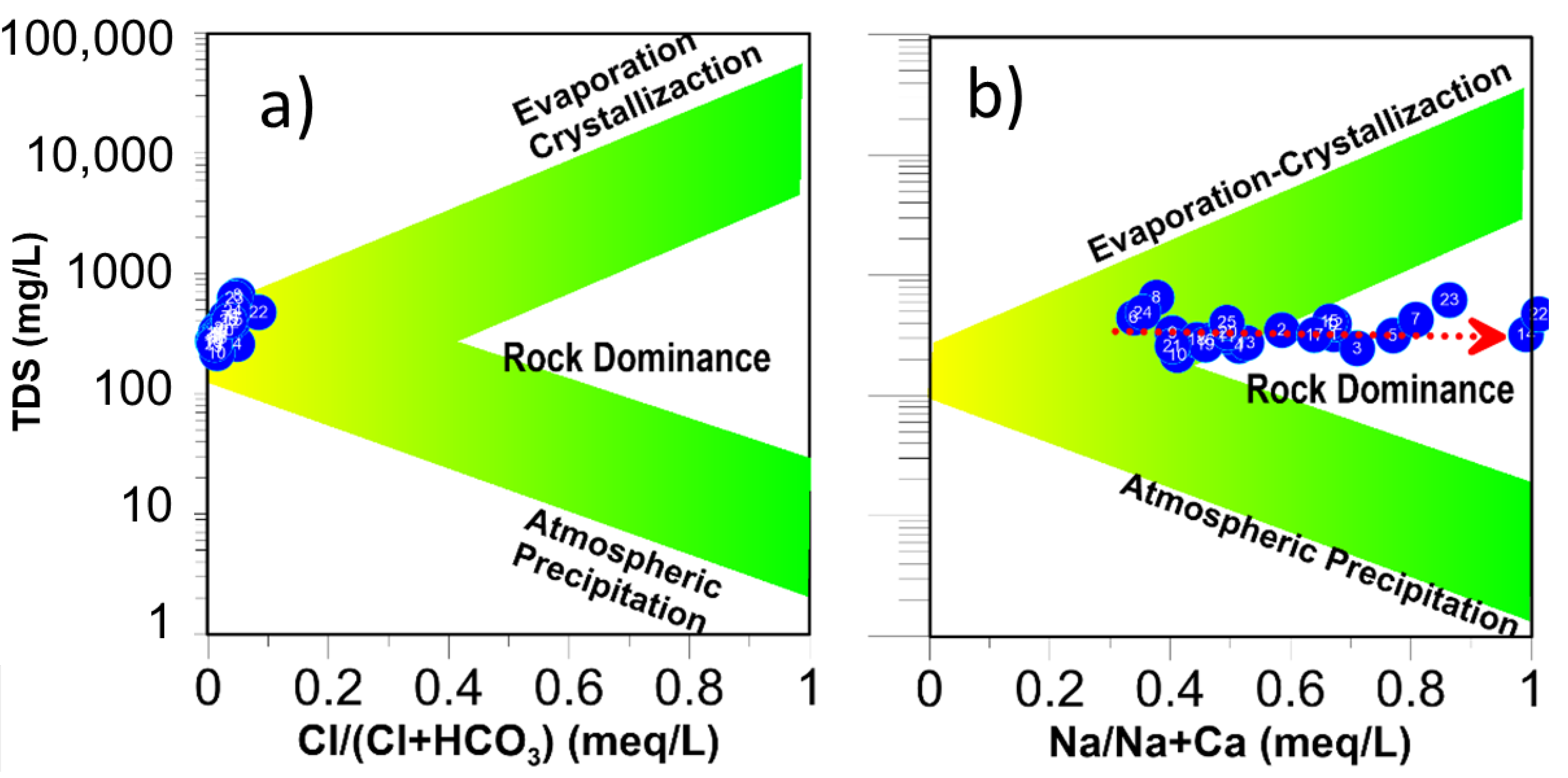
3.5. Evaporation
3.6. Mineral Weathering
3.7. Flow System Classification
3.8. Statistical Analysis
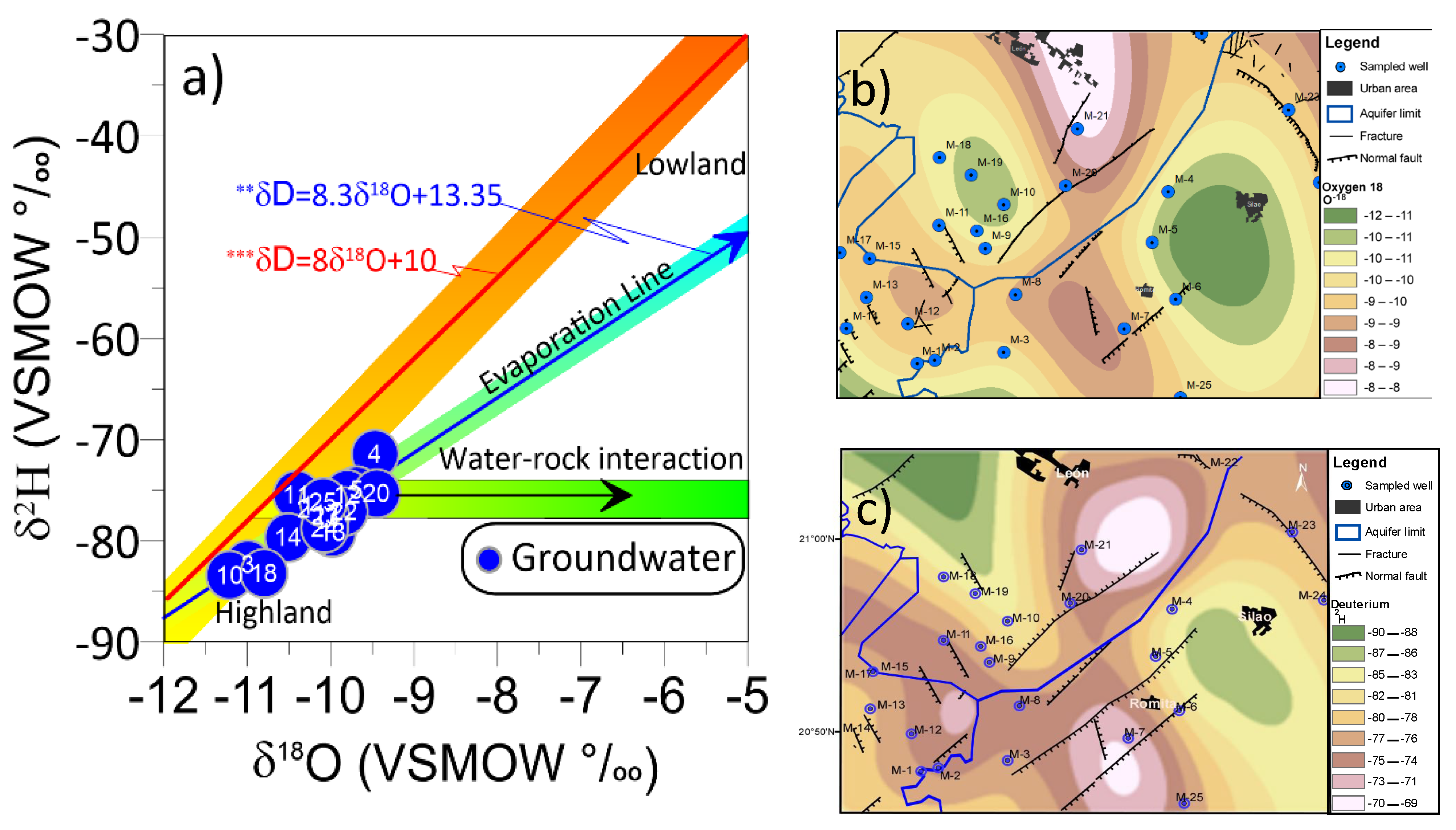
3.9. Deuterium and Oxygen Isotopic Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNESCO. Informe mundial de las Naciones Unidas sobre el desarrollo de los recursos hídricos 2022: Aguas subterráneas: Hacer visible el recurso invisible. Organ. Nac. Unidas Educ. Cienc. Cult. 2022, 1–12. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000380726_spa?fbclid=IwAR37K3mY5OKdAaGGOS_9Foou2Kpk9S87EnDLf_MztKU0uGHVDjTKFmcwGPk (accessed on 20 July 2023).
- Valencia, J. Importancia de las Aguas Subterráneas. Apus Agua. 2014. Available online: https://apusdelagua.blogspot.com/2014/11/importancia-de-las-aguas-subterraneas.html (accessed on 25 July 2023).
