Determination of Heavy Metals and Health Risk Assessment in Tap Water from Wuhan, China, a City with Multiple Drinking Water Sources
Abstract
:1. Introduction
2. Materials and Methods
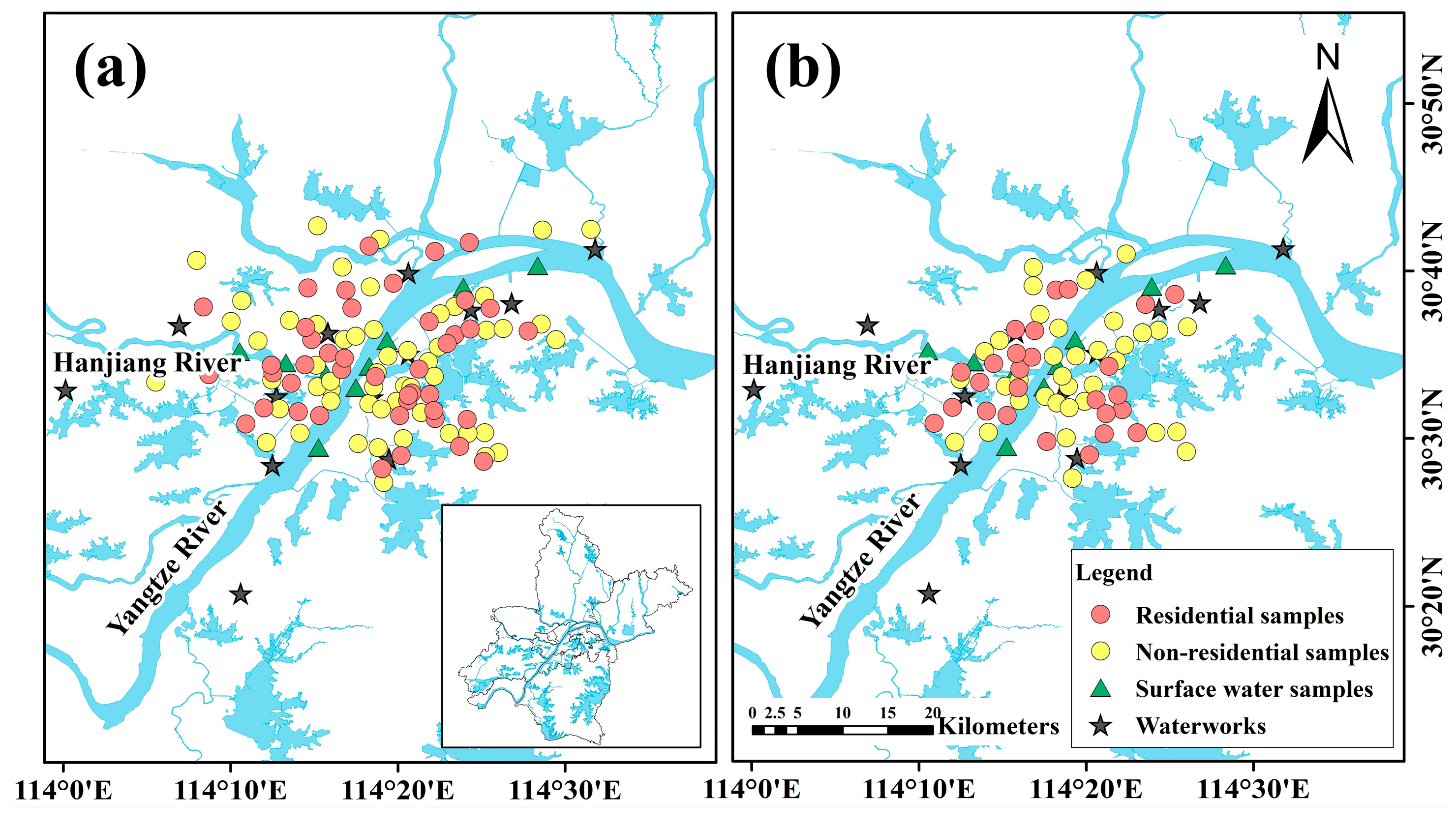
2.1. Study Area
2.2. Sample Collection and Measurement Method
2.3. Statistical Analysis
2.4. Health Risk Assessment Model
| Metals | Rfdoral (mg·kg−1·d−1) | Rfddermal (mg·kg−1·d−1) | k (m/h) | CSF (mg·kg−1·d−1)−1 |
|---|---|---|---|---|
| Fe | 0.7 | 0.14 | 0.00001 | |
| Al | 1 | 0.2 | 0.00001 | |
| Mn | 0.024 | 0.00096 | 0.00001 | |
| Co | 0.0003 | 0.00006 | 0.00004 | |
| Ni | 0.02 | 0.0008 | 0.00004 | |
| Cu | 0.04 | 0.012 | 0.00001 | |
| Se | 0.005 | 0.00015 | 0.00001 | |
| Cd | 0.0005 | 0.000025 | 0.00001 | 6.1 |
| Cr | 0.003 | 0.000075 | 0.00003 | 0.5 |
| Pb | 0.0014 | 0.00042 | 0.00001 | 0.0085 |
2.5. Heavy Metal Pollution Index (HPI)
3. Results and Discussion
3.1. Concentrations of Heavy Metals in Surface Water and Tap Water
| Metals | Summer | Winter | Chinese National Standard Limits [46] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surface Water Environmental Quality | Surface Drinking Water Sources | |||||||||
| Yangtze River | Hanjiang River | Yangtze River | Hanjiang River | I | II | III | IV | V | ||
| Fe | 304.10 ± 42.93 | 146.13 ± 42.65 | 1014.87 ± 271.94 | 832.12 ± 93.19 | 300 | |||||
| Al | 364.70 ± 117.17 | 65.82 ± 47.48 | 1417.82 ± 669.92 | 788.10 ± 144.65 | ||||||
| Mn | 5.01 ± 1.33 | 2.83 ± 1.44 | 26.67 ± 5.24 | 37.83 ± 9.95 | 100 | |||||
| Co | 0.19 ± 0.02 | 0.14 ± 0.02 | 0.67 ± 0.18 | 0.66 ± 0.34 | 1000 | |||||
| Ni | 1.95 ± 0.20 | 2.16 ± 0.32 | 4.74 ± 0.97 | 5.00 ± 0.23 | 20 | |||||
| Cu | 1.92 ± 0.15 | 2.27 ± 0.11 | 3.68 ± 0.58 | 3.10 ± 0.13 | 10 | 1000 | 1000 | 1000 | 1000 | |
| Se | 0.30 ± 0.08 | 0.56 ± 0.05 | 0.78 ± 0.17 | 0.88 ± 0.06 | 10 | 10 | 10 | 20 | 20 | |
| Cd | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.02 | 0.02 ± 0.01 | 1 | 5 | 5 | 5 | 10 | |
| Cr | 3.37 ± 0.29 | 3.46 ± 0.50 | 1.38 ± 0.39 | 0.77 ± 0.26 | 10 | 50 | 50 | 50 | 100 | |
| Pb | 0.21 ± 0.12 | 0.04 ± 0.01 | 1.48 ± 0.37 | 0.70 ± 0.25 | 10 | 10 | 50 | 50 | 100 | |
