Abstract
Tyrosinase enzyme in a crude extract was immobilized in granular activated carbon (GAC) and activated chitosan beads (ACBs), two low-cost supports. It was possible to immobilize up to 70% of the enzymes in GAC under the conditions of 20 g/L support, stirring of 15.7 rad/s, contact time of 120 min, and up to 100% of enzymes in ACBs under the same conditions. In enzymatic oxidation tests, tyrosinase immobilized in GAC (T-GAC) was able to achieve a final phenol concentration below the limit required by the Brazilian legislation (0.5 mg/L) for solutions with initial concentrations of 10 mg/L, while the enzyme immobilized in ACBs (T-ACBs) was able to conform solutions with initial concentrations of phenol of 40 mg/L. It was possible to reuse the T-GAC two times, maintaining the same phenol removal efficiency, while the T-ACBs maintained up to 98% of its efficiency after five cycles of enzymatic oxidation of solutions of 10 mg/L phenol. It was possible to achieve the same phenol removal efficiency, with immobilized enzymes stored for up to 2 weeks. Such results suggest that both materials are effective for phenol removal from water samples, especially T-ACBs, representing promising alternatives for mitigating the effects of this compound in industrial wastewater.
1. Introduction
Water fit for use, that is, free of pollutants and pathogens, is essential for life on the planet. However, the availability of such a resource is threatened by pollution, generated mainly via human activities [1]. Its scarcity is alarming and it tends to be aggravated, affecting around 1.8 billion people by 2025 [1,2]. Considering water use by industrial activities, the generation of effluents with complex compositions is also concerning, and a wide variety of pollutants can be found in such wastewater. Among them, there are phenolic compounds, which are found in aqueous effluents from various industries, such as petrochemical, textile, plastics, resins, cellulose, and paper industries [3,4].
Phenols’ hydrophobicity and, therefore, their solubility in water govern the way they interact with all cell types and tissue structures [5,6,7], since it may facilitate their entrance in the cell tissue via absorption and ingestion through skin [8], causing injuries that could be fatal. In addition, trace concentrations of phenols are already sufficient to alter the organoleptic properties of water [9]. Due to its toxic properties and endocrine-disrupting effects, phenol is on the list of priority pollutants in the United States Environmental Protection Agency (USEPA) [10,11,12], and Canada through the National Pollutant Release Inventory (NPRI) [8]. In Brazil, the National Council for the Environment (Conselho Nacional do Meio Ambiente—CONAMA) legislation imposes low phenol concentration limits in treated industrial effluents. CONAMA Resolution 430/2011 of 30 May 2011 [13] is very restrictive and limits the maximum content of total phenols (4-amino-antipyrine-reacting substances) to 0.5 mg/L for release in freshwater bodies. This motivates the creation of efficient techniques for treating effluents containing this compound.
In general, conventional methods for removing phenolic compounds from effluents, such as distillation, adsorption and extraction, biological treatment, and chemical oxidation, present several limitations related to phenols’ potential toxicity, to its formation of azeotropic mixtures with water, or to its transfer to another phase [8], increasing the interest in developing more efficient and competitive routes. One promising option is phenol and phenolic compound degradation through the use of polyphenol oxidases (PPOs), such as tyrosinase and polyperoxidases. The main advantages of using these enzymes for the degradation of phenol and its derivatives are their high availability, degradation speed, selectivity, and efficiency, even under low concentrations [8,14,15].
The enzymatic treatment consists of a first step, where enzymatic oxidation occurs, and a second one, comprising the separation or adsorption of the generated products [16]. The main limiting factor for this enzyme treatment use is enzyme inactivation [17,18]. In most cases, the addition of chemical additives (such as oxidizing agents, including hydrogen peroxide, for example) is necessary to overcome the loss in activity [8], making this process more expensive. In order to reduce the cost of their use in industrial processes, a significant limitation for their large-scale use, it is possible to use enzymes derived from crude extracts, which are cheaper than commercial enzymes, and to apply immobilized enzymes, which allows for their recuperation and reuse [8,19,20,21,22]. Immobilization also improves cell stability, reducing the inhibition effect and enhancing their tolerance to temperature and pH fluctuations [8,23], the main advantage over using whole cells.
Thus, the present work aimed to study the immobilization of tyrosinase enzyme from a crude enzymatic extract on two types of low-cost supports, granular activated carbon (GAC) and activated chitosan beads (ACBs), in order to produce new materials for the treatment of wastewater containing phenolic compounds. In addition, it also sought to evaluate their application for the removal of phenol in the presence of oxygen, as well as their reuse and post-storage stability, with the purpose of obtaining an effective alternative treatment for removing this compound from industrial effluents.
To the best of our knowledge, this study is the first one to assess the immobilization of tyrosinase directly from a crude extract and not from a purified enzyme sample, which enables the use of agricultural waste as a source of such an enzyme and may reduce the production costs associated with the purification steps. Additionally, the immobilization was performed on low-cost supports, which tends to make the technology more accessible and economically viable for application in different scenarios, including the treatment of industrial wastewater and contaminated effluents [24,25]. Finally, this study also presented stability results in relation to storage, which has been hardly studied for these supports, thus, presenting valuable results for the literature.
2. Materials and Methods
2.1. Materials
The crude enzyme extract containing the tyrosinase enzyme (EC 1.14.18.1) was produced from Agaricus bisporus macrofungus, purchased in lots at a local market.
For the immobilization and enzymatic oxidation assays, two support types were used, namely the commercial Carbotrat AP Granular Activated Carbon, supplied by Indústria Química Carbonífera Criciuma S.A, and chitosan beads, produced in the laboratory. All chemicals (of analytical purity) were purchased from Sigma-Aldrich.
2.2. Tyrosinase Enzyme Obtaining Methodology
The enzymatic crude extract was obtained following the methodology developed by Kameda et al. [26], in which the fruiting bodies of Agaricus bisporus are ground in 1:1 (g/mL) chilled acetone, at a maximum of 500 g at a time. The mixture was vacuum filtered, and the residue was stored at 273 K for 24 h. Then, the mushroom paste was re-suspended in distilled water and stored again at 273 K for 24 h, following a centrifugation step at 418.9 rad/s for 10 min to obtain the first enzyme extract. The process of re-suspension and centrifuging the slurry was performed again to obtain a second extract. The tyrosinase enzymatic activity was established following the adapted procedure of dos Santos et al. [27], where a unit of enzymatic activity (U) is defined as the amount of enzyme that causes a 0.001 increase in absorbance at 280 nm per minute. Equation (1) was used to calculate the enzymatic activity:
where A—enzymatic activity (U/mL), Abs1—initial absorbance, Abs2—final absorbance, t1—initial time, t2—final time, VE—enzyme solution volume, and DE—enzyme solution dilution factor.
2.3. Supports and Immobilization of Enzymes in the Crude Extract
Granular activated carbon (GAC) was used as a support for immobilization of the enzyme using the adsorption method, which was developed by Silva et al. [28]. For it to occur, a specific amount of support and 50.0 mL of enzymatic crude extract solution (in 0.1 mol/L phosphate buffer, pH 7.0, and average enzymatic activity of 3000 U) were placed in an Erlenmeyer. The mixture was kept in contact for 120 min under constant stirring. After that, the immobilized enzymes were vacuum filtered. The enzyme immobilization degree in the GAC was indirectly assessed via the residual enzymatic activity (U) of the permeate solution, since it was not possible to determine the enzymatic activity of the enzyme immobilized on the support [28,29,30], as it was established through a colorimetric method in spectrophotometry, and, therefore, the measurement obtained in the cuvette would not refer to reliable values due to the presence of particles. It was calculated using Equation (2):
where ID—immobilization degree, Ui—initial enzymatic activity of the solution, colorimetrically measured, and Uf—remaining enzymatic activity of the solution after immobilization, colorimetrically measured.
Activated chitosan beads (ACBs) were prepared based on the modified procedure of Zhou et al. [31], where 2.0 g of medium molar weight chitosan was added to 100 mL of 5.0% (v/v) acetic acid solution under stirring for 30 min. The solution was then dripped through a 1 mm plastic tip coupled to a peristaltic pump into 1000 mL of 2.0 mol/L sodium hydroxide solution and ethanol (4:1) throughout the period of 24 h. The formed beads were kept in contact with distilled water for 24 h to remove traces of sodium acetate, sodium hydroxide, and acetic acid and were filtered and washed with distilled water until reaching a neutral pH value. The chitosan beads (CBs) were kept in a watch glass for 24 h to be dehydrated and, consequently, had their mechanical strength increased. For more efficient immobilization, the dry CBs were activated with glutaraldehyde (GA). Activation was performed by keeping the CBs in contact with a solution containing up to 3.0% GA (m/V) under gentle stirring for 90 min. After this time, the beads were vacuum filtered and stored under refrigeration. For covalent immobilization, 5.0 g of ACBs and 20.0 mL of enzymatic crude extract solution (in 0.1 mol/L phosphate buffer, pH 7.0, and enzymatic activity of 2000 U) were placed in an Erlenmeyer. The enzyme solution and beads were kept in contact for up to 120 min, with constant stirring of 15.7 rad/s and room temperature. After the contact period, vacuum filtration was performed to separate the residual enzyme solution from the support containing the adsorbed enzyme. The granules were activated after drying. The enzyme immobilization degree was indirectly determined, as described previously.
The materials’ characterization was performed via scanning electron microscopy (SEM) analysis, providing the surface micrographs, and through Fourier-transform infrared (FTIR) spectroscopy.
2.4. Enzymatic Oxidation of Phenol Using Immobilized Enzymes
The enzymatic oxidation assays were performed by adding 10.0 g of immobilized enzyme on granular activated carbon (T-GAC) or 5.0 g of immobilized enzyme on activated chitosan beads (T-ACBs), both with an average activity of 1500 U, in aqueous solutions of phenol, at concentrations ranging from 10.0 to 100.0 mg/L, at pH 7.0, maintained under constant stirring of 15.7 rad/s. As the supports had different immobilization degrees, in order to compare their efficiencies, the same initial enzymatic activity (1500 U) was established for both supports and, for that reason, the reactions were carried out with different amounts of T-GAC and T-ACBs.
The assays took 120 min at room temperature (298 K). Samples were collected every 15 min to analyze the remaining phenol in the solutions. For comparison purposes, phenol removal was also evaluated via adsorption methods on GAC and ACBs, as well as oxidation by free tyrosinase, following the same test conditions. The phenol concentration of the solutions was determined using the direct colorimetric method, described in the standard methods [32], applying 4-amino-antipyrine as a complexing agent. This method enables the quantification of both single phenol and substituted phenols, except in the para position. The assays were carried out in triplicate.
Phenol removal efficiency was calculated in percent for a clearer understanding of the results found, using Equation (3):
where PhR—phenol removal efficiency (%), Phi—initial phenol concentration in the aqueous solution (mg/L), and Phf—final phenol concentration in the aqueous solution (mg/L).
2.5. Reuse and Post-Storage Stability of the Materials
The T-GAC and T-ACBs, after the first enzymatic oxidation assays, were reused up to seven times. After each assay, the materials were washed with distilled water and phosphate buffer (pH 7.0) and subjected to vacuum filtration. Assays were performed on solutions at concentrations of 10.0 and 60.0 mg/L phenol.