- Wang, S.; Chen, J.; Zhang, S.; Zhang, X.; Chen, D.; Zhou, J. Hydrochemical evolution characteristics, controlling factors, and high nitrate hazards of shallow groundwater in a typical agricultural area of Nansi Lake Basin, North China. Environ. Res. 2023, 223, 115430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, P.; He, J.; Liu, D.; Wang, M.; Wang, M.; Xia, S. Hydrochemical Characteristics, Water Quality, and Evolution of Groundwater in Northeast China. Water 2023, 15, 2669. [Google Scholar] [CrossRef]
- Paíz, R.; Low-Calle, J.F.; Molina-Estrada, A.G.; Gil-Villalba, S.; Condesso de Melo, M.T. Combining spectral analysis and geochemical tracers to investigate surface water–groundwater interactions: A case study in an intensive agricultural setting (southern Guatemala). Sci. Total Environ. 2023, 899, 165578. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, H.; Wang, H.; Chen, J.; Ren, W.; Yang, F. Assessment of background levels and pollution sources for arsenic and fluoride in the phreatic and confined groundwater of Xi’an city, Shaanxi, China. Environ. Sci. Pollut. Res. Int. 2020, 27, 34702–34714. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.C.; Bauer, J.; Börsig, N.; Ho, J.; Vu Huu, L.; Tran Viet, H.; Dörr, F.; Norra, S. Groundwater Use Habits and Environmental Awareness in Ca Mau Province, Vietnam: Implications for Sustainable Water Resource Management. Environ. Chall. 2023, 13, 100742. [Google Scholar] [CrossRef]
- Cerasoli, S.; Porporato, A. California’s groundwater overdraft: An environmental Ponzi scheme? J. Hydrol. 2023, 617, 129081. [Google Scholar] [CrossRef]
- González, V.P.; Avilés, R.M.; Anguiano, J.H.H.; Knappett, P.S.; Martinez, J.L.M.; Alquiza, M.D.J.P.; Naves, A.; Bian, J.; Liu, J.; González, L.M.R.; et al. Influence of geological faults on dissolved arsenic concentrations in an overexploited aquifer with shallow geothermal heat. Appl. Geochem. 2022, 144, 25–28. [Google Scholar] [CrossRef]
- Absametov, M.; Sagin, J.; Adenova, D.; Smolyar, V.; Murtazin, E. Assessment of the groundwater for household and drinking purposes in central Kazakhstan. Groundw. Sustain. Dev. 2023, 21, 100907. [Google Scholar] [CrossRef]
- Saldaña-Robles, A.; Saldana-Robles, N.; Saldaña-Robles, A.; Ana-Zanor, G.; Ruiz-Aguilar, G.M.; Gutiérrez-Vaca, C. Phosphorus effect on arsenic acumulation of barley (Hordeum vulgare L.) through irrigation with contaminated water. Agrociencia 2018, 52, 407–418. [Google Scholar]
- Rodríguez, R.; Morales-Arredondo, I.; Rodríguez, I. Geological differentiation of groundwater threshold concentrations of arsenic, vanadium and fluorine in El Bajio Guanajuatense, Mexico. Geofis. Int. 2016, 55, 5–15. [Google Scholar] [CrossRef]
- Morales-Arredondo, J.I.; Armienta, M.A.; Rodríguez, R. Estimation of exposure to high fluoride contents in groundwater supply in some villages in Guanajuato, Mexico. Tecnol. Ciencias Agua 2018, 9, 156–179. [Google Scholar] [CrossRef]
- Piña González, V.; Fuentes Hernández, V.; Bian, J.; Zhao, Z.; Li, Y. The presence of total inorganic arsenic (iAs) and fluoride (F−) in the groundwater of the center-west of the state of Guanajuato: A hydro-chemical and spatial distribution analysis. Sustain. Water Resour. Manag. 2022, 8, 18. [Google Scholar] [CrossRef]
- Morán-Ramírez, J.; Ledesma-Ruiz, R.; Mahlknecht, J.; Ramos-Leal, J.A. Rock-water interactions and pollution processes in the volcanic aquifer system of Guadalajara, Mexico, using inverse geochemical modeling. Appl. Geochem. 2016, 68, 79–94. [Google Scholar] [CrossRef]
- Morales-Arredondo, I.; Flores-Ocampo, I.Z.; Armienta, M.A.; Morán-Ramírez, J.; Hernández-Hernández, M.A.; Landa-Arreguin, J.F. Identificacion de las fuentes de nitratos mediante métodos hidrogeoquimicos e isotópicos en el agua subterránea del Bajio Guanajuatense. Geofis. Int. 2020, 58, 169–194. [Google Scholar] [CrossRef]
- Ramos-Leal, J.A.; Durazo, J.; González-Morán, T.; Juárez-Sánchez, F.; Cortés-Silva, A.; Johannesson, K.H. Evidencias hidrogeoguímicas de mezcla de flujos regionales en el acuífero de La Muralla, Guanajuato. Rev. Mex. Cienc. Geol. 2007, 24, 293–305. [Google Scholar]
- Cloutier, V.; Lefebvre, R.; Therrien, R.; Savard, M.M. Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J. Hydrol. 2008, 353, 294–313. [Google Scholar] [CrossRef]
- Sajil Kumar, P.J.; James, E.J. Identification of hydrogeochemical processes in the Coimbatore district, Tamil Nadu, India. Hydrol. Sci. J. 2016, 61, 719–731. [Google Scholar] [CrossRef]
- Barrera-de-Calderón, M.; Garfias, J.; Martel, R.; Salas-García, J. Controles geológicos en el flujo de agua subterránea y distribución de volúmenes de extracción asociados a una estructura volcánica compleja. Boletín Soc. Geol. Mex. 2021, 73, A170820. [Google Scholar] [CrossRef]
