Occurrence Assessment of Pharmaceuticals in Various Sewage Treatment Plants and Effluent-Receiving Streams in Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Target STPs
2.2. Target Pharmaceuticals and Chemical Reagents
2.3. Target Pharmaceutical Analysis
2.4. Quality Control
2.5. Organic Carbon Analysis
3. Results and Discussion
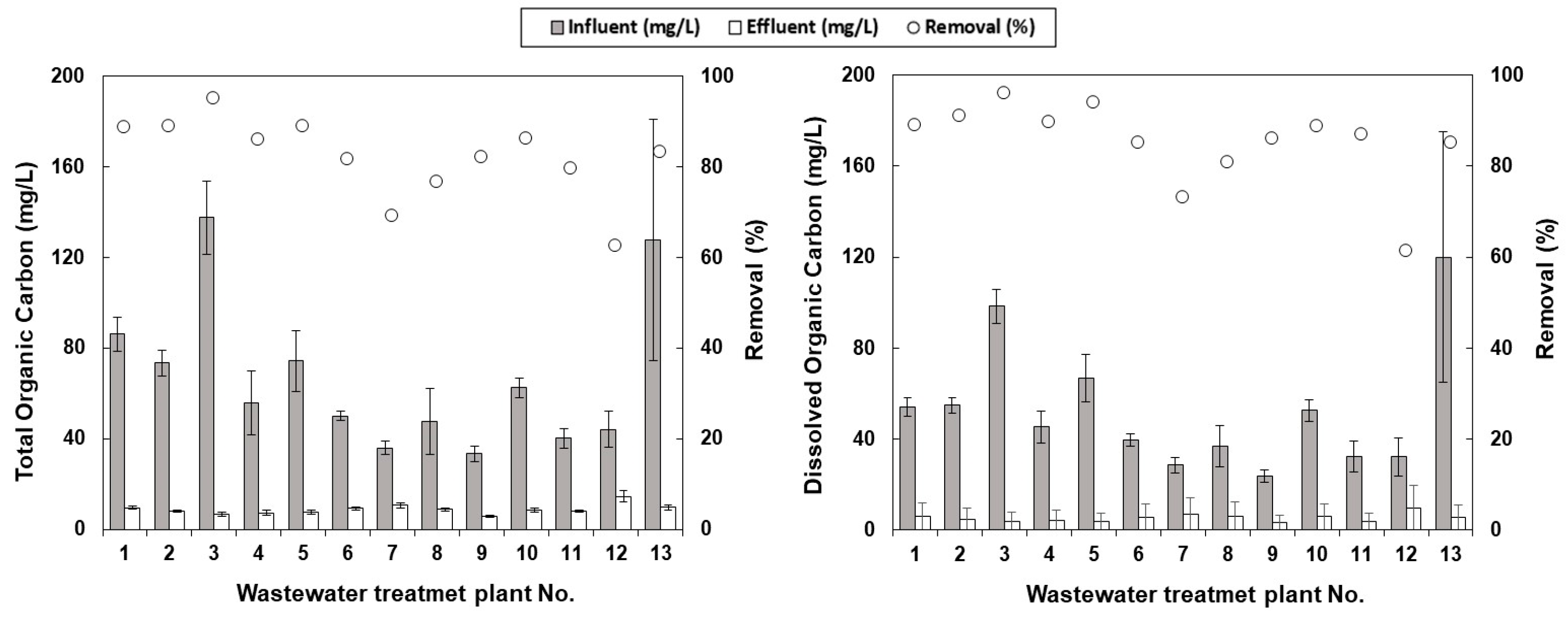
3.1. Organic Carbon Removal of Selected STPs
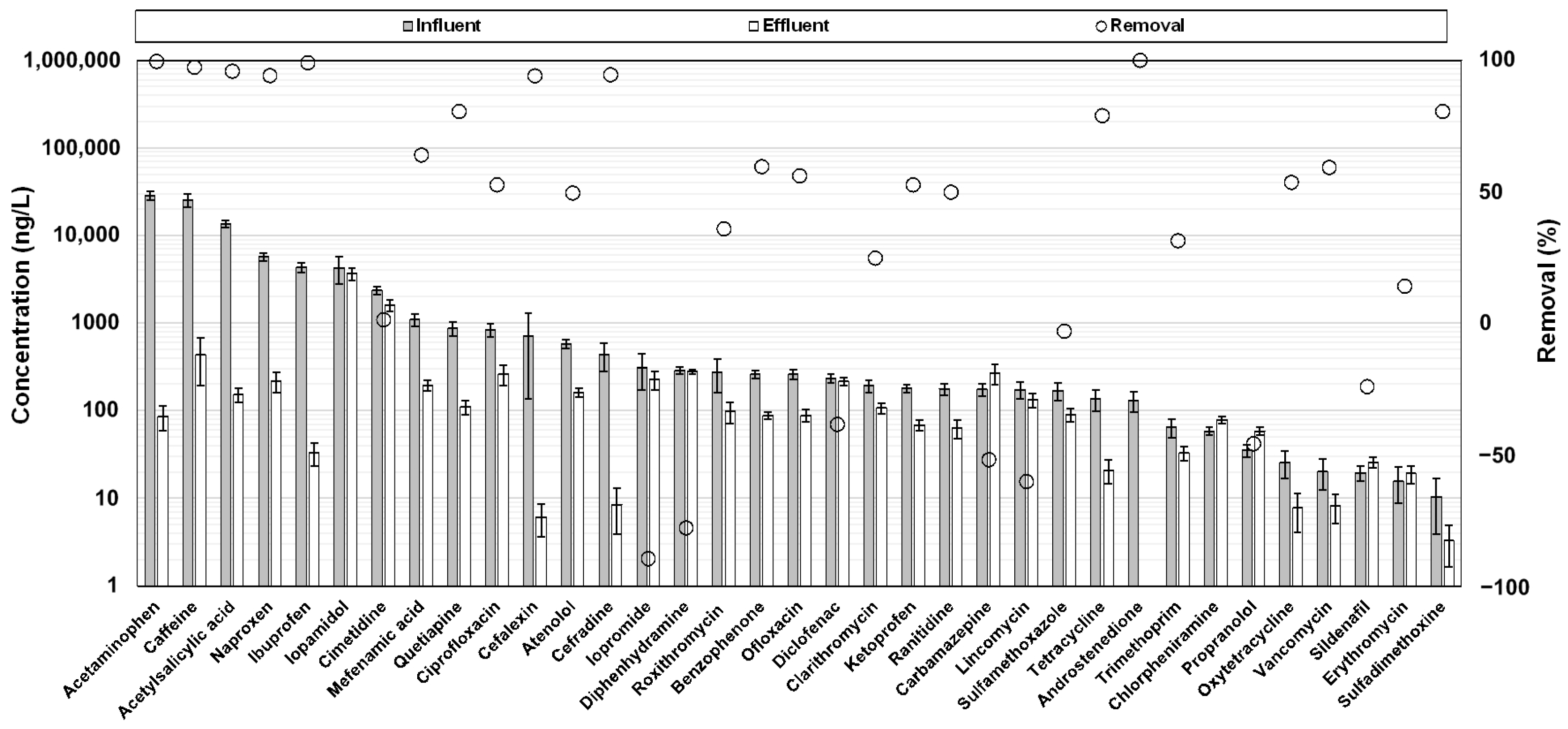
3.2. Occurrence and Fate of Target Pharmaceuticals in Each STP
3.3. Occurrence of Target Pharmaceuticals in STPs Based on Regional Differences
3.3.1. Urban Area
3.3.2. Urban and Rural Complex Area
3.3.3. Rural Area
3.4. Occurrence of Target Pharmaceuticals in STPs Based on Linked Wastewater Differences
3.4.1. Industrial
3.4.2. Livestock
3.4.3. Leachate
3.4.4. Food Wastewater
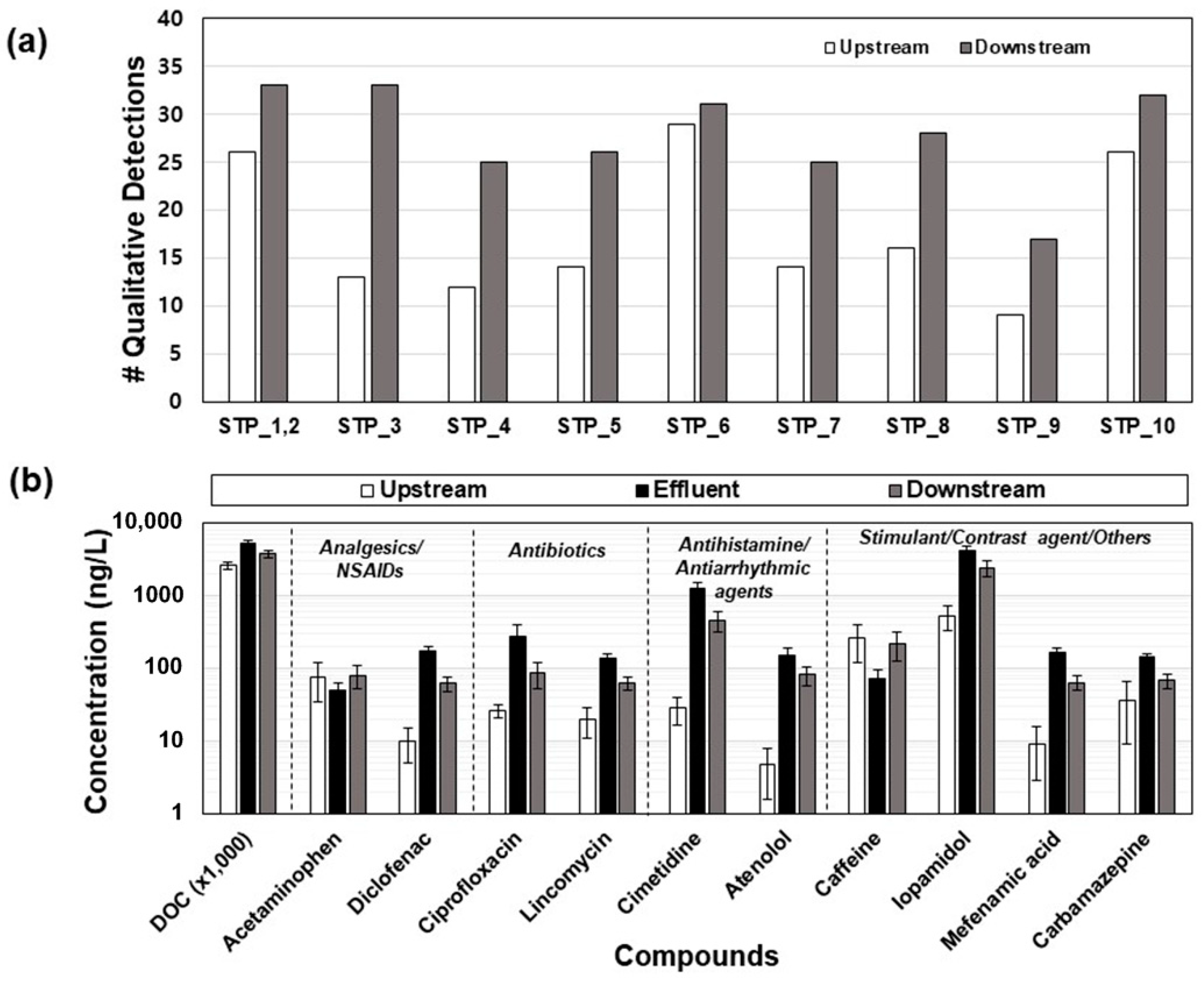
3.5. Occurrence of Pharmaceuticals in Effluent-Receiving Streams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and Fate of Emerging Contaminants in Water Environment: A Review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, Endocrine Disruptors, Personal Care Products, Nanomaterials and Perfluorinated Pollutants: A Review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and Removal of Pharmaceuticals and Personal Care Products (PPCPs) in Drinking Water and Water/Sewage Treatment Plants: A Review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef]
- Blair, B.D.; Crago, J.P.; Hedman, C.J.; Treguer, R.J.F.; Magruder, C.; Royer, L.S.; Klaper, R.D. Evaluation of a Model for the Removal of Pharmaceuticals, Personal Care Products, and Hormones from Wastewater. Sci. Total Environ. 2013, 444, 515–521. [Google Scholar] [CrossRef]
- Hedgespeth, M.L.; Sapozhnikova, Y.; Pennington, P.; Clum, A.; Fairey, A.; Wirth, E. Pharmaceuticals and Personal Care Products (PPCPs) in Treated Wastewater Discharges into Charleston Harbor, South Carolina. Sci. Total Environ. 2012, 437, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, L.; Chang, A.C. Seasonal Variation of Endocrine Disrupting Compounds, Pharmaceuticals and Personal Care Products in Wastewater Treatment Plants. Sci. Total Environ. 2013, 442, 310–316. [Google Scholar] [CrossRef]