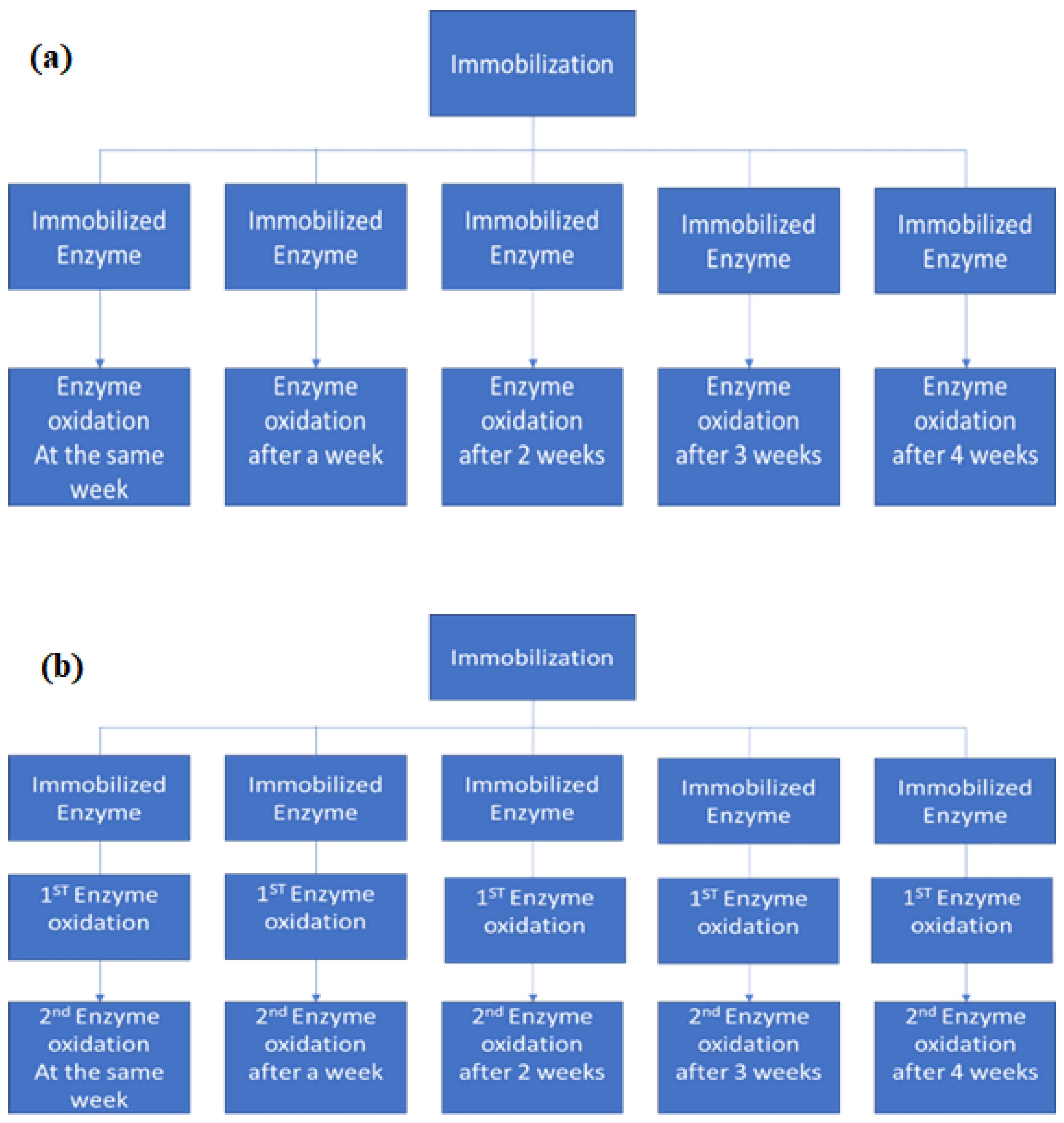
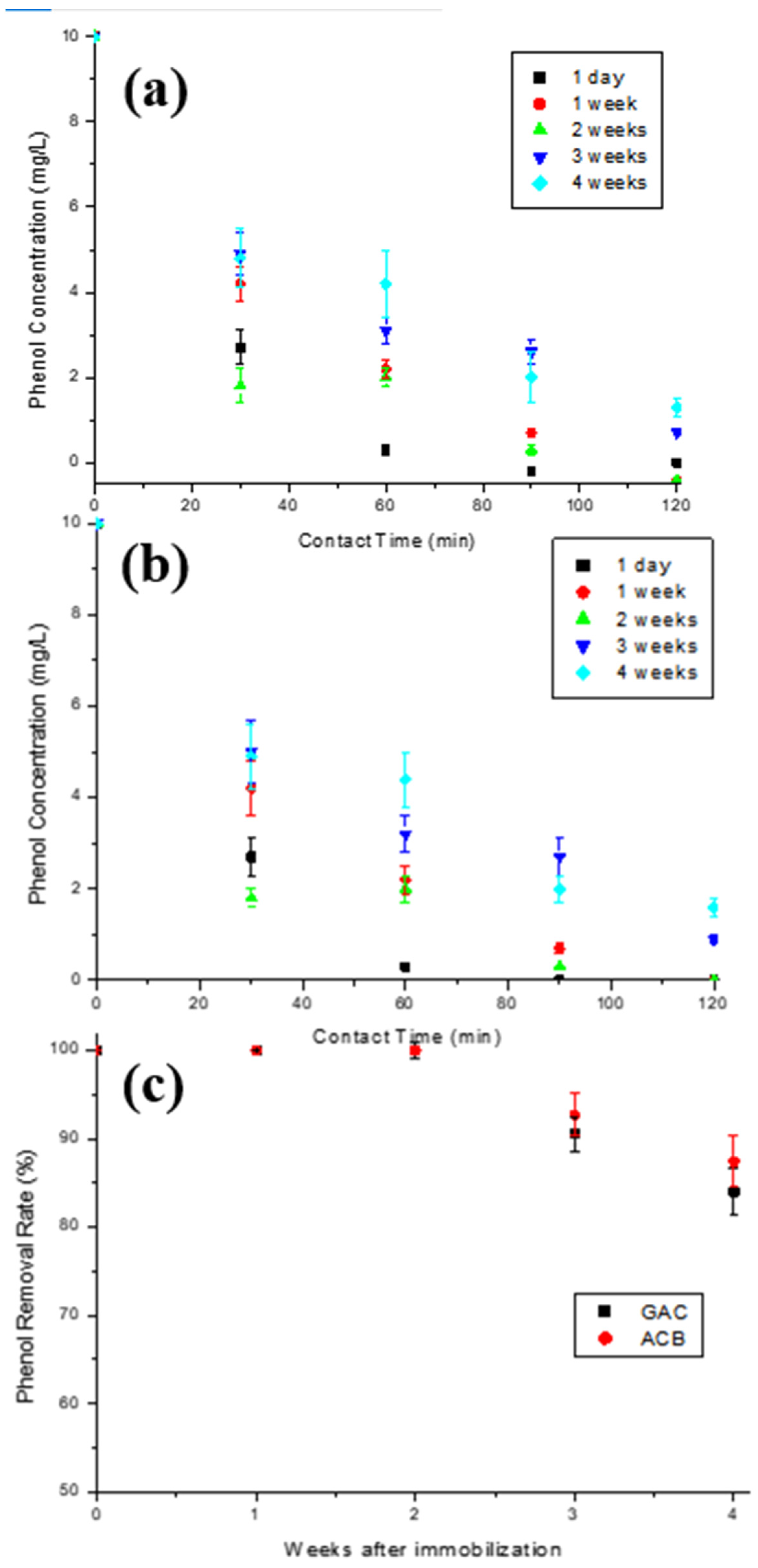
To verify the behavior of the enzymatic activity concerning the storage time of the materials, five immobilizations were performed under the same conditions for each support used, with activities of approximately 1500 U. Both materials were stored under refrigeration for later use in enzymatic oxidation assays for different periods: 1 day, 1 week, 2 weeks, 3 weeks, or 4 weeks after immobilization, as described in Figure 1a.
Figure 1.
Schematic representation of the oxidation assays performed to evaluate the enzymatic activity after storage time under refrigeration (a) before its first use, and (b) after its first use and before its second use.
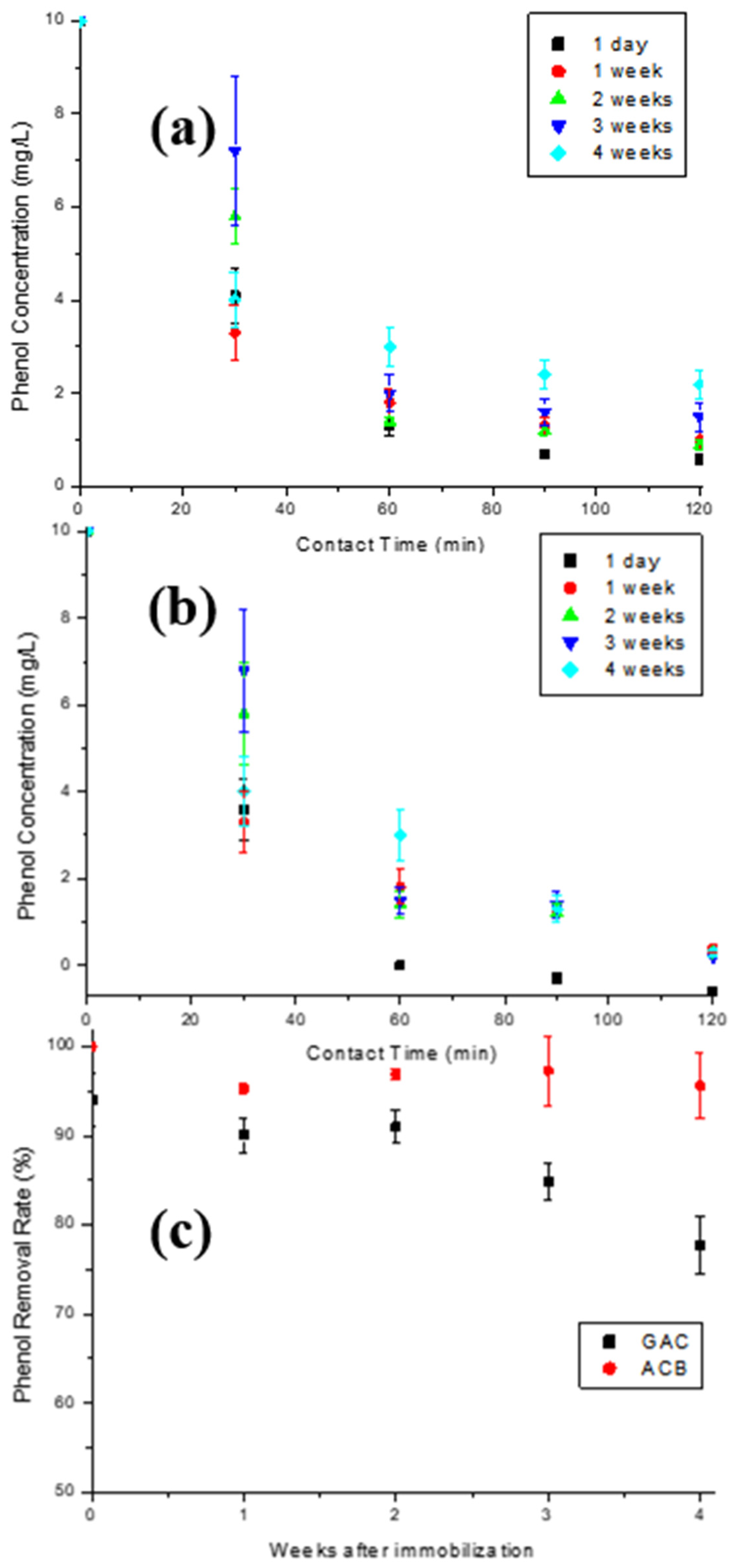
The stability of the T-GAC and T-ACBs concerning the storage time between its first and second use in the enzymatic oxidation assays was also investigated. Once again, five immobilizations were performed for each support type under the same operating conditions, generating five enzyme support systems containing an initial enzymatic activity of 1500 U. Each material was then used in enzymatic oxidation of an aqueous 10.0 mg/L phenol solution and stored under refrigeration after the assay. Once reserved, each material was reused for a second oxidation after 1 day, 1 week, 2 weeks, 3 weeks, or 4 weeks after their first use, as described in Figure 1b.
3. Results and Discussion
3.1. Materials’ Characterization
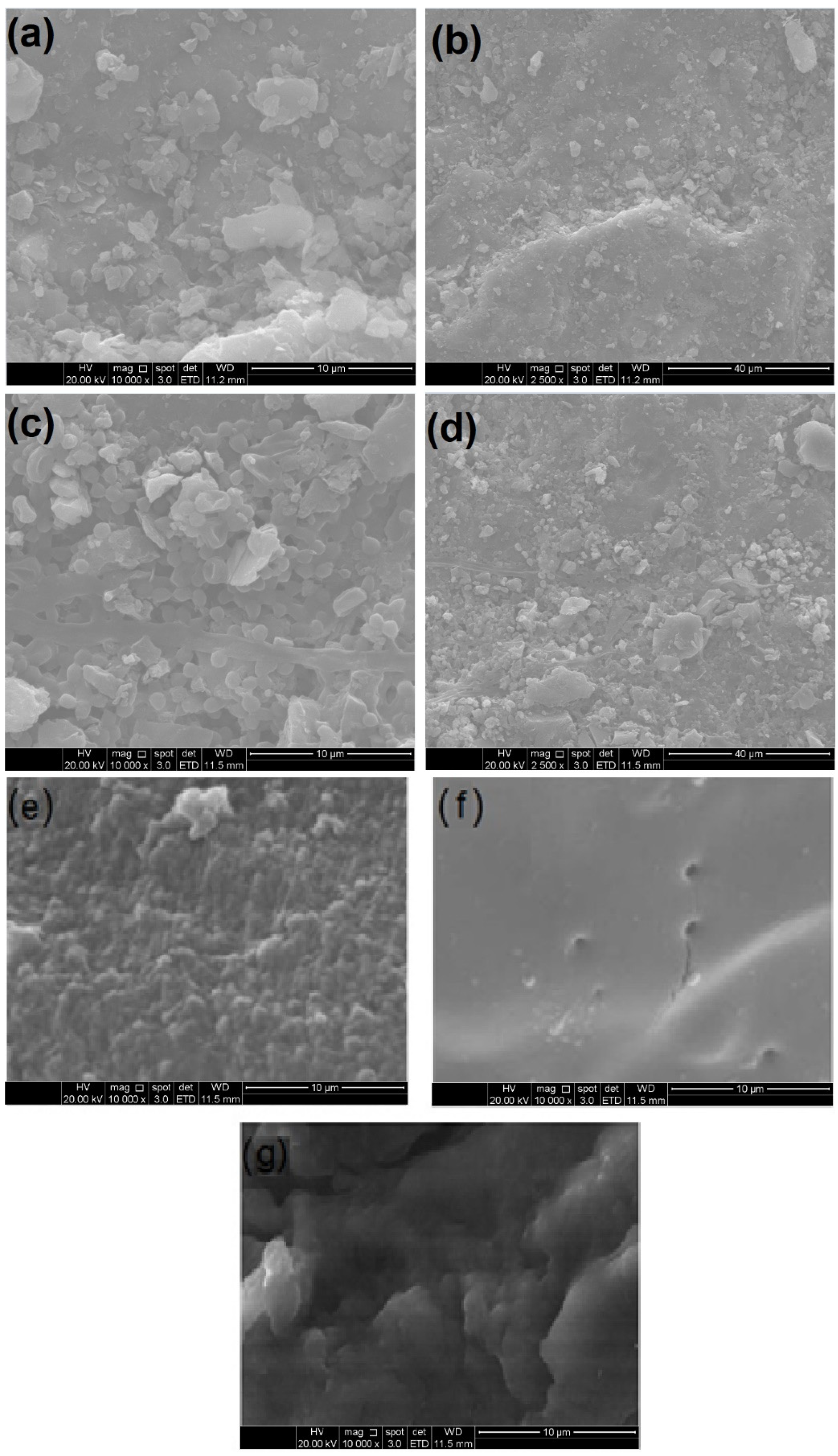
Figure 2 displays the scanning electron microscopy (SEM) images of the GAC, ACBs, T-GAC, and T-ACB used and produced in this work.
