Potentially Toxic Cyanobacteria in a Eutrophic Reservoir in Northern Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Sampling and Analysis
2.3. Isolation and Species Identification
2.4. Alpha and Beta Diversity Indexes
3. Results
3.1. Analysis of Physicochemical Parameters
3.2. Analysis of Cyanobacterial Abundance
3.3. Cyanobacteria Diversity Analysis
Beta Diversity: Cyanobacterial Turnover and Nestedness
3.4. Non-Parametric Multidimensional Scaling Analysis
4. Discussion
4.1. Toxic Blooms in El Guájaro Reservoir
4.2. Reservoir Conditions That Favor the Presence of Potentially Toxic Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The Rise of Harmful Cyanobacteria Blooms: The Potential Roles of Eutrophication and Climate Change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Wagner, C.; Adrian, R. Cyanobacteria Dominance: Quantifying the Effects of Climate Change. Limnol. Oceanogr. 2009, 54, 2460–2468. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate Change: A Catalyst for Global Expansion of Harmful Cyanobacterial Blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial Blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.I.; Raeder, U.; Geist, J.; Zwirglmaier, K. Influence of Temperature, Mixing, and Addition of Microcystin-LR on Microcystin Gene Expression in Microcystis Aeruginosa. Microbiologyopen 2017, 6, e00393. [Google Scholar] [CrossRef]
- Shan, K.; Song, L.; Chen, W.; Li, L.; Liu, L.; Wu, Y.; Jia, Y.; Zhou, Q.; Peng, L. Analysis of Environmental Drivers Influencing Interspecific Variations and Associations among Bloom-Forming Cyanobacteria in Large, Shallow Eutrophic Lakes. Harmful Algae 2019, 84, 84–94. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Duelling ‘CyanoHABs’: Unravelling the Environmental Drivers Controlling Dominance and Succession among Diazotrophic and Non-N2-Fixing Harmful Cyanobacteria. Environ. Microbiol. 2016, 18, 316–324. [Google Scholar] [CrossRef]
- Chia, M.A.; Jankowiak, J.G.; Kramer, B.J.; Goleski, J.A.; Huang, I.-S.; Zimba, P.V.; do Carmo Bittencourt-Oliveira, M.; Gobler, C.J. Succession and Toxicity of Microcystis and Anabaena (Dolichospermum) Blooms Are Controlled by Nutrient-Dependent Allelopathic Interactions. Harmful Algae 2018, 74, 67–77. [Google Scholar] [CrossRef]
- Ma, H.; Zheng, L.; Song, L.; Gan, N.; Li, T.; Wu, Y. Growth Inhibitory Effect of Microcystis on Aphanizomenon Flos-Aquae Isolated from Cyanobacteria Bloom in Lake Dianchi, China. Harmful Algae 2015, 42, 43–51. [Google Scholar] [CrossRef]
- Hu, L.; Shan, K.; Huang, L.; Li, Y.; Zhao, L.; Zhou, Q.; Song, L. Environmental Factors Associated with Cyanobacterial Assemblages in a Mesotrophic Subtropical Plateau Lake: A Focus on Bloom Toxicity. Sci Total Environ. 2021, 777, 146052. [Google Scholar] [CrossRef]
- Park, B.; LI, Z.; Kang, Y.-H.; Shin, H.H.; Joo, J.-H.; Han, M.-S. Distinct Bloom Dynamics of Toxic and Non-Toxic Microcystis (Cyanobacteria) Subpopulations in Hoedong Reservoir (Korea). Microb. Ecol. 2018, 75, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and Harmful Algal Blooms: A Scientific Consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Otten, T.G.; Xu, H.; Qin, B.; Zhu, G.; Paerl, H.W. Spatiotemporal Patterns and Ecophysiology of Toxigenic Microcystis Blooms in Lake Taihu, China: Implications for Water Quality Management. Environ. Sci. Technol. 2012, 46, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Rigosi, A.; Hanson, P.; Hamilton, D.P.; Hipsey, M.; Rusak, J.A.; Bois, J.; Sparber, K.; Chorus, I.; Watkinson, A.J.; Qin, B.; et al. Determining the Probability of Cyanobacterial Blooms: The Application of Bayesian Networks in Multiple Lake Systems. Ecol. Appl. 2015, 25, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Fristachi, A.; Sinclair, J.L.; Hall, S.; Berkman, J.A.H.; Boyer, G.; Burkholder, J.; Burns, J.; Carmichael, W.; DuFour, A.; Frazier, W.; et al. Cyanobacterial Harmful Algal Blooms: Chapter 3: Occurrence of Cyanobacterial Harmful Algal Blooms Workgroup Report. 2008. U.S. Environmental Protection Agency Papers. 40. Available online: https://digitalcommons.unl.edu/usepapapers/40 (accessed on 25 September 2023).
- Sant’Anna, C.L.; Melcher, S.S.; Carvalho, M.d.C.; Gelmego, M.P.; Azevedo, M.T.d.P. Planktic Cyanobacteria from Upper Tietê Basin Reservoirs, SP, Brazil. Braz. J. Bot. 2007, 30, 1–17. [Google Scholar] [CrossRef]
- Torres-Bejarano, F.; Padilla Coba, J.; Rodríguez Cuevas, C.; Ramírez León, H.; Cantero Rodelo, R. La modelación hidrodinámica para la gestión hídrica del embalse del Guájaro, Colombia. Rev. Int. De Métodos Numéricos Para Cálculo Y Diseño En Ing. 2016, 32, 163–172. [Google Scholar] [CrossRef]
- Corporación Autónoma Regional del Atlántico, C.R.A. Monitoreo Fisicoquímico, Microbiológico e Hidrobiológico Sobre la Calidad y Estado Actual de los Cuerpos de Agua las Fuentes Hídricas del Departamento del Atlántico y la Caracterización de los Humedales Sabanagrande, Santo Tomás Y Palmar De Varela En Cumplimiento de lo Establecido en el Plan de Acción Institucional 2016–2019. 2017. Available online: https://crautonoma.gov.co/documentos/Monitoreos%20de%20calidad%20de%20agua/INFORME%20%20CRA%202016.pdf (accessed on 23 March 2023).
