Changes in Extracellular Microcystins (MCs) Accompanying Algae/Cyanobacteria Removal during Three Representative Algae/Cyanobacteria Inactivation Processes and an MC Diffusion Model in Still Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Water
2.2. Algae/Cyanobacteria Removal Experiments
2.3. Simulation of MC Diffusion in Still Water
2.4. Analytic Methods
2.5. Assessment of Changes in Extracellular MCs Accompanying Algae/Cyanobacteria Removal
3. Results and Discussion
3.1. Dynamic Change in Extracellular MCs and Chlorophyll a Concentrations during the Three Algae/Cyanobacteria Removal Processes
3.2. Comparison of the Three Algae/Cyanobacteria Removal Processes
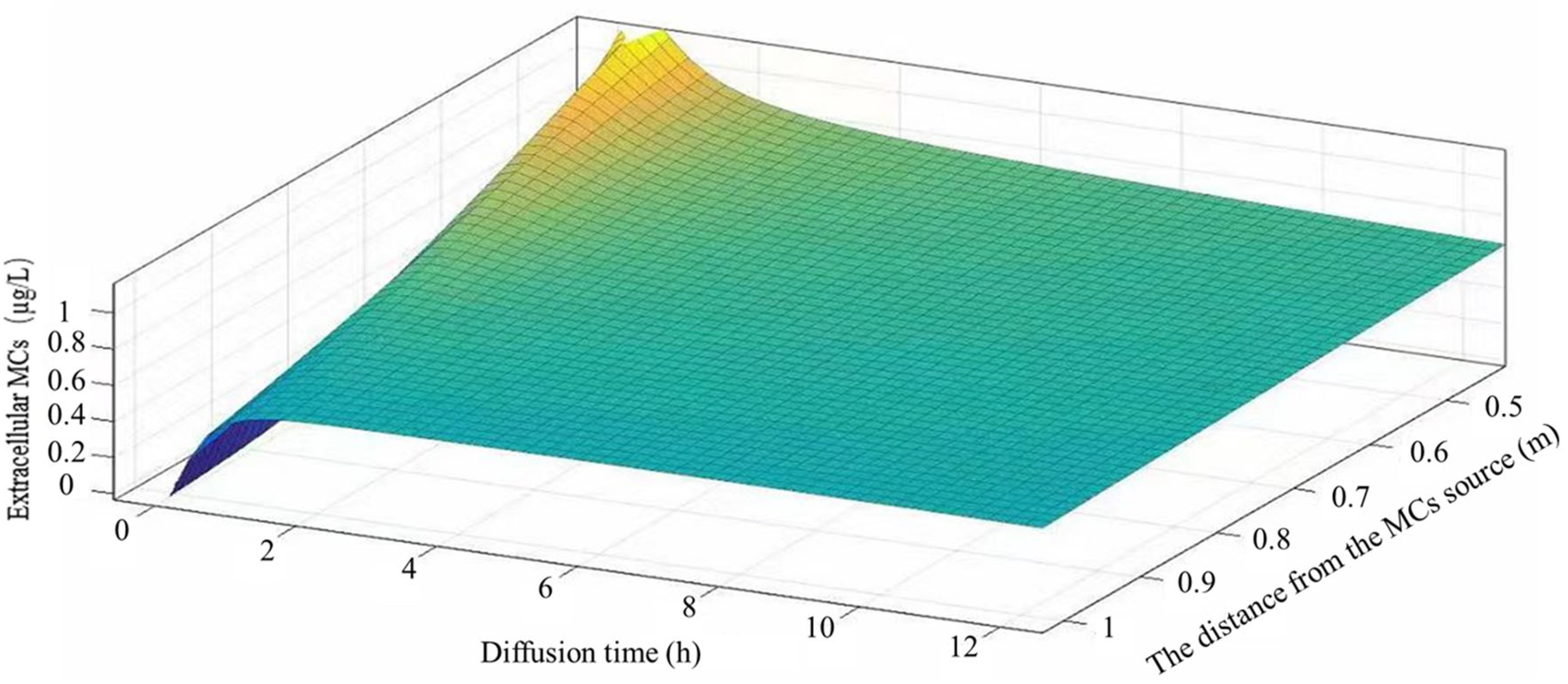
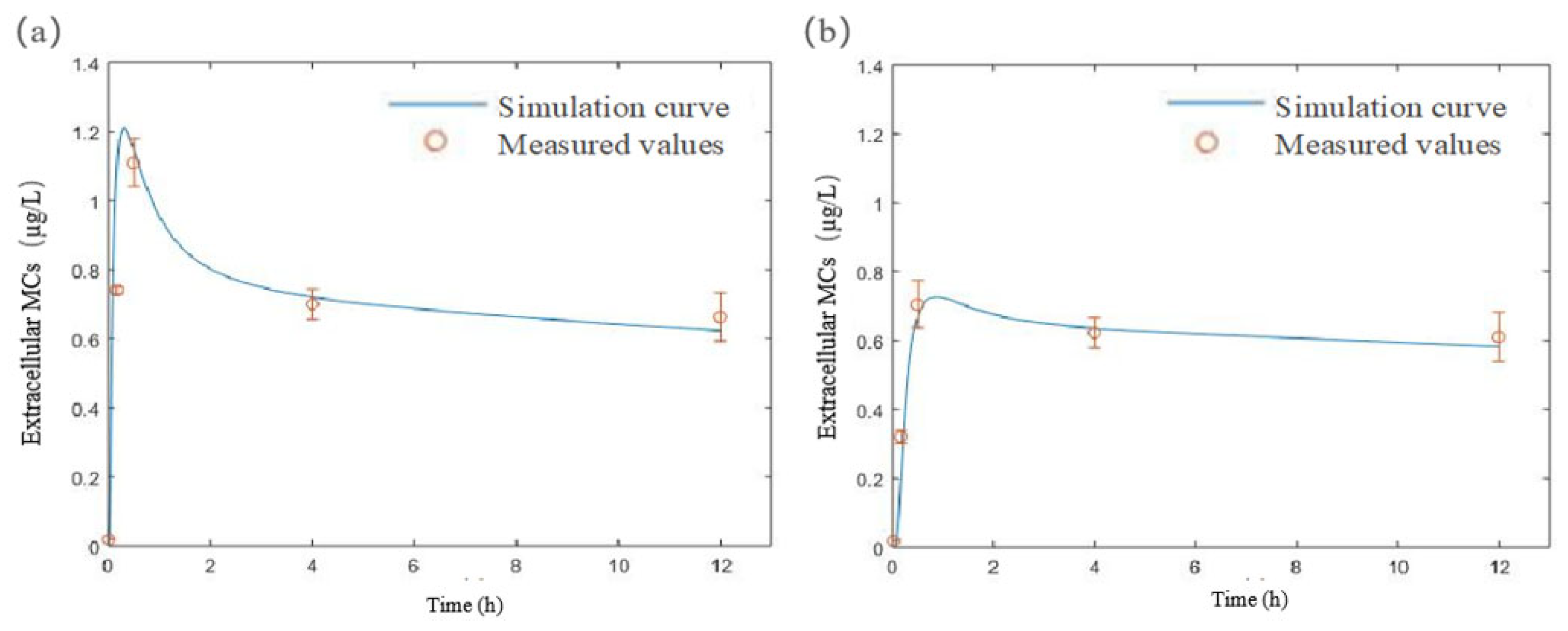
3.3. Development and Validation of an MC Diffusion Model in Still Water
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuellar-Bermudez, S.P.; Aleman-Nava, G.S.; Chandra, R. Nutrients utilization and contaminants removal. A review of two approaches of algae and cyanobacteria in wastewater. Algal Res. 2017, 24, 438–449. [Google Scholar] [CrossRef]
- Gutiérrez, J.E.; Nohora, G.; Gutierrez Benedetti, J.S. Clarification of cyanotoxins in El Guajaro Reservoir, Colombia using a microalgae-based consortium MPMC. J. Chem. Technol. Biotechnol. 2022, 97, 1468–1481. [Google Scholar] [CrossRef]
- Shinde, R.G.; Gawande, S. Eutrophication and aquatic life. Int. J. Adv. Sci. Eng. Technol. 2016, 4, 238–243. [Google Scholar]
- Chen, L.; Giesy, J.P.; Adamovsky, O. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.; Walker, D.; Chicana, R. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Ma, J.; Li, X. MicroRNAs are involved in the toxicity of microcystins. Toxin Rev. 2016, 362, 165–175. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Williams, C.D.; Ni, H. Microcystin-LR induced liver injury in mice and in primary human hepatocytes is caused by oncotic necrosis. Toxicon 2017, 125, 99–109. [Google Scholar] [CrossRef]
- Shartau, R.B.; Snyman, H.N.; Turcotte, L. Acute microcystin exposure induces reversible histopathological changes in Chinook Salmon (Oncorhynchus tshawytscha) and Atlantic Salmon (Salmo salar). J. Fish Dis. 2022, 45, 729–742. [Google Scholar] [CrossRef]