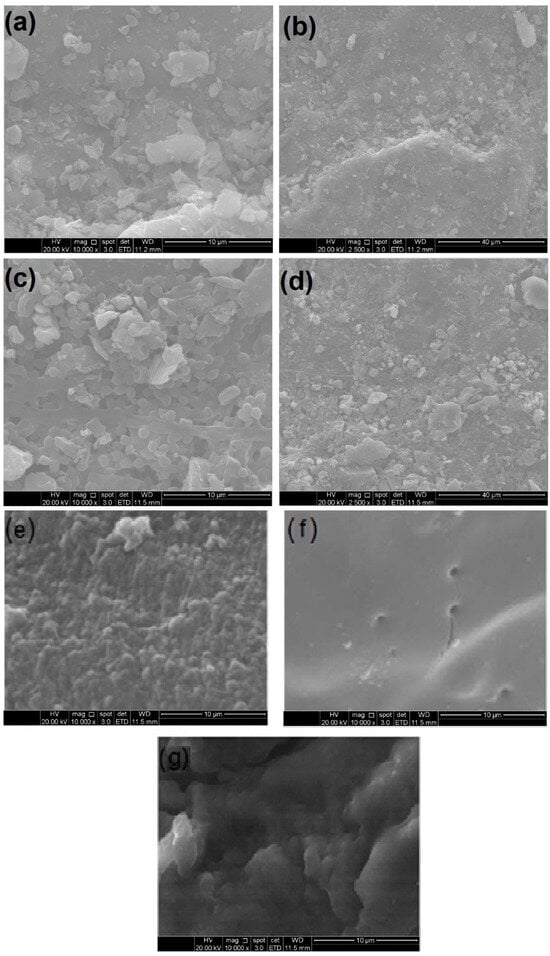
Figure 2.
Scanning electron microscopy images of granular activated carbon (GAC) before adsorption immobilization at (a) 10,000-fold magnification and (b) 2500-fold magnification; GAC after adsorption immobilization (T-GAC) at (c) 10,000-fold magnification and (d) 2500-fold magnification; (e) chitosan beads before activation at 10,000-fold magnification; (f) chitosan beads after activation (ACBs) at 10,000-fold magnification; (g) ACBs after immobilization (T-ACBs) at 10,000-fold magnification.
Figure 2a,b show the SEM images of the GAC before immobilization at magnifications of 10,000 and 2500 times, and Figure 2c,d present the SEM images for GAC after immobilization, also at magnifications of 10,000 and 2500 times. The image analyses of Figure 2a,b, most clearly at the magnification of 10,000, display a homogeneous and regular structure of activated carbon. Considering the images in Figure 2c,d, it can be observed that significant numbers of new particles are present on the surface of the material, indicating that the adsorption of the enzyme indeed occurred on the surface of the carbon.
In immobilization using the chitosan support, the tyrosinase enzyme was immobilized on the outside of the beads via covalent bonding with the amino groups of the support followed by crosslinking with the bifunctional agent, GA. The beads presented diameters between 3.30 mm and 4.15 mm. Figure 2e,f show the SEM images of the chitosan beads before and after activation with GA, and Figure 2g shows the SEM image of the ACBs after immobilization of the tyrosinase contained in the crude enzyme extract. The images’ analyses indicated that the chitosan beads, prior to their activation with GA, had a rougher and non-uniform surface, whereas activation made the bead surface more uniform and smoother, favoring the fixation of the enzyme on the support when using the covalent bonding method.
Although these SEM micrographs are not direct evidence of tyrosinase immobilization, it is a valuable analysis of the materials’ surfaces in micro- or nano-scopic scales, highlighting the differences presented by the materials after immobilization. Also, when associated to FTIR analysis, SEM micrographs may provide a wide comprehension of the immobilization process and its effects on the enzyme.
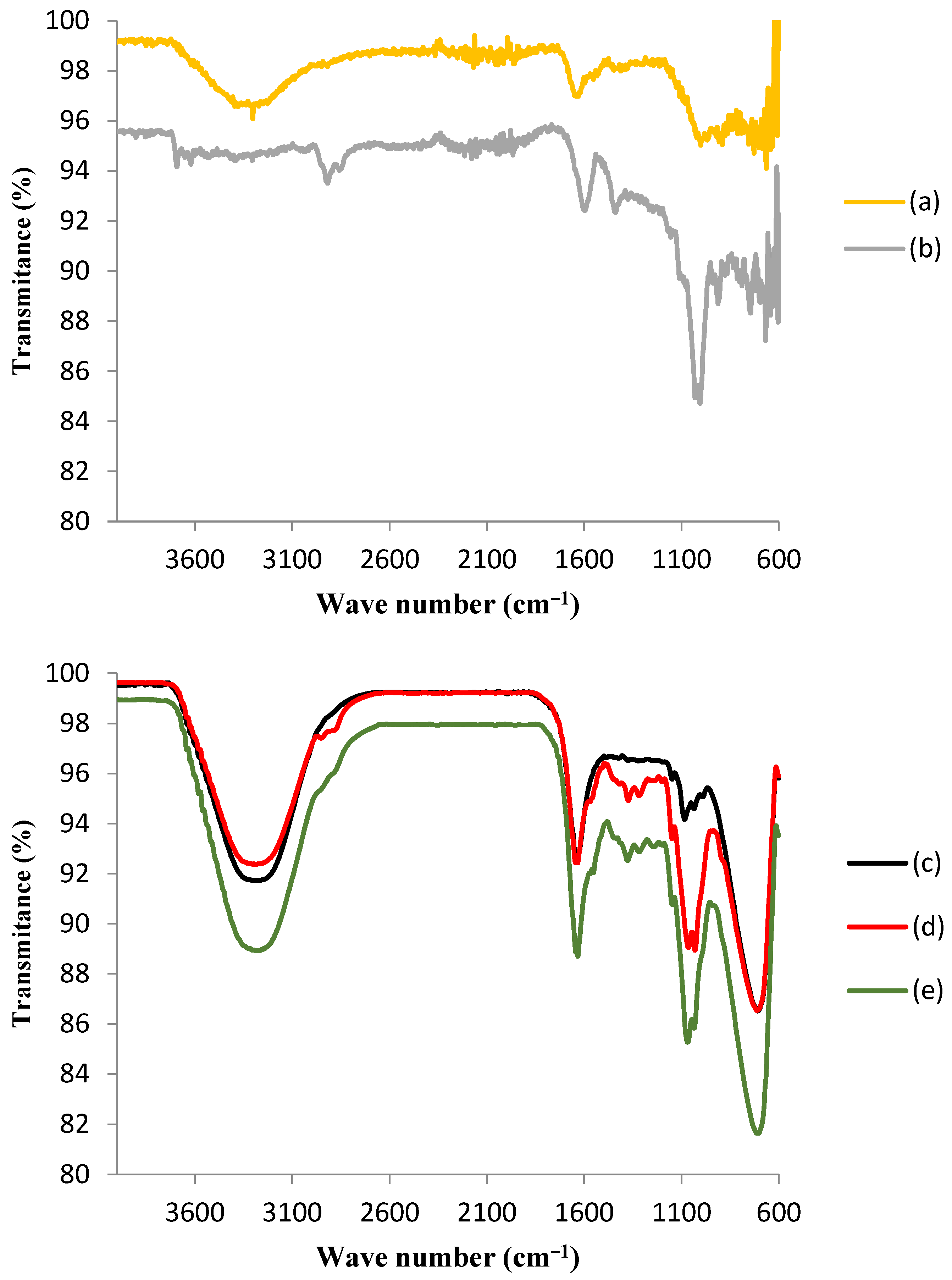
Figure 3 shows the FTIR spectra for the GAC and the ACBs before and after the immobilization of the tyrosinase enzyme.
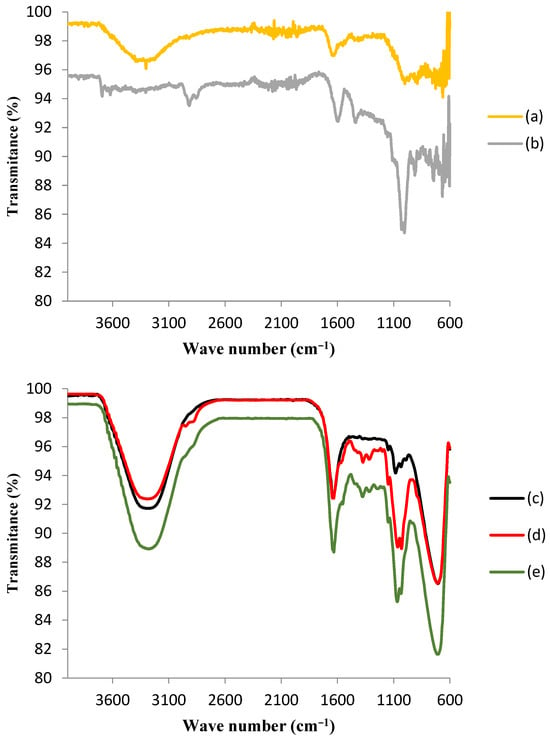
Figure 3.
Fourier-transform infrared spectroscopy (FTIR) spectra of (a) granular activated carbon before immobilization (GAC); (b) GAC after immobilization of tyrosinase (T-GAC); (c) chitosan beads before activation; (d) chitosan beads after activation (ACBs); and (e) ACBs after immobilization of tyrosinase (T-ACBs).
The upper portion of Figure 3 shows the comparison between the FTIR spectra of GAC before immobilization (a) and after the immobilization of the tyrosinase enzyme (b), and some differences may be observed. The spectrum of support after immobilization presented peaks indicating the presence of histidine, which is the most significant component of the tyrosinase enzyme [29]. It is also noteworthy that the adsorption of the enzyme did not change the activated carbon’s structure. The appearance of two bands in the 3700–3600 cm−1 region in the FTIR spectrum of GAC after immobilization (b), corresponding to the stretching frequency of N-H binding, was likely due to the interaction between tyrosinase and the activated carbon matrix [29]. In addition, the band that appeared near 1400 cm−1 in the GAC spectrum after immobilization (b) corresponded to C-N bonding, which is also indicative of the presence of histidine on the matrix. It was noted that this band was absent in the GAC spectrum before immobilization (a). The band near 1000 cm−1 in this spectrum (b) also suggests the presence of histidine, as it corresponds to the C-O vibration of carboxylic acid [33]. Although these results might suggest that chemical adsorption occurred during the immobilization of the enzyme on granular activated carbon, later tests showed a large desorption of this enzyme, which was proven through the determination of the enzymatic activity in the aqueous solution used in the phenol removal tests, which points that, although there may have been some chemical adsorption, the immobilization took place mainly by means of physical adsorption.
The lower portion of Figure 3 presents the comparison among the FTIR spectra of chitosan beads before activation (c), after activation (d), and after tyrosinase enzyme immobilization (e). The infrared spectrum of chitosan beads was characterized by the presence of a large band between 3400 and 3200 cm−1, which was attributed to the axial deformation of the O-H group associated with other polar groups through intra- and inter-molecular hydrogen bonds and to the axial deformation of N-H, normally obscured by hydrogen bonds with OH groups. The activation with GA may be confirmed by the appearance of a peak in the region around 2900 cm−1 and another one in the region near 1600 cm−1, as a result of the interaction of GA with chitosan functional groups. As it was observed for the GAC FTIR spectrum after immobilization (b), the presence of tyrosinase was suggested via the existence of the most pronounced peak in the region of 1100 cm−1, which is indicative of the presence of histidine. Immobilization was also responsible for the disappearance of the peak in the region around 2900 cm−1, indicating the interaction between tyrosinase and the modified chitosan surface [33].