- Ramírez Umaña, D.; García Torres, D.; Vargas Cuervo, G.; Bermúdez Gómez, H.; Castellanos Giovanini, S.; Amor Beltrán, H.; Blanco, C.; Henao Calad, M. El Renacer del Sur. 2019. Gobernación del Atlántico. Available online: https://atlanticolider.com/libros/LIBRO-EL-SUR-DEL-ATLANTICO.pdf (accessed on 23 March 2023).
- Torregroza-Espinosa, A.C.; Martínez-Mera, E.; Castañeda-Valbuena, D.; González-Márquez, L.C.; Torres-Bejarano, F. Contamination Level and Spatial Distribution of Heavy Metals in Water and Sediments of El Guájaro Reservoir, Colombia. Bull. Environ. Contam. Toxicol. 2018, 101, 61–67. [Google Scholar] [CrossRef]
- Corporación Autónoma Regional Del Atlántico, C.R.A. Caracterización Fisicoquímica de los Vertimientos de Aguas Residuales Hacia los Cuerpos de Agua del Departamento del Atlántico Y Monitoreo de la Calidad Y Estado Actual de las Fuentes Hídricas del Departamento Año 2011. 2011. Available online: https://www.crautonoma.gov.co/documentos/pomcas/Recurso%20Hidrico/INFORME%20FINAL.pdf (accessed on 17 March 2023).
- Informe Técnico Laboratorio LIMA Ltd.a. Caracterización Fisicoquímica, Microbiológica e Hidrobiológica de Algunos Cuerpos de Agua Lénticos del Departamento del Atlántico. 2014. Available online: https://www.crautonoma.gov.co/documentos/Monitoreos%20de%20calidad%20de%20agua/INFORME1.PDF (accessed on 17 March 2023).
- García-Alzate, C.; Gutierrez Moreno, L.; De la Parra, A. El emablse El Guájaro: Diagnostico ambiental y estrategias de rehabilitación. In Sur del Atlántico Una Nueva Oportunidad. Capitulo 5. Alvarado, M., Ed.; Fundación Promigas: Bogota, Colombia, 2016; pp. 148–181. Available online: http://repositorio.gestiondelriesgo.gov.co/bitstream/handle/20.500.11762/20493/Sur_del_Atl%c3%a1ntico.pdf?sequence=6&isAllowed=y (accessed on 25 September 2023).
- Corporación Autónoma Regional del Atlántico, C.R.A. Monitoreo Fisicoquímico, Microbiológico e Hidrobiológico Sobre la Calidad y Estado de Los Cuerpos de Agua, las Fuentes Hídricas del Departamento del Atlántico y la Caracterización de los Humedales Sabanagrande, Santo Tomás y Palmar de Varela en Cumplimiento de lo Establecido en el Plan de Acción Institucional 2012–2015, Parte 2. 2015. Available online: https://crautonoma.gov.co/documentos/Monitoreos%20de%20calidad%20de%20agua/Infofinal-CRA-Parte%202%20de%203%202015.pdf (accessed on 23 March 2023).
- Corporación Autónoma Regional del Atlántico, C.R.A. Monitoreo Fisicoquímico, Microbiológico e Hidrobiológico Sobre la Calidad y Estado Actual de Los Cuerpos de Agua Las Fuentes Hídricas del Departamento del Atlántico y la Caracterización de los Humedales Sabanagrande, Santo Tomás, y Palmar de Valera en Cumplimiento de lo Establecido en el Plan de Acción Institucional 2016–2019, Tomo 2. 2019. Available online: https://www.crautonoma.gov.co/documentos/Monitoreos%20de%20calidad%20de%20agua/INFORME%20TOMO%20II%20-%20CIENAGAS%20-%202019.pdf (accessed on 23 March 2023).
- Corporación Autónoma Regional del Atlántico, C.R.A. Caracterización Fisicoquímica, Microbiológica, e Hidrobiológica de tres lagunas costeras en el Depaartamento del Atlántico y Desarrollo de Índice de Calidad del Agua para su Gestión. 2018. Available online: https://crautonoma.gov.co/documentos/Monitoreos%20de%20calidad%20de%20agua/INFORME%20Caracterizacion%20%20ICA%20%20C%20000294.pdf (accessed on 23 March 2023).
- Corporación Autónoma Regional del Atlántico, C.R.A. Monitoreo Fisicoquímico, Microbiológico e Hidrobiológico Sobre la Calidad y Estado de los Cuerpos de Agua, las Fuentes Hídricas del Departamento Del Atlántico y la Caracterización de los Humedales Sabanagrande, Santo Tomas y Palmar De Varela en Cumplimiento de lo Establecido en el Plan de Acción Institucional 2012–2015 Informe Final. 2015. Parte 3. Available online: https://crautonoma.gov.co/documentos/Monitoreos%20de%20calidad%20de%20agua/Infofinal-CRA-Parte%203%20de%203%202015.pdf (accessed on 17 March 2023).
- Instituto de Hidrología Metereología y Estudios Ambientales IDEAM. Boletín de Predicción Climática y Recomendación Sectorial Para la Planear y Decidir. 2019. Available online: http://www.ideam.gov.co/documents/21021/79336843/09_Bolet%C3%ADn_Predicci%C3%B3n_Climatica_Septiembre_2019.pdf/44f2bf35-bb43-42e7-a768-c0025eb572bc?version=1.1 (accessed on 27 March 2023).
- IDEAM—Instituto de Hidrología, Meteorología y Estudios Ambientales. 2015—Boletín Predicción Climática y Alertas—IDEAM. Available online: http://www.ideam.gov.co/web/tiempo-y-clima/boletin-prediccion-climatica-y-alertas/-/document_library_display/AWmAtbtiD5qY/view/413023 (accessed on 27 March 2023).
