1. Introduction
One of the popular methods of food-processing wastewater (FPWW) treatment is land application, which relies heavily on microbial metabolism to oxidize organic waste [
1,
2]. During land application, especially under continuous saturation and anoxic conditions, metals in native soil become reduced and mobile and can contaminate groundwater. Thus, heavy metals are often found in excess of the permissible levels in groundwater [
3]. Heavy metals are defined generally as metals with high densities (relative to water) and high atomic weights (greater than that of iron) that are toxic at even minute concentrations (ppb). In contrast, metalloids are elements with properties that are intermediates between metals and non-metals. One of the most common contaminants in groundwater systems is trace elements, more specifically, metallic elements such as arsenic, cadmium, lead, chromium, copper, nickel, and mercury [
4].
Due to natural, agricultural, industrial, domestic, and other sources, heavy metal pollution has been a problem that has been plaguing the environment and negatively impacting human health for some time. The increase in urbanization and industrialization has led to the rise in heavy metal pollution [
4]. In North Carolina, 50% of well waters have Mn levels above the state’s maximum contaminant level (MCL) [
5]. Even more concerning is the levels of arsenic in North Carolina’s groundwater [
6].
Once a groundwater source is contaminated, the water can be unsafe for human consumption. This is because these metals enter the body through ingestion through food and water [
7]. Increasing human exposure through food and water due to the mobility and bioavailability of heavy metals is a global concern [
8]. Exposure to heavy metal or metalloid contaminants such as Pb, Cd, Zn, Cu, Hg, and As threaten human health. Of all trace elements, Pb and Cu are some of the most hazardous environmental pollutants. Duffus (2002) noted that even at minor concentrations and low levels of exposure, some metalloids, like arsenic, are able to induce toxicity [
9].
But it is not only humans that are at risk. Excess concentrations of heavy metals in soils can lead to a loss of microbial activity, which leads to reduced soil fertility, resulting in yield loss. That is why it is vital to protect groundwater from contamination from these toxins. If contaminated by these metals, the soil and water are very difficult and expensive to remediate because they are immutable by biochemical reactions, unlike organic contaminants that may be broken down into smaller molecules.
For decades, researchers have studied soil–microbe–metal interactions to deduce the best method for solving this environmental crisis. The metals of interest in this study were iron, arsenic, and manganese. The bioavailability and toxicity of iron to a plant relies on the specificity of the site’s soil conditions (pH, moisture, ORP) [
10]. These metals and metalloids (arsenic, iron, and manganese) are redox-sensitive metals, meaning they are reduced under anoxic conditions. The reduced species are more soluble, more mobile, and more toxic. Therefore, we need to prevent the formation of reducing conditions in the soil during the land application of wastewaters.
Plants are likely to reduce the formation of such conditions due to several processes, including oxygenation, rhizostimulation, and evapotranspiration. Plant roots can leak oxygen into the soil atmosphere, thus influencing the soil redox potential and the metal fate in the soil. Additionally, plants and bacteria can form specific associations where the plants provide C as an energy source for the microbes, which in turn allows the microbes to reduce the phytotoxicity of the contaminated soils and/or to immobilize the pollutants. Alternatively, plants and bacteria can form nonspecific associations in which normal plant processes stimulate the microbial community, which, in the course of normal metabolic activity, degrades contaminants in soil. Plants’ roots can provide root exudates as well as increase ion solubility. These biochemical mechanisms increase the remediation activity of bacteria associated with plant roots.
The solution to the problem of contaminant mobilization in soils can be understood if plant–soil–metal–microbe interactions are studied more intensely. This is important because the interaction of the plant, soil, and contaminant influences the success of plant-based remediation systems [
11]. Therefore, the goal of this project was to evaluate the plant–soil–microbe processes during land application of food processing wastewaters. The specific objectives during land application of food processing wastewater to poplar planted soil were as follows:
- I.
Evaluate plant-related processes (i) evapotranspiration, (ii) plant uptake, (iii) oxygenation, and (iv) rhizostimulation;
- II.
Assess the combined effects of the studied processes on the mobilization of metals and nitrates.
2. Materials and Methods
Six columns (6 in. diameter × 18.32 in. length) were made of polyvinyl chloride (PVC) pipes, capped with PVC caps, and sealed with sealant in a temperature-controlled indoor laboratory. Spigots were inserted in holes drilled an inch from the bottom of the columns, and ¼ in. tubing was connected to the spigots to allow for water samples’ collection during experimentation. After construction, the columns were filled with a mixture of soil and silica sand with a bulk density of 1.43 × 10
3 kg/m
3 spiked with heavy metal(loids) up to 16.32 inches (
Figure 1). The bulk density of the sand–soil mixture and the volume of the cylinder were taken into account to determine the mass of the heavy metals needed to spike the soil.