3.2. Enzyme Immobilization
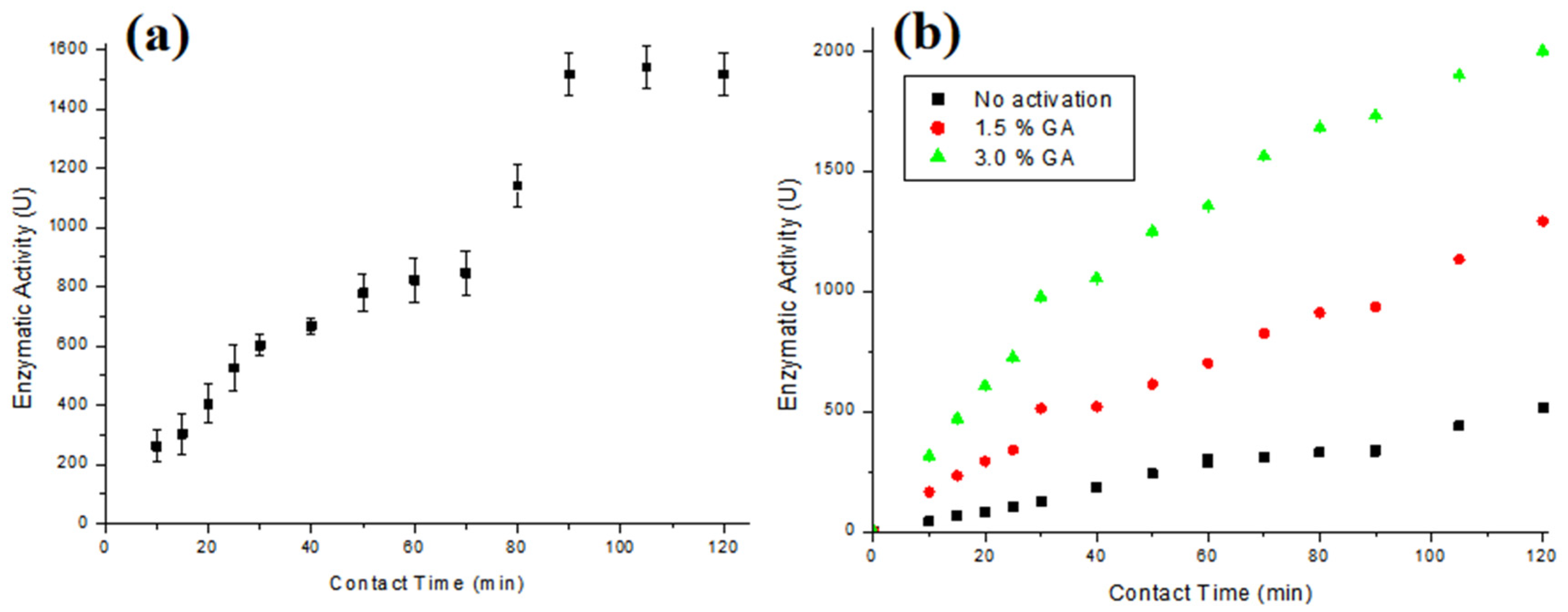
Figure 4a presents the enzymatic activity results obtained using T-GAC, and Figure 4b illustrates the results of the enzymatic activity of the T-ACBs with different GA concentrations and chitosan beads without activation.
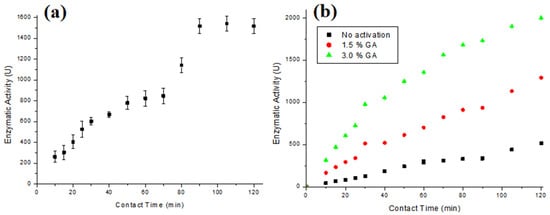
Figure 4.
Enzymatic activity of (a) T-GAC and (b) T-ACBs with 1.5 % of GA, 3.0 % GA, and without activation. Experimental conditions: enzymatic activity up to 3000 U (a) or 2000 U (b), stirring at 15.7 rad/s, and 298 K.
The immobilizations in GAC and chitosan beads mainly occurred via physical adsorption, so enzyme desorption is more accessible in case of operational changes. The ACBs’ support was subjected to a chemical activation process with glutaraldehyde, a cross-linking agent, which introduces aldehyde functional groups into the chitosan spheres that form covalent bonds with the amine (NH2) and thiol (SH) functional groups of tyrosinase [34]. Some authors have observed that during the immobilization of enzymes in GAC, the percentage of immobilized enzymes usually stabilizes under less than 120 min of contact between the enzyme and the support [28,29,30].
It can be observed that the immobilization degree increases over contact time for all the supports (Figure 4a,b). According to the test, the best immobilization time was 120 min for both supports, with immobilization on GAC presenting an immobilization degree up to 55% of the possible 3000 U. Kennedy et al. [29] managed to immobilize tyrosinase extracted from potatoes, with an enzymatic activity close to 3.0 × 105 U/L on a modified GAC, whereas Kumar et al. [30] and Silva et al. [28] managed to immobilize almost 100% of enzymes on different types of GAC, working with acid protease and pancreatin enzymes, respectively. The results for the immobilization with chitosan beads, Figure 4b, show that the immobilization on the beads without activation with GA presented an immobilization degree around 25% of the possible 2000 U, while the activation with GA made it possible to immobilize up to 100% of the enzymes when the beads were activated with 3% GA, and close to 60% when they were activated with 1.5% GA. The results of the ACBs’ support that was activated with the 3% GA solution are similar to those found by Chavita [35] and Miyaguti [36], and higher than the one found by Santos et al. [27].
The best stirring condition for the physical adsorption of the enzyme from the extract in GAC was investigated. Table 1 presents the immobilization degrees found for different stirring speeds after the contact time between the enzymatic solution and the support.

Table 1.
Variation of the immobilization degree and the enzymatic activity of the immobilized enzyme on GAC according to the stirring speed.
According to Table 1, the worst immobilization degree was obtained using a constant stirring of 10.5 rad/s. Under 15.7 and 20.9 rad/s, it was possible to obtain an immobilization degree above 0.50; while stirring at 31.4 rad/s dropped the immobilization degree to 0.40. The low immobilization observed at a low stirring speed was deemed to be due to insufficient tyrosinase contact with the support. For higher stirring values, the mechanical inactivation of the enzyme occurred due to the increase in the contact area of the enzyme with air and with the Erlenmeyer walls. This phenomenon was reported by Colombiè et al. [37], Gikanga et al. [38], Menoncin et al. [39], and Wiesbauer et al. [40]. Since both phenomena were expected to take place, regardless of the type of support, all immobilizations in ACBs were performed under the same optimum stirring speed as for GAC (15.7 rad/s).
3.3. Enzymatic Oxidation Assays
The phenol enzymatic oxidations using T-GAC and T-ACBs were compared with other phenol removal methods, such as enzymatic oxidation using free tyrosinase, also originated from the crude extract, and phenol adsorption using only the GAC or ACBs’ supports. All tests were performed under the same conditions established in the Materials and Methods section. The results regarding phenol removal from a solution containing 10 mg/L phenol are present in Figure 5.
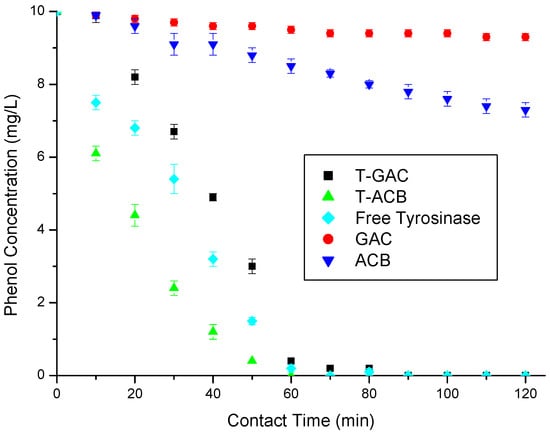
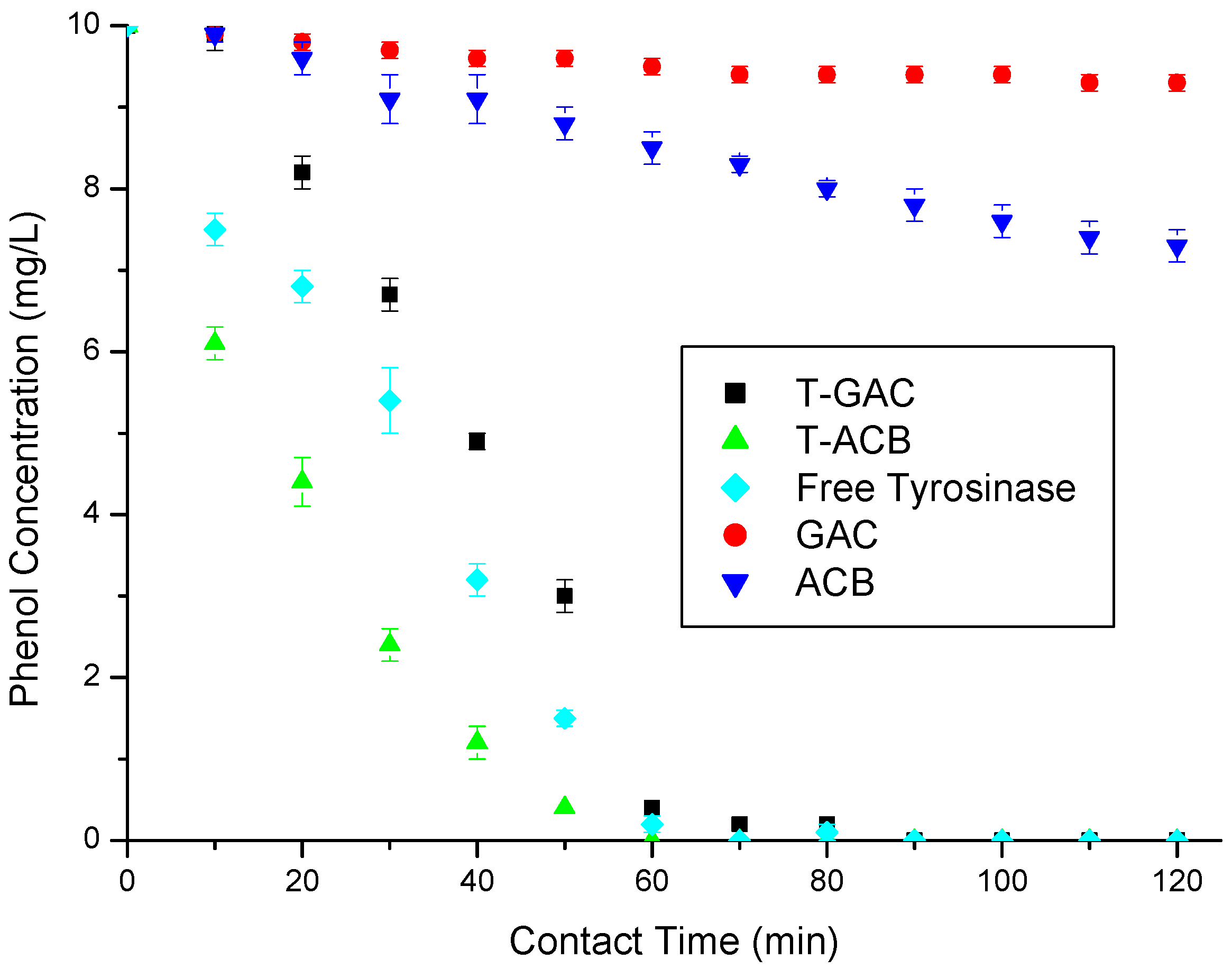
Figure 5.
Phenol removal from a 10 mg/L aqueous solution via enzymatic oxidation with T-GAC, T-ACBs and free tyrosinase, all with enzyme activities of 1500 U, and via adsorption on GAC and ACBs. Experimental conditions: support concentration of 20.0 g/L; stirring of 15.7 rad/s; 298 K.
According to Figure 5, the enzymatic oxidations using free enzymes, T-GAC, and T-ACBs all reached 100% of phenol removal after 60 min, while the adsorption using the ACBs and GAC removed just 14.7% and 5% of phenol in the same contact time, respectively. At the end of the assays (120 min), these adsorption methods removed 20.5% and 7% of the total phenol, respectively, still quite below the results obtained in the enzymatic oxidation tests. The curves referring to the oxidation using the T-ACBs present a quicker reduction in the concentration of phenol in the solution than the free enzymes, which may have been caused by the availability of the enzyme in the support to come into contact with the phenol molecules in the solution. A plateau in the first five minutes of the enzyme oxidation test was reported by other authors (Botelho 2010; Santos 2012; Silva 2010), which might be explained by the adaptation time required for the enzyme to act on low concentrations of phenol.
The higher activity of the free enzyme can be explained by some factors, such as conformation and accessibility, as when the enzyme is immobilized on a solid support its active conformation can be altered due to the bonds that hold it to the support, restriction of movement, since immobilizing the enzyme on a solid support can restrict its movement and its ability to access substrates, limited diffusion, as when the enzyme is immobilized, the diffusion of the substrates and products to and from the enzyme’s active site can be limited, and steric inhibition, where the immobilization of the enzyme on a solid support can result in steric inhibition, where the solid support directly interferes with the conformation and activity of the enzyme. However, the main focus of enzyme immobilization is not to enhance the enzymatic oxidation of tyrosinase when compared to the free enzymes, but to allow its reuse, enhance its long-term stability and the ability to easily recover the enzyme from the reaction medium.
Oxidation using immobilized enzymes are preferable for several reasons: immobilization offers greater stability, preserving the enzymatic activity and enabling its use in many oxidation cycles. The facility of separation after oxidation provided by the supports simplifies the oxidation process and the product’s purification. Additionally, immobilization provides higher enzyme tolerance to high substrate concentrations, reduction in the enzymatic activity inhibition by scavenger compounds, and reduction in final product contamination.
The first phenol enzymatic oxidation assays were performed using both materials in solutions of 10, 20, 40, 60, and 100 mg/L. All assays were performed under the same stirring conditions (15.7 rad/s, 298 K, and 120 min). The results, in terms of the final average concentrations of phenol in the aqueous solution and phenol removals, are shown in Table 2.