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota: Teil 1/Part 1: Chroococcales; Süßwasserflora von Mitteleuropa Series; Spektrum Akademischer Verlag: Heidelberg/Berlin, Germany, 2008; Volume 19/1, p. 548. [Google Scholar]
- Komárek, J. Recent Changes (2008) in Cyanobacteria Taxonomy Based on a Combination of Molecular Background with Phenotype and Ecological Consequences (Genus and Species Concept). Hydrobiologia 2010, 639, 245–259. [Google Scholar] [CrossRef]
- Komárek, J.; Kopecký, J.; Cepák, V. Generic Characters of the Simplest Cyanoprokaryotes Cyanobium, Cyanobacterium and Synechococcus. Cryptogam. Algol. 1999, 20, 209–222. [Google Scholar] [CrossRef]
- Dworkin, M.; Falkow, S.; Rosenberg, E.; Schleifer, K.-H.; Stackebrandt, E. The Prokaryotes: Volume 4: Bacteria: Firmicutes, Cyanobacteria; Springer: New York, NY, USA, 2006; p. 1140. [Google Scholar] [CrossRef]
- Komarek, J.; Kastovsky, J.; Jezberova, J. Phylogenetic and taxonomic delimitation of the cyanobacterial genus Aphanothece and description of Anathece gen. nov. Eur. J. Phycol. 2011, 46, 315–326. [Google Scholar] [CrossRef]
- Edler, L.; Elbrächter, M. The Utermöhl Method for Quantitative Phytoplankton Analysis. In Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis; Intergovernmental Oceanographic Commission, UNESCO: Ostende, Belgium, 2010; pp. 13–20. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. INEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (Hill Numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2023. Available online: https://www.R-project.org/ (accessed on 1 June 2023).
- Chao, A.; Jost, L. Coverage-Based Rarefaction and Extrapolation: Standardizing Samples by Completeness Rather than Size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Ma, K.; Hsieh, T.C. User’s Guide for INEXT Online: Software for Interpolation and Extrapolation of Species Diversity. Code 2016, 30043, 1–14. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the Turnover and Nestedness Components of Beta Diversity: Partitioning Beta Diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Package Betapart. Available online: https://cran.r-project.org/web/packages/betapart/betapart.pdf (accessed on 1 June 2023).
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4ª ed., 4th ed.; Springer Science & Business Media: Berlin, Germany, 2003; p. 498. Available online: http://staff.ustc.edu.cn/~houbo/course/Modern%20Applied%20Statistics%20with%20Splus%20(Fourth%20edition).pdf (accessed on 25 September 2023).
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Mesa-Fúquen, E.; Hernández, J.S.; Camperos, J.E.; Mesa-Fúquen, E.; Hernández, J.S.; Camperos, J.E. Uso de modelos lineales generalizados en el conteo de Leptopharsa gibbicarina (Hemiptera: Tingidae) en palma de aceite. Rev. Colomb. De Entomol. 2021, 47, e7661. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-04882021000100009 (accessed on 25 September 2023). [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Domingos, P.; Rubim, T.K.; Molica, R.J.R.; Azevedo, S.M.F.O.; Carmichael, W.W. First Report of Microcystin Production by Picoplanktonic Cyanobacteria Isolated from a Northeast Brazilian Drinking Water Supply. Environ. Toxicol. 1999, 14, 31–35. [Google Scholar] [CrossRef]
- Cirés Gómez, S.; Quesada de Corral, A. Catálogo de Cianobacterias Planctónicas Potencialmente Tóxicas de Las Aguas Continentales Españolas; Ministerio de Medio Ambiente, y Medio Rural y Marino, Secretaría General Técnica: Madrid, Spain, 2011; p. 86. Available online: https://www.sergas.es/saudepublica/documents/1477/cat%C3%81logo%20cires%20quesada.pdf (accessed on 31 July 2023).
- Wood, S.A.; Kelly, L.T.; Bouma-Gregson, K.; Humbert, J.-F.; Laughinghouse IV, H.D.; Lazorchak, J.; McAllister, T.G.; McQueen, A.; Pokrzywinski, K.; Puddick, J.; et al. Toxic Benthic Freshwater Cyanobacterial Proliferations: Challenges and Solutions for Enhancing Knowledge and Improving Monitoring and Mitigation. Freshw. Biol. 2020, 65, 1824–1842. [Google Scholar] [CrossRef]
- Lombardo, M.; Pinto, F.C.R.; Vieira, J.M.S.; Honda, R.Y.; Pimenta, A.M.C.; Bemquerer, M.P.; Carvalho, L.R.; Kiyota, S. Isolation and Structural Characterization of Microcystin-LR and Three Minor Oligopeptides Simultaneously Produced by Radiocystis Feernandoi (Chroococcales, Cyanobacteriae): A Brazilian Toxic Cyanobacterium. Toxicon 2006, 47, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Molica, R.J.R.; Oliveira, E.J.A.; Carvalho, P.V.V.C.; Costa, A.N.S.F.; Cunha, M.C.C.; Melo, G.L.; Azevedo, S.M.F.O. Occurrence of Saxitoxins and an Anatoxin-a(s)-like Anticholinesterase in a Brazilian Drinking Water Supply. Harmful Algae 2005, 4, 743–753. [Google Scholar] [CrossRef]
- Falconer, I.R.; Humpage, A.R. Health Risk Assessment of Cyanobacterial (Blue-Green Algal) Toxins in Drinking Water. Int. J. Environ. Res. Public Health 2005, 2, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.M.d.S.; Azevedo, M.T.d.P.; Azevedo, S.M.F.d.O.; Honda, R.Y.; Corrêa, B. Toxic Cyanobacteria and Microcystin Concentrations in a Public Water Supply Reservoir in the Brazilian Amazonia Region. Toxicon 2005, 45, 901–909. [Google Scholar] [CrossRef]
- Van Hassel, W.H.R.; Andjelkovic, M.; Durieu, B.; Marroquin, V.A.; Masquelier, J.; Huybrechts, B.; Wilmotte, A. A Summer of Cyanobacterial Blooms in Belgian Waterbodies: Microcystin Quantification and Molecular Characterizations. Toxins 2022, 14, 61. [Google Scholar] [CrossRef]
- Molica, R.; Onodera, H.; García, C.; Rivas, M.; Andrinolo, D.; Nascimento, S.; Meguro, H.; Oshima, Y.; Azevedo, S.; Lagos, N. Toxins in the Freshwater Cyanobacterium Cylindrospermopsis Raciborskii (Cyanophyceae) Isolated from Tabocas Reservoir in Caruaru, Brazil, Including Demonstration of a New Saxitoxin Analogue. Phycologia 2002, 41, 606–611. [Google Scholar] [CrossRef]
- Mihali, T.K.; Kellmann, R.; Neilan, B.A. Characterisation of the Paralytic Shellfish Toxin Biosynthesis Gene Clusters in Anabaena Circinalis AWQC131C and Aphanizomenon Sp. NH-5. BMC Biochem. 2009, 10, 8. [Google Scholar] [CrossRef]
- Kapkov, V.I.; Vasilieva, S.G.; Lobakova, E.S. Growth of Toxic Cyanobacteria Dolichospermum Flos-Aquae (Anabaena Flos-Aquae) in the Waters of the Boreal Zone. Mosc. Univ. Biol.Sci. Bull. 2019, 74, 15–20. [Google Scholar] [CrossRef]
- Li, X.; Dreher, T.; Li, R. An Overview of Diversity, Occurrence, Genetics and Toxin Production of Bloom-Forming Dolichospermum (Anabaena) Species. Harmful Algae 2016, 54, 54–68. [Google Scholar] [CrossRef]
- Frazão, B.; Martins, R.; Vasconcelos, V. Are Known Cyanotoxins Involved in the Toxicity of Picoplanktonic and Filamentous North Atlantic Marine Cyanobacteria? Mar. Drugs 2010, 8, 1908–1919. [Google Scholar] [CrossRef]
- Freudenthal, M.P.L. Cianobacterias Tóxicas y Mortandades en Masa de Fauna Salvaje en las Marismas de Doñana. Ph.D. Tesis, Universidad Complutense de Madrid, Servicio de Publicaciones, Madrid, Spain, 2007; p. 161. Available online: http://webs.ucm.es/BUCM/tesis/vet/ucm-t30117.pdf (accessed on 31 July 2023).