Heavy metals and metalloids such as arsenic (As), manganese (Mn), and iron (Fe) were spiked in the soil and sand mixture to stimulate the relevant environmental concentrations of each metal in North Carolina soil, which were 40, 900, and 2 × 10
4 mg kg
−1, respectively [
12]. The metal concentrations were chosen to represent a range of low, medium, and high contaminant levels in the environment (
Table 1). For spiking, a standard solution of iron(III) oxide or disodium hydrogen arsenate or manganese sulfate was prepared with a known concentration, a specific volume of standard solution was pipetted and added to the soils, and soil was homogenized for even distribution. To ensure that soil–metal concentrations increased, soil samples were taken before and after spiking and then analyzed for metal concentration using ICP-OES, the results of which are presented in
Figure 2.
Columns were fitted with a Hanna Instruments HI3133B ORP (Smithfield, RI, USA) probe with a Fisherbrand™ accumet™ Epoxy Body Mercury-Free Reference Electrode (Waltham, MA, USA) and an Apogee Instrument (SO-110) (Logan, UT, USA) soil oxygen probe to monitor redox potential and soil oxygen levels, respectively. Data was collected from the sensors every 15 min and stored on Campbell Scientific CR1000× dataloggers. Data was collected from the dataloggers periodically throughout the experiment. ORP data was corrected and converted to a standard hydrogen electrode.
In this experiment, there were three planted columns and three no-plant control columns. In the planted columns, tall shade 2–3 ft poplar plants
Populus deltoides ×
Populus nigra OP367 sourced from Fast Growing Trees, LLC were planted in a completely randomized design, and tap water was applied during the establishment phase. Hybrid poplar trees were chosen for this experiment because they possess the ability to achieve rapid above and below-ground biomass, have high evapotranspiration, deep roots, and are one of the most used species in phytoremediation. Additionally, poplars are able to remediate soil and groundwater through removal, stabilization, and break down of contaminants [
13,
14]. Synthetic, carbon-rich wastewater was prepared in the laboratory using sucrose, soluble starch, and salts, which emulated the average characteristics of factory food processing water (
Table 2). The wastewater application started 14 days after planting to ensure plants were properly established. The daily local rate of application in non-coastal environments was taken into consideration when applying wastewater [
15]. The synthetic wastewater was applied at a rate of 15.4 mm per day to each column for five months. Additionally, the season was taken into consideration, with WW application taking place less frequently during periods of reduced evapotranspiration when plants shed their leaves, which coincided with winter. Columns were allowed to drain freely both during and after wastewater application to avoid waterlogging. Leachate volume was measured weekly, and evapotranspiration was calculated by subtracting the total leachate volume from the volume of WW applied weekly.
Leachate water samples were collected every other week and the water quality, including COD, pH, ORP, NH4-N, NO3-N, and TN, was tested according to EPA-approved standard methods. 24-mL water samples were collected in glass vials and analyzed using various instruments, which included a Hach DR6000 UV/VIS Spectrophotometer (Loveland, CO, USA) with RFID Technology in conjunction with a Hach—DRB200-04 for COD; a Thermo Scientific™ Orion Star™ A211 Benchtop pH Meter (Singapore) for pH; and a Thermo Scientific™ Dionex™ ICS-6000 Capillary HPIC™ for nitrogen species concentrations. COD was measured using a HACH method 8000. As, Fe, and Mn in leachate water samples were analyzed using a PerkinElmer Optima 8000 ICP-OES (Shelton, CT, USA) with an S10 Autosampler & PolyScience Chiller. During sample preparation, the samples were filtered with a 0.45 or 0.22 μm PTFE filter when necessary. Nitrate-N and total-N were analyzed as anions and ammonium-N was analyzed as cations using a Thermofisher Scientific Ion Chromatography (IC), ICS-6000. Anions were separated using the 2 × 250 mm analytical AS11-HC-4 μm column with an AG11-HC-4 µm guard along with an EGC of 35 mM KOH at a flow rate of 0.25 mL/min and detected with a conductivity detector. Similarly, cations were separated using the 2 × 250 mm analytical CS19-4 µm column with a CG19-4 μm guard along with an EGC of 20 mM MSA at a flow rate of 0.25 mL/min and detected with a conductivity detector. For quality assurance and quality control, samples on the IC were analyzed along with replicates, anion and cation standards, and blanks. A linear calibration curve with at least six standard levels was used for calibration and R2 for the calibration was at least 0.99.