- Arévalo, L.A.P.; Joel, J.; Rivera, C. Definición de zonas de recarga y descarga de agua subterránea a partir de indicadores superficiales: Centro-sur de la Mesa Central, México. Investigaciones. Geográficas 2013, 81, 18–32. [Google Scholar]
- Erwin, R. Landforms of Mexico, Scale 1:3,000,000; Office of Naval Research Geography Branch: Cambridge, MA, USA, 1959; Volume 1. [Google Scholar]
- Comisión Nacional del Agua. Actualización de la Disponibilidad Media Anual de Agua en el Acuífero Silao-Romita (1110), Estado de Guanajuato; Comisión Nacional del Agua: Ciudad de México, México, 2020. [Google Scholar]
- Comisión Nacional del Agua. Actualización de la Disponibilidad Media Anual de Agua en el Acuífero la Muralla; Comisión Nacional del Agua: Ciudad de México, México, 2015. [Google Scholar]
- Río Varela, P.; Nieto-Samaniego, Á.F.; Alaniz-Álvarez, S.A.; Ángeles-Moreno, E.; de Jesús Escalona-Alcázar, F.; del Pilar-Martínez, A. Geología y estructura de las sierras de Guanajuato y Codornices, Mesa Central, México. Boletín Soc. Geol. Mex. 2020, 72, 1–20. [Google Scholar] [CrossRef]
- Comisión Estatal del agua de Guanajuato. Integración de Información Geológica-Geofísica al Sistema de Integración Geográfica (SIG); Comisión Estatal del agua de Guanajuato: Guanajuato, México, 2001. [Google Scholar]
- Nieto-Samaniego, A.F.; Del Pilar-Martínez, A.; Suárez-Arias, A.M.; Angeles-Moreno, E.; Alaniz-Álvarez, S.A.; Levresse, G.; Xu, S.; Olmos-Moya, M.J.P.; Báez-López, J.A. Una revisión de la geología y evolución tectónica cenozoicas de la Mesa Central de México. Rev. Mex. Cienc. Geol. 2023, 40, 187–213. [Google Scholar] [CrossRef]
- Miranda-Avilés, R.; Puy-Alquiza, M.J.; Omaña, L.; Loza-Aguirre, I. Los depósitos clásticos pos-laramide de la sierra de Guanajuato: Implicaciones de su composición en la evolución tectono-sedimentaria y paleogeográfica. Estud. Geol. 2016, 72, e058. [Google Scholar] [CrossRef][Green Version]
- Botero-Santa, P.A.; Alaniz-Álvarez, S.A.; Nieto-Samaniego, Á.F.; López-Martínez, M.; Levresse, G.; Xu, S.; Ortega-Obregón, C. Origen y desarrollo de la cuenca El Bajío en el sector central de la Faja Volcánica Transmexicana. Rev. Mex. Cienc. Geol. 2015, 32, 84–98. [Google Scholar]
- Comisión Estatal del Agua de Guanajuato. Estudio Geohidrológico Acuífero la Muralla; Comisión Estatal del Agua de Guanajuato: Guanajuato, México, 2003. [Google Scholar]
- NOM-014-SSA1-1993; Secretaría de Salud Procedimientos Sanitarios para el Muestreo de Agua para Uso y Consumo Humano en Sistemas de Abastecimiento de Agua Públicos y Privados. Fondo para la Comunicación y la Educación Ambiental: Mexico City, México, 1993; pp. 1–8.
- American Society for Testing and Materials. Annual Book of ASTM Standards, Section 11, Water and Environmental Technology: Volume 11.01: Water (I); American Society for Testing and Materials: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Wali, S.U.; Umar, K.J.; Abubakar, S.D.; Ifabiyi, I.P.; Dankani, I.M.; Shera, I.M.; Yauri, S.G. Hydrochemical Characterization of Shallow and Deep Groundwater in Basement Complex Areas of Southern Kebbi State, Sokoto Basin, Nigeria. Appl. Water Sci. 2019, 9, 169. [Google Scholar] [CrossRef]
- Morris, P.J.; Baird, A.J.; Eades, P.A.; Surridge, B.W.J. Controls on Near-Surface Hydraulic Conductivity in a Raised Bog. Water Resour. Res. 2019, 55, 1531–1543. [Google Scholar] [CrossRef]
- Ríos-Vásquez, L.C.; Colegial-Gutiérrez, J.D.; Collazo-Caraballo, M.P. Caracterización hidrogeoquímica de las aguas subterráneas en la ciudad de Minas, departamento de Lavalleja, Uruguay. Boletín Geol. 2023, 45, 103–117. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Zhan, H.; Chen, X.; Liu, M.; Wang, M. Identification of hydrogeochemical processes and transport paths of a multi-aquifer system in closed mining regions. J. Hydrol. 2020, 589, 125344. [Google Scholar] [CrossRef]
- Poznanović Spahić, M.; Marinković, G.; Spahić, D.; Sakan, S.; Jovanić, I.; Magazinović, M.; Obradović, N. Water–Rock Interactions across Volcanic Aquifers of the Lece. Water 2023, 15, 3653. [Google Scholar] [CrossRef]
- Jacks, G.; Sharma, V.P. Geochemistry of Calcic horizons in relation to hillslope processes Southern India. Geoderma 1995, 67, 203–214. [Google Scholar] [CrossRef]
- Jalali, M. Major ion chemistry of groundwaters in the Bahar area, Hamadan, western Iran. Environ. Geol. 2005, 47, 763–772. [Google Scholar] [CrossRef]
- Mifflin, M.D. Delineation of Ground-Water Flow Systems in Nevada. Ph.D. Thesis, University of Nevada, Reno, NV, USA, 1968; 112p. Available online: http://scholarworks.unr.edu:8080/bitstream/handle/11714/1519/Mackay442_Mifflin.pdf?sequence=1&isAllowed=y (accessed on 30 November 2022).