- Chen, L.; Hu, Y.; He, J. Responses of the Proteome and Metabolome in Livers of Zebrafish Exposed Chronically to Environmentally Relevant Concentrations of Microcystin-LR. Environ. Sci. Technol. 2017, 511, 596–607. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Liu, B. Removal of Microcystis aeruginosa and microcystin-LR by UV/Fenton system: Characteristics and degradation pathways. Sep. Purif. Technol. 2023, 306, 122596. [Google Scholar] [CrossRef]
- Lin, J.-L.; Nugrayanti, M.S.; Ika, A.R. Removal of Microcystis aeruginosa by oxidation-assisted coagulation: Effect of algogenic organic matter fraction changes on algae destabilization with Al hydrates. J. Water Process Eng. 2021, 42, 102142. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Chen, M. Microcystis aeruginosa removal by peroxides of hydrogen peroxide, peroxymonosulfate and peroxydisulfate without additional activators. Water Res. 2021, 201, 117263. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Chen, Z.; Gu, S. Self-floating photocatalytic hydrogel for efficient removal of Microcystis aeruginosa and degradation of microcystins-LR. Chemosphere 2021, 284, 131283. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, Z.; Kong, Y. Effects of ultrasound on Microcystis aeruginosa cell destruction and release of intracellular organic matter. Ultrason. Sonochem. 2020, 63, 104909. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, D.; Niu, X. Effective removal of Microcystis aeruginosa and microcystins by integrated pre-oxidation and coagulation: An environmental and economical way. Int. J. Environ. Sci. Technol. 2020, 178, 3761–3770. [Google Scholar] [CrossRef]
- Wu, H.; Wei, G.; Tan, X. Species-dependent variation in sensitivity of Microcystis species to copper sulfate: Implication in algal toxicity of copper and controls of blooms. Sci. Rep. 2017, 7, 40393. [Google Scholar] [CrossRef]
- Liu, H.; Chen, S.; Zhang, H. Effects of copper sulfate algaecide on the cell growth, physiological characteristics, the metabolic activity of Microcystis aeruginosa and raw water application. J. Hazard. Mater. 2023, 445, 130604. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, G.; Mao, F. Use of an integrated metabolomics platform for mechanistic investigations of three commonly used algaecides on cyanobacterium, Microcystis aeruginosa. J. Hazard. Mater. 2019, 367, 120–127. [Google Scholar] [CrossRef]