Soil ORP was measured using an HI3133B ORP probe in conjunction with a Fisherbrand™ accumet™ Epoxy Body Mercury-Free Reference Electrode for ORP, whereas soil oxygen concentration was measured using an Apogee Instrument (SO-110).
At the conclusion of the experiment, trees were sacrificed and the columns were deconstructed. Soil samples were taken from various depths—the “topsoil” sample was taken from 0 to 4”, the “root zone soil” sample was taken from 10 to 14” and the “composite soil” sample was taken from 6 to 8” below the surface. The soil samples were dried for 36 h using a Thermo Scientific Heratherm OGS60 General Protocol Oven and ground using a laboratory blender and a mortar and pestle. Triplicate soil samples from each depth and treatment were analyzed for metal and microbial populations. Microbial analysis was conducted using a 16s rRNA Illumina Amplicon Sequencing at the UNC Chapel Hill School of Medicine Microbiome Core Facility. DNA extraction and purification were performed following standard protocols and procedures. Taxonomic information at the genus/species level, calculations of alpha and beta diversity, and the basic analysis of the over or under-representation of microbial groups were determined. The data was provided by targeting the V3–V4 variable regions in the 16S ribosomal gene to analyze the complexity of the microbial communities present in the soil samples. Visualization of microbial data was conducted using the Visualization and Analysis of Microbial Population Structures (VAMPS) program to generate KRONA charts.
The trees were removed from the columns, rinsed thoroughly, separated into roots and shoots, and the mass was measured. The plant samples were dried for 24 h at 104 °C, and the dry mass was recorded. Plant tissues, water, and soil samples were analyzed by NCATSU Analytical Services Laboratory (ASL) for concentration of metals after solid samples were processed with acid digestion. ICP-OES was used to determine the concentration of metals in both soil and plant samples. For acid digestion, 0.3 g of sample was weighted, and a predetermined amount of concentrated nitric acid (67–70%, Fisher Scientific) and hydrofluoric acid (48–51%, VWR Chemicals) were added to the digestion tube. The acid-treated samples were left for 10 min, and they were subjected to automated sequential microwave digestion using the CEM Microwave Technology Ltd. (Matthews, NC, USA) MARS 6 digester.
All data was plotted in Microsoft Excel 2016, and the standard error was determined. During visualization, the replicates collected were averaged. Statistical analysis included using SigmaPlot version 14.5 to run the non-normal data test Mann–Whitney U test of medians and Kruskal–Wallis one-way analysis of variance (ANOVA) on ranks. For normal data, the Brown–Forsythe test of variance was used.
4. Discussion
4.1. Phytoprocesses
Phytoremediation of land applied FPWW is successful due to the different attributes poplar plants possess, which support plant processes to remove nutrients and pollutants from the soil. The phytoprocesses that were studied during this experiment included plant uptake, evapotranspiration, root oxygenation, and rhizostimulation. These processes are facilitated by plants, soil microbes, and root exudates.
Evapotranspiration was a key process as the overall crop coefficient was 1.88, which indicated accelerated loss of water from planted columns than from controls by a factor of 1.88. Therefore, plants can act as a “pump”, contributing to less leachate and less loading of pollutants to the groundwater.
Land application of FPWW can be a very rich source of carbon for soils. Extra inputs of carbon, either through plant roots or FPWW, can lead to increased microbial activity and lower oxygen availability in soils, forcing denitrifiers in the rhizosphere to find alternate energy sources [
19]. The microbes present in the study included nitrifiers (ammonia-oxidizing bacteria:
Nitrosomonas spp.,
Nitrosococcus spp.,
Nitrosospira spp.,
Nitrosolobus spp., and
Nitrosovibrio spp.; nitrite-oxidizing bacteria:
Nitrobacter spp.,
Nitrococcus spp.,
Nitrospira spp., and
Nitrospina spp., and
Nitrobacteraceae), metal reducers (
Geobacter (
Deltaproteobacteria), Geothrix fermentans (
Acidobacteria),
Thermoanaerobacter (
Firmicutes),
Aeromonas, Escherichia, Bacteroidetes, Clostridium, Lysinibacillus, Micrococcus), C degraders (
Achromobacter, Acinetobacter, Alkanindiges, Alteromonas, Burkholderia, Enterobacter, Mycobacterium, Pandoraea, Pseudomonas, Staphylococcus, Rhodococcus, Alkanindiges sp.,
Brevundimonas olei, Xanthomonas sp.), denitrifiers (
Actinobacteria, Pseudomonas, Bacillus, Alcaligenes, and
Rhizobium), methanotrophs (
Methylobacter, Methylomonas) and, sulfate reducers
(Chromatiaceae, Chlorobiaceae, Ignavibacterium).