| Metals | Summer | Winter | Detection Frequency (%) | WHO Limits [49] | Chinese National Standard Limits [44] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Residential 43 Samples | Non-Residential 54 Samples | Total 97 Samples | Range | Residential 26 Samples | Non-Residential 35 Samples | Total 61 Samples | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||
| Fe | 64.58–159.61 | 102.72 | 18.6 | 99.94 | 17.96 | 101.17 | 18.3 | 23.18–175.97 | 101.48 | 37.87 | 113.80 | 33.23 | 108.55 | 35.8 | 100.00 | 300 | |
| Al | 22.77–176.99 | 76.25 | 36.3 | 64.24 | 28.43 | 69.56 | 32.71 | 4.18–296.74 | 74.96 | 74.20 | 66.33 | 48.45 | 70.01 | 60.93 | 100.00 | 200 | |
| Mn | n.d.–3.26 | 0.3 | 0.61 | 0.24 | 0.51 | 0.27 | 0.56 | n.d.–2.35 | 0.27 | 0.29 | 0.30 | 0.46 | 0.29 | 0.39 | 36.08 | 100 | |
| Co | n.d.–0.45 | 0.09 | 0.05 | 0.08 | 0.06 | 0.11 | 0.04 | n.d.–0.23 | 0.08 | 0.05 | 0.09 | 0.05 | 0.09 | 0.05 | 97.47 | 50 | |
| Ni | 0.39–10.89 | 1.53 | 1.01 | 1.22 | 1.48 | 1.36 | 1.3 | n.d.–2.95 | 1.58 | 0.84 | 1.62 | 0.75 | 1.6 | 0.79 | 98.10 | 70 | 20 |
| Cu | n.d.–10.02 | 1.27 | 2.14 | 0.89 | 2 | 1.04 | 2.08 | n.d.–2.99 | 0.62 | 0.84 | 0.21 | 0.27 | 0.39 | 0.62 | 49.37 | 2000 | 1000 |
| Se | n.d.–0.68 | 0.38 | 0.11 | 0.38 | 0.11 | 0.38 | 0.11 | n.d.–1.01 | 0.67 | 0.19 | 0.67 | 0.19 | 0.67 | 0.19 | 94.94 | 40 | 10 |
| Cd | n.d.–0.10 | 0.03 | 0.01 | 0.03 | 0.01 | 0.03 | 0.01 | n.d.–0.33 | 0.03 | 0.01 | 0.04 | 0.05 | 0.04 | 0.04 | 51.27 | 3 | 5 |
| Cr | n.d.–6.65 | 1.18 | 1.52 | 0.27 | 0.33 | 0.68 | 1.14 | n.d.–1.28 | 0.24 | 0.29 | 0.16 | 0.13 | 0.2 | 0.22 | 55.06 | 50 | 50 |
| Pb | n.d.–8.10 | 2.12 | 1.77 | 1.29 | 1.15 | 1.66 | 1.52 | n.d.–6.09 | 0.87 | 1.06 | 0.81 | 1.27 | 0.84 | 1.19 | 73.42 | 10 | 10 |
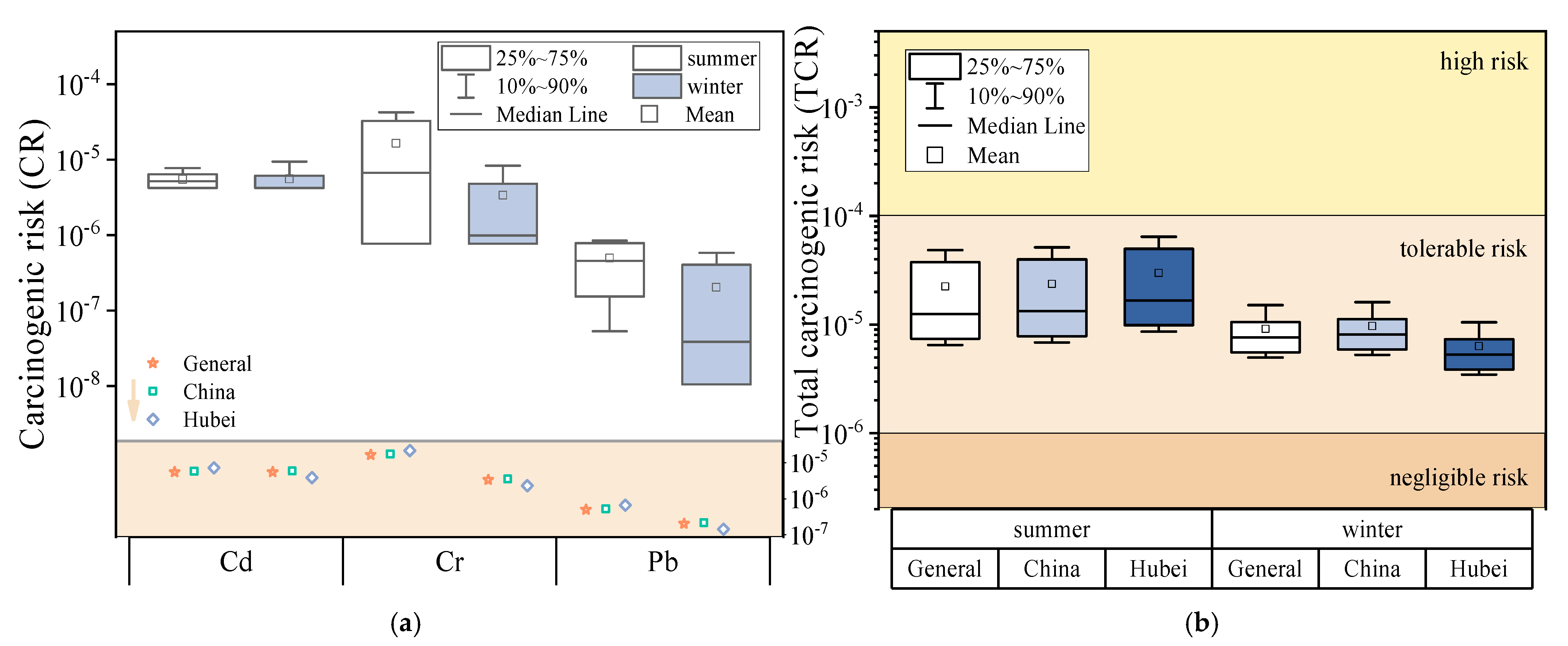
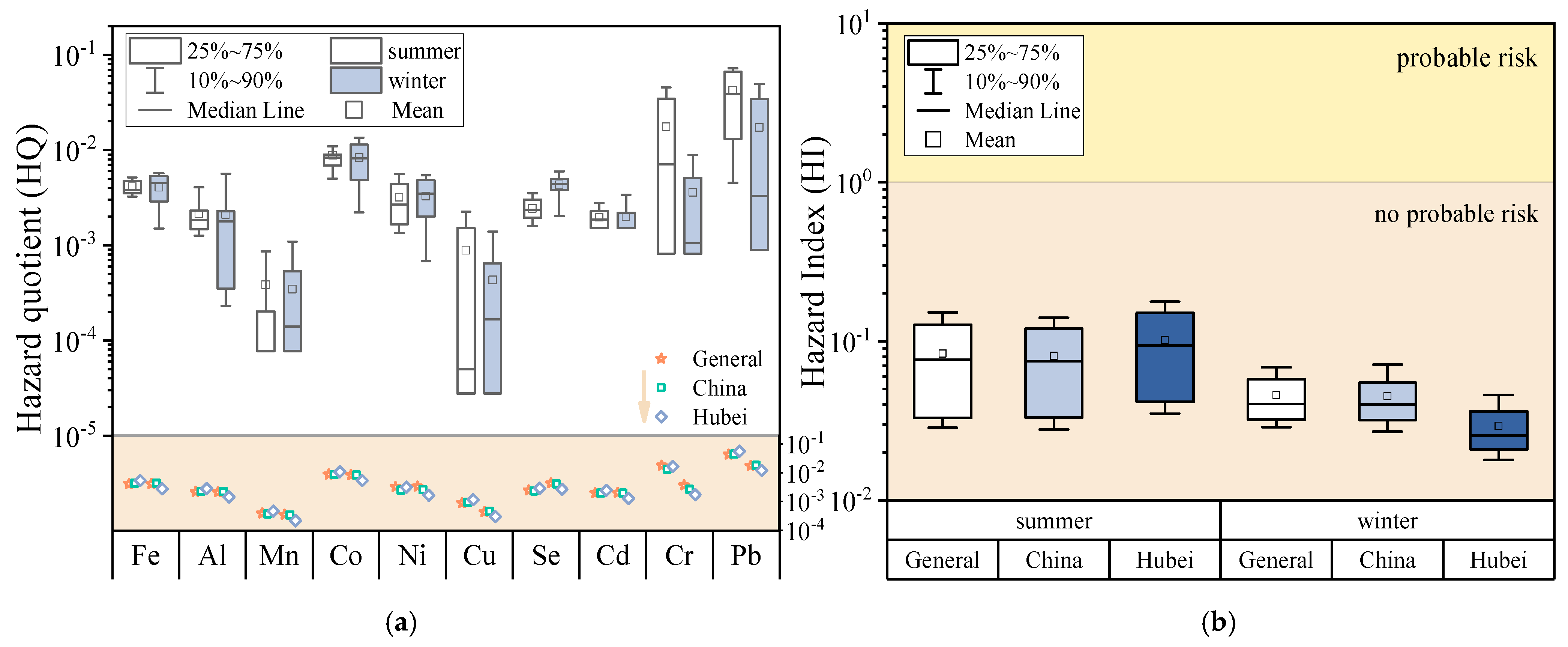
3.2. Health Risk Assessment on Human Health
3.3. Potential Contamination of Heavy Metals from Water Supply Pipelines
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bichai, F.; Smeets, P.W.M.H. Using QMRA-based regulation as a water quality management tool in the water security challenge: Experience from the netherlands and australia. Water Res. 2013, 47, 7315–7326. [Google Scholar] [CrossRef] [PubMed]
