Interaction between Groundwater and Surface Water in the Qujiang River Basin in China: Evidence from Chemical Isotope Measurements
Abstract
:1. Introduction
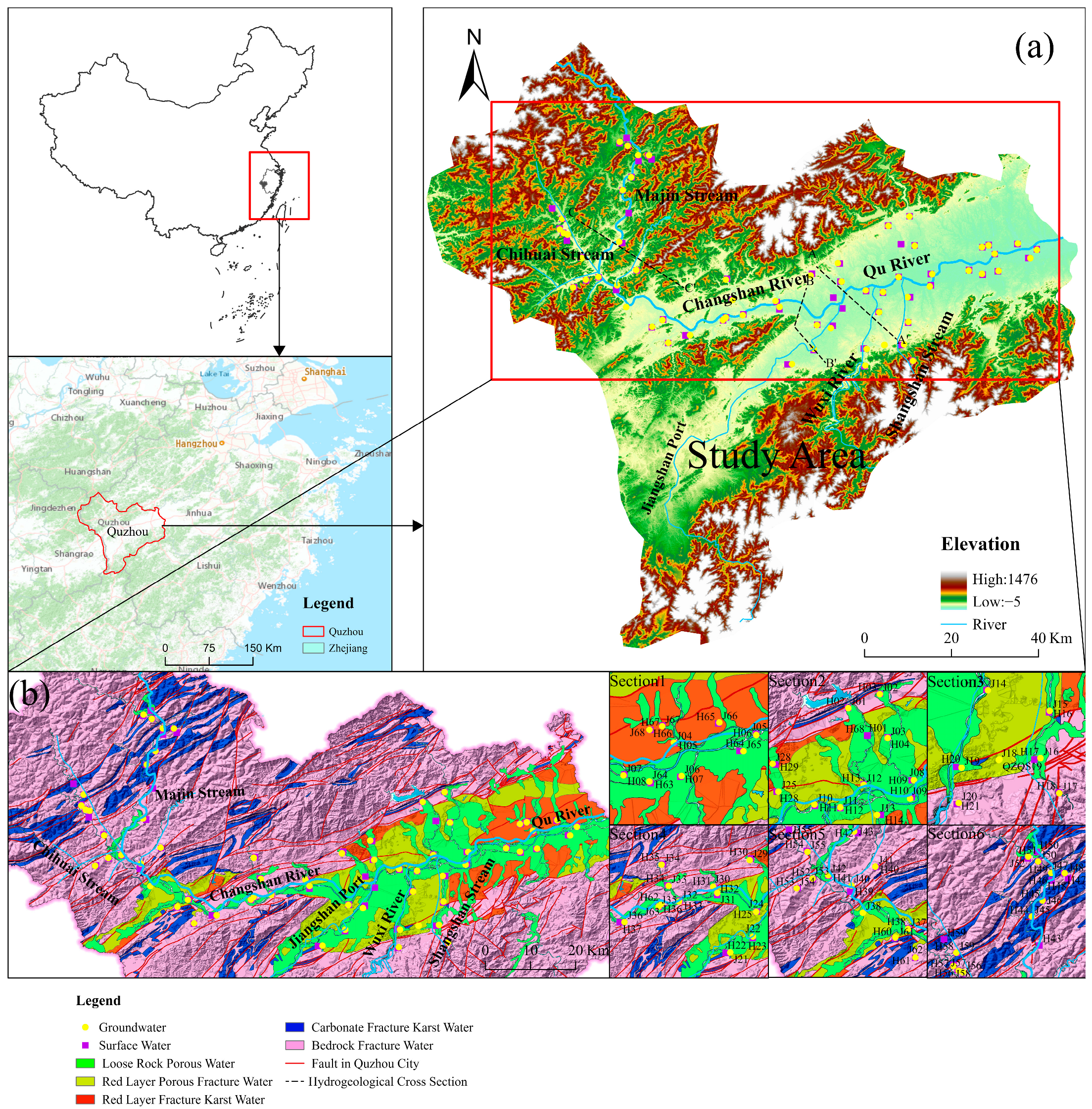
2. Case Study Area
2.1. Natural Geographical Location
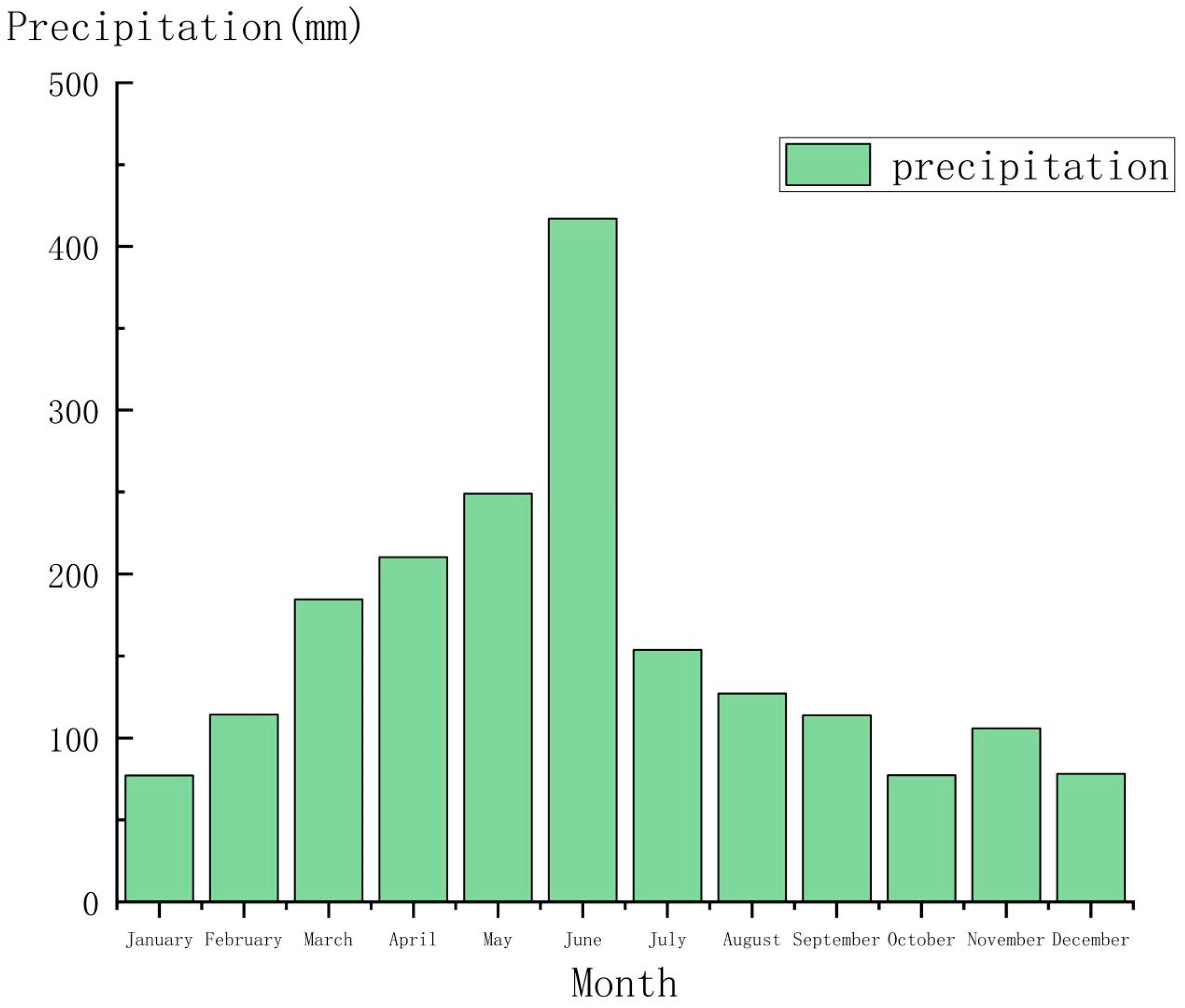
2.2. Climatic Conditions
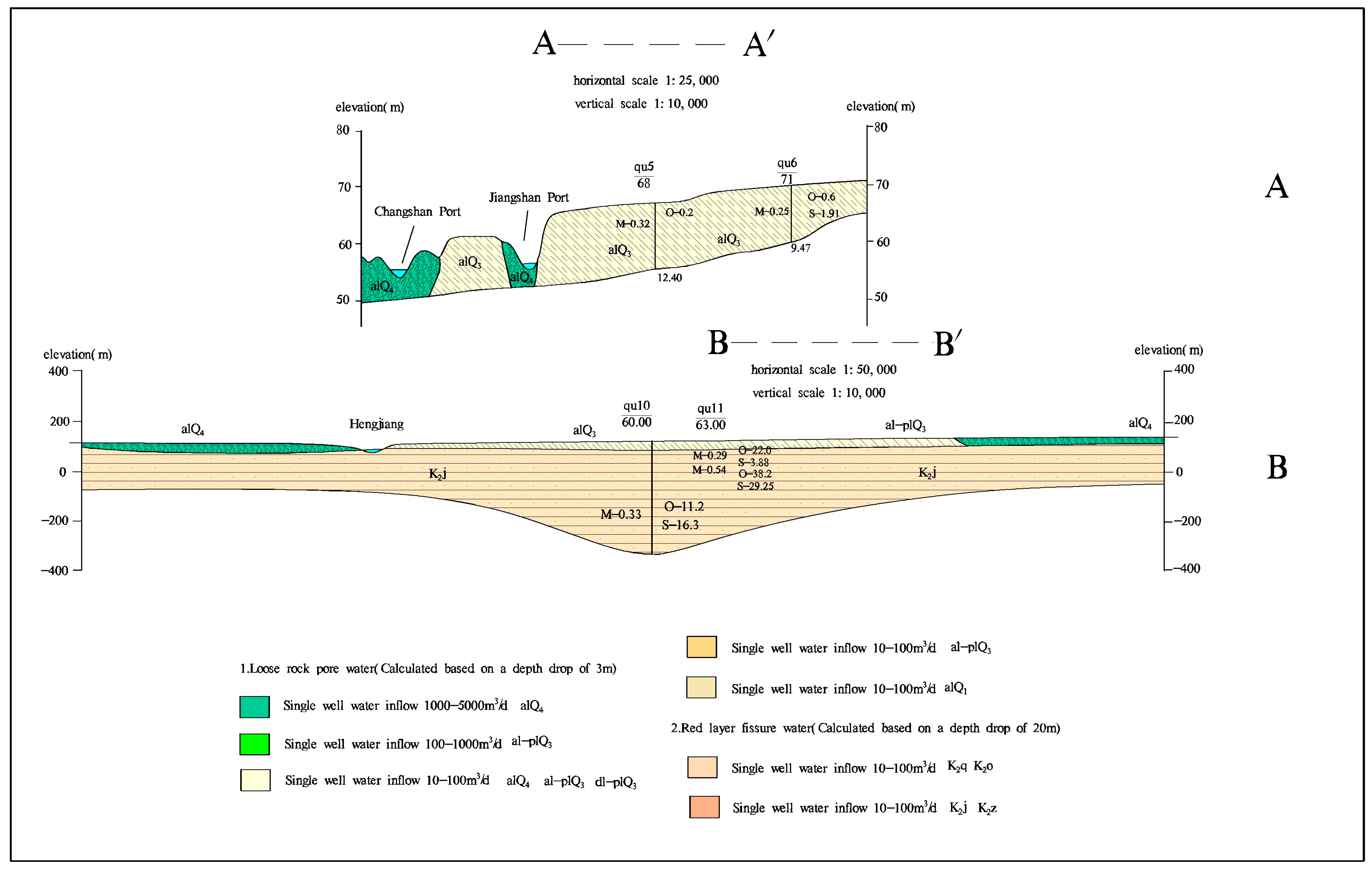
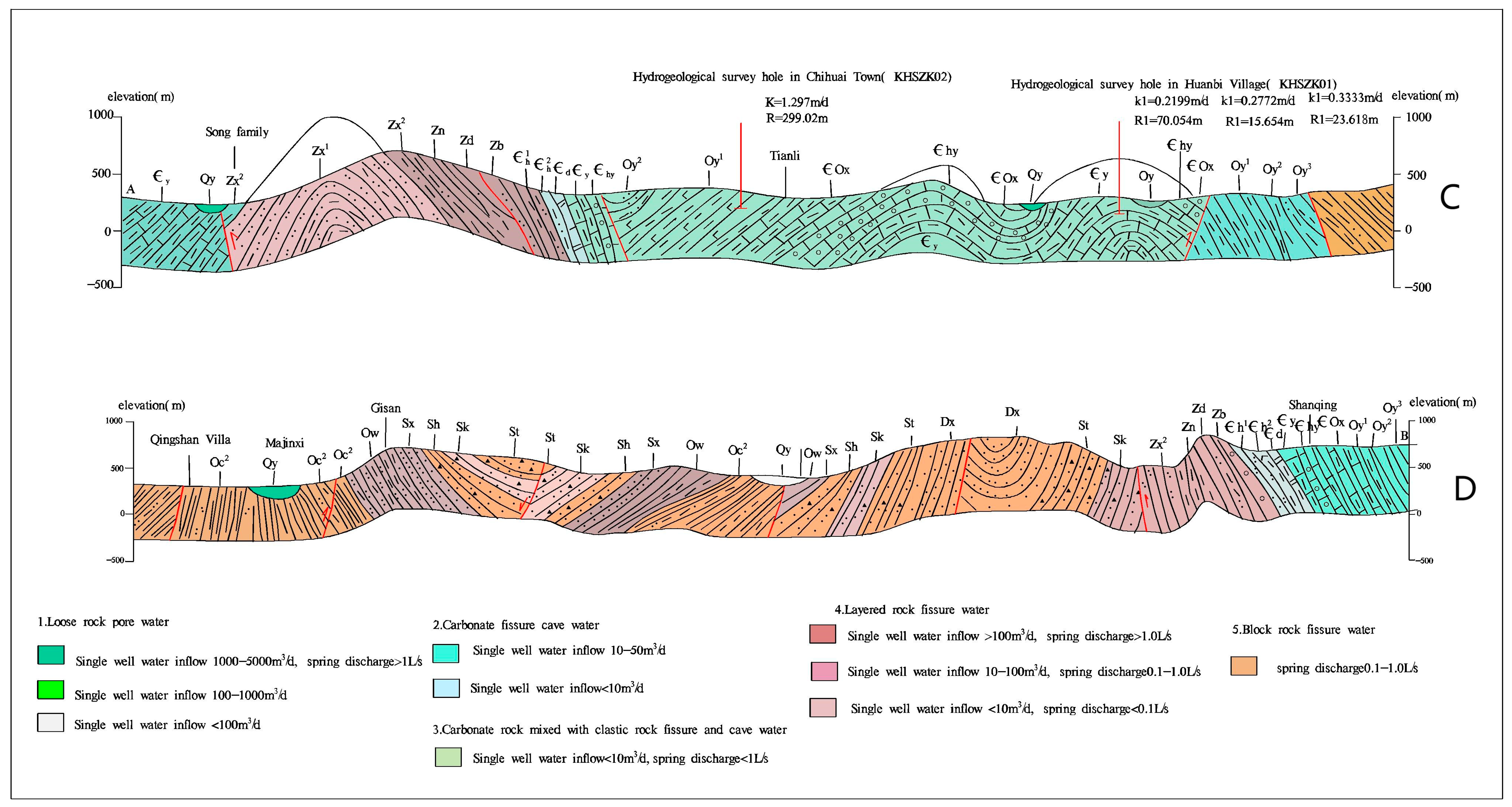
2.3. Geological and Hydrogeological Conditions
3. Materials and Methods
4. Results
