1. Introduction
Nasim Mine is a copper mine located in Bardeskan city in the northeastern province of Khorasan-e-Razavi, Iran. The mine features a factory that produces cathode copper and cement copper using tank leaching to extract the copper [
1]. However, this process results in a high percentage of moisture retained in the soil, posing environmental concerns and necessitating moisture removal. Metallurgical processes typically involve various techniques to extract valuable materials from ores, with tank leaching being a commonly used method. Tank leaching is a hydrometallurgical technique in which crushed ores are placed in open tanks and stirred by agitators under atmospheric pressure to extract precious metals from the ore at an accelerated rate. This process, also known as a “semi-closed system”, requires tailings impoundments for storing processed materials. If a heap leaching facility is present, the dewatered tailings might be sent to the leach pad for a second round of leaching or return to tank leaching after pressure oxidation or roasting to capture any residual metal [
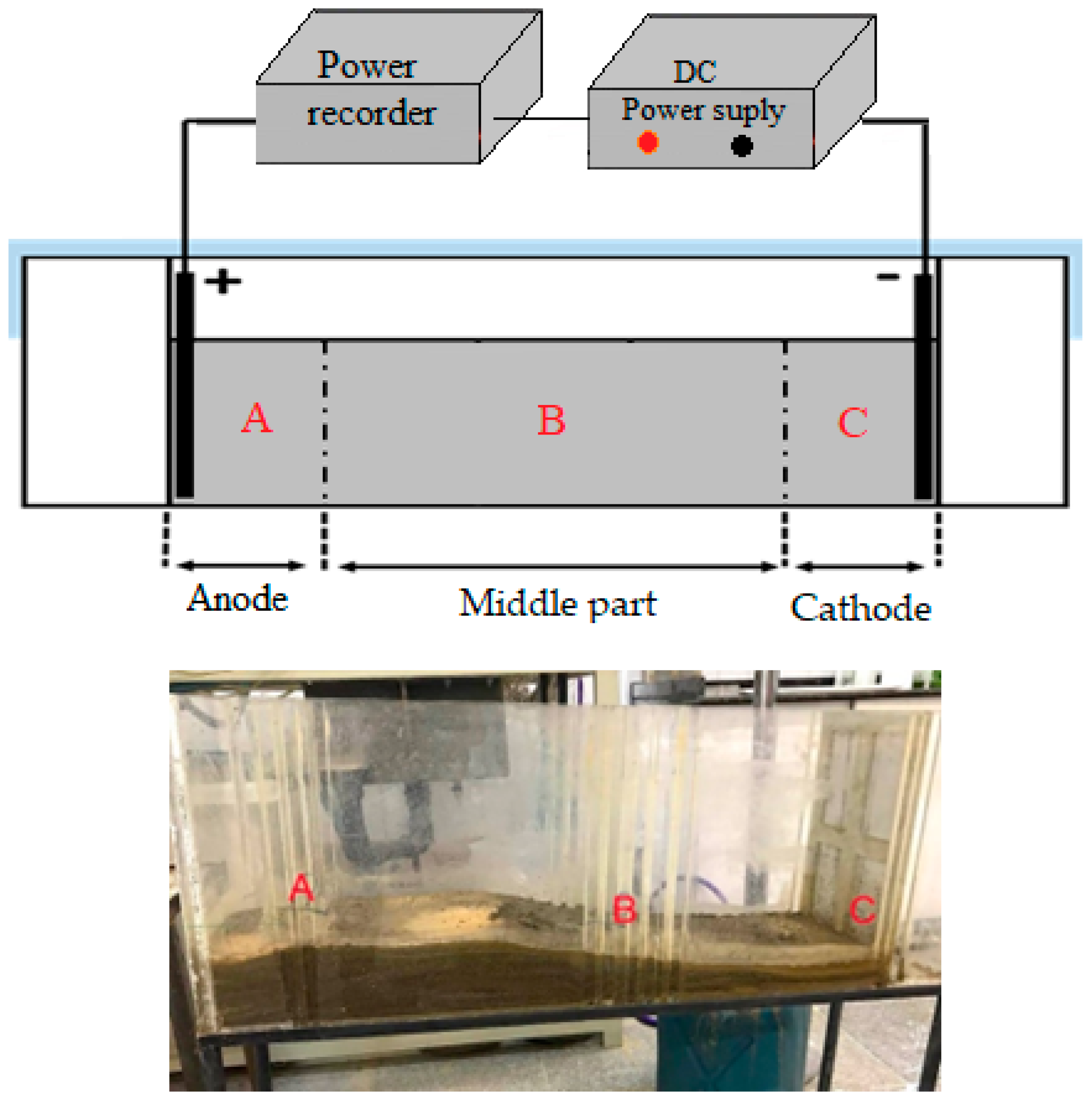
2]. In the tank leaching process, water and sulfuric acid are mixed with soil in tanks according to a specific ratio. The mixture is then drained into ponds with a designated gradient (as shown in
Figure 1) to extract the desired metal.