- Puchol-Salort, P.; Boskovic, S.; Dobson, B.; van Reeuwijk, M.; Mijic, A. Water neutrality framework for systemic design of new urban developments. Water Res. 2022, 219, 118583. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Anik, A.H.; Khan, R.; Ahmed, F.T.; Siddique, M.A.B.; Khan, A.H.A.N.; Saha, N.; Idris, A.M.; Alam, M. Public Health Vulnerability Due to the Exposure of Dissolved Metal(oid)s in Tap Water from a Mega City (Dhaka, Bangladesh): Source and Quality Appraisals. Expo. Health 2022, 14, 713–732. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zheng, J.; Liang, J.; Li, R.; Gong, Z. Source tracing and health risk assessment of phthalate esters in household tap-water: A case study of the urban area of Quanzhou, Southeast China. Ecotoxicol. Environ. Saf. 2022, 248, 114277. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, E.; Lima, A.; Albanese, S.; Birke, M.; Cicchella, D.; Giaccio, L.; Valera, P.; De Vivo, B. Major and trace elements in tap water from Italy. J. Geochemical Explor. 2012, 112, 54–75. [Google Scholar] [CrossRef]
- Fernández-Navarro, P.; Villanueva, C.M.; García-Pérez, J.; Boldo, E.; Goñi-Irigoyen, F.; Ulibarrena, E.; Rantakokko, P.; García-Esquinas, E.; Pérez-Gómez, B.; Pollán, M.; et al. Chemical quality of tap water in Madrid: Multicase control cancer study in Spain (MCC-Spain). Environ. Sci. Pollut. Res. 2017, 24, 4755–4764. [Google Scholar] [CrossRef]
- Wang, G.; Shen, J.; Wei, S.; Cai, D.; Liu, J. Identification of Heavy Metals and Organic Micropollutants in Drinking Water Sources in Typical Villages and Towns in Northeast China. Molecules 2022, 27, 8033. [Google Scholar] [CrossRef]
- Zhao, L.; Gong, D.; Zhao, W.; Lin, L.; Yang, W.; Guo, W.; Tang, X.; Li, Q. Spatial-temporal distribution characteristics and health risk assessment of heavy metals in surface water of the Three Gorges Reservoir, China. Sci. Total Environ. 2020, 704, 134883. [Google Scholar] [CrossRef]
- Li, M.S.; Luo, Y.P.; Su, Z.Y. Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environ. Pollut. 2007, 147, 168–175. [Google Scholar] [CrossRef]