- Anjos, F.M.d.; Bittencourt-Oliveira, M.d.C.; Zajac, M.P.; Hiller, S.; Christian, B.; Erler, K.; Luckas, B.; Pinto, E. Detection of Harmful Cyanobacteria and Their Toxins by Both PCR Amplification and LC-MS during a Bloom Event. Toxicon 2006, 48, 239–245. [Google Scholar] [CrossRef]
- Betancourt Aguilar, C.R.; Labaut, Y. La Calidad Físicoquímica Del Agua En Embalses, Principales Variables a Considerar. Physical-Chemical Water Quality of Reservoirs: A Review of the Main Variables. Agroecosistemas 2013, 1, 78–103. [Google Scholar]
- González-Márquez, L.C.; Torres-Bejarano, F.M.; Torregroza-Espinosa, A.C.; Hansen-Rodríguez, I.R.; Rodríguez-Gallegos, H.B. Use of LANDSAT 8 Images for Depth and Water Quality Assessment of El Guájaro Reservoir, Colombia. J. South Am. Earth Sci. 2018, 82, 231–238. [Google Scholar] [CrossRef]
- Brasil, J.; Attayde, J.L.; Vasconcelos Francisco, R.; Dantas Danyhelton, D.F.; Huszar Vera, L.M. Drought-Induced Water-Level Reduction Favors Cyanobacteria Blooms in Tropical Shallow Lakes. Hydrobiologia 2015, 770, 145–164. [Google Scholar] [CrossRef]
- Dalu, T.; Wasserman Ryan, J. Cyanobacteria Dynamics in a Small Tropical Reservoir: Understanding Spatio-Temporal Variability and Influence of Environmental Variables. Sci. Total Environ. 2018, 643, 835–841. [Google Scholar] [CrossRef]
- Dörr, F.A.; Pinto, E.; Soares, R.M.; Azevedo, S.M.F.d.O.e. Microcystins in South American Aquatic Ecosystems: Occurrence, Toxicity and Toxicological Assays. Toxicon 2010, 56, 1247. [Google Scholar] [CrossRef]
- Salomón, S.; Rivera-Rondón, C.A.; Zapata, A.M. Cyanobacterial Blooms in Colombia: State of Knowledge Andresearch Needs in the Context of Climate Global Change. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 2020, 44, 376–391. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0370-39082020000200376 (accessed on 25 September 2023). [CrossRef]
- Mowe Maxine, A.D.; Mitrovic, S.M.; Lim, R.P.; Furey, A.; Yeo, D. Tropical Cyanobacterial Blooms: A Review of Prevalence, Problem Taxa, Toxins and Influencing Environmental Factors. J. Limnol. 2014, 74, 205–224. [Google Scholar] [CrossRef]
- Sprőber, P.; Shafik, H.M.; Présing, M.; Kovács, A.W.; Herodek, S. Nitrogen Uptake and Fixation in the Cyanobacterium Cylindrospermopsis raciborskii under Different Nitrogen Conditions. Hydrobiologia 2003, 506, 169–174. [Google Scholar] [CrossRef]
- Sitoki, L.; Kurmayer, R.; Rott, E. Spatial Variation of Phytoplankton Composition, Biovolume, and Resulting Microcystin Concentrations in the Nyanza Gulf (Lake Victoria, Kenya). Hydrobiologia 2012, 691, 109–122. [Google Scholar] [CrossRef]
- Wan, L.; Chen, X.; Deng, Q.; Yang, L.; Li, X.; Zhang, J.; Song, C.; Zhou, Y.; Cao, X. Phosphorus Strategy in Bloom-Forming Cyanobacteria (Dolichospermum and Microcystis) and Its Role in Their Succession. Harmful Algae 2019, 84, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; Dantas, Ê.W.; Moura, A.d.N. Modeling Cyanobacterial Blooms in Tropical Reservoirs: The Role of Physicochemical Variables and Trophic Interactions. Sci. Total Environ. 2020, 744, 140659. [Google Scholar] [CrossRef] [PubMed]
- Algas Tóxicas Siguen Causando Muerte de Animales al Beber Agua de Embalse. 2019. Available online: https://www.catorce6.com/actualidad-ambiental/regionales/16918-algas-toxicas-siguen-causando-muerte-de-animales-al-beber-agua-de-embalse (accessed on 19 July 2023).
- Algas Tóxicas, Causa de Muerte de 40 Reses en Manatí. 2019. Available online: https://www.atlantico.gov.co/index.php/noticias/prensa-desarrollo/11584-algas-toxicas-causa-de-muerte-de-40-reses-en-manati (accessed on 19 July 2023).