Tank leaching and vat leaching differ in several aspects, including the size of the materials used, the equipment employed, system continuity, and retention time. Vat leaching uses coarser materials than does tank leaching, making size reduction more cost-effective. Tank leaching systems typically involve tanks with agitators and baffles, while vat leaching systems have only agitators. Tank leaching is usually a continuous process, whereas vat leaching can operate either continuously or in batches. However, vat leaching requires a longer retention time to achieve comparable recovery rates of valuable materials [
3].
During the leaching process, a significant amount of waste is generated, which includes leaching residue with a high concentration of pore water. This pore water exhibits different behavior compared to ordinary water and can be categorized into free water, interstitial or capillary water, vicinal water, and chemically bound or water of hydration [
4,
5,
6,
7,
8,
9,
10]. Dold and Fontboté [
11] studied the mineralogy, geochemistry, and microbiology of three mine tailings impoundments and found that all flotation tailings have the potential to produce acid, posing risks to both the environment and groundwater. Consequently, removing excess moisture from these materials is crucial to mitigate environmental hazards. Valenzuela et al. [
12] addressed this issue by employing electroosmotic drainage and compared its efficacy to gravity drainage. Through their tests of various electrode configurations, voltage levels, and electrode distances, they found that introducing an electric potential to the solid residue from copper leaching speeds up the drainage process.
Alshawabkeh et al. [
13] proposed a method to evaluate the performance of 1-D and 2-D electrokinetic remediation using different electrode configurations. They compared these configurations based on time, energy consumption, and optimal spacings. The electrokinetic phenomena encompass various processes, such as electrophoresis, electroosmosis, diffusiophoresis, capillary osmosis, sedimentation potential, streaming potential/current, colloid vibration current, and electric sonic amplitude [
14]. Electroosmotic flow, defined as the flow of a fluid in a porous medium under the influence of an electric field, was explained by Eykholt and Daniel [
15]. By applying electric currents and placing electrodes in the system, contaminants can be removed from the soil. Typically, this fluid flow aligns with the direction of the applied electric potential, moving from the anode to the cathode [
15]. Zhou et al. [
16] reported that by enhancing the electric field strength, water can be more efficiently removed from sludge. They suggested that electro-dewatering could extract both capillary water and free water, while vacuum filtration was only effective in removing free water from the sludge.
Despite the promising advancements in tank leaching, the adoption of electrokinetic techniques is not without its challenges. These include the substantial heterogeneity of the material [
17], which can disrupt the uniformity of the electrical field, hindering the technique’s effectiveness. Power consumption due to the continuous electric field application is a significant operational cost, posing a barrier to its broad implementation. Scaling up from laboratory settings to larger, real-world scenarios is another pivotal issue. The degradation and corrosion of electrode materials over time not only reduce the process’s efficiency but also elevate maintenance costs [
18]. Additionally, ion migration resulting from the electrical field can create acidic or alkaline conditions at the electrodes, which could affect the process or the electrodes’ structural integrity. The heat generated by electrokinetic methods might lead to evaporation [
19], reducing the process’s efficiency and posing risks to equipment. The environmental impact cannot be overlooked, as while electrokinetics may boost leaching efficiency, they might also unintentionally spread contaminants. Hence, future studies on the application of electrokinetic techniques in tank leaching should tackle these challenges to optimize their effectiveness and relevance.
Valenzuela et al. [
12] carried out a pilot study employing electroosmotic drainage to treat solid waste from copper leaching. They assessed the process using various electrode configurations, voltage levels, and distances between electrodes. Specifically, they tested three electrode configurations: hexagonal, linear, and alternate linear, alongside two voltage levels (12 V and 24 V). The best results were achieved using the alternate linear electrode configuration at 12 V. Their findings revealed that while electrokinetic processes can expedite the drainage in leaching systems, performance is sensitive to multiple conditions and parameters. Thus, conducting various tests can ascertain the ideal conditions for achieving optimal results.