- Qian, Y.; Cheng, C.; Feng, H.; Hong, Z.; Zhu, Q.; Kolenčík, M.; Chang, X. Assessment of metal mobility in sediment, commercial fish accumulation and impact on human health risk in a large shallow plateau lake in southwest of China. Ecotoxicol. Environ. Saf. 2020, 194, 110346. [Google Scholar] [CrossRef]
- Khan, S.; Shah, I.A.; Muhammad, S.; Malik, R.N.; Shah, M.T. Arsenic and Heavy Metal Concentrations in Drinking Water in Pakistan and Risk Assessment: A Case Study. Hum. Ecol. Risk Assess. 2015, 21, 1020–1031. [Google Scholar] [CrossRef]
- Le Bot, B.; Lucas, J.P.; Lacroix, F.; Glorennec, P. Exposure of children to metals via tap water ingestion at home: Contamination and exposure data from a nationwide survey in France. Environ. Int. 2016, 94, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Çelebi, A.; Şengörür, B.; Kløve, B. Human health risk assessment of dissolved metals in groundwater and surface waters in the Melen watershed, Turkey. J. Environ. Sci. Health Part A 2014, 49, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Zhang, H.M.; Sojinu, S.O.; Liu, G.H.; Zhang, J.Q.; Ni, H.G. Trace elements contamination and human health risk assessment in drinking water from Shenzhen, China. Environ. Monit. Assess. 2015, 187, 4220. [Google Scholar] [CrossRef]
- Ranjbar, Z.; Pourhadadi, D.; Montazeri, S.; Roshanzamir Modaberi, M. Lead compounds in paint and coatings: A review of regulations and latest updates. Prog. Org. Coatings 2023, 174, 107247. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Yin, K.; Lv, Y.; Zhang, D. Environmental-geochemical characteristics of Cu in the soil and water in copper-rich deposit area of southeastern Hubei Province, along the middle Yangtze River, Central China. Environ. Pollut. 2009, 157, 2957–2963. [Google Scholar] [CrossRef]
- Tao, S.; Zhang, X.; Xu, J.; Pan, G.; Gu, F. Anthropogenic impacts on isotopic and geochemical characteristics of urban streams: A case study in Wuhan, China. Environ. Sci. Pollut. Res. 2021, 28, 39186–39198. [Google Scholar] [CrossRef]