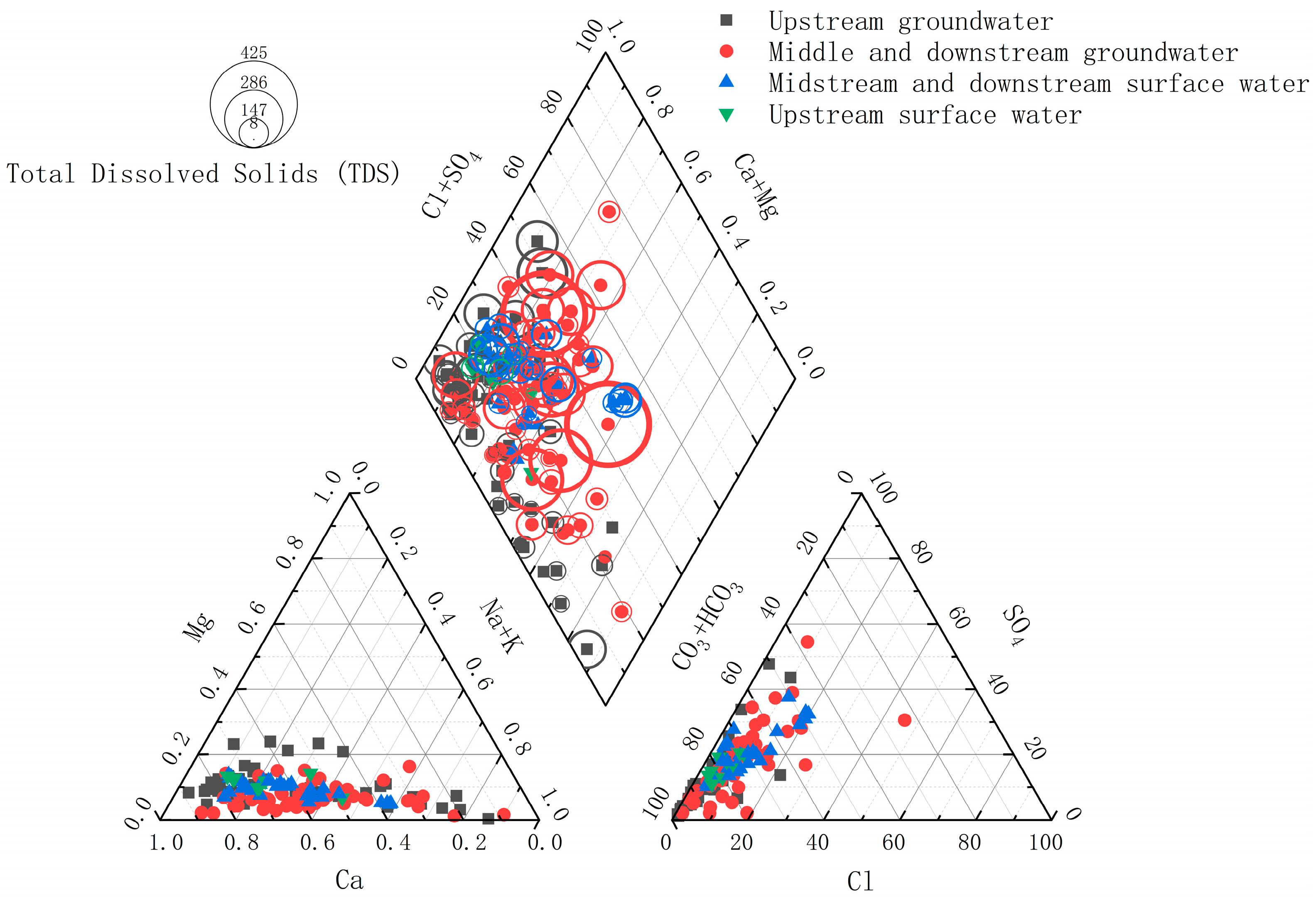
4.1. Hydrochemistry
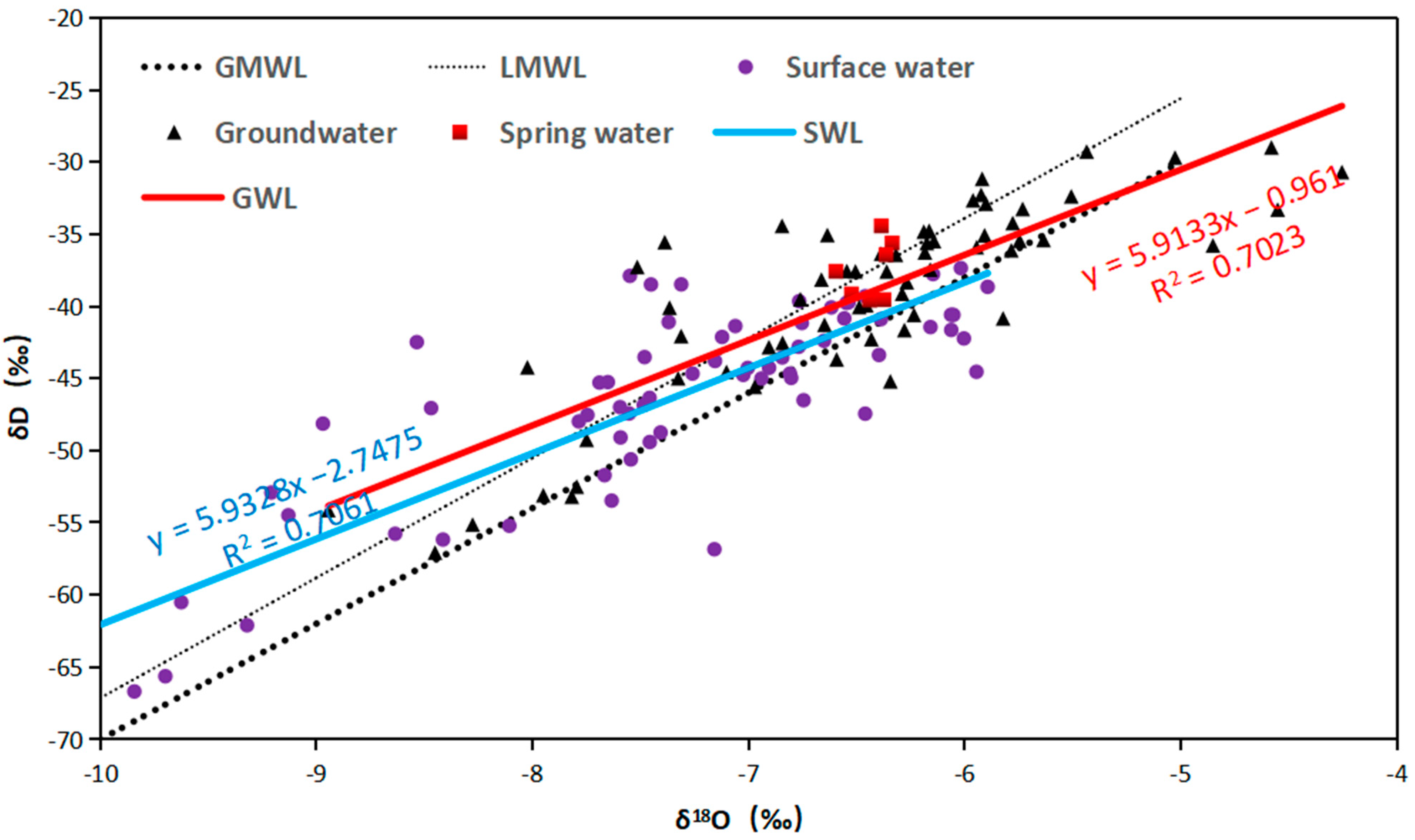
4.2. Hydrogen and Oxygen Stable Isotopes
5. Discussion
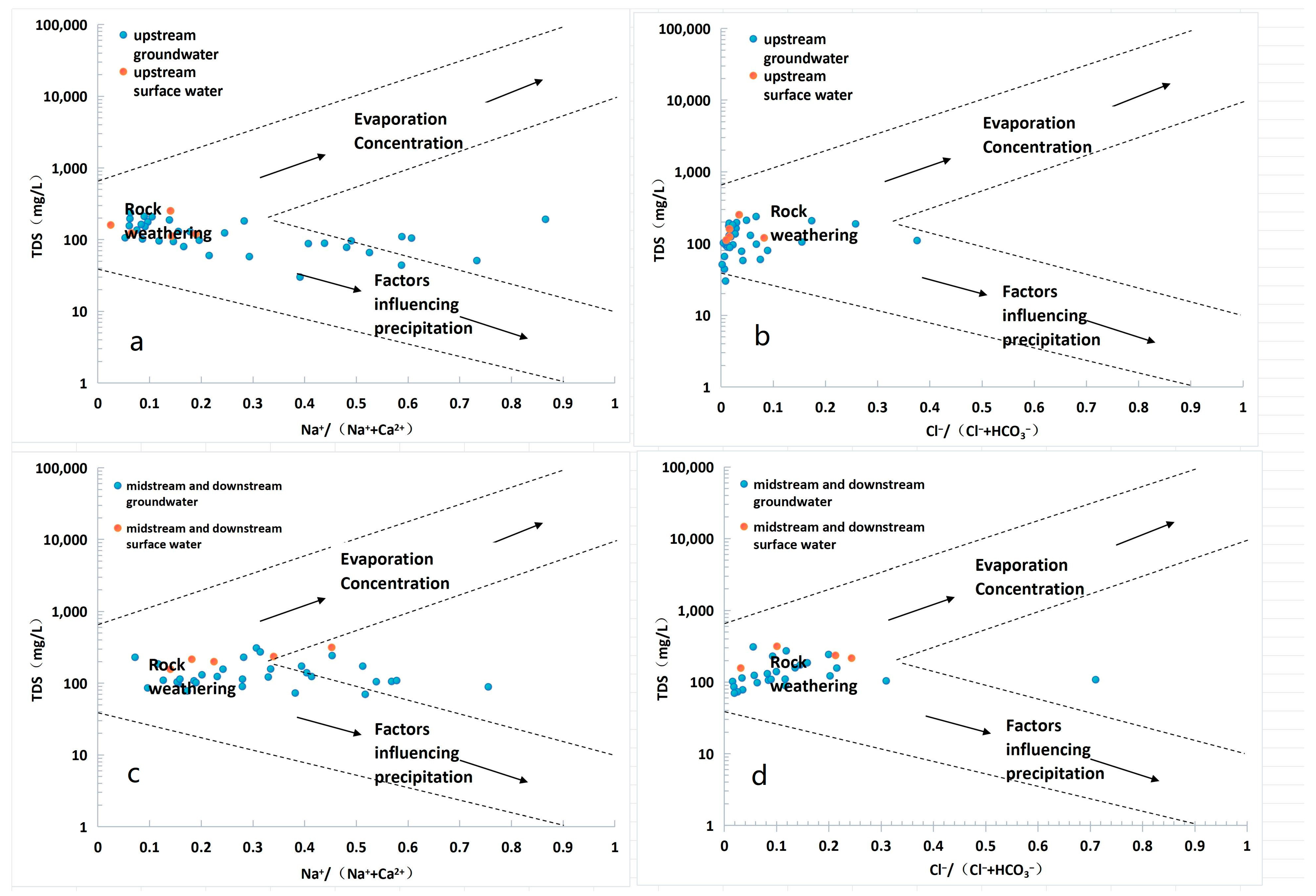
5.1. The Main Control Factors of Hydrochemistry
5.2. Indicative Significance of Stable Isotopes
5.3. Interaction between Groundwater and Surface Water
5.3.1. Analysis of TDS Change Rule along the Way
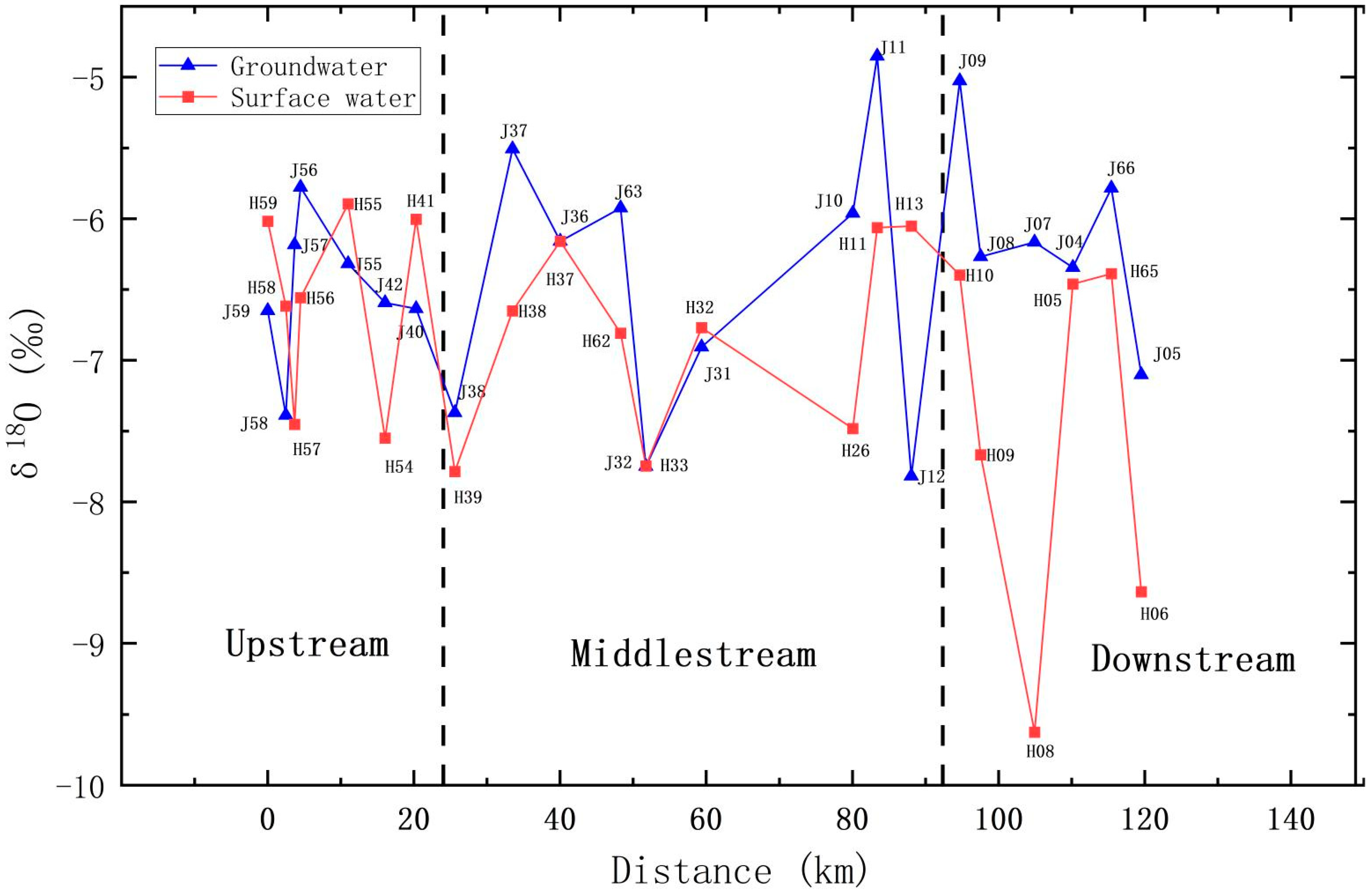
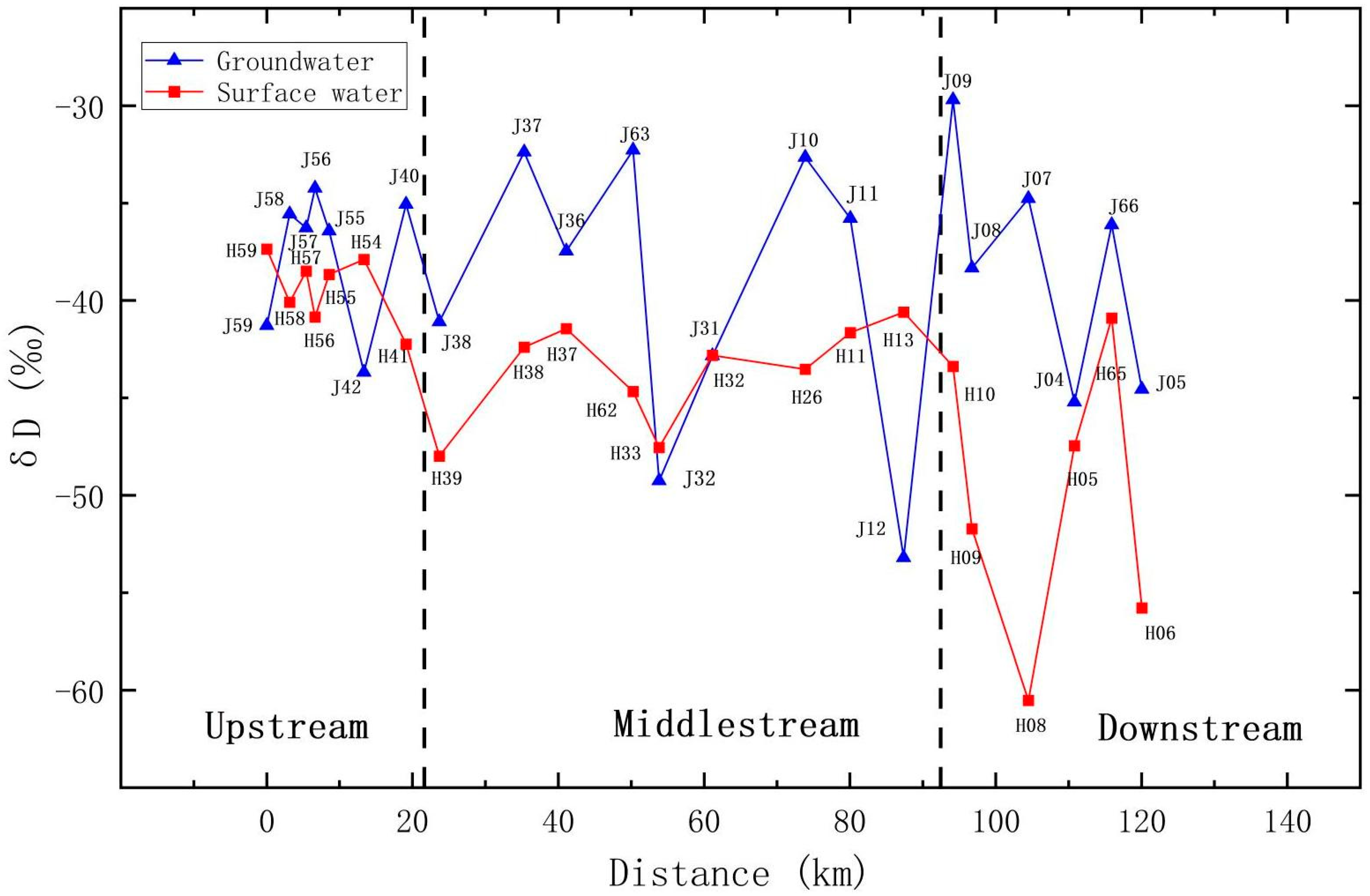
5.3.2. Analysis of Stable Isotopes Change Rule along the Way
5.3.3. Transformational Relationships between Surface Water and Groundwater
- (1)
- Spatial distribution: The deuterium (D) and oxygen-18 (18O) compositions within groundwater and river water exhibited fluctuations across distinct geographical locations. As one moved from the upstream to the downstream sections of the Qujiang River, the D and 18O values within river water displayed an oscillating pattern. In the middle and lower reaches, which are characterized by flat terrain, the δD and δ18O values were generally lower than in the upstream areas, which are mountainous. Nonetheless, the plain regions exhibited more pronounced variations. In general, the δD and δ18O values of the groundwater and surface water gradually decreased along the river from the upper reaches to the middle and lower reaches. This suggests frequent water exchanges between groundwater in the middle and lower reaches and river water, implying a close hydrological connection and favorable conditions for groundwater recharge and runoff.
- (2)
- Water body comparison: While both river water and groundwater manifested fluctuating trends in D and 18O along their flow paths, the δD and δ18O values, along with their averages, in groundwater were typically higher than those in river water in most areas. This indicates that the primary source of groundwater is the infiltration of atmospheric precipitation.