Electrokinetics is a promising technology for the dewatering and remediation of fine-grained soil. Shafaei et al. [
20] investigated the impact of the electrokinetic process on extracting trapped water from the tailings slurry of a copper mine and assessed the effect of the dewatering process on copper remediation. They utilized a one-dimensional cylindrical cell to measure the electroosmotic permeability, which ranged from 1.23 × 10
−9 to 1.36 × 10
−9 m
2/V·s. The highest efficiency in water extraction was achieved by applying a voltage gradient of 1 V/cm over a period of 24 h.
Shafaei et al. [
18] studied the potential of mechanical–electrical dewatering in recovering water from the tailings slurry of the Miduk copper mine. Their research examined the effects of both voltage levels and mechanical pressures on the dewatering process, with experiments conducted over 120 min. Results revealed that mechanical filtration at pressures of 3 and 6 bars extracted 63 and 71% of the process water, respectively. An increase in the applied voltage led to a higher water recovery rate, underscoring the efficiency of the combined mechanical–electrical dewatering approach. Additionally, the average moisture content of the filtered tailings dropped to between 3.7 and 12.7%, confirming successful dewatering.
Although many previous experimental and modeling studies investigated the transport of electrophobic water and the migration of copper ions in electrokinetic remediation processes from an environmental perspective, the use of electrokinetic systems in increasing metal recovery from ores in hydrometallurgical systems, including heap and tank leaching, has not been fully investigated. Therefore, there is a need to model and conduct experiments incorporating both economic aspects and environmental considerations in order to increase metal extraction and minimize water and soil pollution. In this study, the role of the electrokinetic process in increasing the efficiency of copper recovery in a tank leaching system is evaluated from an economic standpoint, in addition to considering the environmental aspect. Moreover, the model presented here takes into account both hydraulic and electroosmotic flow in the transport of copper ions from the anode to the cathode. This method can be effectively applied in the copper industry, especially in the tank leaching system, which mainly deals with high-grade ores compared to the heap leaching process. Furthermore, the leaching of very low-grade ores may be investigated using this method to increase copper recovery via the electrokinetic process, which is essential for maximizing profitability in mining.