- Aydin, S.; Aydin, M.E.; Ulvi, A.; Kilic, H. Antibiotics in Hospital Effluents: Occurrence, Contribution to Urban Wastewater, Removal in a Wastewater Treatment Plant, and Environmental Risk Assessment. Environ. Sci. Pollut. Res. 2019, 26, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of Emerging Pollutants in Urban Wastewater and Their Removal through Biological Treatment Followed by Ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Kim, S.G.; Kim, S.H. Removal of Antibiotics by Coagulation and Granular Activated Carbon Filtration. J. Hazard. Mater. 2008, 151, 38–43. [Google Scholar] [CrossRef]
- Tewari, S.; Jindal, R.; Kho, Y.L.; Eo, S.; Choi, K. Major Pharmaceutical Residues in Wastewater Treatment Plants and Receiving Waters in Bangkok, Thailand, and Associated Ecological Risks. Chemosphere 2013, 91, 697–704. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions-a Review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Tran, N.H.; Chen, H.; Reinhard, M.; Mao, F.; Gin, K.Y.H. Occurrence and Removal of Multiple Classes of Antibiotics and Antimicrobial Agents in Biological Wastewater Treatment Processes. Water Res. 2016, 104, 461–472. [Google Scholar] [CrossRef]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, Fate and Transformation of Emerging Contaminants in Water: An Overarching Review of the Field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Ng, C.; Tran, N.H.; Chen, H.; Gin, K.Y.H. Removal of Antibiotic Residues, Antibiotic Resistant Bacteria and Antibiotic Resistance Genes in Municipal Wastewater by Membrane Bioreactor Systems. Water Res. 2018, 145, 498–508. [Google Scholar] [CrossRef] [PubMed]
- WHO. Integrated Surveillance of Antimicrobial Resistance in Foodborne Bacteria: Application of a One Health Approach. Available online: https://iris.who.int/bitstream/handle/10665/255747/9789241512411-eng.pdf?sequence=1 (accessed on 31 October 2023).