- (1)
- Upstream of Qujiang: In the upstream region, the TDS, δD, and δ18O values in groundwater exhibited fluctuations, indicating diverse recharge sources and circulation processes in different regions. For instance, at the J56 sampling point, groundwater’s TDS value diminished rapidly, while river water’s TDS exhibited a declining trend. Groundwater’s δD and δ18O values were enriched and higher than those of river water, implying that groundwater in this area primarily receives recharge from atmospheric precipitation and river infiltration. Conversely, at the J42 sampling point, groundwater’s TDS value rose rapidly, while river water’s TDS remained relatively stable. Groundwater’s δD and δ18O values depicted a swift depletion trend, suggesting that groundwater in this area primarily receives lateral flow recharge from depleted groundwater, with a weak hydrological connection to the river.
- (2)
- Midstream of Qujiang: In the midstream area, groundwater’s TDS, δD, and δ18O values fluctuated, exhibiting more significant variations compared to the upstream. This indicates diverse recharge sources in different midstream regions, along with a more complex hydrological connection to other water bodies and potential human interference. For example, at the J37 sampling point, groundwater’s TDS value varied slightly, and the δD and δ18O values were enriched and higher than those of river water, indicating a transformational relationship between groundwater and river water. Groundwater in this area might be influenced by groundwater extraction and human activities. In contrast, at the J32 sampling point, groundwater’s TDS value continually rose, while river water’s TDS decreased. Groundwater’s δD and δ18O values depicted a rapid depletion trend closely resembling those of river water, implying that the groundwater in this area receives recharge from the river.
5.3.4. Calculation of Conversion Rate between Groundwater and Surface Water
6. Conclusions
- (1)
- Hydrochemical characteristics: The examination of spatial distribution patterns and hydrochemical attributes demonstrated that the water quality within the research area is predominantly slightly alkaline. The principal hydrochemical categories which were identified were Ca-HCO3 and Ca-HCO3·Cl. These waters manifest low mineralization levels, signifying that atmospheric precipitation serves as the principal source of water in the basin. The resemblance in hydrochemical types between the surface water and groundwater implies a certain hydraulic connection between these two components. The Gibbs diagram suggests that ion concentrations are primarily influenced by rock weathering, with the additional influence of precipitation factors in the upstream river sections. The spatial distribution patterns of TDS and pH indicators demonstrated similar variations in both surface water and groundwater, indicating frequent exchange between them. In particular, groundwater mainly recharged surface water in the upstream area, whereas surface water primarily replenished groundwater in the middle and downstream areas.
- (2)
- Isotopic characteristics: Spatial distribution patterns and characteristics of stable hydrogen and oxygen isotopes show that the isotopic content increases along the flow direction in the upper reaches, particularly in areas with fewer tributaries. Furthermore, an elevation effect of −0.15‰/100 m for oxygen isotopes was established through the correlation between the elevation of upstream springs and δ18O values. The connection between hydrogen and oxygen isotopes and the atmospheric water line further corroborates that both groundwater and surface water in the study area are recharged by atmospheric precipitation and influenced by evaporation during the mutual replenishment process.
- (3)
- Contribution of surface water to groundwater: By utilizing the mass conservation equation for δ18O in water transformation, the quantitative calculation of the contribution rate of surface water to groundwater offered insights into the transformation dynamics between these two elements. The results indicate that in the upstream region of the study area, groundwater primarily recharges surface water, with an average contribution rate of 19.67% and a daily replenishment volume of 2.73 × 106 m3/d. Conversely, in the middle and downstream regions, surface water predominantly recharges groundwater, with an average contribution rate of 22.77% and a daily replenishment volume of 3.49 × 106 m3/d.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Song, X.; Zhang, Y.; Han, D.; Tang, C.; Yu, Y.; Ma, Y. Hydrochemical characteristics and water quality assessment of surface water and groundwater in Songnen plain, Northeast China. Water Res. 2012, 46, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Lasagna, M.; De Luca, D.A.; Franchino, E. Nitrate contamination of groundwater in the western Po Plain (Italy): The effects of groundwater and surface water interactions. Environ. Earth Sci. 2016, 75. [Google Scholar] [CrossRef]
- Kanduc, T.; Grassa, F.; McIntosh, J.; Stibilj, V.; Ulrich-Supovec, M.; Supovec, I.; Jamnikar, S. A geochemical and stable isotope investigation of groundwater/surface-water interactions in the Velenje Basin, Slovenia. Hydrogeol. J. 2014, 22, 971. [Google Scholar] [CrossRef]
- Tsuchihara, T.; Yoshimoto, S.; Shirahata, K.; Ishida, S. 17O-excess and stable isotope compositions of rainwater, surface water and groundwater in paddy areas in Ibaraki, Japan. IDRE J. 2016, 84, 185–194. [Google Scholar]
- Han, Z.; Zhang, Y.; Wang, H.; Jiao, R.; Liang, W.; Ji, G. Characteristics of stable isotopes in precipitation, surface water and groundwater in the arid and semi-arid desertification steppe: A case study in the Darhan Maomingan Joint Banner. Sci. Technol. Eng. 2020, 20, 11463–11471. [Google Scholar]
- Zheng, J. Research on identification and quantification methods of groundwater and surface water interaction. J. Anhui Agric. Sci. 2015, 43, 203–205. [Google Scholar] [CrossRef]