- Ali, M.M.; Rahman, S.; Islam, M.S.; Rakib, M.R.J.; Hossen, S.; Rahman, M.Z.; Kormoker, T.; Idris, A.M.; Phoungthong, K. Distribution of heavy metals in water and sediment of an urban river in a developing country: A probabilistic risk assessment. Int. J. Sediment Res. 2022, 37, 173–187. [Google Scholar] [CrossRef]
- Nduka, J.K.; Kelle, H.I.; Umeh, T.C.; Okafor, P.C.; Iloka, G.C.; Okoyomon, E. Ecological and health risk assessment of radionuclides and heavy metals of surface and ground water of Ishiagu–Ezillo quarry sites of Ebonyi, Southeast Nigeria. J. Hazard. Mater. Adv. 2023, 10, 100307. [Google Scholar] [CrossRef]
- Selvam, S.; Jesuraja, K.; Roy, P.D.; Venkatramanan, S.; Khan, R.; Shukla, S.; Manimaran, D.; Muthukumar, P. Human health risk assessment of heavy metal and pathogenic contamination in surface water of the Punnakayal estuary, South India. Chemosphere 2022, 298, 134027. [Google Scholar] [CrossRef]
- Najafzadeh, M.; Niazmardi, S. A Novel Multiple-Kernel Support Vector Regression Algorithm for Estimation of Water Quality Parameters. Nat. Resour. Res. 2021, 30, 3761–3775. [Google Scholar] [CrossRef]
- Najafzadeh, M.; Homaei, F.; Farhadi, H. Reliability assessment of water quality index based on guidelines of national sanitation foundation in natural streams: Integration of remote sensing and data-driven models. Artif. Intell. Rev. 2021, 54, 4619–4651. [Google Scholar] [CrossRef]
- Najafzadeh, M.; Basirian, S. Evaluation of River Water Quality Index Using Remote Sensing and Artificial Intelligence Models. Remote Sens. 2023, 15, 2359. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Lee, D.; Gibson, J.M.; Brown, J.; Habtewold, J.; Murphy, H.M. Burden of disease from contaminated drinking water in countries with high access to safely managed water: A systematic review. Water Res. 2023, 242, 120244. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J. Formation and prevention of pipe scale in water supply pipelines with anti-corrosion lining. Water Supply 2022, 22, 4006–4014. [Google Scholar] [CrossRef]
- Sun, H.; Shi, B.; Yang, F.; Wang, D. Effects of sulfate on heavy metal release from iron corrosion scales in drinking water distribution system. Water Res. 2017, 114, 69–77. [Google Scholar] [CrossRef]