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Elimination of Pharmaceuticals in Sewage Treatment Plants in Finland. Water Res. 2007, 41, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Kim, H.W.; Oh, J.E.; Park, H.S. Occurrence and Removal of Antibiotics, Hormones and Several Other Pharmaceuticals in Wastewater Treatment Plants of the Largest Industrial City of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kim, Y.; Park, J.; Park, C.K.; Kim, M.Y.; Kim, H.S.; Kim, P. Seasonal Variations of Several Pharmaceutical Residues in Surface Water and Sewage Treatment Plants of Han River, Korea. Sci. Total Environ. 2008, 405, 120–128. [Google Scholar] [CrossRef]
- Ghosh, G.C.; Nakada, N.; Yamashita, N.; Tanaka, H. Occurrence and Fate of Oseltamivir Carboxylate (Tamiflu) and Amantadine in Sewage Treatment Plants. Chemosphere 2010, 81, 13–17. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Mass Flows and Removal of Antibiotics in Two Municipal Wastewater Treatment Plants. Chemosphere 2011, 83, 1284–1289. [Google Scholar] [CrossRef]
- Sim, W.J.; Lee, J.W.; Lee, E.S.; Shin, S.K.; Hwang, S.R.; Oh, J.E. Occurrence and Distribution of Pharmaceuticals in Wastewater from Households, Livestock Farms, Hospitals and Pharmaceutical Manufactures. Chemosphere 2011, 82, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Balakrishna, K.; Joshua, D.I.; Kannan, K. Mass Loading and Removal of Pharmaceuticals and Personal Care Products Including Psychoactives, Antihypertensives, and Antibiotics in Two Sewage Treatment Plants in Southern India. Chemosphere 2017, 167, 429–437. [Google Scholar] [CrossRef]
- Sun, Q.; Li, M.; Ma, C.; Chen, X.; Xie, X.; Yu, C.P. Seasonal and Spatial Variations of PPCP Occurrence, Removal and Mass Loading in Three Wastewater Treatment Plants Located in Different Urbanization Areas in Xiamen, China. Environ. Pollut. 2016, 208, 371–381. [Google Scholar] [CrossRef]
- Tran, N.H.; Gin, K.Y.H. Occurrence and Removal of Pharmaceuticals, Hormones, Personal Care Products, and Endocrine Disrupters in a Full-Scale Water Reclamation Plant. Sci. Total Environ. 2017, 599–600, 1503–1516. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Du, J.; Qu, Y.; Shen, C.; Tan, F.; Chen, J.; Quan, X. Occurrence, Removal, and Risk Assessment of Antibiotics in 12 Wastewater Treatment Plants from Dalian, China. Environ. Sci. Pollut. Res. 2017, 24, 16478–16487. [Google Scholar] [CrossRef]