- Jin, S.; Jiang, J.; Chi, B.; Li, Y.; Han, Q. Analyses of groundwater circulation patterns based on environmental isotopes and hydrochemistry in the Huocheng Plain. Hydrogeol. Eng. Geol. 2016, 43, 43–51. [Google Scholar] [CrossRef]
- Wang, H.; Gu, H.; Jiang, J.; Chi, B.; Kong, H. Isotopes, hydrochemical characteristics and genesis of river water in the Yili river basin, Xinjiang. Quat. Sci. 2016, 36, 1383–1392. [Google Scholar] [CrossRef]
- Zhang, R. Application of Isotope Method in Hydrogeology; Geology Press: Beijing, China, 1983. [Google Scholar]
- Salesse, K.; Dufour, É.; Wurster, C.; Bruzek, J.; Giuliani, R.; Castex, D. Contributions of phenetic relationship and stable isotope analysis (δ13C, δ15N, δ18O) to the study of a mortality crisis in the catacomb of Saints Peter and Marcellinus in Rome (1st–3rd century AD). Am. J. Phys. Anthropol. 2012, 147, 80–311. [Google Scholar] [CrossRef]
- Gázquez, F.; Calaforra, J.M.; Evans, N.P.; Hodell, D.A. Using stable isotopes (δ^{18}O and δ$D) of gypsum hydration water to unravel the mode of gypsum speleothem formation in semi-arid caves. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 17–22 April 2016; p. EPSC2016-8911. [Google Scholar]
- Midhun, M.; Lekshmy, P.R.; Ramesh, R. Hydrogen and oxygen isotopic compositions of water vapor over the Bay of Bengal during monsoon. Geophys. Res. Lett. 2013, 40, 6324–6328. [Google Scholar] [CrossRef]
- Lu, C.; Chen, S.; Jiang, Y.; Shi, J.; Yao, C.; Su, X. Quantitative analysis of riverbank groundwater flow for the Qinhuai River, China, and its influence factors. Hydrol. Process. 2018, 32, 2734–2747. [Google Scholar] [CrossRef]
- Niyazi, B.; Khan, A.A.; Masoud, M.; Elfeki, A.; Basahi, J. Variability of the geomorphometric characteristics of Makkah Al-Mukaramah basins in Saudi Arabia and the impact on the hydrologic response. J. Afr. Earth Sci. 2020, 168, 103842. [Google Scholar] [CrossRef]
- Tang, X. Transformation of Surface Water and Groundwater in the Plain Area of Liuhe Basin, Liaoning Province. Master’s Thesis, Jilin University, Changchun, China, 2022. [Google Scholar]
- Wang, X.; Xu, H.; Yan, J.; Ling, H.; Zhao, X. Study on the transformation of lower Tarim River Water into groundwater based on oxygen isotope (δ18 O). J. Water Resour. Water Eng. 2018, 29, 84–89. [Google Scholar] [CrossRef]
- Sun, C. Characteristics of Runoff Components and Water Vapor Sources in Typical Mountainous Areas of Tarim River Basin; University of Chinese Academy of Sciences: Beijing, China, 2015. [Google Scholar]
- Shen, Z.; Zhu, W.; Zhong, Z. Foundation of Hydro-Geochemistry; Geological Publishing House: Beijing, China, 1993. [Google Scholar]
- Sun, C.; Chen, Y.; Li, W.; Li, X.; Yang, Y. Isotopic time series partitioning of streamflow components under regional climate change in the Urumqi River, northwest China. Hydrol. Sci. J. 2016, 61, 1443–1459. [Google Scholar]
- Gu, W. Isotopic Hydrology; Science Press: Beijing, China, 2011. [Google Scholar]
- Zhang, Y.; Su, X.; Wang, Q.; Yang, F.; Ren, W.; Zhao, Z. Research on the relationship between surface water and groundwater transformation in the western Plain of Yili River Valley. J. Beijing Normal Univ. Nat. Sci. Ed. 2020, 56, 664–674. [Google Scholar] [CrossRef]
- Marandim, A.; Shand, P. Groundwater chemistry and the Gibbs Diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar]
- Lei, M.; Liu, Y.; Ma, Q.; Zhu, Z.; Zhang, S. Research on water cycle in Jinqu Basin based on isotope technique. Yellow River 2020, 42, 88–92,99. [Google Scholar] [CrossRef]
- Bailey, A.; Aemisegger, F.; Villiger, L.; Los, S.A.; Reverdin, G.; Quiñones Meléndez, E.; Acquistapace, C.; Baranowski, D.B.; Böck, T.; Bony, S. Isotopic measurements in water vapor, precipitation, and seawater during EUREC 4 A. Earth Syst. Sci. Data 2023, 15, 465–495. [Google Scholar] [CrossRef]
- Tharammal, T.; Bala, G.; Nusbaumer, J.M. Sources of water vapor and their effects on water isotopes in precipitation in the Indian monsoon region: A model-based assessment. Sci. Rep. 2023, 13, 708. [Google Scholar] [CrossRef]
- Fang, L.; Gao, R.; Wang, X.; Liu, T. Isotopes-based characterization of precipitation compositions and atmospheric water vapor sources over typical Eurasian steppes in south mongolian Plateau. J. Hydrol. 2022, 615, 128724. [Google Scholar] [CrossRef]
- Liu, Y.; Man, W.; Zhou, T.; Peng, D. A tracing study on influence factors of East Asian stable isotopes in atmospheric water vapor. Chin. J. Atmos. Sci. 2023, 47, 616–630. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Yang, L.; Duan, L.; Chu, S.; Zhang, G.; Zhang, B.; Liu, T. Study on the transformation relationship between precipitation, river water, and groundwater in the upper reaches of the Xilin River during the rainy season. Environ. Sci. 2023, 1–16. [Google Scholar] [CrossRef]
- Liu, Z.; Yoshimura, K.; Kennedy, C.D.; Wang, X.; Pang, S. Water vapor δD dynamics over China derived from SCIAMACHY satellite measurements. Sci. China Earth Sci. 2014, 57, 813–823. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Cai, Y.; Sinha, A.; Spötl, C.; Baker, J.; Kathayat, G.; Liu, Z.; Ye, T.; Lu, J.; et al. A data-model comparison pinpoints Holocene spatiotemporal pattern of East Asian summer monsoon. Quat. Sci. Rev. 2021, 261, 106911. [Google Scholar] [CrossRef]
- Abd-Elaty, I.; Sallam, G.A.H.; Pugliese, L.; Negm, A.M.; Straface, S.; Scozzari, A.; Ahmed, A. Managing coastal aquifer salinity under sea level rise using rice cultivation recharge for sustainable land cover. J. Hydrol. Reg. Stud. 2023, 48, 101466. [Google Scholar] [CrossRef]
- Adjovu, G.E.; Stephen, H.; James, D.; Ahmad, S. Measurement of Total Dissolved Solids and Total Suspended Solids in Water Systems: A Review of the Issues, Conventional, and Remote Sensing Techniques. Remote Sens. 2023, 15, 3534. [Google Scholar] [CrossRef]