- Toth, J. Las aguas subterráneas como agente geológico: Causas, procesos y manifestaciones. Bol. Geol. Min. 2000, 111, 9–25. [Google Scholar]
- Landa-Arreguín, J.F.A.; Villanueva-Estrada, R.E.; Morales-Arredondo, J.I.; Salvatore, I.; Rodríguez-Díaz, A.A.; Pérez-Martínez, I.; Rocha-Miller, R.; Bernard-Romero, R.; Tello-Hinojosa, E. Hydrogeochemistry and isotopic assessment of a new geothermal prospect in rancho Nuevo (Guanajuato-Central Mexico). J. Geochem. Explor. 2023, 253, 107294. [Google Scholar] [CrossRef]




| ID | Coordinates | Physico-Chemical Parameters | Cations (mg/L) | Anions (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Latitude | Longitude | PH | T °C | EC (µS/cm) | TDS (mg/L) | Ca2+ | Mg2+ | Na+ | K+ | ||||
| M-1 | 20°48′24″ | 101°42′47″ | 7.55 | 30.50 | 502.00 | 321.28 | 36.67 | 5.49 | 79.12 | 4.04 | 290.00 | 3.51 | 51.77 |
| M-2 | 20°48′34″ | 101°41′53″ | 7.56 | 29.10 | 547.00 | 350.08 | 46.49 | 9.42 | 67.93 | 5.43 | 319.00 | 3.34 | 35.28 |
| M-3 | 20°49′01″ | 101°38′20″ | 7.61 | 28.70 | 384.00 | 245.76 | 31.08 | 5.67 | 75.70 | 6.00 | 298.00 | 2.86 | 36.36 |
| M-4 | 20°57′07″ | 101°29′58″ | 7.58 | 25.00 | 402.00 | 257.28 | 39.76 | 9.01 | 29.26 | 15.80 | 262.00 | 7.70 | 13.14 |
| M-5 | 20°54′36″ | 101°30′46″ | 7.61 | 25.80 | 491.00 | 314.24 | 27.33 | 6.49 | 72.07 | 12.99 | 302.00 | 2.36 | 22.63 |
| M-6 | 20°51′47″ | 101°29′30″ | 7.34 | 23.80 | 695.00 | 444.80 | 76.40 | 30.34 | 41.68 | 3.88 | 375.00 | 9.68 | 46.25 |
| M-7 | 20°50′17″ | 101°32′07″ | 7.51 | 30.90 | 671.00 | 429.44 | 29.75 | 6.90 | 125.78 | 6.01 | 296.00 | 5.28 | 135.83 |
| M-8 | 20°51′54″ | 101°37′46″ | 7.28 | 25.00 | 1023.00 | 654.72 | 139.01 | 1.52 | 93.24 | 3.63 | 304.00 | 8.97 | 316.01 |
| M-9 | 20°54′10″ | 101°39′22″ | 7.29 | 21.40 | 517.00 | 330.88 | 57.43 | 11.84 | 35.33 | 9.57 | 300.00 | 4.28 | 31.23 |
| M-10 | 20°56′21″ | 101°38′27″ | 7.70 | 24.50 | 340.00 | 217.60 | 38.60 | 7.17 | 24.83 | 6.27 | 232.00 | 1.94 | 6.28 |
| M-11 | 20°55′17″ | 101°41′46″ | 7.58 | 38.60 | 484.00 | 309.76 | 49.09 | 2.09 | 48.30 | 5.76 | 206.00 | 2.48 | 94.66 |
| M-12 | 20°50′ 22″ | 101°43′18″ | 7.49 | 31.40 | 614.00 | 392.96 | 40.62 | 7.82 | 86.45 | 5.16 | 298.00 | 6.55 | 87.21 |
| M-13 | 20°51′38″ | 101°45′27″ | 7.28 | 30.40 | 428.00 | 273.92 | 41.61 | 6.45 | 44.15 | 7.23 | 302.00 | 1.33 | 7.79 |
| M-14 | 20°50′04″ | 101°46′27″ | 9.03 | 41.30 | 502.00 | 321.28 | 1.49 | 0.06 | 117.34 | 1.24 | 292.00 | 2.13 | 11.56 |
| M-15 | 20°53′34″ | 101°45′20″ | 7.46 | 28.70 | 641.00 | 410.24 | 44.44 | 6.12 | 89.98 | 6.42 | 305.00 | 7.75 | 102.84 |
| M-16 | 20°55′01″ | 101°39′48″ | 7.60 | 19.50 | 449.00 | 287.36 | 43.21 | 14.02 | 33.63 | 7.85 | 295.00 | 1.87 | 13.31 |