Table 2.
The final average phenol concentrations and phenol removals from aqueous solutions at different concentrations after enzymatic oxidation. Experimental conditions: 15.7 rad/s, 298 K, and 120 min.
Using T-GAC, the phenol limit required by CONAMA 430/2011 [13] was only reached via enzymatic oxidation on solutions with an initial concentration of 10 mg/L phenol (Table 2). However, with this material, it was possible to reach phenol removal above 50%, even for solutions containing 100 mg/L phenol. It is essential to point out that, although the complete mineralization was not achieved, the product generated by this oxidation, o-quinone, is not within the list of compounds with limitations for disposal [13], and its removal from the aqueous solution via adsorption on the solid supports studied was observed during the tests. As for the T-ACBs, they were able to reach final phenol concentrations below the limit required by the Brazilian legislation for solutions, with an initial concentration between 10 and 40 mg/L (Table 2). Also, for solutions of 60 mg/L, it was possible to remove up to 95.8% of phenol, and even in solutions containing 100 mg/L phenol, it was possible to achieve a removal of 67.9%. All of these results are greater than those found for T-GAC and using lower amounts of support.
The decrease in removal efficiency, with the increase in the initial concentration of phenol, may be explained not only by the smaller proportion of immobilized enzyme available for oxidation but also by the fact that o-quinone, formed during the degradation of phenol through tyrosinase, inactivates this enzyme when in higher concentrations [41,42]. Another reason for solutions with high initial phenol concentrations not attending the limit established by CONAMA 430/2011 [13] is the short contact time, which was set at 120 min in order to avoid prolonged contact of o-quinone with the immobilized enzyme, thus avoiding its inactivation. Other authors needed 8 h (480 min) to remove practically 100% of phenol from solutions initially containing 100 mg/L, presenting a great loss in their material’s enzymatic activities in latter oxidation cycles [26,35].
3.4. Reuse of Materials and Supports
3.4.1. Immobilized Enzyme Reuse
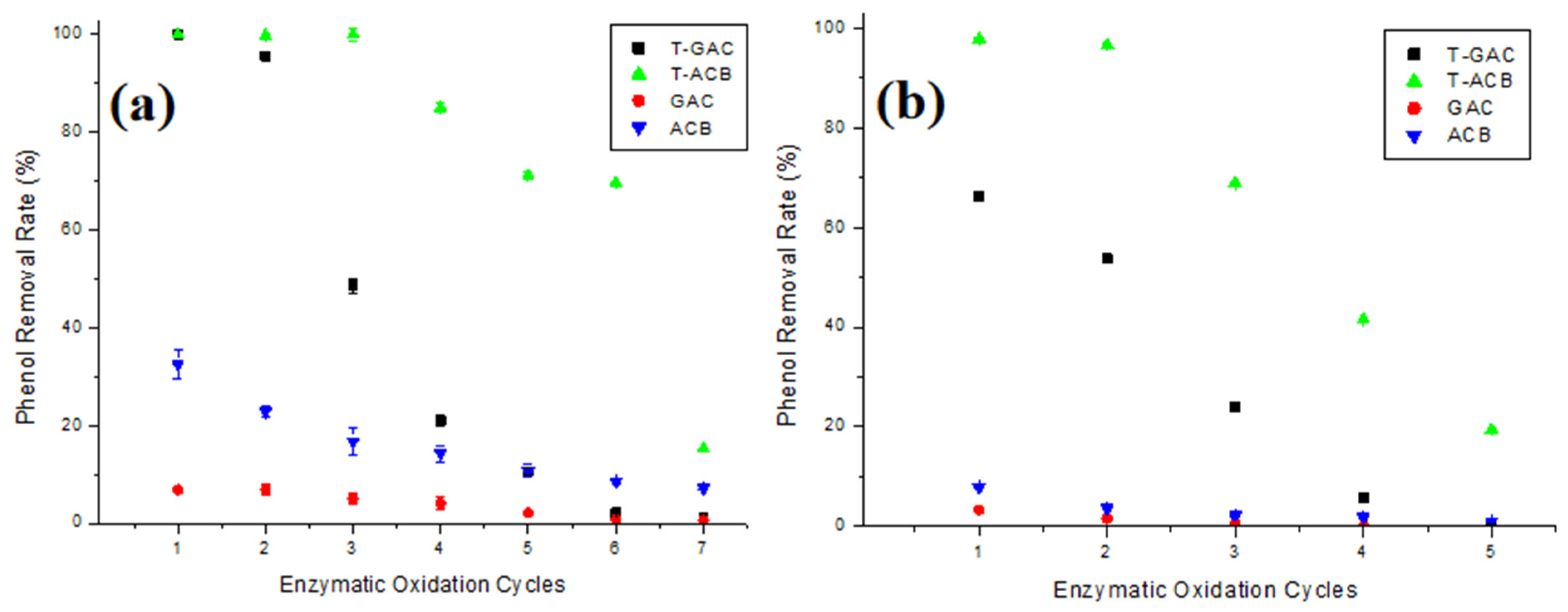
Figure 6a,b present phenol removal from aqueous solutions of 10 mg/L and 60 mg/L, respectively, via enzymatic oxidation with T-GAC, T-ACBs, and adsorption on GAC and ACBs, according to the number of reuse cycles of the support.
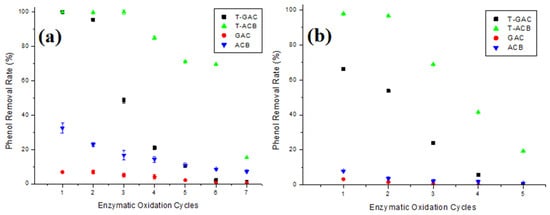
Figure 6.
Phenol removal from aqueous solutions of (a) 10.0 mg/L and (b) 60.0 mg/L via enzymatic oxidation with T-GAC, T-ACBs, and adsorption on GAC and ACBs, according to the number of reuse cycles of the support. Experimental conditions: initial activity of 1500 U, support concentration of 20.0 g/L, stirring of 15.7 rad/s, 298 K, and contact time of 120 min.
According to Figure 6a, removals using immobilized enzymes, T-GAC and T-ACBs, were higher than those presented by the GAC and ACBs, as expected. For phenol removal on 10 mg/L solutions using T-GAC, it was observed that, in the second cycle of use, there was a slight yield drop over the 100% removal yield of the first cycle, and it further dropped to less than 50% in its third use. T-ACBs were able to remove up to 100% of phenol in their first three uses. From the fourth use onwards, there was a 15.0% drop in the yield. The T-ACBs showed lower reductions than the T-GAC, maintaining the phenol removal efficiency close to 70% until their sixth use.
Considering the removal in 60 mg/L phenol solutions (Figure 6b), it is clear, once again, that the reductions using immobilized enzymes, T-GAC and T-ACB, were higher than those presented by the GAC and ACBs. Moreover, the use of T-GAC presented significant yield drops from the second cycle onwards, with an 18.9% decrease compared to the 66.2% yield during the first cycle, and at the third use, the removal yield dropped to less than 30%. T-ACBs were able to remove around 98.0% of phenol in their first use, and, in its second use, there was a yield drop of 1.2% and, differently from removal using T-GAC, just in the fourth cycle of use, the yield of phenol removal dropped to less than 50%.
The drop in enzymatic oxidation yield may be explained by a prolonged exposure of the enzyme to the phenol degradation product (which explains a higher yield drop in more concentrated phenol solutions, Figure 6b) by the adsorption of this degradation product on the support (which causes the inactivation of the tyrosinase enzyme) and also by the partial loss of the support that occurs between uses either during enzymatic oxidation or during the washing and filtration process after testing [41,42]. In addition, the sharpest drop in the yield during the reuse of T-GAC was likely caused by the enzyme’s desorption due to the stirring of the system during enzymatic oxidation, as reported by Colombié et al. [37], Gikanga et al. [38], Menoncin et al. [39], and Wiesbauer et al. [40].
3.4.2. Support Reuse
Since the immobilization in GAC mainly occurred via physical adsorption, enabling the removal of the immobilized enzyme after its activity loss, the reuse of this support was evaluated. The degree of immobilization dropped from 0.52 in the first immobilization cycle to 0.43 after three cycles, as shown in Table 3, which can be considered a very positive result. Reusing this support would help lower the cost of using the immobilized enzyme, providing higher economic viability.

Table 3.
Immobilization degrees of reused GAC supports.
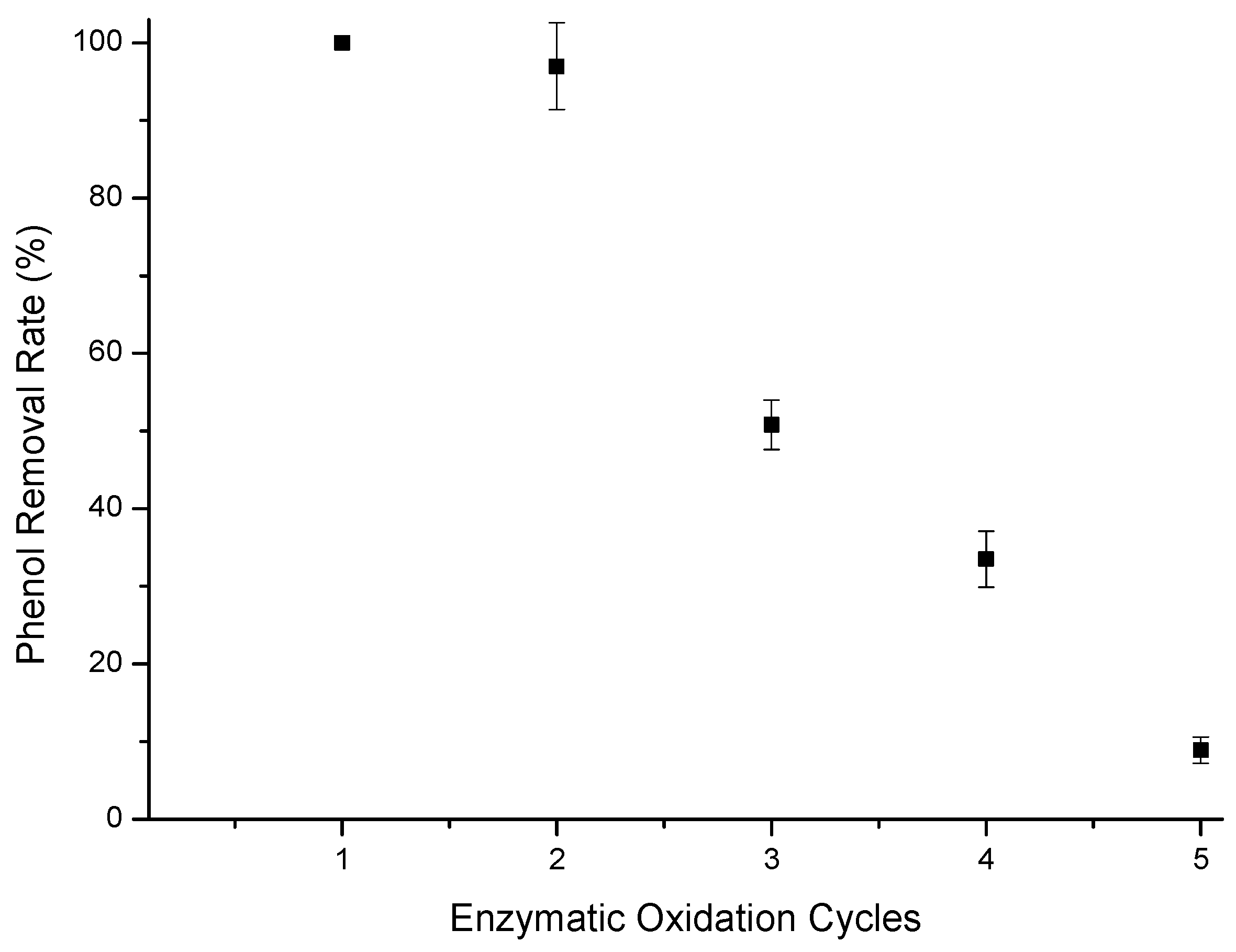
Enzymatic oxidation assays were performed using the T-GAC on a reused support (second immobilization on the support), also evaluating the removal of phenol from aqueous solutions containing 10 mg/L. The results of the enzymatic oxidation for phenol removal are represented in Figure 7.
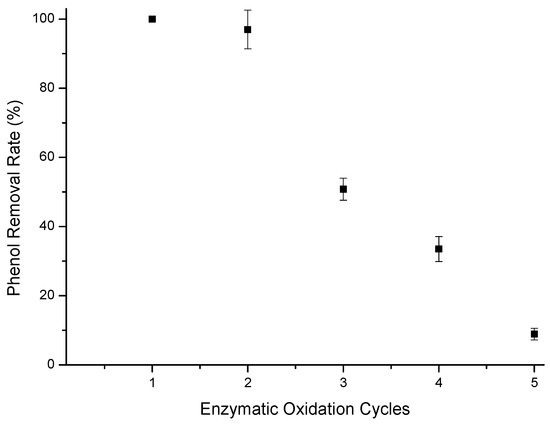
Figure 7.
Phenol removal efficiency from a 10 mg/L aqueous solution using T-GAC with a reused support. Experimental conditions: initial activity of 1500 U, constant stirring of 15.7 rad/s, 298 K, and contact time of 120 min.