- Heraldo, E. Muerte de Reses en el Guájaro fue por Algas Tóxicas. EL HERALDO. Available online: https://www.elheraldo.co/atlantico/muerte-de-reses-en-el-guajaro-fue-por-algas-toxicas-618022 (accessed on 19 July 2023).
- Bonilla, S.; Aubriot, L.; Haakonsson, S.; Illarze, M.; Isasa, I.D.; Brena, B.M. Las floraciones de cianobacterias tóxicas comprometen el uso del agua del Río Negro, Uruguay. Innotec 2021, 22, e577. [Google Scholar] [CrossRef]
- Kramer, B.J.; Hem, R.; Gobler, C.J. Elevated CO2 Significantly Increases N2 Fixation, Growth Rates, and Alters Microcystin, Anatoxin, and Saxitoxin Cell Quotas in Strains of the Bloom-Forming Cyanobacteria, Dolichospermum. Harmful Algae 2022, 120, 102354. [Google Scholar] [CrossRef] [PubMed]
- Global Climate in 2015–2019: Climate Change Accelerates. Available online: https://public.wmo.int/en/media/press-release/global-climate-2015-2019-climate-change-accelerates (accessed on 23 March 2023).
- Dokulil, M.; Teubner, K. Cyanobacterial Dominance in Lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Mendoza-Lera, C.; Federlein, L.L.; Knie, M.; Mutz, M. The Algal Lift: Buoyancy-Mediated Sediment Transport. Water Resour. Res. 2016, 52, 108–118. [Google Scholar] [CrossRef]
- Bubak, I.; Śliwińska-Wilczewska, S.; Głowacka, P.; Szczerba, A.; Możdżeń, K. The Importance of Allelopathic Picocyanobacterium Synechococcus Sp. on the Abundance, Biomass Formation, and Structure of Phytoplankton Assemblages in Three Freshwater Lakes. Toxins 2020, 12, 259. [Google Scholar] [CrossRef]
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Kaufononga, S.A.F.; Cary, S.C.; Hamilton, D.P. High Levels of Structural Diversity Observed in Microcystins from Microcystis CAWBG11 and Characterization of Six New Microcystin Congeners. Mar. Drugs 2014, 12, 5372–5395. [Google Scholar] [CrossRef]
- Koreivienė, J.; Belous, O.; Kasperovičienė, J. Variations of Microcystins in Freshwater Ecosystems. Bot. Lith. 2013, 19, 139–148. [Google Scholar] [CrossRef]
- Lu, X.; Tian, C.; Pei, H.; Hu, W.; Xie, J. Environmental Factors Influencing Cyanobacteria Community Structure in Dongping Lake, China. J. Environ. Sci. 2013, 25, 2196–2206. [Google Scholar] [CrossRef]
- Krausfeldt, L.E.; Farmer, A.T.; Castro Gonzalez, H.F.; Zepernick, B.N.; Campagna, S.R.; Wilhelm, S.W. Urea is both a carbon and nitrogen source for Microcystis aeruginosa: Tracking 13C incorporation at bloom pH conditions. Front. Microbiol. 2019, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Aulnois, M.; Roux, P.; Caruana, A.; Réveillon, D.; Briand, E.; Hervé, F.; Amzil, Z. Physiological and metabolic responses of freshwater and brackish strains of Microcystis aeruginosa acclimated to a salinity gradient: Insight into salt tolerance. Appl. Environ. Microbiol. 2019, 85, e01614–e01619. [Google Scholar] [CrossRef]
- Prihantini, N.B.; Pertiwi, Z.D.; Yuniati, R.; Sjamsuridzal, W.; Putrika, A. The effect of temperature variation on the growth of Leptolyngbya (cyanobacteria) HS-16 and HS-36 to biomass weight in BG-11 medium. Biocatal. Agric. Biotechnol. 2019, 19, 101105. [Google Scholar] [CrossRef]
- Otero, A.; Vincenzini, M. Extracellular polysaccharide synthesis by Nostoc strains as affected by N source and light intensity. J. Biotechnol. 2003, 102, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.; Vincenzini, M. Nostoc (Cyanophyceae) goes nude: Extracellular polysaccharides serve as a sink for reducing power under unbalanced C/N metabolism. J. Phycol. 2004, 40, 74–81. [Google Scholar]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms—A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
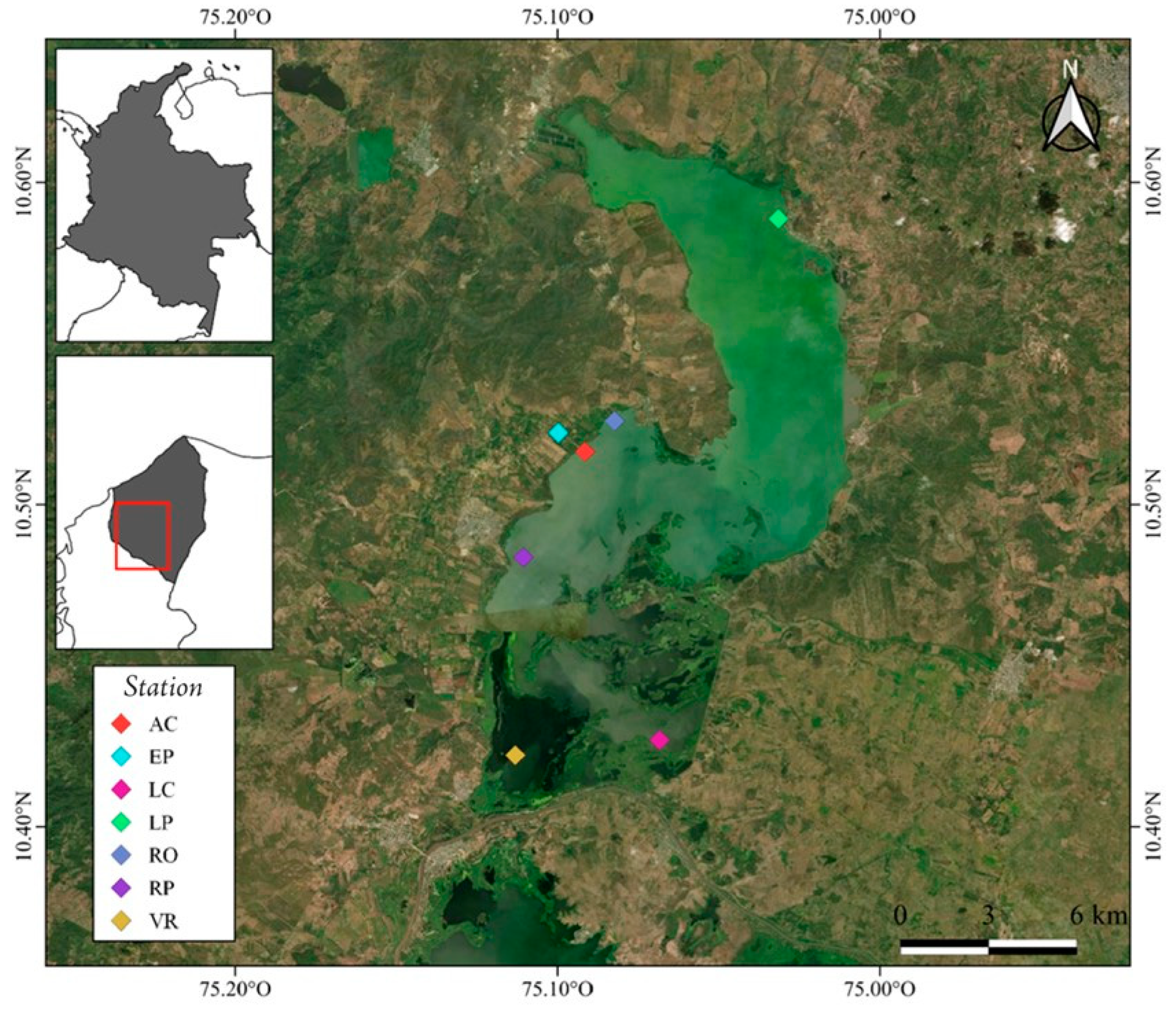