- Sarin, P.; Snoeyink, V.L.; Bebee, J.; Jim, K.K.; Beckett, M.A.; Kriven, W.M.; Clement, J.A. Iron release from corroded iron pipes in drinking water distribution systems: Effect of dissolved oxygen. Water Res. 2004, 38, 1259–1269. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, Y.; Dai, Z.; Ren, A.; Fang, J.; Li, X.; van der Meer, W.; Medema, G.; Rose, J.B.; Liu, G. Building water quality deterioration during water supply restoration after interruption: Influences of premise plumbing configuration. Water Res. 2023, 241, 120149. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Gao, S.; Wang, Z.; He, S. Distribution, source, water quality and health risk assessment of dissolved heavy metals in major rivers in Wuhan, China. PeerJ 2021, 9, 11853. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Xu, J.; Yang, J.; Zhang, X.; Tao, S. Characterization of Dissolved Organic Matter of Sediments in Urban Streams Using EEMs–PARAFAC and Absorption Spectroscopy: A Case Study in Wuhan, China. Water 2022, 14, 3181. [Google Scholar] [CrossRef]
- Rao, K.; Zhang, X.; Yi, X.J.; Li, Z.S.; Wang, P.; Huang, G.W.; Guo, X.X. Interactive effects of environmental factors on phytoplankton communities and benthic nutrient interactions in a shallow lake and adjoining rivers in China. Sci. Total Environ. 2018, 619–620, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhou, X.; Cheng, W. Water effects on urban heat islands in summer using WRF-UCM with gridded urban canopy parameters—A case study of Wuhan. Build. Environ. 2022, 225, 109528. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Z.; Shen, Z.; Niu, J.; Cai, Y. Residues of organochlorine pesticides in water and suspended particulate matter from the Yangtze River catchment of Wuhan, China. Environ. Monit. Assess. 2008, 137, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Water Quality—Determination of 65 Elements—Inductively Coupled Plasma-Mass Spectrometry (HJ 700-2014); Ministry of Environmental Protection; China Environmental Press: Beijing, China, 2014.