- Wang, H. Study on the Formation and Evolution of Groundwater in the Changbai Mountain Area under Seasonal Freeze-Thaw Action. Ph.D. Thesis, Jilin University, Changchun, China, 2023. [Google Scholar]
- Smail, R.Q.S.; Dişli, E. Assessment and validation of groundwater vulnerability to nitrate and TDS using based on a modified DRASTIC model: A case study in the Erbil Central Sub-Basin, Iraq. Environ. Monit. Assess. 2023, 195, 567. [Google Scholar] [CrossRef]
- Zou, J. Study on the Hydrochemical Variation Characteristics and Influencing Factors of Groundwater in a Typical Water Pressure Mining Area in Heilonggang; Hebei University of Engineering: Handan, China, 2022. [Google Scholar]
- Han, C.; Wang, Z.; Tian, H.; Niu, Q.; Liu, L.; Zhu, Y.; Ding, L.; Zhao, L.; Zhao, H.; Zhao, C.; et al. Study on the hydrochemical characteristics and genesis of groundwater in Hanzhong Basin. Northwest. Geol. 2023, 56, 263–273. [Google Scholar] [CrossRef]
- Yu, J.; Song, X.; Liu, X.; Cong, Y.; Tang, C.; Li, F.; Yasuo, S.; Akihiko, K. A Study of Groundwater Cycle in Yongding River Basin by Using δD, δ18O and Hydrochemical Data. J. Nat. Resour. 2007, 22, 415–423. [Google Scholar] [CrossRef]

| Hydrochemical Indicators | Loose Pore Phreatic Water | Red Layer Pore Fissure Water | Bedrock Fissure Water | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVG | Min | Max | SD | Cov | AVG | Min | Max | SD | Cov | AVG | Min | Max | SD | Cov | |
| pH | 6.33 | 5.05 | 6.95 | 0.46 | 0.07 | 6.36 | 5.12 | 6.89 | 0.48 | 0.08 | 5.4 | 5.05 | 6.01 | 0.43 | 0.1 |
| F− | 0.2 | 0.05 | 0.55 | 0.13 | 0.64 | 0.2 | 0.05 | 0.55 | 0.17 | 0.9 | 0.53 | 0.05 | 1.43 | 0.64 | 1.49 |
| COD | 1.24 | 0.18 | 4.63 | 1.04 | 0.85 | 1.35 | 0.25 | 3.83 | 1.04 | 0.8 | 0.58 | 0.18 | 1.18 | 0.43 | 0.9 |
| TDS | 179.36 | 70 | 426 | 102.01 | 0.58 | 148.42 | 98 | 273 | 56.38 | 0.4 | 74.33 | 43 | 102 | 24.23 | 0.4 |
| NH4+ | 0.23 | 0.02 | 2.17 | 0.5 | 2.22 | 0.25 | 0.02 | 1.87 | 0.5 | 2.12 | 0.03 | 0.02 | 0.04 | 0.01 | 0.43 |
| N03-N | 4.28 | 0.2 | 18.6 | 4.79 | 1.14 | 5.09 | 0.2 | 13.2 | 4.46 | 0.91 | 1.52 | 0.85 | 2.76 | 0.88 | 0.71 |
| N02-N | 0.05 | 0 | 0.44 | 0.12 | 2.41 | 0.07 | <0.001 | 0.4 | 0.13 | 1.97 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| total hardness | 112.94 | 43 | 191 | 51.86 | 0.47 | 104.23 | 66.6 | 195 | 44.03 | 0.44 | 38.67 | 15.8 | 57.2 | 17.18 | 0.54 |
| SO42− | 27.6 | 1.99 | 100 | 23.36 | 0.86 | 20.38 | 3.78 | 45 | 13.25 | 0.68 | 3.55 | 2.82 | 4.62 | 0.77 | 0.27 |
| Cl− | 14.86 | 1.45 | 67.1 | 16.55 | 1.14 | 15.17 | 1.48 | 49 | 12.32 | 0.85 | 4.58 | 2.73 | 6.95 | 1.76 | 0.47 |
| CO32− | 5 | 5 | 5 | <5 | <5 | 5 | 5 | 5 | <5 | <5 | 5 | 5 | 5 | <5 | <5 |
| HCO3− | 91.44 | 31 | 243 | 43.28 | 0.48 | 85.92 | 20 | 222 | 50.86 | 0.62 | 44.33 | 20 | 75 | 22.9 | 0.63 |
| Ca | 34.4 | 13.7 | 54.4 | 12.12 | 0.36 | 37.48 | 14.1 | 70.1 | 16.5 | 0.46 | 7.4 | 1.58 | 18.2 | 7.65 | 1.27 |
| Mg | 5.06 | 1.19 | 15.3 | 3.95 | 0.8 | 3.02 | 1.06 | 6.05 | 1.73 | 0.6 | 0.65 | 0.13 | 1.53 | 0.63 | 1.18 |
| Zn | 0.07 | 0.02 | 0.2 | 0.05 | 0.64 | 0.05 | 0.02 | 0.08 | 0.02 | 0.47 | 0.02 | 0.01 | 0.03 | 0.01 | 0.82 |
| Fe | 0.07 | 0.01 | 1.17 | 0.23 | 3.47 | 0.07 | 0.01 | 0.62 | 0.17 | 2.7 | 0.05 | 0.01 | 0.12 | 0.05 | 1.22 |
| Mn | 0.22 | 0.01 | 3.14 | 0.66 | 3.07 | 0.02 | 0.01 | 0.15 | 0.04 | 1.87 | 0.01 | 0.01 | 0.01 | <0.01 | <0.01 |
| K | 8.04 | 1.33 | 35.2 | 8.46 | 1.07 | 6.72 | 0.66 | 18.4 | 5.16 | 0.8 | 2.72 | 2.15 | 3.85 | 0.8 | 0.36 |
| Na | 14.04 | 2.32 | 49.8 | 10.88 | 0.79 | 8.49 | 2.16 | 14.7 | 4.19 | 0.52 | 7.4 | 3.15 | 12.7 | 3.97 | 0.66 |
| Al | 0.11 | 0.04 | 1.2 | 0.26 | 2.32 | 0.16 | 0.04 | 1.49 | 0.4 | 2.6 | 0.21 | 0.11 | 0.3 | 0.07 | 0.44 |
| acidity | 18.01 | 8.71 | 27.8 | 5.87 | 0.33 | 20.05 | 8.91 | 43.6 | 9 | 0.47 | 13.24 | 8.11 | 16.7 | 3.7 | 0.34 |
| alkalinity | 87.14 | 25.6 | 200 | 32.55 | 0.38 | 82.43 | 16.8 | 183 | 40.72 | 0.52 | 42.83 | 16.6 | 80.7 | 27.43 | 0.78 |
| CO2 | 16.25 | 7.81 | 26.3 | 5.53 | 0.35 | 17.79 | 7.99 | 39.2 | 8.06 | 0.47 | 11.79 | 7.27 | 14.7 | 3.24 | 0.34 |
| PO43− | 0.18 | 0.01 | 1.3 | 0.31 | 1.78 | 0.16 | 0.01 | 0.45 | 0.18 | 1.19 | 0.03 | 0.01 | 0.07 | 0.03 | 1.35 |
| Type of Water Body | Location | δD | δO18 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Max. | Min. | Average | SD. | Max. | Min. | Average | SD. | ||
| Surface water | Upstream | −37.36 | −53.48 | −42.26 | 3.99 | −5.89 | −7.69 | −6.92 | 0.54 |
| mid and downstream | −37.36 | −75.45 | −45.51 | 7.99 | −5.95 | −10.89 | −7.73 | 1.21 | |
| Groundwater | Upstream | −28.99 | −53.11 | −38.39 | 5.37 | −4.58 | −7.95 | −6.42 | 0.72 |
| mid and downstream | −28.99 | −57.08 | −39.86 | 7.31 | −4.25 | −8.95 | −6.62 | 1.02 | |
| River Section | Qs/m3·s−1 | Cs/‰ | Cg/‰ | Cb/‰ | f/% |
|---|---|---|---|---|---|
| Chihuixi River | 23.5 | −6.85 | −6.31 | −6.58 | 500.80% |
| Majin River | 39.8 | −7.12 | −6.57 | −6.84 | 516.52% |
| Changshan River | 56.8 | −7.24 | −6.38 | −7.83 | 40.96% |
| Jiangshangang River | 26.3 | −7.15 | −6.16 | −7.24 | 8.23% |
| Wuxi River | 35.4 | −7.52 | −6.74 | −7.79 | 25.80% |
| Shangshanxi River | 18.4 | −7.72 | −6.72 | −8.13 | 28.95% |
| Qujiang River | 65.2 | −7.71 | −6.57 | −8.10 | 25.69% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, C.; Jiang, J.; Zhang, Y.; Wang, G.; Xu, L.; Qu, Z. Interaction between Groundwater and Surface Water in the Qujiang River Basin in China: Evidence from Chemical Isotope Measurements. Water 2023, 15, 3932. https://doi.org/10.3390/w15223932
Liu Y, Zhang C, Jiang J, Zhang Y, Wang G, Xu L, Qu Z. Interaction between Groundwater and Surface Water in the Qujiang River Basin in China: Evidence from Chemical Isotope Measurements. Water. 2023; 15(22):3932. https://doi.org/10.3390/w15223932
Chicago/Turabian StyleLiu, Yi, Chaoyu Zhang, Jiyi Jiang, Ying Zhang, Guanghao Wang, Liangliang Xu, and Zhihui Qu. 2023. "Interaction between Groundwater and Surface Water in the Qujiang River Basin in China: Evidence from Chemical Isotope Measurements" Water 15, no. 22: 3932. https://doi.org/10.3390/w15223932