| Station Code | Location | Coordinates | Description |
|---|---|---|---|
| North zone | |||
| LP | La Peña | 10°35′19″ N 75°01′53″ W | Area adjacent to the town of La Peña, with fish farming, shrimp farming, and domestic sewage. |
| Central zone | |||
| RO | Rotinet | 10°31′33″ N 75°04′56″ W | Area adjacent to the town of Rotinet-Repelón, with high impact due to limestone mining, agriculture and livestock. |
| RE | Repelón | 10°29′01″ N 75°06′38″ W | Zone close to fish farming in an area of approximately 500 ha, livestock, artisanal fishing and rice, banana and citrus crops. |
| AC | Aqueduct system of the Repelón área | 10°30′59″ N 75°05′29″ W | Catchment and aqueduct for human consumption. |
| EP | Aquaculture station | 10°31′20″ N 75°05′ 59″ W | Fish station reservoir of the National Aquaculture and Fisheries Authority. |
| South zone | |||
| LC | Floodgates El Limón | 10°25′37″ N 75°04′06″ W | System of four radial gates with a capacity of up to 250 m3/s. Area with high content of suspended solids and organic matter. |
| VR | Floodgates Villa Rosa | 10°25′20″ N 75°06′47″ W | Villa Rosa earth barrier (5.5 km long) separates the El Guájaro reservoir from the Dique channel. Zone with high presence of macrophytes. |
| Year | pH | Temperature °C | Dissolved Oxygen (mg/L) | Conductivity (µS/cm) | BOD5 (mgO2/L) | TSS (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry | Rain | Dry | Rain | Dry | Rain | Dry | Rain | Dry | Rain | Dry | Rain | ||
| 2015 | Min | 7.24 | 6.21 | 27.77 | 25.23 | 4.44 | 5.93 | 544.77 | 642.00 | 3.44 | 5.04 | 9.87 | 17.27 |
| Max | 8.47 | 8.31 | 31.41 | 28.57 | 7.45 | 6.59 | 1184.67 | 877.33 | 5.93 | 6.04 | 33.04 | 36.38 | |
| Mean | 7.88 | 6.94 | 30.01 | 27.06 | 5.90 | 6.21 | 930.83 | 763.10 | 4.57 | 5.60 | 21.45 | 30.30 | |
| St.des | 0.39 | 0.76 | 1.10 | 1.36 | 0.97 | 0.26 | 217.89 | 91.84 | 0.93 | 0.41 | 8.64 | 6.23 | |
| 2016 | Min | 7.49 | 6.09 | 29.76 | 25.45 | 3.63 | 5.21 | 736.33 | 345.72 | 4.15 | 3.50 | 28.08 | 9.20 |
| Max | 8.73 | 8.31 | 31.14 | 27.95 | 6.07 | 7.91 | 1263.33 | 838.67 | 6.64 | 4.11 | 48.00 | 24.41 | |
| Mean | 8.22 | 7.03 | 30.55 | 26.20 | 5.17 | 6.25 | 940.10 | 609.69 | 5.17 | 3.86 | 37.17 | 15.36 | |
| St.des | 0.47 | 0.82 | 0.53 | 0.83 | 1.27 | 0.88 | 184.34 | 193.69 | 0.86 | 0.26 | 7.04 | 5.09 | |
| 2017 | Min | 7.06 | 7.81 | 27.98 | 29.87 | 3.56 | 4.72 | 172.21 | 727.33 | 3.86 | 3.01 | 8.53 | 27.38 |
| Max | 8.39 | 9.23 | 31.35 | 31.13 | 6.56 | 6.92 | 1089.00 | 918.00 | 5.68 | 5.87 | 34.13 | 35.45 | |
| Mean | 7.47 | 8.77 | 28.78 | 30.36 | 4.72 | 6.21 | 430.38 | 802.10 | 4.29 | 4.57 | 16.01 | 29.26 | |
| St.des | 0.48 | 0.53 | 1.15 | 0.42 | 1.26 | 0.77 | 306.34 | 88.89 | 0.63 | 1.09 | 9.56 | 2.77 | |
| 2018 | Min | 6.90 | 7.52 | 29.56 | 30.13 | 3.52 | 3.50 | 181.44 | 324.67 | 3.64 | 2.41 | 8.49 | 16.02 |
| Max | 8.79 | 8.88 | 32.10 | 31.15 | 6.62 | 5.59 | 1080.33 | 1147.33 | 5.56 | 2.80 | 32.70 | 28.37 | |
| Mean | 7.62 | 8.31 | 30.58 | 30.46 | 4.74 | 5.11 | 547.89 | 723.29 | 4.22 | 2.65 | 16.66 | 24.44 | |
| St.des | 0.90 | 0.43 | 0.90 | 0.37 | 1.17 | 0.73 | 374.56 | 265.60 | 0.63 | 0.19 | 9.13 | 4.23 | |
| 2019 | Min | 7.62 | 7.86 | 29.42 | 30.13 | 4.21 | 4.08 | 499.11 | 292.67 | 4.99 | 2.34 | 28.01 | 18.67 |
| Max | 8.53 | 8.93 | 31.28 | 33.80 | 5.36 | 5.65 | 1034.66 | 1138.33 | 7.26 | 2.90 | 43.10 | 28.00 | |
| Mean | 7.97 | 8.38 | 30.40 | 32.80 | 4.70 | 4.82 | 730.15 | 720.24 | 5.92 | 2.61 | 34.40 | 22.81 | |
| St.des | 0.34 | 0.34 | 0.65 | 1.32 | 0.45 | 0.91 | 259.18 | 272.53 | 0.73 | 0.22 | 7.55 | 4.34 | |
| Order | Specie | Relative Abundance (%) | Relative Frequency (%) | Toxin Type * | Ref. |
|---|---|---|---|---|---|