| M-17 | 20°53′50″ | 101°46′51″ | 7.49 | 35.90 | 497.00 | 318.08 | 35.31 | 8.57 | 59.39 | 7.81 | 298.00 | 3.45 | 15.04 |
| M-18 | 20°58′38″ | 101°41′48″ | 7.47 | 26.20 | 455.00 | 291.20 | 40.88 | 19.63 | 29.14 | 8.00 | 306.00 | 2.94 | 13.69 |
| M-19 | 20°57′47″ | 101°40′08″ | 7.47 | 23.60 | 414.00 | 264.96 | 39.56 | 14.86 | 30.27 | 7.57 | 299.00 | 1.76 | 11.02 |
| M-20 | 20°57′21″ | 101°35′15″ | 7.36 | 25.30 | 524.00 | 335.36 | 52.90 | 9.07 | 48.56 | 9.73 | 308.00 | 5.08 | 28.65 |
| M-21 | 21°00′11″ | 101°34′42″ | 7.60 | 26.20 | 402.00 | 257.28 | 45.78 | 8.98 | 25.66 | 10.10 | 278.00 | 1.71 | 8.16 |
| M-22 | 21°05′01″ | 101°28′22″ | 8.71 | 95.00 | 743.00 | 475.52 | 2.37 | 0.05 | 148.23 | 7.88 | 310.00 | 16.38 | 27.96 |
| M-23 | 21°01′16″ | 101°23′48″ | 7.53 | 21.80 | 973.00 | 622.72 | 28.43 | 23.59 | 178.78 | 6.95 | 605.00 | 16.10 | 14.26 |
| M-24 | 20°57′41″ | 101°22′09″ | 7.17 | 26.20 | 765.00 | 489.60 | 76.11 | 34.94 | 42.02 | 6.67 | 453.00 | 11.21 | 22.69 |
| M-25 | 20°46′54″ | 101°29′10″ | 7.56 | 25.90 | 633.00 | 405.12 | 56.43 | 19.48 | 54.38 | 7.83 | 308.00 | 6.43 | 52.41 |
| N | Range | Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|---|---|
| PH | 25 | 1.86 | 7.17 | 9.03 | 7.5932 | 0.40795 |
| T °C | 25 | 75.50 | 19.50 | 95.00 | 30.4280 | 14.39793 |
| EC | 25 | 683.00 | 340.00 | 1023.00 | 563.8400 | 173.37019 |
| TDS | 25 | 437.00 | 218.00 | 655.00 | 360.8400 | 111.05730 |
| Ca | 25 | 137.52 | 1.49 | 139.01 | 44.8300 | 25.97602 |
| Mg | 25 | 34.89 | 0.05 | 34.94 | 10.7832 | 8.82665 |
| Na | 25 | 153.95 | 24.83 | 178.78 | 67.2488 | 40.53269 |
| K | 25 | 14.56 | 1.24 | 15.80 | 7.1928 | 2.98369 |
| HCO3 | 25 | 399.00 | 206.00 | 605.00 | 313.6400 | 74.63460 |
| Cl | 25 | 15.05 | 1.33 | 16.38 | 5.4836 | 4.25592 |
| SO4 | 25 | 309.73 | 6.28 | 316.01 | 48.2412 | 65.31975 |
| pH | T °C | EC | TDS | Ca | Mg | Na | K | HCO3 | Cl | SO4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.00 | ||||||||||
| T °C | 0.08 | 1.00 | |||||||||
| EC | −0.38 | −0.15 | 1.00 | ||||||||
| TDS | −0.38 | −0.15 | 1.00 ** | 1.00 | |||||||
| Ca | −0.65 ** | −0.14 | 0.31 | 0.31 | 1.00 | ||||||
| Mg | −0.51 * | −0.47 * | 0.640 ** | 0.64 ** | 0.63 ** | 1.00 | |||||
| Na | 0.11 | 0.06 | 0.706 ** | 0.70 ** | −0.39 | 0.04 | 1.00 | ||||
| K | 0.13 | −0.29 | −0.29 | −0.29 | −0.23 | −0.16 | −0.25 | 1.00 | |||
| HCO3 | −0.36 | −0.37 | 0.86 ** | 0.86 ** | 0.12 | 0.69 ** | 0.68 ** | −0.16 | 1.00 | ||
| Cl | −0.33 | −0.25 | 0.90 ** | 0.90 ** | 0.30 | 0.64 ** | 0.61 ** | −0.08 | 0.83 ** | 1.00 | |
| SO4 | 0.01 | 0.49* | 0.25 | 0.25 | 0.17 | −0.24 | 0.27 | −0.42 | −0.19 | 0.15 | 1.00 |
| Dependent Variable | Aquifer 1-Aquifer 2 | Test Statistic | Std. Error | Std. Test Statistic | Sig. | Adj. Sig. a |
|---|---|---|---|---|---|---|