We apply the electrokinetic process to solid waste from tank leaching at the Nasim mine to reduce the moisture content of the solid residue and transport inert copper cations. The high moisture content of these wastes poses an environmental hazard, as they can remain in place for extended periods, leading to acid mine drainage (AMD) with high concentrations of copper and other toxic metals. Furthermore, the residual copper in the waste is not economically viable. Therefore, we conducted three sets of experiments to evaluate drainage efficiency, comparing moisture reduction to natural drainage and modeling the transport of copper cations.
3. Results and Discussion
3.1. Experiment
Table 3,
Table 4 and
Table 5 display the experimental results.
Table 3 documents the outcomes from the anode section of the soil;
Table 4 pertains to the middle section, while
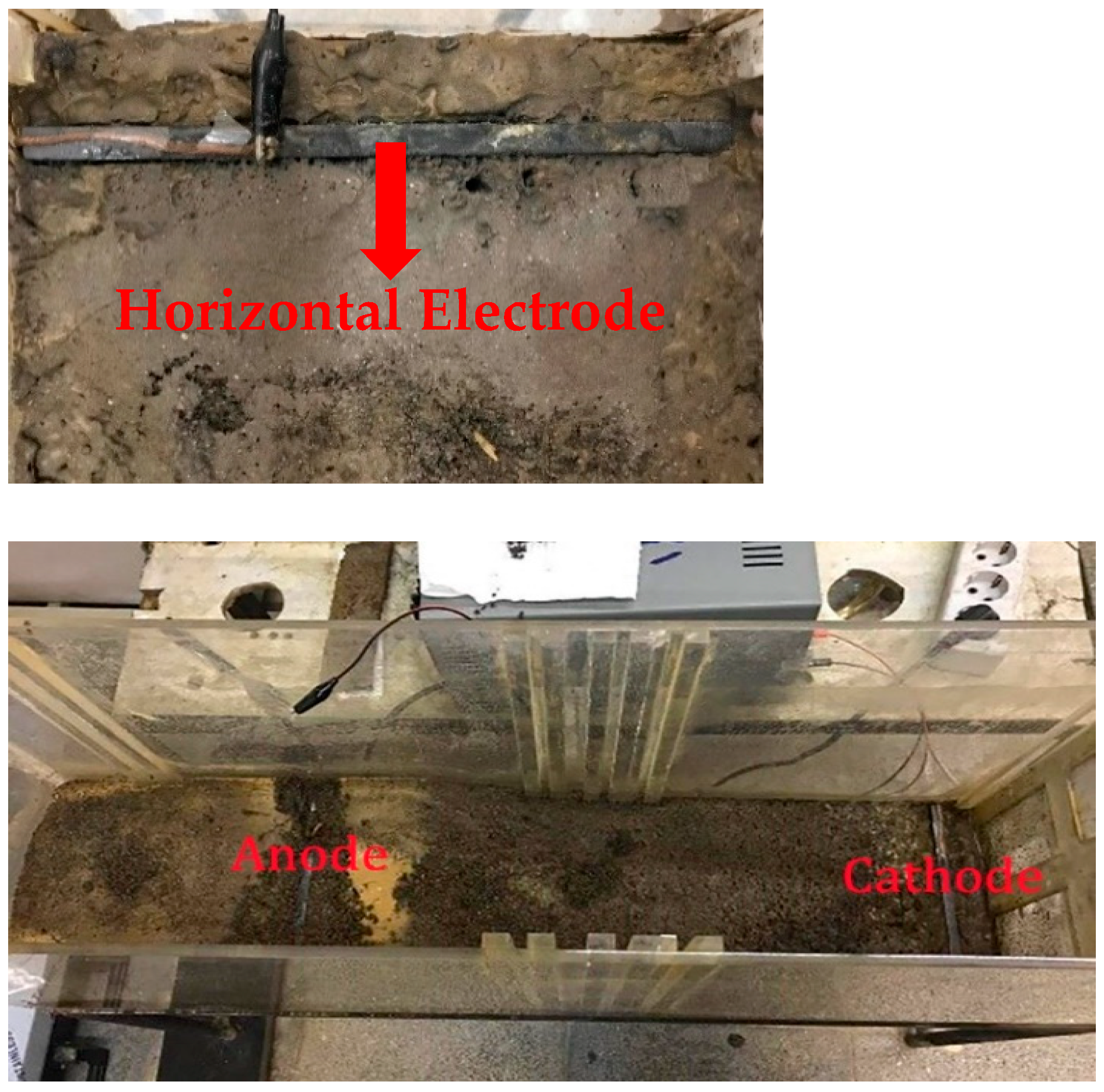
Table 5 presents the findings from the cathode section. These tables juxtapose the residual moisture content under three conditions: natural drainage of solid residue from tank leaching and drainage induced by the electrokinetic process, comparing two distinct electrode arrangements—one with horizontally placed electrodes in the soil and the other with vertically positioned electrodes within the soil.
At the beginning of the tests, the water content in all cases was almost the same. In the anode part, after 14 days of applying the electrokinetic process with vertical electrodes, the moisture content of the soil decreased by only 3.66%. However, with horizontal electrodes, it reduced by 8.8%, and naturally, it decreased by 1.73%. After 14 days of testing with horizontal electrodes, it was observed that the soil had nearly dried up, causing a decrease in current until it reached zero. Therefore, the application of the electrokinetic procedure in the system was terminated. On the other hand, this process took 34 days during the test with vertical electrodes. To compare these tests with natural drainage, a system without applying an electrokinetic process was used for a period of 34 days. Measurements were conducted in the middle part of the soil, where the electrokinetic process with horizontal electrodes took 16 days, and the moisture content of the soil decreased by 14.47%. In contrast, in the test with vertical electrodes, this value was reduced by 6.48% for the same time period. Additionally, the highest moisture reduction occurred in the cathode part: 38.4% with horizontal electrodes after 20 days of applying an electrokinetic process in the system, 12.28% with vertical electrodes after 20 days, and 31.48% after 34 days. It is evident from all the tests that the most significant reduction in moisture content occurred with horizontal electrodes because the electrodes have the most contact surface with the soil, making it easier for the current to penetrate the wet soil.