| Chroococcales | Aphanocapsa delicatissima | 2.72 | 0.66 | microcystin | [47] |
| Aphanocapsa grevillei | 2.22 | 0.93 | |||
| Aphanocapsa sp. | 3.23 | 1.72 | microcystin | [48] | |
| Merismopedia sp. | 1.23 | 2.12 | |||
| Snowella lacustris | 0.51 | 0.66 | |||
| Synechocystis sp. | 2.08 | 0.93 | |||
| Aphanothece sp. | 2.11 | 1.32 | microcystin | [49] | |
| Aphanothece stagnina | 0.65 | 0.26 | |||
| Chroococcus dispersus | 1.01 | 1.06 | |||
| Gloeocapsa sp. | 0.74 | 1.59 | |||
| Microcystis aeruginosa | 3.78 | 2.25 | microcystin | [4] | |
| Microcystis flos-aquae | 0.08 | 0.13 | microcystin | [48] | |
| Microcystis sp. | 0.46 | 0.40 | microcystin | [48] | |
| Radiocystis fernandoi | 0.55 | 0.66 | microcystin | [50] | |
| Chroococcus dispersus | 1.01 | 1.06 | |||
| Synechococcales | Synechococcus rubescens | 0.95 | 0.66 | ||
| Synechococcus sp. | 2.26 | 1.72 | microcystin | [48] | |
| Jaaginema sp. | 0.13 | 0.53 | |||
| Nostocales | Anabaena sp. | 0.06 | 0.66 | anatoxin-a (S) | [51] |
| Anabaenopsis sp. | 0.17 | 0.40 | microcistyn | [48] | |
| Aphanizomenon flos-aquae | 0.41 | 0.53 | saxitoxin | [52] | |
| Aphanizomenon gracile | 0.02 | 0.13 | microcystin | [53] | |
| Aphanizomenon sp. | 0.19 | 0.40 | microcystin saxitoxins | [52,54] | |
| Calothrix sp. | 0.46 | 0.79 | microcystin | [49] | |
| Cylindrospermopsis sp. | 2.00 | 2.12 | cylindrospermopsin | [52] | |
| Raphidiopsis raciborskii | 3.29 | 2.51 | saxitoxins cylindrospermopsin | [48,55] | |
| Raphidiopsis curvata | 3.37 | 2.51 | cylindrospermopsin | [48] | |
| Rhaphidiopsis sp. | 0.40 | 0.53 | microcystin cylindrospermopsin | [48] | |
| Dolichospermum circinale | 0.34 | 1.06 | microcystin saxitoxin anatoxin-a (S) | [56] | |
| Dolichospermum crassum | 0.99 | 0.53 | microcystin anatoxin-a (S) | [48,56] | |
| Dolichospermum flos-aquae | 0.55 | 0.26 | anatoxin-a (S) | [48,57] | |
| Dolichospermum sigmoideum | 0.55 | 0.53 | microcystin anatoxin | [58] | |
| Dolichospermum affinis | 0.62 | 0.40 | microcystin anatoxin | [48] | |
| Dolichospermum sp. | 0.91 | 1.19 | microcystin | [48] | |
| Dolichospermum spiroides | 0.20 | 0.26 | anatoxin-a (S) | [58] | |
| Cylindrospermum sp. | 0.07 | 0.26 | |||
| Nodularia sp. | 0.02 | 0.13 | microcystin nodularin | [48] | |
| Nostoc commune | 3.90 | 3.97 | |||
| Nostoc muscorum | 2.00 | 1.59 | microcystin | [49] | |
| Nostoc sp. | 1.29 | 1.72 | microcystin anatoxin-a | [4] | |
| Scytonema sp. | 0.53 | 1.32 | BMAA saxitoxin | [4,49] | |
| Tolypothrix sp. | 0.44 | 0.66 | microcystin | [51] | |
| Stigonema sp. | 0.46 | 1.19 | |||
| Leptolyngbyales | Leptolyngbya rivulariarum | 0.11 | 0.13 | ||
| Leptolyngbya sp. | 4.68 | 5.16 | microcystin | [59] | |
| Leptolyngbya subtilis | 1.69 | 0.93 | |||
| Leptolyngbya valderiana | 1.12 | 1.32 | |||
| Pseudophormidium tenue | 1.04 | 0.79 | |||
| Pseudophormidium viride | 0.54 | 0.79 | |||
| Pseudophormidium sp. | 0.86 | 1.46 | |||
| Pseudophormidium purpureum | 0.12 | 0.13 | |||
| Pseudanabaenales | Limnothrix planktonica | 1.40 | 2.12 | ||
| Limnothrix redekei | 0.75 | 1.32 | microcystin | [48] | |
| Limnothrix sp. | 0.07 | 0.66 | |||
| Pseudanabaena catenata | 8.85 | 6.22 | microcystin | [60] | |
| Pseudanabaena galeata | 0.54 | 0.13 | |||
| Pseudanabaena limnetica | 0.33 | 0.40 | |||
| Pseudanabaena mucicola | 0.70 | 0.66 | microcystin | [60] | |
| Oscillatoriales | Arthrospira jenneri | 0.20 | 0.93 | ||
| Arthrospira platensis | 0.11 | 0.66 | |||
| Arthrospira skujae | 0.02 | 0.26 | |||
| Arthrospira sp. | 0.04 | 0.40 | |||
| Cyanothece sp. | 1.26 | 1.19 | |||
| Oscillatoria limosa | 0.27 | 0.93 | microcystin | [4,49] | |
| Oscillatoria sp. | 0.99 | 3.70 | anatoxin-a microcystin | [49] | |