As for Figure 7, it was possible to reach the limit established by CONAMA 430 [13] for phenol-containing effluent disposal in the first two uses of the immobilized enzyme on the reused GAC support. There was also a sharp drop in phenol removal from the first use to the third one, similarly to what was observed for the immobilized enzymes in the new GAC support. The drop in removal efficiency can still be explained by the loss of enzyme during the test and its inactivation by o-quinone [38,41,42].
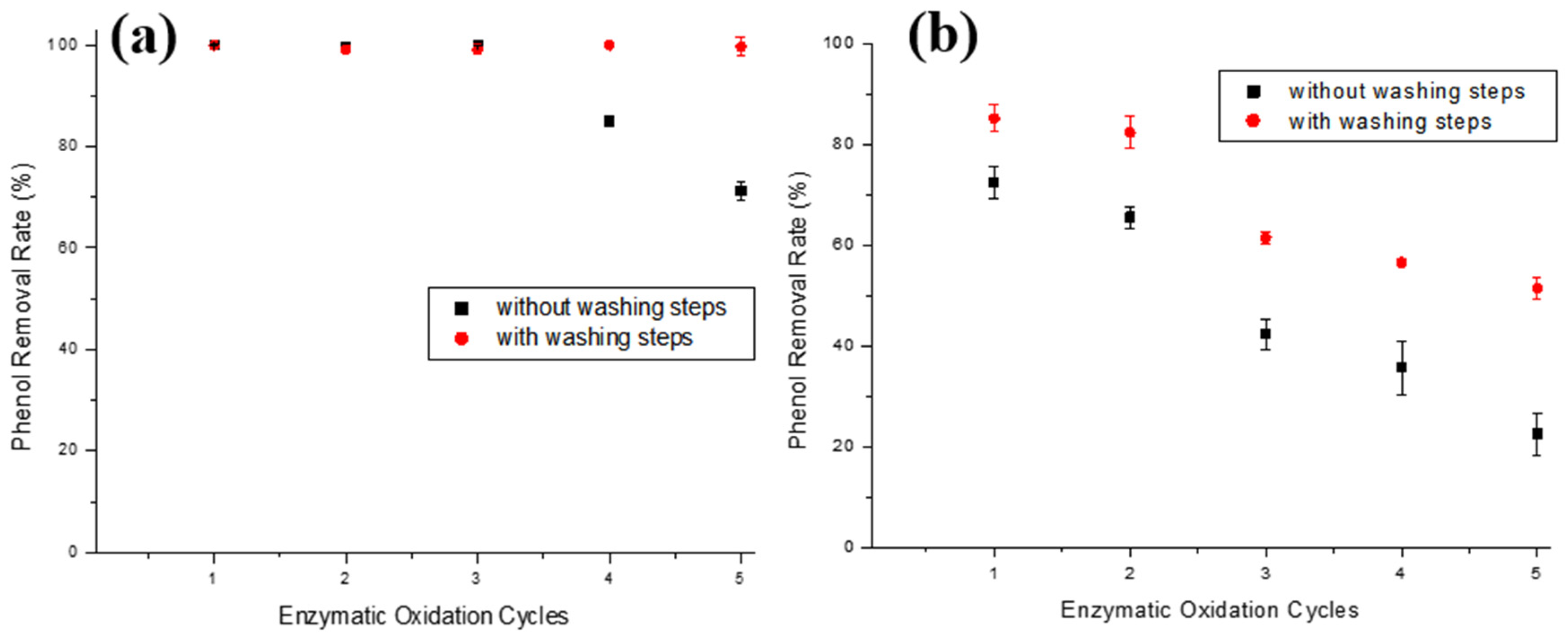
Since it was not possible to reuse the ACBs’ support, as adsorption occurs via covalent bonding (chemically joining the enzyme to the support surface), a way of decreasing the yield drop of the immobilized enzyme in this support type was studied. A probable cause for the drop in phenol removal yield is the adsorption of o-quinone on the bead surface, which inactivates tyrosinase; successive washing steps were added to the enzymatic oxidation test after each oxidation cycle with pH 6.7 and 8.0 buffers and distilled water. To verify the efficiency of these new steps, enzymatic oxidation assays were carried out using the T-ACBs material for five cycles in both 10 and 100 mg/L phenol solutions. Figure 8a,b show five enzymatic oxidation cycles reusing T-ACBs in aqueous solutions of 10 and 100 mg/L phenol, respectively, with and without the addition of washing steps.
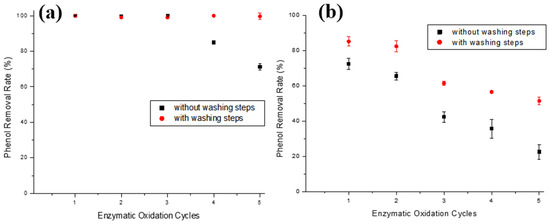
Figure 8.
Phenol removal efficiencies during enzymatic oxidation cycles using T-ACBs of aqueous phenol solutions of (a) 10.0 mg/L and (b) 100.0 mg/L, without or with intermediate washing steps. Experimental conditions: initial activity of 1500 U, T-ACBs concentration of 20 g/L, stirring of 15.7 rad/s, 298 K, and contact time of 120 min.
In the 10.0 mg/L phenol solution (Figure 8a), washing steps after each assay allowed for the phenol removal yield to only decline by 0.9% after five cycles, unlike the enzymatic oxidation cycles without washing steps that reduced 28.8% in the fifth cycle. It is worth stressing that the main limiting factor for the use of enzymatic treatment is the inactivation of the enzyme [18]. Still, Figure 8a shows that the ACBs-supported tyrosinase has a low drop even after five cycles, suggesting that this material is a promising alternative for phenol removal. For the phenol removal of 100 mg/L solution (Figure 8b), although the use of successive washing steps after each cycle was not sufficient for the maintenance of the initial enzymatic activity, its application still had a positive impact on the decrease in the phenol removal yield in the initial cycles, allowing for the immobilized enzyme to be used five times, with a phenol removal above 50%. There was a 33.7% yield drop after five uses of the T-ACBs, whereas, without the washing steps, this drop was almost 60%.
3.5. Immobilized Enzyme Storage
In order to verify the loss in enzymatic activity during the materials’ storage time, five immobilizations were performed under the same conditions for each support used, as described in Figure 1a. Figure 9a,b show the phenol concentration decay over contact time of the aqueous solution initially containing 10 mg/L phenol with T-GAC and T-ACBs after different storage times. The graph in Figure 9c shows the yield of the enzyme oxidation reaction related to the storage times of the T-GAC and T-ACBs before their first use. The data in Figure 9c were calculated using the graphs in Figure 9a,b.
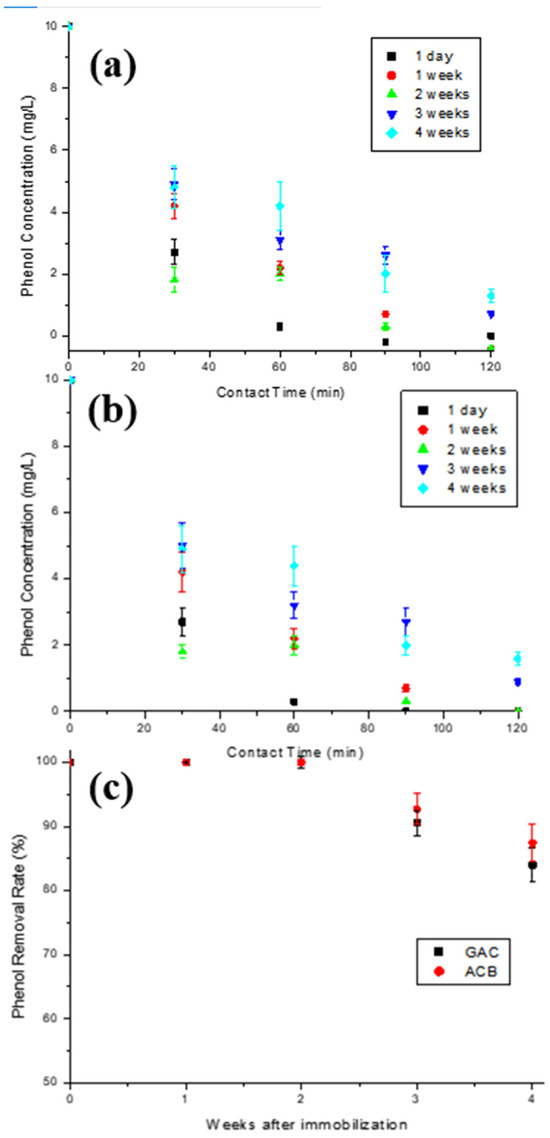
Figure 9.
Enzymatic oxidation of phenol solutions containing 10 mg/L using (a) T-GAC and (b) T-ACB, and (c) phenol removal efficiency after enzyme oxidation concerning the time between immobilization and its first use with the T-GAC or T-ACBs. Experimental conditions: initial activity of 1500 U, constant stirring of 15.7 rad/s, and 298 K.
Enzymatic oxidation using immobilized tyrosinase one day after immobilization reached 100% of phenol removal within 60 min for both materials (Figure 9a,b), similarly to what was found by other authors, such as Chavita [35] and Pigatto [43]. Analyzing the graphs, it is shown that phenol removal decreases as the time between immobilization and enzymatic oxidation increases, and that, after the fourth week of storage, the activity drop was more accentuated in both cases. According to the results, the immobilized enzymes on both supports removed 100% of phenol from a 10 mg/L solution after 2 weeks of storage in refrigeration. After 3 and 4 weeks of storage, the enzyme showed an efficiency drop of 9.5% and 16.0%, respectively, when immobilized on GAC, and 7.3% and 12.6%, respectively, when immobilized on ACBs.
The stability of the T-GAC and T-ACBs concerning the storage time between their first and second use in the enzymatic oxidation assays was also investigated. Once again, five immobilizations were performed for each support type under the same operating conditions. Each material was then used in enzymatic oxidation of an aqueous 10 mg/L phenol solution and stored under refrigeration after the assay, as described in Figure 1b. The graphs shown in Figure 10a,b illustrate the phenol concentration decay over contact time of the aqueous 10 mg/L phenol solution with the T-GAC (Figure 10a) and T-ACBs (Figure 10b) on their second use. Figure 10c shows the yield of the enzyme oxidation reaction related to the storage time of the T-GAC and T-ACBs between their first and second uses.
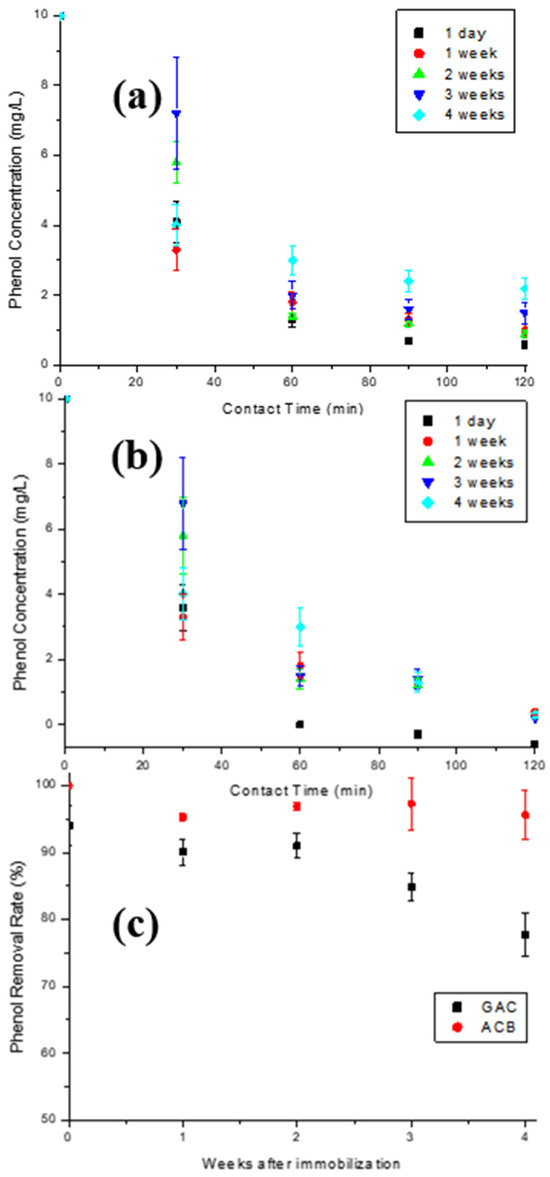
Figure 10.
Enzymatic oxidation of phenol solutions containing 10 mg/L using, for a second time, (a) T-GAC and (b) T-ACB, and (c) phenol removal efficiency after enzyme oxidation concerning the storage time of the T-GAC or T-ACBs between their first and second uses. Experimental conditions: initial activity of 1500 U, constant stirring of 15.7 rad/s, and 298 K.
The second enzymatic oxidation with the same immobilized enzyme one day after the first use was able to remove 94.0% and 100% of phenol from the solution on the GAC and ACB supports (Figure 10a,b), respectively, shortly after 60 min of contact, similarly to what occurred with the first use of the immobilized enzyme after one day of storage. Analyzing the graphs, it is clear that the enzymatic oxidation assay curves for the second use of the T-ACBs two, three, and four weeks after the first use are very similar, indicating a stabilization of the immobilized enzyme, while the same results for T-GAC showed similar decays when compared to the first use of the immobilized enzyme.