- Guerra, P.; Kim, M.; Shah, A.; Alaee, M.; Smyth, S.A. Occurrence and Fate of Antibiotic, Analgesic/Anti-Inflammatory, and Antifungal Compounds in Five Wastewater Treatment Processes. Sci. Total Environ. 2014, 473–474, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Buleté, A.; Vulliet, E.; Deshayes, S.; Zedek, S.; Mirande-Bret, C.; Eudes, V.; Bressy, A.; et al. Removal of a Wide Range of Emerging Pollutants from Wastewater Treatment Plant Discharges by Micro-Grain Activated Carbon in Fluidized Bed as Tertiary Treatment at Large Pilot Scale. Sci. Total Environ. 2016, 542, 983–996. [Google Scholar] [CrossRef]
- Thiebault, T.; Boussafir, M.; Le Milbeau, C. Occurrence and Removal Efficiency of Pharmaceuticals in an Urban Wastewater Treatment Plant: Mass Balance, Fate and Consumption Assessment. J. Environ. Chem. Eng. 2017, 5, 2894–2902. [Google Scholar] [CrossRef]
- de Franco, M.A.E.; de Carvalho, C.B.; Bonetto, M.M.; de Pelegrini Soares, R.; Féris, L.A. Diclofenac Removal from Water by Adsorption Using Activated Carbon in Batch Mode and Fixed-Bed Column: Isotherms, Thermodynamic Study and Breakthrough Curves Modeling. J. Clean. Prod. 2018, 181, 145–154. [Google Scholar] [CrossRef]
- Gardner, M.; Jones, V.; Comber, S.; Scrimshaw, M.D.; Coello-Garcia, T.; Cartmell, E.; Lester, J.; Ellor, B. Performance of UK Wastewater Treatment Works with Respect to Trace Contaminants. Sci. Total Environ. 2013, 456–457, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Keen, O.S.; Baik, S.; Linden, K.G.; Aga, D.S.; Love, N.G. Enhanced Biodegradation of Carbamazepine after UV/H2O 2 Advanced Oxidation. Environ. Sci. Technol. 2012, 46, 6222–6227. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Feng, Y.; Li, X.; Suo, N.; Chen, H.; Wang, Z.; Yu, Y. Removal of Diclofenac by Three-Dimensional Electro-Fenton-Persulfate (3D Electro-Fenton-PS). Chemosphere 2019, 219, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, M.; Ghaffari, E.; Aminzadeh, B. Removal of Carbamazepine from Municipal Wastewater Effluent Using Optimally Synthesized Magnetic Activated Carbon: Adsorption and Sedimentation Kinetic Studies. J. Environ. Chem. Eng. 2016, 4, 3309–3321. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Degradation of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solution, a Direct Comparison of Ozonation, Photocatalysis, and Non-Thermal Plasma. Chem. Eng. J. 2017, 313, 1033–1041. [Google Scholar] [CrossRef]
- Komtchou, S.; Dirany, A.; Drogui, P.; Bermond, A. Removal of Carbamazepine from Spiked Municipal Wastewater Using Electro-Fenton Process. Environ. Sci. Pollut. Res. 2015, 22, 11513–11525. [Google Scholar] [CrossRef] [PubMed]

| STP | Capacity (m3/d) | Process | Regional Feature | Linked Wastewater | ||
|---|---|---|---|---|---|---|
| Flowrate (m3/d) | Percentage (%) | Wastewater Type | ||||
| 1 | 22,000 | HDF 1 | Urban and rural complex area | - | - | - |
| 2 | 17,000 | HDF 1 | Urban and rural complex area | - | - | - |
| 3 | 47,000 | Bio-SAC | Urban | - | - | - |
| 4 | 1500 | SMMIAR 2 | - | 124.6 | 14.7 | Industrial |
| 5 | 25,000 | Activated sludge, DNR 3 | - | 1916.6 | 8.9 | Industrial |
| 6 | 12,900 | HDF 1 | - | 163.7 | 1.3 | Livestock |
| 7 | 10,000 | NPR 4 | - | 315.7 | 4.0 | Livestock |
| 8 | 30,000 | SDPR 5 | - | 296 | 1.1 | Leachate |
| 9 | 2000 | DeNiPho | - | 59.6 | 2.8 | Leachate |
| 10 | 16,000 | CSBR 6 | - | 209.9 | 2.3 | Food waste |
| 11 | 3000 | DeNiPho | - | 68.2 | 2.5 | Food waste |
| 12 | 200 | KSBNR 7 | Rural | - | - | - |
| 13 | 300 | CF-SBR 8 | Rural | - | - | - |
| No. | Pharmaceuticals | CAS No. | Molecular Formula | Molecular Weight (g/mol) | pKa | Water Solubility (mg/mL) | log Kow |
|---|---|---|---|---|---|---|---|
| Analgesics/Non-Steroidal Anti-Inflammatory Drugs | |||||||
| 1 | Acetaminophen | 103-90-2 | C8H9NO2 | 151.165 | 9.38 | 14 (at 20 °C) | 0.46 |
| 2 | Acetylsalicylic acid | 50-78-2 | C9H8O4 | 180.159 | 3.49 | 3.3 (at 20 °C) | 1.19 |
| 3 | Diclofenac | 15307-86-5 | C14H11Cl2NO2 | 296.147 | 4.15 | 2.37 × 10−3 (at 25 °C) | 4.51 |
| 4 | Ibuprofen | 15687-27-1 | C13H18O2 | 206.285 | 5.3 | 2.1 × 10−2 (at 25 °C) | 3.97 |
| 5 | Ketoprofen | 22071-15-4 | C16H14O3 | 254.28 | 4.45 | 5.1 × 10−2 (at 25 °C) | 3.12 |