| pH | La Muralla-Valle de León | −2.536 | 3.445 | −0.736 | 0.462 | 1.000 |
| La Muralla-Silao-Romita | −3.688 | 3.344 | −1.103 | 0.270 | 0.811 | |
| Valle de León-Silao-Romita | 1.152 | 3.205 | 0.359 | 0.719 | 1.000 | |
| T °C | Valle de León-Silao-Romita | 3.759 | 3.206 | 1.172 | 0.241 | 0.723 |
| Valle de León-La Muralla | 10.988 | 3.446 | 3.188 | 0.001 | 0.004 | |
| Silao-Romita-La Muralla | 7.229 | 3.346 | 2.161 | 0.031 | 0.092 | |
| EC | Valle de León-Silao-Romita | 5.330 | 3.210 | 1.660 | 0.097 | 0.290 |
| Valle de León-La Muralla | 5.643 | 3.451 | 1.635 | 0.102 | 0.306 | |
| Silao-Romita-La Muralla | 0.313 | 3.350 | 0.093 | 0.926 | 1.000 | |
| TDS | Valle de León-Silao-Romita | 5.330 | 3.210 | 1.660 | 0.097 | 0.290 |
| Valle de León-La Muralla | 5.643 | 3.451 | 1.635 | 0.102 | 0.306 | |
| Silao-Romita-La Muralla | 0.313 | 3.350 | 0.093 | 0.926 | 1.000 | |
| Ca | La Muralla-Silao-Romita | −1.625 | 3.351 | −0.485 | 0.628 | 1.000 |
| La Muralla-Valle de León | −2.643 | 3.452 | −0.766 | 0.444 | 1.000 | |
| Silao-Romita-Valle de León | −1.018 | 3.211 | −0.317 | 0.751 | 1.000 | |
| Mg | La Muralla-Silao-Romita | −5.417 | 3.351 | −1.616 | 0.106 | 0.318 |
| La Muralla-Valle de León | −6.310 | 3.452 | −1.828 | 0.068 | 0.203 | |
| Silao-Romita-Valle de León | −0.893 | 3.211 | −0.278 | 0.781 | 1.000 | |
| Na | Valle de León-Silao-Romita | 7.232 | 3.211 | 2.252 | 0.024 | 0.073 |
| Valle de León-La Muralla | 10.857 | 3.452 | 3.145 | 0.002 | 0.005 | |
| Silao-Romita-La Muralla | 3.625 | 3.351 | 1.082 | 0.279 | 0.838 | |
| K | La Muralla-Silao-Romita | −3.917 | 3.351 | −1.169 | 0.242 | 0.727 |
| La Muralla-Valle de León | −8.024 | 3.452 | −2.324 | 0.020 | 0.060 | |
| Silao-Romita-Valle de León | −4.107 | 3.211 | −1.279 | 0.201 | 0.603 | |
| HCO3 | Valle de León-La Muralla | 1.702 | 3.445 | 0.494 | 0.621 | 1.000 |
| Valle de León-Silao-Romita | 3.411 | 3.205 | 1.064 | 0.287 | 0.862 | |
| La Muralla-Silao-Romita | −1.708 | 3.344 | −0.511 | 0.609 | 1.000 | |
| Cl | Valle de León-La Muralla | 4.190 | 3.452 | 1.214 | 0.225 | 0.674 |
| Valle de León-Silao-Romita | 6.982 | 3.211 | 2.174 | 0.030 | 0.089 | |
| La Muralla-Silao-Romita | −2.792 | 3.351 | −0.833 | 0.405 | 1.000 | |
| SO4 | Valle de León-Silao-Romita | 6.304 | 3.211 | 1.963 | 0.050 | 0.149 |
| Valle de León-La Muralla | 7.095 | 3.452 | 2.055 | 0.040 | 0.120 | |
| Silao-Romita-La Muralla | 0.792 | 3.351 | 0.236 | 0.813 | 1.000 |
| ID | Coordinates | Physico-Chemical Parameters | Isotopes | |||||
|---|---|---|---|---|---|---|---|---|
| Latitude | Longitude | pH | T (°C) | EC (µS/cm) | TDS (mg/L) | δ18O (‰) | δ2H (‰) | |
| M-1 | 20°48′24″ | 101°42′47″ | 7.55 | 30.5 | 502 | 321.28 | −76.520915 | −10.284116 |