T-ACBs are more stable than T-GAC (Figure 10c). The stability of the immobilized enzyme in ACBs may be caused by two factors: covalent immobilization, which keeps the enzyme in a stable position compared to the free enzyme, and a stabilizing effect provided by the support, which minimizes possible distortion effects imposed by the aqueous solution on the active site of the enzyme. One of the most critical parameters to be considered in enzymatic immobilization is storage stability, since storing enzymes in solutions usually makes them unstable and leads to activity decays. As presented in this work, both materials are promising, presenting the possibility to be reused and the stability needed over storage time.
3.6. O-Quinone Production and Adsorption via the Supports
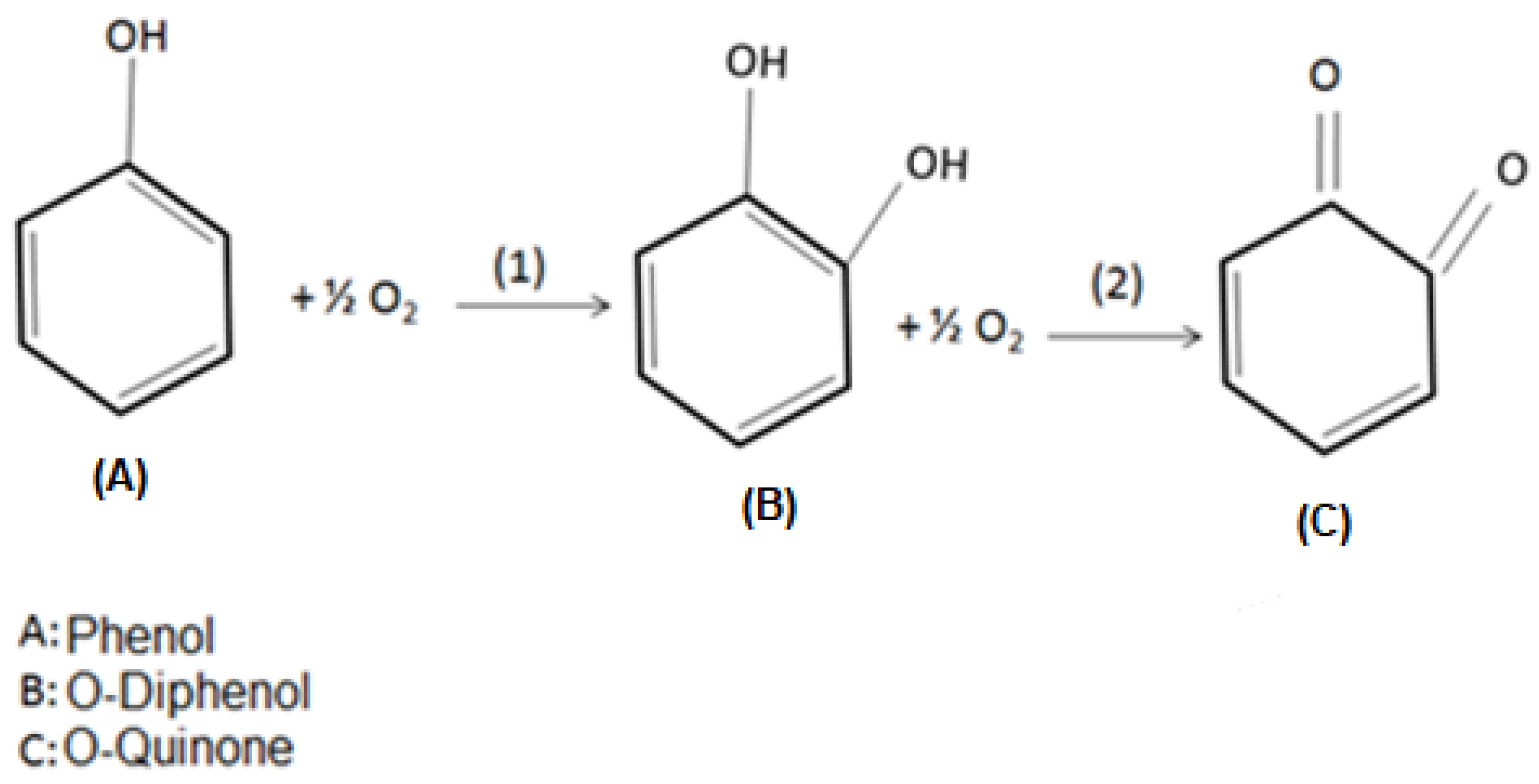
Tyrosinase may present three oxidation states (oxi, met, and deoxi), which are related to its active site. In the oxy state, the monofenolase activity takes place, while difenolase occurs under the met and deoxy states. Under monofelonase activity, the phenol molecules bind to Cu atoms in the enzyme’s active site, leading to its hydroxylation, producing o-diphenols. During difenolase, the oxidation of o-diphenols results in the formation of o-quinones [44,45,46]. Figure 11 illustrates such enzymatic oxidation reactions.
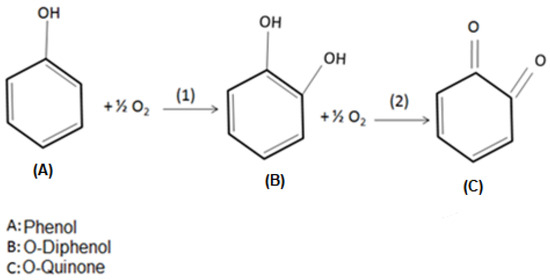
Figure 11.
Stages of phenol enzymatic oxidation to o-quinone. (1) is the monofenolase catalytic activity, and (2) is the difenolase catalytic activity.
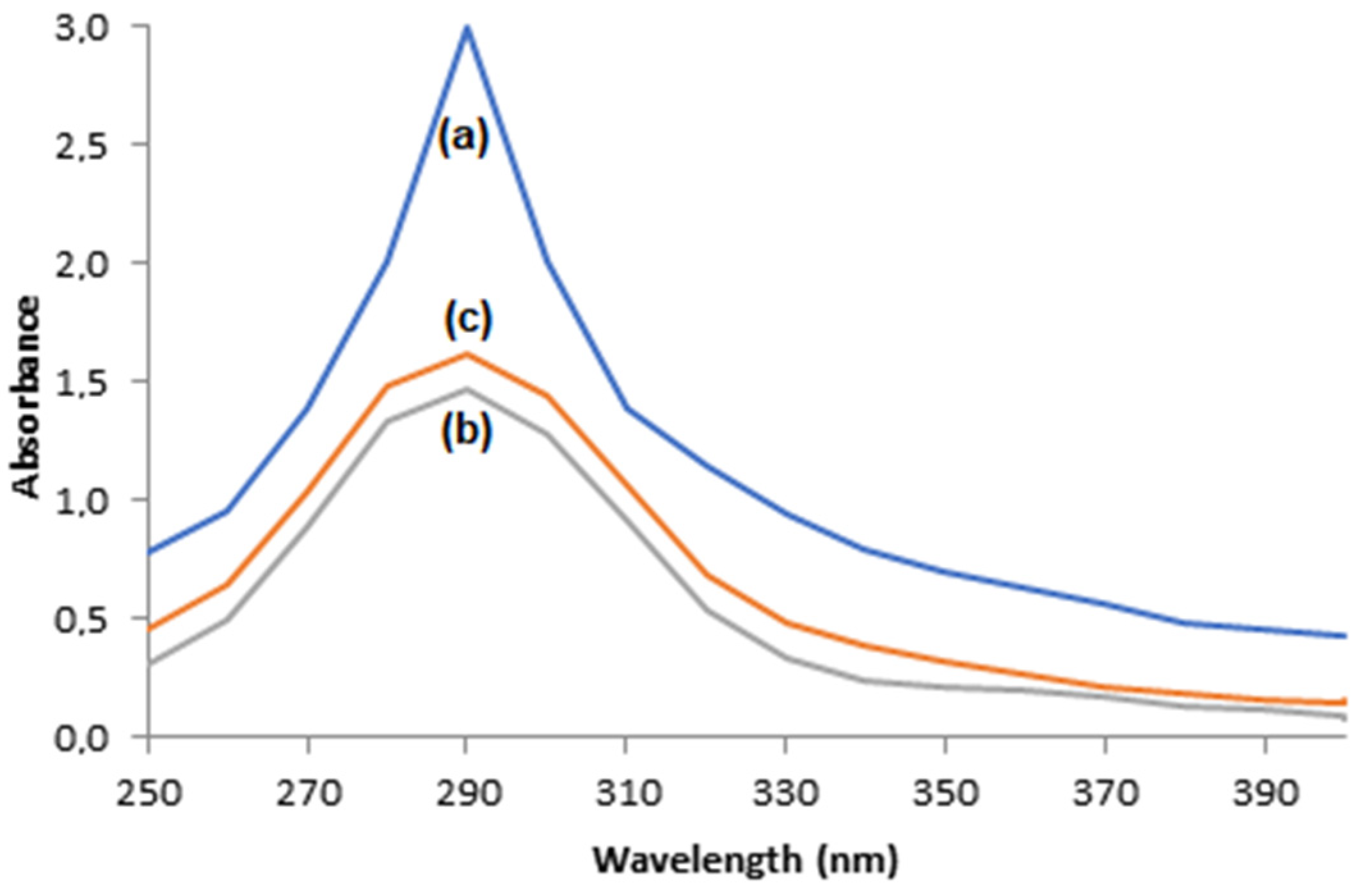
Phenol’s enzymatic oxidation and its consequent production of o-quinone is evidenced by the appearance of a dark color, which is characteristic of o-quinone solutions, and by the presence of an absorbance peak close to 290 nm in the scanning mode of the UV–visible electron spectrophotometer of phenol solutions after enzymatic oxidation [47,48,49].
The photos displayed in Figure 12 show that the dark color is less intense when oxidation took place through immobilized enzymes when compared to the free enzymes, which is an indication the that supports, that are adsorbent materials, were able to adsorb the o-quinone produced. This indication is corroborated by the UV–visible electron spectrophotometer scanning of solutions after enzymatic oxidation with free enzymes or immobilized ones, illustrated in Figure 13, where the absorbance peaks at 290 nm are smaller for oxidations with immobilized enzymes, suggesting the adsorption of o-quinone via the supports.

Figure 12.
Color of 60 mg/L phenol solutions after 2 h of enzymatic oxidation using (a) free enzymes, (b) T-ACBs, and (c) T-GAC.
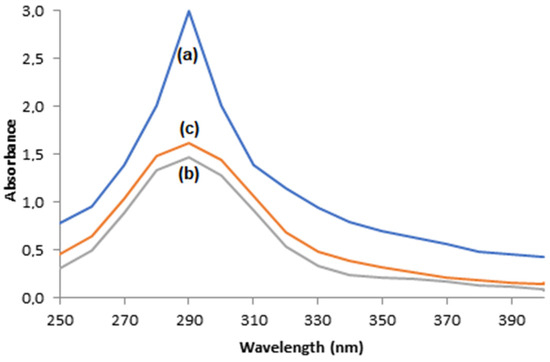
Figure 13.
UV–visible electron spectrophotometer scanning of 60 mg/L phenol solutions after enzymatic oxidation using (a) free enzymes, (b) T-ACBs, and (c) T-GAC.
Once both supports seemed to present a decent capacity of adsorbing o-quinone, the products of the oxidation of phenols could be further removed from the solution by additional ACBs or GAC, without tyrosinase.
4. Conclusions
The tyrosinase enzyme from a crude extract was immobilized on granular activated carbon (T-GAC) and activated chitosan beads (T-ACBs). It was possible to obtain enzymatic immobilizations of up to 70% on GAC and up to 100% on ACB in a contact time of 120 min, keeping the temperature at 298 K and the pH at 7.0. Overall, ACBs seem to be a better support for the immobilization of tyrosinase than GAC, presenting better results of phenol oxidation efficiencies, material’s reuse, and stability over storage.
In initial concentrations of 10 mg/L, the phenol oxidation assays returned final concentrations below the one required by the Brazilian legislation for the disposal of wastewater in the tests developed in the presence of both T-GAC and T-ACBs. Particularly, in the case of the T-ACBs, this limit was achieved for initial phenol concentrations of up to 40 mg/L. Regarding the materials’ reuse, it was possible to notice that T-GAC can be used up to three times, and in the T-ACBs case, up to six times before the phenol removal decreased more of 50%.
The addition of washing steps with different buffers had a positive impact on the yield drop that occurred with repeated T-ACBs use, improving the yield decay from 28.8% to 1.3% after five uses in solutions with a phenol concentration of 10 mg/L. The phenol removal yield remained unchanged using the T-GAC and T-ACBs that were stored under refrigeration for up to 2 weeks. After their first use, the T-ACBs were more stable during storage, presenting just a 4.4% drop in phenol removal yield after 4 weeks.
Such results suggest that both materials are effective for phenol removal, especially the T-ACBs, representing promising alternatives for mitigating the effects of this compound in industrial effluents. It is worth highlighting the materials’ capacity of achieving final phenol concentrations below that required by the Brazilian legislation and their great stability and possibility of reuse.