- Zhou, S.; Shao, Y.; Gao, N. Effects of different algaecides on the photosynthetic capacity, cell integrity and microcystin-LR release of Microcystis aeruginosa. Sci. Total Environ. 2013, 463–464, 111–119. [Google Scholar] [CrossRef]
- Tsai, K.P. Effects of two copper compounds on Microcystis aeruginosa cell density, membrane integrity, and microcystin release. Ecotoxicol. Environ. Saf. 2015, 120, 428–435. [Google Scholar] [CrossRef]
- Ma, J.; Jia, B.; Li, S.; Kong, Y.; Nie, Y.; Zhang, H.; Xiao, M.; Gao, T. Enhanced coagulation of covalent composite coagulant with potassium permanganate oxidation for algae laden water treatment: Algae and extracellular organic matter removal. Chem. Eng. J. Adv. 2023, 13, 100427. [Google Scholar] [CrossRef]
- Nguyen, H.V.-M.; Kim, J.K.; Chang, S.W. A case study of low pressure air flotation ferryboat for algae removal in Korean rivers and lakes. J. Ind. Eng. Chem. 2019, 69, 32–38. [Google Scholar] [CrossRef]
- Wu, X.; Joyce, E.M.; Mason, T.J. Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Res. 2012, 469, 2851–2858. [Google Scholar] [CrossRef]
- Chen, G.; Ding, X.; Zhou, W. Study on ultrasonic treatment for degradation of Microcystins (MCs). Ultrason. Sonochem. 2020, 63, 104900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, P.; Wang, B. Ultrasonic frequency effects on the removal of Microcystis aeruginosa. Ultrason. Sonochem. 2006, 135, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Peng, Y.; Zhang, Z. Removal of Microcystis aeruginosa by ultrasound: Inactivation mechanism and release of algal organic matter. Ultrason. Sonochem. 2019, 56, 447–457. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, S.; Qin, L. Isolation, identification of algicidal bacteria and contrastive study on algicidal properties against Microcystis aeruginosa. Biochem. Eng. J. 2022, 185, 108525. [Google Scholar] [CrossRef]
- Jia, L.; Cheng, X.; Fang, L. Flocculation and lysis of Microcystis aeruginosa by Paebubacillus sp. A9 and inhibition of microcystin release. Environ. Technol. Innov. 2023, 31, 103152. [Google Scholar] [CrossRef]
- Liu, F.; Qin, L.; Zhu, S.; Chen, H.; Al-Haimi, A.A.N.M.; Xu, J.; Zhou, W.; Wang, Z. Applications-oriented algicidal efficacy research and in-depth mechanism of a novel strain Brevibacillus sp. on Microcystis aeruginosa. Environ. Pollut. 2023, 330, 121812. [Google Scholar] [CrossRef]