- USEPA. Risk Management Guidance for Superfund Volume I Environmental Evaluation Manual (Part A); USEPA: Washington, DC, USA, 1989. [Google Scholar]
- Fakhri, Y.; Saha, N.; Ghanbari, S.; Rasouli, M.; Miri, A.; Avazpour, M.; Rahimizadeh, A.; Riahi, S.M.; Ghaderpoori, M.; Keramati, H.; et al. Carcinogenic and non-carcinogenic health risks of metal(oid)s in tap water from Ilam city, Iran. Food Chem. Toxicol. 2018, 118, 204–211. [Google Scholar] [CrossRef]
- Duan, X.; Zhao, X.; Wang, B.; Chen, Y.; Cao, S. Highlights of the Chinese Exposure Factors Handbook (Adults); Science Press: Beijing, China, 2014. [Google Scholar]
- Qu, L.; Huang, H.; Xia, F.; Liu, Y.; Dahlgren, R.A.; Zhang, M.; Mei, K. Risk analysis of heavy metal concentration in surface waters across the rural-urban interface of the Wen-Rui Tang River, China. Environ. Pollut. 2018, 237, 639–649. [Google Scholar] [CrossRef]
- Rasic Misic, I.D.; Tosic, S.B.; Pavlovic, A.N.; Pecev-Marinkovic, E.T.; Mrmosanin, J.M.; Mitic, S.S.; Stojanovic, G.S. Trace element content in commercial complementary food formulated for infants and toddlers: Health risk assessment. Food Chem. 2022, 378, 132113. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q. Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the Upper Han River, China. J. Hazard. Mater. 2010, 181, 1051–1058. [Google Scholar] [CrossRef]
- Tiwari, A.K.; De Maio, M.; Singh, P.K.; Mahato, M.K. Evaluation of Surface Water Quality by Using GIS and a Heavy Metal Pollution Index (HPI) Model in a Coal Mining Area, India. Bull. Environ. Contam. Toxicol. 2015, 95, 304–310. [Google Scholar] [CrossRef]
- Roșca, O.M.; Dippong, T.; Marian, M.; Mihali, C.; Mihalescu, L.; Hoaghia, M.-A.; Jelea, M. Impact of anthropogenic activities on water quality parameters of glacial lakes from Rodnei mountains, Romania. Environ. Res. 2020, 182, 109136. [Google Scholar] [CrossRef]
- GB 5749—2022; Standards for Drinking Water Quality. State Administration for Market Regulation; Standards Press of China: Beijing, China, 2022.
- Zhang, Y.; Zhang, M.; Yu, W.; Li, J.; Kong, D. Ecotoxicological risk ranking of 19 metals in the lower Yangtze River of China based on their threats to aquatic wildlife. Sci. Total Environ. 2022, 812, 152370. [Google Scholar] [CrossRef] [PubMed]
- GB 3838-2002; Environmental Quality Standards for Surface Water. State Environmental Protection Administration of China; Standards Press of China: Beijing, China, 2002.
- Islam, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Habibullah -Al- Mamun, M.; Islam, M.K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country. Ecol. Indic. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Liu, N.; Ni, T.; Xia, J.; Dai, M.; He, C.; Lu, G. Non-carcinogenic risks induced by metals in drinking source water of Jiangsu Province, China. Environ. Monit. Assess. 2011, 177, 449–456. [Google Scholar] [CrossRef]
- Guidelines for Drinking Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011.