| 6 | Naproxen | 22204-53-1 | C14H14O3 | 230.263 | 4.15 | 15.9 × 10−3 (at 25 °C) | 3.18 |
| Antibiotics | |||||||
| 7 | Amoxicillin | 26787-78-0 | C16H19N3O5S | 365.404 | 3.23 * 7.43 ** | 3.43 (at 25 °C) | 0.87 |
| 8 | Cefalexin | 15686-71-2 | C16H17N3O4S | 347.389 | 3.26 * 7.23 ** | 10 | 0.65 |
| 9 | Cefradine | 38821-53-3 | C16H19N3O4S | 349.4 | 3.46 * 7.6 ** | 21.3 | −1.5 |
| 10 | Chlortetracycline | 57-62-5 | C22H23CIN2O8 | 478.882 | 2.99 * 9.04 ** | 0.259 *** | −0.62 |
| 11 | Ciprofloxacin | 85721-33-1 | C17H18FN3O3 | 331.4 | 6.09 | <1 | 0.28 |
| 12 | Clarithromycin | 81103-11-9 | C38H69NO13 | 747.964 | 8.99 | 0.33 × 10−3 | 3.16 |
| 13 | Cloxacillin | 61-72-3 | C19H18ClN3O5S | 435.9 | 2.78 | 13.9 × 10−3 | 2.48 |
| 14 | Demeclocycline | 127-33-3 | C21H21ClN2O8 | 464.9 | −2.6 * 8.23 ** | 1.52 (at 21 °C) | 0.2 |
| 15 | Erythromycin | 114-07-8 | C37H67NO13 | 733.937 | 8.88 | 2 | 3.06 |
| 16 | Florfenicol | 73231-34-2 | C12H14Cl2FNO4S | 358.2 | 8.49 * −3.4 ** | 0.219 *** | |
| 17 | Flumequine | 42835-25-6 | C14H12FNO3 | 261.25 | 6.5 | 2.19 *** (at 25 °C) | 1.6 |
| 18 | Lincomycin | 154-21-2 | C18H34N2O6S | 406.5 | 7.6 | 0.927 *** (at 25 °C) | 0.29 |
| 19 | Ofloxacin | 82419-36-1 | C18H20FN3O4 | 361.373 | 5.45 * 6.2 ** | 28.3 | −0.39 |
| 20 | Oseltamivir | 196618-13-0 | C16H28N2O4 | 312.4 | 14.03 * 9.31 ** | 1.6 *** (at 25 °C) | 1 |
| 21 | Oseltamivir acid | 187227-45-8 | C14H24N2O4 | 284.35 | 4.19 * 9.33 ** | 0.686*** | 0.95 |
| 22 | Oxytetracycline | 79-57-2 | C22H24N2O9 | 460.439 | 3.27 | 0.313 (at 25 °C) | −0.90 |
| 23 | Pefloxacin | 70458-92-3 | C17H20FN3O3 | 333.36 | 5.66 * 6.47 ** | 11.4 (at 25 °C) | 0.27 |
| 24 | Penicillin G | 61-33-6 | C16H18N2O4S | 334.4 | 2.74 | 0.21 | 1.83 |
| 25 | Penicillin V | 87-08-1 | C16H18N2O5S | 350.4 | 2.79 | <1 | 2.09 |
| 26 | Roxithromycin | 80214-83-1 | C41H76N2O15 | 837.058 | 12.45 * 9.08 ** | 0.187 | 1.7 |
| 27 | Sulfachlorpyridazine | 80-32-0 | C10H9ClN4O2S | 284.72 | 6.6 * 2.02 ** | 0.035 | 0.31 |
| 28 | Sulfadimethoxine | 122-11-2 | C12H14N4O4S | 310.3 | 6.91 * 1.95 ** | 0.343 | 1.63 |
| 29 | Sulfamethazine | 57-68-1 | C12H14N4O2S | 278.330 | 7.59 | 1.5 (at 29 °C) | 0.89 |