| Phormidium articulatum | 1.86 | 2.25 | |||
| Phormidium breve | 0.06 | 0.13 | |||
| Phormidium arthrospiroides | 1.11 | 1.06 | |||
| Phormidium formosum | 0.18 | 0.13 | |||
| Phormidium papyraceum | 4.26 | 3.57 | |||
| Phormidium sp. | 4.91 | 4.10 | anatoxin-a homoanatoxin | [4] | |
| Phormidium granulatum | 0.06 | 0.13 | |||
| Planktothrix agardhii | 4.32 | 0.13 | microcystin | [61] | |
| Planktothrix sp. | 0.79 | 3.70 | microcystin anatoxin-a | [54] | |
| Coleofasciculales | Symploca dubia | 0.04 | 0.13 | ||
| Symploca sp. | 0.22 | 0.53 | |||
| Spirulinales | Spirulina sp. | 0.02 | 0.13 | ||
| Spirulina subsalsa | 0.08 | 0.40 | |||
| Gomontiellales | Komvophoron sp. | 0.31 | 0.66 | ||
| Komvophoron crassum | 0.13 | 0.13 | |||
| Nodosilineales | Romeria leopoliensis | 2.58 | 1.46 | ||
| Romeria sp. | 2.73 | 2.12 | |||
| Geitlerinematales | Geitlerinema sp. | 0.24 | 0.40 | ||
| Geitlerinema unigranulatum | 0.04 | 0.13 | |||
| Pleurocapsales | Pleurocapsa sp. | 1.30 | 1.85 | ||
| Hyella sp. | 0.16 | 0.40 |
| Response Variable | Factor/Explanatory Variable | lr ×2 | Df | Pr(>Chisq) |
|---|---|---|---|---|
| N (Abundance) | Season | 248.020 | 1 | 2.20 × 10−16 * |
| Site | 380.940 | 6 | 2.20 × 10−16 * | |
| Year | 32.610 | 1 | 1.13 × 10−8 * | |
| Oxygen | 16.560 | 1 | 4.71 × 10−5 * | |
| Temperature | 135.950 | 1 | 2.20 × 10−16 * | |
| BOD5 | 49.560 | 1 | 1.93 × 10−12 * | |
| Season * Year | 82.050 | 1 | 2.20 × 10−16 * | |
| Season * Oxygen | 2.890 | 1 | 0.089 | |
| Season * Temperature | 141.260 | 1 | 2.20 × 10−16 * | |
| Site * BOD5 | 205.030 | 6 | 2.20 × 10−16 * | |
| Site * Temperature | 157.210 | 6 | 2.20 × 10−16 * | |
| 0D (Richness) | Season | 4.045 | 1 | 0.044 * |
| Year | 1.051 | 1 | 0.305 | |
| Site | 4.901 | 6 | 0.556 | |
| Conductivity | 0.221 | 1 | 0.637 | |
| BOD5 | 0.021 | 1 | 0.883 | |
| pH | 0.007 | 1 | 0.931 | |
| Temperature | 0.042 | 1 | 0.836 | |
| Season * Year | 0.855 | 1 | 0.355 | |
| Year * Site | 4.029 | 6 | 0.672 | |
| Season * Conductivity | 1.106 | 1 | 0.292 | |
| Season * BOD5 | 0.288 | 1 | 0.591 | |
| Site * Conductivity | 2.929 | 6 | 0.817 | |
| Season * pH | 1.390 | 1 | 0.238 | |
| Season * Temperature | 2.285 | 1 | 0.130 | |
| 1D (Common species) | Year | 1.140 | 1 | 0.285 |
| Season | 0.150 | 1 | 0.698 | |
| Conductivity | 2.991 | 1 | 0.084 | |
| pH | 0.818 | 1 | 0.365 | |
| Temperature | 1.244 | 1 | 0.264 | |
| Year * Season | 12.974 | 1 | 0.003 * | |
| Season * Conductivity | 2.353 | 1 | 0.125 | |
| Season * pH | 8.329 | 1 | 0.004 * | |
| Season * Temperature | 4.280 | 1 | 0.039 * | |
| Year * Conductivity | 2.603 | 1 | 0.106 | |
| 2D (Dominant species) | Season | 5.910 | 1 | 0.015 * |
| pH | 1.619 | 1 | 0.203 | |
| Temperature | 1.544 | 1 | 0.214 | |
| Conductivity | 2.277 | 1 | 0.131 | |
| Year | 2.741 | 1 | 0.097 | |
| Conductivity * Year | 4.405 | 1 | 0.035 * | |
| Season * Year | 17.345 | 1 | 0.031 * | |
| Temperature * Year | 3.537 | 1 | 0.059 | |
| Season * pH | 4.203 | 1 | 0.040 * | |
| Season * Temperature | 3.416 | 1 | 0.064 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claudia, T.-L.; Jesus, O.-V. Potentially Toxic Cyanobacteria in a Eutrophic Reservoir in Northern Colombia. Water 2023, 15, 3696. https://doi.org/10.3390/w15203696
Claudia T-L, Jesus O-V. Potentially Toxic Cyanobacteria in a Eutrophic Reservoir in Northern Colombia. Water. 2023; 15(20):3696. https://doi.org/10.3390/w15203696
Chicago/Turabian StyleClaudia, Tapia-Larios, and Olivero-Verbel Jesus. 2023. "Potentially Toxic Cyanobacteria in a Eutrophic Reservoir in Northern Colombia" Water 15, no. 20: 3696. https://doi.org/10.3390/w15203696