Author Contributions
Conceptualization (A.C.C.d.M., A.M.S. and F.V.d.F.), data curation (A.C.C.d.M.), formal analysis (A.C.C.d.M.), investigation (A.C.C.d.M.), methodology (A.C.C.d.M., A.M.S. and F.V.d.F.), visualization (A.C.C.d.M., F.P.d.S. and E.G.), resources (A.C.C.d.M., A.M.S. and F.V.d.F.), supervision (A.M.S. and F.V.d.F.), validation (F.P.d.S. and E.G.), writing—original draft (A.C.C.d.M.), writing—review and editing (F.P.d.S., E.G., A.M.S. and F.V.d.F.). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (funding number: 140526/2017-0), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Brazil (funding number: E-26/200.981/201).
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hasan, M.K.; Shahriar, A.; Jim, K.U. Water pollution in Bangladesh and its impact on public health. Heliyon 2019, 5, e02145. [Google Scholar] [CrossRef] [PubMed]
- WWAP. The United Nations World Water Development Report 4: Managing Water under Uncertainty and Risk; WWAP: Paris, France, 2012. [Google Scholar]
- Motamedi, M.; Yerushalmi, L.; Haghighat, F.; Chen, Z. Recent developments in photocatalysis of industrial effluents ։ A review and example of phenolic compounds degradation. Chemosphere 2022, 296, 133688. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Kargari, A.; Sanaeepur, H.; Abbassian, K.; Najafi, A.; Mofarrah, E. Phenol removal from industrial wastewaters: A short review. Desalin. Water Treat. 2013, 53, 2215–2234. [Google Scholar] [CrossRef]
- Hansch, C.; McKarns, S.C.; Smith, C.J.; Doolittle, D.J. Comparative QSAR evidence for a free-radical mechanism of phenol-induced toxicity. Chem. Biol. Interact. 2000, 127, 61–72. [Google Scholar] [CrossRef]
- Boyd, E.M.; Killham, K.; Meharg, A. Toxicity of mono-, di- and tri-chlorophenols to lux marked terrestrial bacteria, Burkholderia species Rasc c2 and Pseudomonas fluorescens. Chemosphere 2001, 43, 157–166. [Google Scholar] [CrossRef]
- Ertürk, M.D.; Saçan, M.T.; Novic, M.; Minovski, N. Quantitative structure–activity relationships (QSARs) using the novel marine algal toxicity data of phenols. J. Mol. Graph. Model. 2012, 38, 90–100. [Google Scholar] [CrossRef]
- Said, K.A.M.; Ismail, A.F.; Karim, Z.A.; Abdullah, M.S.; Hafeez, A. A review of technologies for the phenolic compounds recovery and phenol removal from wastewater. Process. Saf. Environ. Prot. 2021, 151, 257–289. [Google Scholar] [CrossRef]
- Ilavský, J.; Hrivnák, J.; Barlokov, D. Analysis of chlorinated phenols in water. Food Environ. Saf. 2012, 11, 5–14. [Google Scholar]
- Chae, Y.; Kim, L.; Kim, D.; Cui, R.; Lee, J.; An, Y.-J. Deriving hazardous concentrations of phenol in soil ecosystems using a species sensitivity distribution approach. J. Hazard. Mater. 2020, 399, 123036. [Google Scholar] [CrossRef]
- Li, C.; Deng, W.; Gao, C.; Xiang, X.; Feng, X.; Batchelor, B.; Li, Y. Membrane distillation coupled with a novel two-stage pretreatment process for petrochemical wastewater treatment and reuse. Sep. Purif. Technol. 2019, 224, 23–32. [Google Scholar] [CrossRef]
- USEPA. Federal Register; USEPA: Washington, DC, USA, 1987. [Google Scholar]
- CONAMA. Brazilian National Council for the Environment, Resolution #430; CONAMA: Brasília, Brazil, 2011; 9p. (In Portuguese) [Google Scholar]
- Pandey, K.; Singh, B.; Pandey, A.K.; Badruddin, I.J.; Pandey, S.; Mishra, V.K.; Jain, P.A. Application of Microbial Enzymes in Industrial Waste Water Treatment. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1243–1254. [Google Scholar] [CrossRef]
- Xu, D.-Y.; Yang, Z. Cross-linked tyrosinase aggregates for elimination of phenolic compounds from wastewater. Chemosphere 2013, 92, 391–398. [Google Scholar] [CrossRef]
- Erhan, E.; Keskinler, B.; Akay, G.; Algur, O. Removal of phenol from water by membrane-immobilized enzymes: Part I. Dead-end filtration. J. Membr. Sci. 2002, 206, 361–373. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, R.; Sharma, R.S.; Mishra, V. Phenol remediation by peroxidase from an invasive mesquite: Turning an environmental wound into wisdom. J. Hazard. Mater. 2017, 334, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef]
- Yamada, K.; Inoue, T.; Akiba, Y.; Kashiwada, A.; Matsuda, K.; Hirata, M. Removal of p-Alkylphenols from Aqueous Solutions by Combined Use of Mushroom Tyrosinase and Chitosan Beads. Biosci. Biotechnol. Biochem. 2006, 70, 2467–2475. [Google Scholar] [CrossRef][Green Version]
- Wu, J.C.Y.; Hutchings, C.H.; Lindsay, M.J.; Werner, C.J.; Bundy, B.C. Enhanced Enzyme Stability Through Site-Directed Covalent Immobilization. J. Biotechnol. 2015, 193, 83–90. [Google Scholar] [CrossRef]
- Abdollahi, K.; Yazdani, F.; Panahi, R. Covalent immobilization of tyrosinase onto cyanuric chloride crosslinked amine-functionalized superparamagnetic nanoparticles: Synthesis and characterization of the recyclable nanobiocatalyst. Int. J. Biol. Macromol. 2017, 94, 396–405. [Google Scholar] [CrossRef]
- Mollaei, M.; Abdollahpour, S.; Atashgahi, S.; Abbasi, H.; Masoomi, F.; Rad, I.; Lotfi, A.S.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Enhanced phenol degradation by Pseudomonas sp. SA01: Gaining insight into the novel single and hybrid immobilizations. J. Hazard. Mater. 2010, 175, 284–292. [Google Scholar] [CrossRef]
- El-aziz, S.M.A.; Hassan, A.; Faraag, I. Tyrosinase Enzyme Purification and Immobilization from Pseudomonas sp. EG22 Using Cellulose Coated Magnetic Nanoparticles : Characterization of Bioactivity in Melanin Product. World J. Microbiol. Biotechnol. 2023; preprints. [Google Scholar] [CrossRef]
- Liu, D.-M.; Dong, C. Recent advances in nano-carrier immobilized enzymes and their applications. Process. Biochem. 2020, 92, 464–475. [Google Scholar] [CrossRef]
- Kameda, E.; Langone, M.A.P.; Coelho, M.A.Z. Tyrosinase Extract from Agaricus bisporus Mushroom and itsin Natura Tissue for Specific Phenol Removal. Environ. Technol. 2006, 27, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, V.P.S.; Silva, L.M.C.; Salgado, A.M.; Pereira, K.S. Application of agaricus bisporus extract for benzoate sodium detection based on tyrosinase inhibition for biosensor development. Chem. Eng. Trans. 2013, 32, 1831–1836. [Google Scholar] [CrossRef]
- Silvestre, M.P.C.; Silva, V.D.M.; de Marco, L.M.; Delvivo, F.M.; Coelho, J.V. Imobilização da pancreatina em carvão ativado e em alumina para o preparo de hidrolisados de soro de leite. Acta Sci. Health Sci. 2005, 27, 163–169. [Google Scholar] [CrossRef][Green Version]
- Kennedy, L.J.; Selvi, P.; Padmanabhan, A.; Hema, K.; Sekaran, G. Immobilization of polyphenol oxidase onto mesoporous activated carbons-isotherm and kinetic studies. Chemosphere 2007, 69, 262–270. [Google Scholar] [CrossRef]
- Kumar, A.G.; Perinbam, K.; Kamatchi, P.; Nagesh, N.; Sekaran, G. In situ immobilization of acid protease on mesoporous activated carbon packed column for the production of protein hydrolysates. Bioresour. Technol. 2010, 101, 1377–1379. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Pan, S.; Wu, T.; Tang, X. Optimal immobilization of β-glucosidase into chitosan beads using response surface methodology. Electron. J. Biotechnol. 2013, 16. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Tuncay, F.O.; Cakmak, U.; Kolcuoğlu, Y. Rhaphiolepis indica (L.) Lindl. Fruit: LC-HRMS-based phytochemical profile, FTIR spectral, in vitro enzyme inhibition and antioxidant analysis. Food Biosci. 2023, 56, 103228. [Google Scholar] [CrossRef]
- Romero, G.; Contreras, L.M.; Céspedes, C.A.; Wilkesman, J.; Clemente-Jiménez, J.M.; Rodríguez-Vico, F.; Heras-Vázquez, F.J.L. Efficiency Assessment between Entrapment and Covalent Bond Immobilization of Mutant β-Xylosidase onto Chitosan Support. Polymers 2023, 15, 3170. [Google Scholar] [CrossRef]
- Chavita, A.C. Estudo da Remoção de Fenóis de Soluções aquosas através da Adsorção em Quitosana, Degradação Enzimática por Tirosinase e Imobilização de Tirosinase em Matriz de Quitosana. Master’s Thesis, University of São Paulo, São Paulo, Brazil, 2010. (In Portuguese). [Google Scholar]
- Miyaguti, R.M. Oxidação Enzimática de Soluções Fenólicas com Tirosinase Imobilizada em Quitosana Oxidação Enzimática de Soluções Fenólicas com Tirosinase Imobilizada em Quitosana. Master’s Thesis, University of São Paulo, São Paulo, Brazil, 2011. (In Portuguese). [Google Scholar]
- Colombié, S.; Gaunand, A.; Lindet, B. Lysozyme inactivation under mechanical stirring: Effect of physical and molecular interfaces. Enzym. Microb. Technol. 2001, 28, 820–826. [Google Scholar] [CrossRef]
- Gikanga, B.; Hui, A.; Maa, Y.-F. Mechanistic Investigation on Grinding-Induced Subvisible Particle Formation during Mixing and Filling of Monoclonal Antibody Formulations. PDA J. Pharm. Sci. Technol. 2017, 72, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Menoncin, S.; Domingues, N.M.; Freire, D.M.G.; Oliveira, J.V.; Di Luccio, M.; Treichel, H.; de Oliveira, D. Imobilização de lipases produzidas por fermentação em estado sólido utilizando Penicillium verrucosum em suportes hidrofóbicos. Food Sci. Technol. 2009, 29, 440–443. [Google Scholar] [CrossRef]
- Wiesbauer, J.; Cardinale, M.; Nidetzky, B. Shaking and stirring: Comparison of controlled laboratory stress conditions applied to the human growth hormone. Process. Biochem. 2013, 48, 33–40. [Google Scholar] [CrossRef]
- Bevilaqua, J.; Cammarota, M.; Freire, D.M.G.; Sant’Anna, G.L. Phenol removal through combined biological and enzymatic treatments. Braz. J. Chem. Eng. 2002, 19, 151–158. [Google Scholar] [CrossRef]
- Wada, S.; Ichikawa, H.; Tatsumi, K. Removal of phenols from wastewater by soluble and immobilized tyrosinase. Biotechnol. Bioeng. 1993, 42, 854–858. [Google Scholar] [CrossRef]
- Pigatto, G. Study on Tyrosinase Application in the Treatment of Plant Effluent and Verification of Genotoxicity of the Treated Effluent into Plant Cells. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2013. (In Portuguese). [Google Scholar]
- García-Molina, P.; García-Molina, F.; Teruel-Puche, J.A.; Rodríguez-López, J.N.; García-Cánovas, F.; Muñoz-Muñoz, J.L. Considerations about the kinetic mechanism of tyrosinase in its action on monophenols: A review. Mol. Catal. 2022, 518, 112072. [Google Scholar] [CrossRef]
- Choi, S.; Ahn, H.; Kim, S. Tyrosinase-mediated hydrogel crosslinking for tissue engineering. J. Appl. Polym. Sci. 2021, 139, 51887. [Google Scholar] [CrossRef]
- Lee, P.-G.; Lee, S.-H.; Hong, E.Y.; Lutz, S.; Kim, B.-G. Circular permutation of a bacterial tyrosinase enables efficient polyphenol-specific oxidation and quantitative preparation of orobol. Biotechnol. Bioeng. 2018, 116, 19–27. [Google Scholar] [CrossRef]
- Gulaboski, R.; Bogeski, I.; Mirčeski, V.; Saul, S.; Pasieka, B.; Haeri, H.H.; Stefova, M.; Stanoeva, J.P.; Mitrev, S.; Hoth, M.; et al. Hydroxylated derivatives of dimethoxy-1,4-benzoquinone as redox switchable earth-alkaline metal ligands and radical scavengers. Sci. Rep. 2013, 3, srep01865. [Google Scholar] [CrossRef]
- Ito, S.; Sugumaran, M.; Wakamatsu, K. Chemical Reactivities of ortho-Quinones Produced in Living Organisms: Fate of Quinonoid Products Formed by Tyrosinase and Phenoloxidase Action on Phenols and Catechols. Int. J. Mol. Sci. 2020, 21, 6080. [Google Scholar] [CrossRef]
- Qu, Y.; Zhan, Q.; Du, S.; Ding, Y.; Fang, B.; Du, W.; Wu, Q.; Yu, H.; Li, L.; Huang, W. Catalysis-based specific detection and inhibition of tyrosinase and their application. J. Pharm. Anal. 2020, 10, 414–425. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).