| 30 | Sulfamethoxazole | 723-46-6 | C10H11N3O3S | 253.276 | 6.16 * 1.97 ** | 0.61 (at 37 °C) | 0.89 |
| 31 | Sulfathiazole | 72-14-0 | C9H9N3O2S2 | 255.310 | 7.2 | 0.373 (at 25 °C) | 0.05 |
| 32 | Tetracycline | 60-54-8 | C19H28O2 | 288.4 | 3.3 | 0.231 (at 25 °C) | −1.3 |
| 33 | Triclosan | 3380-34-5 | C22H24N2O8 | 444.440 | 7.9 | 0.01 (at 20 °C) | 4.76 |
| 34 | Trimethoprim | 738-70-5 | C14H18N4O3 | 290.3 | 7.12 | 0.4 (at 25 °C) | 0.91 |
| 35 | Tylosin | 1401-69-0 | C46H77NO17 | 916.1 | 7.73 | 5 (at 25 °C) | 1.63 |
| 36 | Vancomycin | 1404-90-6 | C66H75Cl2N9O24 | 1449.2 | 2.99 * 9.93 ** | 0.225 | −3.1 |
| Antiarrhythmic Agents | |||||||
| 37 | Atenolol | 29122-68-7 | C14H22N2O3 | 266.341 | 9.6 | 13.3 (at 25 °C) | 0.16 |
| 38 | Metoprolol | 51384-51-1 | C15H25NO3 | 267.369 | 9.7 | 0.402 *** | 1.88 |
| 39 | Propranolol | 525-66-6 | C16H21NO2 | 259.349 | 9.42 | 0.0617 (at 25 °C) | 3.48 |
| Antihistamines | |||||||
| 40 | Chlorpheniramine | 132-22-9 | C16H19ClN2 | 274.79 | 9.13 | 160 (at 25 °C) | 3.38 |
| 41 | Cimetidine | 51481-61-9 | C10H16N6S | 252.340 | 6.8 | 9.38 (at 25 °C) | 0.40 |
| 42 | Diphenhydramine | 58-73-1 | C17H21NO | 255.35 | 8.98 | 3.06 (at 37 °C) | 3.27 |
| 43 | Ranitidine | 66357-35-5 | C13H22N4O3S | 314.404 | 7.8 ** | 0.0795 *** | 1.93 |
| Contrast Agents | |||||||
| 44 | Iopamidol | 60166-93-0 | C17H22I3N3O8 | 777.1 | 11 * −2.8 ** | 120 *** (at 20 °C) | −2.42 |
| 45 | Iopromide | 73334-07-3 | C18H24I3N3O8 | 791.1 | 11.09 * −1.7 ** | 0.336 *** | −2.05 |
| Hormones | |||||||
| 46 | Androstenedione | 63-05-8 | C19H26O2 | 286.409 | 19.03 * −4.8 ** | 0.0578 (at 25 °C) | 2.75 |
| 47 | Testosterone | 58-22-0 | C19H28O2 | 288.4 | 18.52 * −0.88 ** | 0.0234 (at 25 °C) | 3.32 |
| Stimulant | |||||||
| 48 | Caffeine | 58-08-2 | C8H10N4O2 | 194.19 | 14 | 21.7 | −0.07 |
| Others | |||||||
| 49 | Aripiprazole | 129722-12-9 | C23H27Cl2N3O2 | 448.4 | 7.6 | 0.00777 | 5.3 |
| 50 | Benzophenone | 119-61-9 | C13H10O | 182.22 | −7.5 ** | 0.137 (at 25 °C) | 3.18 |
| 51 | Carbamazepine | 298-46-4 | C15H12N2O | 236.274 | 15.96 * −3.8 ** | 0.152 *** | 2.45 |
| 52 | Fluoxetine | 54910-89-3 | C17H18F3NO | 309.3 | 10.1 | 0.0017 *** | 4.05 |