Overall, the most abundant microbes responsible for each function differed by treatment (
Table 3). There were microbes that were found in the planted treatments but were not present in the control treatments and vice versa. Planted treatments included
Nitrospira spp.,
Pseudomonas,
Actinobacteria, and
Acidobacteria, whereas the no-plant control treatments included
Rhizobium,
Desulfobacterota, and
Methylomonas. The most abundant carbon-degrading populations differed in both treatments. While the no-plant control treatments appeared to be more diverse, there were healthier (abundance and diversity) populations of microorganisms in the planted treatments. This indicated that poplar presence indeed affects the microbial composition of soils (phytostimulation), which may be key in the removal of pollutants by rhizodegradation. The poplar plant uses microorganisms in the rhizosphere, which is enhanced via rhizostimulation, to reduce the metal(loids) and take up the reduced form of metal(loid)s by the roots into the plants, or the immobilization of these metal(loid)s in the soil via phytostabilization.
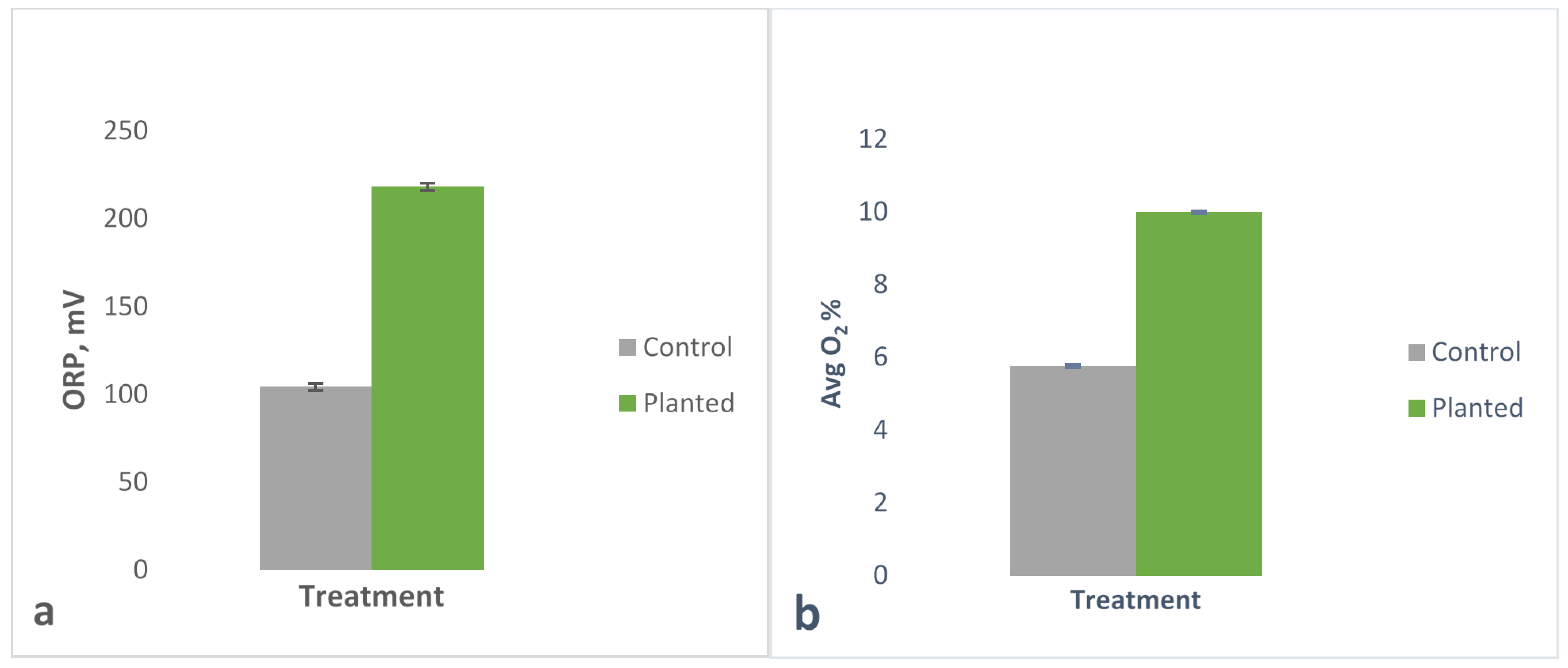
Plants are known to leak oxygen to the rhizosphere. In this experiment, significantly higher ORP and oxygen concentrations in planted columns compared to control columns indicated oxygenation by poplar trees. The results demonstrated the ability of plants to oxygenate the rhizosphere, which can be engineered for the purpose of contaminant remediation. Oxygen is needed by nitrifying bacteria to produce NO
2− and NO
3− during the denitrification process. On the other hand, the metals of interest are more mobile or soluble in reducing conditions. At a pH of 6–8, Mn is soluble at as high as +500 mV [
20], Fe is soluble at +300 to +100 mV [
21], whereas lower redox potentials are conducive with the more water-soluble form of arsenic, As
3+ [
22]. The more soluble the metals are, the more bioavailable for plants during phytoremediation and the higher the potential to leach to groundwater.