3.2. Modeling
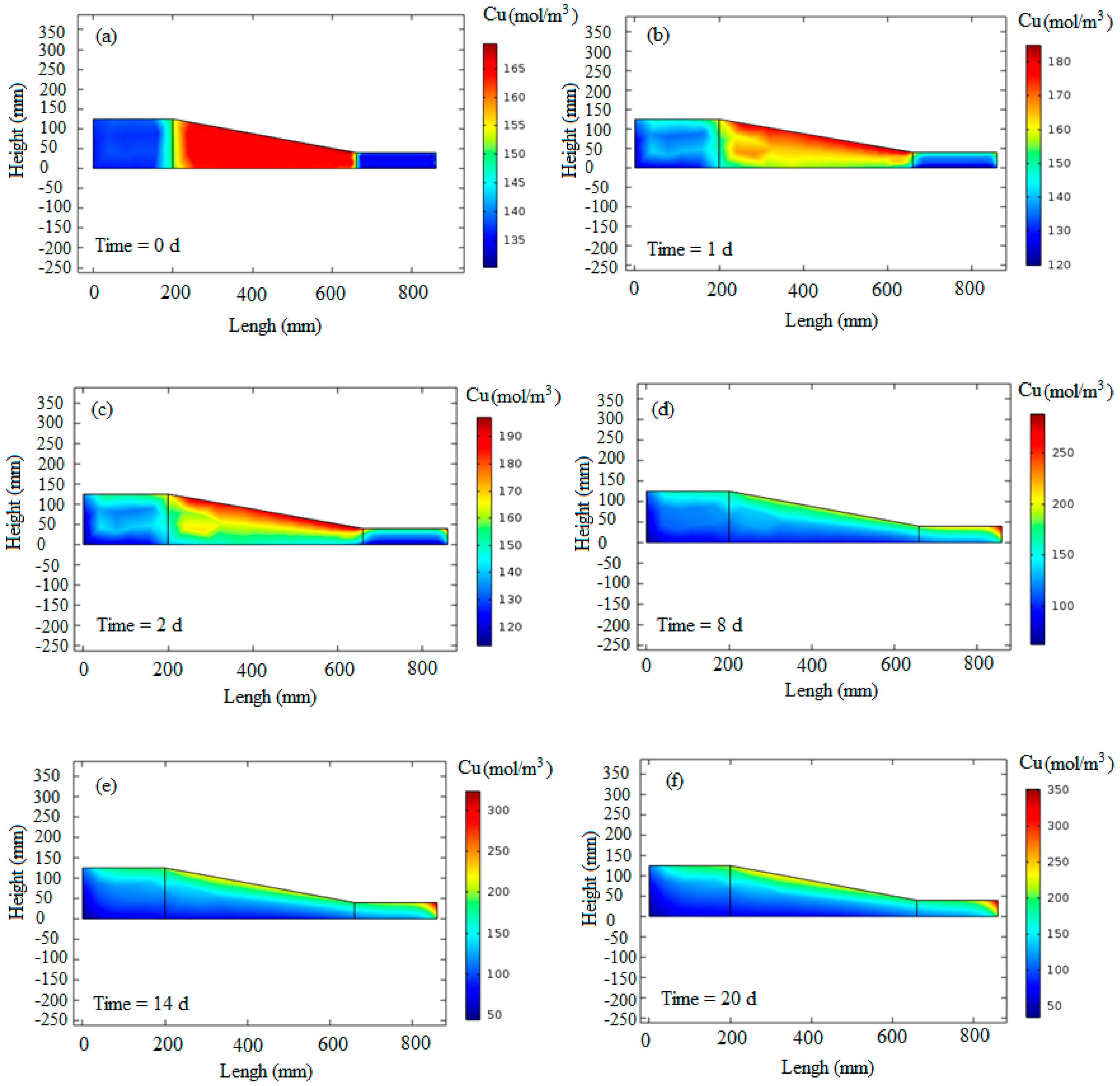
Figure 9 displays the results of copper transport during the electrokinetic process over six different days. At the beginning of the simulation, the copper concentration was set to match the laboratory measurements, which were 137.5 mol/m
3 in the anode part, 165 mol/m
3 in the middle part, and 135 mol/m
3 in the cathode part (
Figure 9a).
Table 11 presents the maximum percentage error in predicting the copper concentration at various measurement points over time [
39].
The highest percentage difference between the model prediction and the experimental data occurs in a period of 20 days, for which the model has overestimated so that the relative error is more than 10%. This relative error may be obtained at various stages of the laboratory experiment or modeling procedure. Both the model and the laboratory work show an increasing trend for Cu ion accumulation on the cathode side. This means that by increasing the processing time, it is possible to increase the concentration of copper in the cathode. In similar research, the effect of increasing the voltage and mechanical pressure of the soil in increasing the electrokinetic efficiency has been concluded [
18].