- Khan, S.; Shahnaz, M.; Jehan, N.; Rehman, S.; Shah, M.T.; Din, I. Drinking water quality and human health risk in Charsadda district, Pakistan. J. Clean. Prod. 2013, 60, 93–101. [Google Scholar] [CrossRef]
- Ong, C.; Ibrahim, S.; Sen Gupta, B. A survey of tap water quality in Kuala Lumpur. Urban Water J. 2007, 4, 29–41. [Google Scholar] [CrossRef]
- Kumar, M.; Rahman, M.M.; Ramanathan, A.L.; Naidu, R. Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: Health risk index. Sci. Total Environ. 2016, 539, 125–134. [Google Scholar] [CrossRef]
- He, L.; Wang, S.; Liu, M.; Chen, Z.; Xu, J.; Dong, Y. Transport and transformation of atmospheric metals in ecosystems: A review. J. Hazard. Mater. Adv. 2023, 9, 100218. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Pandita, S.; Singh, S.; Bhardwaj, R.; Varol, M.; Rodrigo-Comino, J. A global meta-analysis of toxic metals in continental surface water bodies. J. Environ. Chem. Eng. 2023, 11, 109964. [Google Scholar] [CrossRef]
- Van Dijk-Looijaard, A.M.; Van Genderen, J. Levels of exposure from drinking water. Food Chem. Toxicol. 2000, 38, 37–42. [Google Scholar] [CrossRef]
- Hasan, M.M.; Ahmed, M.S.; Adnan, R.; Shafiquzzaman, M. Water quality indices to assess the spatiotemporal variations of Dhaleshwari river in central Bangladesh. Environ. Sustain. Indic. 2020, 8, 100068. [Google Scholar] [CrossRef]
- Kavcar, P.; Sofuoglu, A.; Sofuoglu, S.C. A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int. J. Hyg. Environ. Health 2009, 212, 216–227. [Google Scholar] [CrossRef] [PubMed]

| Parameters | General | China | Hubei Province | |
|---|---|---|---|---|
| Summer | Winter | |||
| IRDW (L/d) | 2 | 1.85 | 2.3 | 1.2 |
| EF (d/a) | 350 | 350 | 350 | 350 |
| ED (a) | 70 | 70 | 70 | 70 |
| BW (kg) | 70 | 60.6 | 60.1 | 60.1 |
| EAT (d) | 25,550 | 25,550 | 25,550 | 25,550 |
| SA (m2) | 1.8 | 1.6 | 1.6 | 1.6 |
| BT (h/d) | 0.58 | 0.12 | 0.17 | 0.08 |
| Waterworks | Fe | Al | Mn | Co | Ni | Cu | Se | Cd | Cr | Pb |
|---|---|---|---|---|---|---|---|---|---|---|
| W1 | 14 | 85 | 0.6 | 0.4 | 0.9 | <0.1 | <4 | <0.1 | ||
| W2 | 17 | 90 | 4.1 | 0.6 | 1.4 | <0.1 | <4 | <0.1 | ||
| W3 | <50 | 94 | <100 | 2 | <100 | <1 | <4 | <5 | ||
| W4 | <50 | 88 | <100 | 0.4 | <100 | <1 | <1 | <5 | ||
| W5 | 13 | 131 | 1.1 | 0.8 | 1.4 | <0.1 | <4 | <0.1 | ||
| W6 | 54 | 119 | 2.4 | 0.4 | 0.8 | <0.1 | <4 | <0.1 | ||
| W7 | 11 | 95 | 0.2 | 0.4 | 0.9 | <0.1 | <4 | <0.1 | ||
| W8 | 12 | 99 | 0.5 | 0.4 | 0.9 | <0.1 | <4 | <0.1 | ||
| W9 | 12 | 59 | <0.1 | 0.6 | 0.6 | <0.1 | <4 | <0.1 | ||
| W10 | 12 | 94 | 0.4 | 0.6 | 0.8 | <0.1 | <4 | <0.1 | ||
| W11 | 16 | 112 | 0.7 | 0.3 | 1 | <0.1 | <4 | <0.1 | ||
| W12 | 13 | 104 | 4.9 | 0.7 | 1.2 | <0.1 | <4 | <0.1 | ||
| W13 | 11 | 103 | 0.2 | 1.5 | 0.8 | <0.1 | <4 | <0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Tao, S.; Sun, Z.; Chen, Y.; Xu, J. Determination of Heavy Metals and Health Risk Assessment in Tap Water from Wuhan, China, a City with Multiple Drinking Water Sources. Water 2023, 15, 3709. https://doi.org/10.3390/w15213709
Liu Z, Tao S, Sun Z, Chen Y, Xu J. Determination of Heavy Metals and Health Risk Assessment in Tap Water from Wuhan, China, a City with Multiple Drinking Water Sources. Water. 2023; 15(21):3709. https://doi.org/10.3390/w15213709
Chicago/Turabian StyleLiu, Zufan, Shiyong Tao, Zuyou Sun, Yilin Chen, and Jing Xu. 2023. "Determination of Heavy Metals and Health Risk Assessment in Tap Water from Wuhan, China, a City with Multiple Drinking Water Sources" Water 15, no. 21: 3709. https://doi.org/10.3390/w15213709
APA StyleLiu, Z., Tao, S., Sun, Z., Chen, Y., & Xu, J. (2023). Determination of Heavy Metals and Health Risk Assessment in Tap Water from Wuhan, China, a City with Multiple Drinking Water Sources. Water, 15(21), 3709. https://doi.org/10.3390/w15213709