Accumulation of metals is another way the poplar trees affect the bioavailable metals in the rhizosphere. The accumulation factors (
Table 4) confirm the uptake of the metal from the soil to the root to the shoots of the plants and storage in their tissues.
4.2. Effects of Phytoprocesses on Metal and Nitrate Mobilization
Phytoremediation is a natural, cost-effective way to remediate soils and prevent groundwater contamination. Jadia and colleagues (2009) noted that the success of phytoremediation relies on plants that can (1) uptake large quantities of heavy metals in their roots, (2) translocate absorbed heavy metals into surface biomass, and (3) rapidly produce large plant biomass. It is also very important that the plants used for phytoremediation have mechanisms that allow them to detoxify the high metal concentrations accumulated in their shoots [
23]. Various plants, such as Indian mustard [
24], water hyacinth, channel grass [
25], herbal plants, and poplars [
26], etc., are used for phytoremediation. Poplars have the ability to reduce the amount of nutrients that are leached from soil systems using phytoprocesses like evapotranspiration, oxygenation, plant uptake, and rhizostimulation.
First, the poplar trees need to grow healthily for any phytoremediation project to be successful. Indeed, the growth of both above and below-ground biomass of the poplar plants was not inhibited by synthetic FPWW application in this experiment.
Second, the primary purpose of FPWW treatment is to reduce carbon loading from the wastewater. The removal of 65–71% C concentration-wise was achieved in both treatments. Although removal in the no-plant controls was higher concentration-wise, the loading was lower from planted treatments. Overall, carbon and nitrogen loading were higher from the no-plant controls than from the planted treatments. The reduction in leachate volume contributed to lower carbon and nitrogen loading. The nitrate concentration as the experiment progressed indicated that the poplars were successful in preventing the mobilization of nitrates from the WW to the leachate, thus protecting ground water.
Third, metal leaching varied greatly between treatments. Redox potential and pH greatly affect the speciation of metals in soil, as observed in the data. Solubility and mobility of metals and metalloids were only favorable during the periods where the pH and ORP were in a specific range. The most prevalent metal varied between samples, with Mn being prevalent in the topsoil and tissue cells of the shoots, whereas Fe was prevalent in the root tissue cells and root–soil samples. Additionally, less As was leached from the planted treatments than the no-plant controls, consistent with oxygen and ORP data. Oxic conditions occurred in the planted treatments more often than the no-plant treatments, resulting in significantly higher ORP, O2 concentration, and nitrates, while ammonium levels were low, indicating that reducing conditions occurred more frequently in control columns than in the treatment columns. Translocation of metals from the soil–root–shoot occurred throughout the experiment. Metal(oid) concentrations were reduced in the planted treatments with a translocation factor of 10.62 and an accumulation factor of ~1.5 for some metals.
Therefore, all the data strongly indicate that poplar plants helped increase oxic conditions in the rhizosphere, increasing available nitrate and manganese. However, control columns were in more reducing conditions and had more bioavailable iron or arsenic. Thus, the highly reducing species like arsenic leached less from the planted columns, while the metals that reduce in slightly reducing conditions, such as manganese, leached higher from the planted columns. This is consistent with our other data that shows that under oxic conditions, the planted leachate contained higher nitrates and lower ammonium due to nitrification.