In
Figure 9b, the concentration of copper is observed to change from 120 to 180 mol/m
3 on the first day of the test. This indicates the transportation of copper from the anode to the cathode side. The anode part exhibited the minimum copper concentration, while the maximum concentration was evident near the cathode across all segments.
Figure 9c, representing the second day of the test, shows a decrease in copper concentration near the anode and an increase near the cathode, highlighting the continued movement of copper cations in the system.
Figure 9d–f depicts the 8th, 14th, and last days of the test, respectively. The simulated copper concentrations align well with the laboratory measurements. A comparison between the experimental measurements and numerical modeling for the anode and cathode parts is presented in
Figure 10a and
Figure 10b, respectively.
The results obtained from modeling help optimize efficiency, cost, and energy. The use of an electrokinetic process facilitated the migration of copper cations from the anode to the cathode in the tank leaching system, which, in turn, reduced the cost and energy consumption.
Furthermore, as
Figure 10 shows, the simulated Cu ion content on the cathode side increased from 135 mol/m
3 to above 220 mol/m
3 after 20 days. Also, in the same period of time, it decreased from 137.5 mol/m
3 to less than 70 mol/m
3 on the anode side. These results show that the electrokinetic process can significantly increase copper transport and its recovery efficiency in the tank leaching process.
As copper ions transport occurs via both electrokinetic and hydraulic mechanisms, this allows relatively rapid movement of ions through low hydraulic conductivity media, thus circumventing the need for permeability enhancement techniques such as ore crushing, which in turn reduces energy and tank washing time.
Both the modeling predictions and the results of the laboratory work show that the optimum time for the tank leaching operation was 20 days. Determining the optimal leaching time will increase the speed of metal recovery from ore, reduce operation time, and reduce related costs.
4. Conclusions and Future Prospects
Laboratory studies have shown that a significant amount of fluid, which contains 5 gr/L of copper, remains trapped in the fine-grained portion of the soil following the copper leaching process and the exit of the PLS. Given this, it becomes imperative for mining investors to devise a method to extract copper from this retained moisture, addressing both economic and environmental concerns. Recognized for its capacity to transport metals and aid water movement in soil, the electrokinetic process presents a potential solution. This research sought to explore the efficacy of the electrokinetic process in accelerating copper transport and extracting copper from soil moisture. This exploration involved both laboratory experiments and numerical modeling. Two electrode configurations—vertical and horizontal—were tested with variable electrode spacing and voltages to diminish the moisture content of the solid residues resulting from tank leaching. The findings revealed that horizontal electrodes were superior in facilitating moisture transfer owing to their enhanced contact with the soil. Specifically, in the cathode portion of the soil, a 20-day electrokinetic treatment led to a moisture content reduction of 12.28% with vertical electrodes, compared to a 38.4% reduction with horizontal ones. Additionally, this study delved into the transport dynamics of copper cations from the anode to the cathode under the influence of the electrokinetic process.
The study’s findings indicated that the copper content in the soil, located near the cathode electrode, rose from 0.54% to 0.77% following a 20-day electrokinetic treatment. Additionally, the experiments revealed that the electrokinetic process expedited the evaporation of pore water, which became evident when comparing scenarios with and without the application of the electrokinetic process. A numerical model was also established to study the transport of copper under the electrokinetic process in an aqueous solution. After a 20-day application of the process, the model predicted the copper cation concentration in the cathode region to be between 150 and 350 mol/m3. Experimental data indicated an actual concentration of 192.5 mol/m3 in the solution extracted from the cathode part, which aligns well with the model’s predictions. It is important to emphasize that while these results underscore the potential for scaling up the electrokinetic process, further research is required to confirm its wider applicability.
We recommend that future studies conduct experiments using various electrode configurations to explore the impact of electrode spacing and voltage on the transport and removal of copper from soil moisture. Such investigations would offer more comprehensive insights and facilitate the optimization of the electrokinetic process for enhanced copper extraction from soil moisture.
The determination and interpretation of electrical resistivity are complex within the tank leaching medium due to the joined impact of moisture variation, temperature changes, and chemical reactions such as hydrolysis, precipitation, and secondary minerals formation. This can increase the future relevance and significance of the research.
In addition, determining the minimum amount of energy consumption for the migration of copper ions remaining on the anode side can be considered in future studies; for this purpose, the optimal voltage should be determined correctly. In addition, a combination of mechanical pressure and the selection of an optimal voltage will enable us to transfer a considerable content of copper from the anode side to the cathode side, which will increase copper recovery, especially in low-grade ores with very low permeability.