| 53 | Gemfibrozil | 25812-30-0 | C15H22O3 | 250.338 | 4.42 * −4.8 ** | 0.0278 *** | 4.387 |
| 54 | Mefenamic acid | 61-68-7 | C15H15NO2 | 241.28 | 4.2 | 0.02 (at 25 °C) | 5.12 |
| 55 | Quetiapine | 111974-69-7 | C21H25N3O2S | 383.51 | 7.06 | 0.0403 | 2.81 |
| 56 | Sildenafil | 139755-83-2 | C22H30N6O4S | 474.6 | 11.14 * 5.99 ** | 3.5 | 2.75 |
| 57 | Tadalafil | 171596-29-5 | C22H19N3O4 | 389.4 | 15.17 * −4.2 ** | 0.25 *** | 1.7 |
| 58 | Warfarin | 81-81-2 | C19H16O4 | 308.333 | 5 | 0.017 (at 20 °C) | 2.70 |
| Parameters | Condition |
|---|---|
| Mobile phase A, C | 0.1% formic acid in water |
| Mobile phase B | Methanol |
| Mobile phase D | Acetonitrile:methanol:IPA:water (1:1:1:1) |
| Gradient elution | 10% B pump (0–5 min)—100% B pump (5–20 min)—10% B pump (20 min–) |
| Injection volume | 900 μL |
| Flow rate | 1.5 mL/min |
| Ionization mode | ESI * negative, positive |
| Gas temperature | 270 °C |
| Gas flow | 12 L/min |
| Nebulizer | 40 psi |
| Sheath gas heater | 375 °C |
| Sheath gas flow | 11 L/min |
| Capillary voltage | (−)3500, (+)3500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, D.-J.; Kim, C.-S.; Lee, J.-H.; Yoon, J.-K.; Lee, S.-H.; Jeong, D.-H. Occurrence Assessment of Pharmaceuticals in Various Sewage Treatment Plants and Effluent-Receiving Streams in Korea. Water 2023, 15, 3897. https://doi.org/10.3390/w15223897
Son D-J, Kim C-S, Lee J-H, Yoon J-K, Lee S-H, Jeong D-H. Occurrence Assessment of Pharmaceuticals in Various Sewage Treatment Plants and Effluent-Receiving Streams in Korea. Water. 2023; 15(22):3897. https://doi.org/10.3390/w15223897
Chicago/Turabian StyleSon, Dong-Jin, Chang-Soo Kim, Jae-Ho Lee, Jeong-Ki Yoon, Soo-Hyung Lee, and Dong-Hwan Jeong. 2023. "Occurrence Assessment of Pharmaceuticals in Various Sewage Treatment Plants and Effluent-Receiving Streams in Korea" Water 15, no. 22: 3897. https://doi.org/10.3390/w15223897
APA StyleSon, D.-J., Kim, C.-S., Lee, J.-H., Yoon, J.-K., Lee, S.-H., & Jeong, D.-H. (2023). Occurrence Assessment of Pharmaceuticals in Various Sewage Treatment Plants and Effluent-Receiving Streams in Korea. Water, 15(22), 3897. https://doi.org/10.3390/w15223897