| M-3 | 20°49′01″ | 101°38′20″ | 7.61 | 28.7 | 384 | 245.76 | −76.8683705 | −10.320088 |
| M-5 | 20°54′36″ | 101°30′46″ | 7.61 | 25.8 | 491 | 314.24 | −82.3333356 | −10.992 |
| M-7 | 20°50′17″ | 101°32′07″ | 7.51 | 30.9 | 671 | 429.44 | −71.430466 | −9.4682802 |
| M-8 | 20°51′54″ | 101°37′46″ | 7.28 | 25 | 1023 | 654.72 | −74.995188 | −9.6851966 |
| M-10 | 20°56′21″ | 101°38′27″ | 7.7 | 24.5 | 340 | 217.6 | −83.4117049 | −11.213424 |
| M-11 | 20°55′17″ | 101°41′46″ | 7.58 | 38.6 | 484 | 309.76 | −75.4597666 | −10.412072 |
| M-12 | 20°50′ 22″ | 101°43′18″ | 7.49 | 31.4 | 614 | 392.96 | −75.4023192 | −9.8022843 |
| M-13 | 20°51′38″ | 101°45′27″ | 7.28 | 30.4 | 428 | 273.92 | −79.1533606 | −9.978976 |
| M-14 | 20°50′04″ | 101°46′27″ | 9.03 | 41.3 | 502 | 321.28 | −79.6393628 | −10.510739 |
| M-17 | 20°53′50″ | 101°46′51″ | 7.49 | 35.9 | 497 | 318.08 | −77.339109 | −10.048801 |
| M-18 | 20°58′38″ | 101°41′48″ | 7.47 | 26.2 | 455 | 291.2 | −83.208591 | −10.804766 |
| M-20 | 20°57′21″ | 101°35′15″ | 7.36 | 25.3 | 524 | 335.36 | −75.3109938 | −9.4467074 |
| M-22 | 21°05′01″ | 101°28′22″ | 8.71 | 95 | 743 | 475.52 | −77.1162215 | −9.8375429 |
| M-23 | 21°01′16″ | 101°23′48″ | 7.53 | 21.8 | 973 | 622.72 | −77.5618975 | −10.059836 |
| M-24 | 20°57′41″ | 101°22′09″ | 7.17 | 26.2 | 765 | 489.6 | −78.7530691 | −10.071817 |
| M-25 | 20°46′54″ | 101°29′10″ | 7.56 | 25.9 | 633 | 405.12 | −76.1983787 | −10.093386 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibarra-Olivares, G.; Miranda-Avilés, R.; Ramos-Leal, J.A.; Morán-Ramirez, J.; Puy-Alquiza, M.J.; Li, Y.; Ángeles-Moreno, E.; Kshirsagar, P. Hydrogeochemical Characterization of Groundwater at the Boundaries of Three Aquifers in Central México. Water 2023, 15, 3948. https://doi.org/10.3390/w15223948
Ibarra-Olivares G, Miranda-Avilés R, Ramos-Leal JA, Morán-Ramirez J, Puy-Alquiza MJ, Li Y, Ángeles-Moreno E, Kshirsagar P. Hydrogeochemical Characterization of Groundwater at the Boundaries of Three Aquifers in Central México. Water. 2023; 15(22):3948. https://doi.org/10.3390/w15223948
Chicago/Turabian StyleIbarra-Olivares, Guadalupe, Raúl Miranda-Avilés, José A. Ramos-Leal, Janete Morán-Ramirez, María Jesús Puy-Alquiza, Yanmei Li, Edgar Ángeles-Moreno, and Pooja Kshirsagar. 2023. "Hydrogeochemical Characterization of Groundwater at the Boundaries of Three Aquifers in Central México" Water 15, no. 22: 3948. https://doi.org/10.3390/w15223948
APA StyleIbarra-Olivares, G., Miranda-Avilés, R., Ramos-Leal, J. A., Morán-Ramirez, J., Puy-Alquiza, M. J., Li, Y., Ángeles-Moreno, E., & Kshirsagar, P. (2023). Hydrogeochemical Characterization of Groundwater at the Boundaries of Three Aquifers in Central México. Water, 15(22), 3948. https://doi.org/10.3390/w15223948