- Wu, Z.; Lai, X.; Li, K. Water quality assessment of rivers in Lake Chaohu Basin (China) using water quality index. Ecol. Indic. 2021, 121, 107021. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Chen, K. Using hydrogen peroxide to control cyanobacterial blooms: A mesocosm study focused on the effects of algal density in Lake Chaohu, China. Environ. Pollut. 2021, 272, 115923. [Google Scholar] [CrossRef]
- Huang, Y.-R.; Li, H.-Z.; Wei, X.-M. The effect of low frequency ultrasonic treatment on the release of extracellular organic matter of Microcystis aeruginosa. Chem. Eng. J. 2020, 383, 123141. [Google Scholar] [CrossRef]
- Huang, H.; Wu, G.; Sheng, C. Improved Cyanobacteria Removal from Harmful Algae Blooms by Two-Cycle, Low-Frequency, Low-Density, and Short-Duration Ultrasonic Radiation. Water 2020, 129, 2431. [Google Scholar] [CrossRef]
- Yamamoto, K.; King, P.M.; Wu, X. Effect of ultrasonic frequency and power on the disruption of algal cells. Ultrason. Sonochem. 2015, 24, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Peng, H.; Zheng, X. Effects of ultrasonic irradiation on organic matter of Microcystis aeruginosa cells. Desalination Water Treat. 2018, 129, 101–115. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Fan, L.; Nguyen, T. Impact of sonication at 20 kHz on Microcystis aeruginosa, Anabaena circinalis and Chlorella sp. Water Res. 2012, 465, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Li, N.; An, J. Fenton-based technologies as efficient advanced oxidation processes for microcystin-LR degradation. Sci. Total Environ. 2021, 753, 141809. [Google Scholar] [CrossRef]
- Garoma, T.; Yazdi, R.E.; Chin, C. Chlorella vulgaris cell disruption using copper sulfate. Biofuels 2019, 128, 1007–1015. [Google Scholar] [CrossRef]
- Iwinski, K.J.; Calomeni, A.J.; Geer, T.D. Cellular and aqueous microcystin-LR following laboratory exposures of Microcystis aeruginosa to copper algaecides. Chemosphere 2016, 147, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, H.; He, X. Evaluating the treatment effectiveness of copper-based algaecides on toxic algae Microcystis aeruginosa using single cell-inductively coupled plasma-mass spectrometry. Anal. Bioanal. Chem. 2019, 41121, 5531–5543. [Google Scholar] [CrossRef]
- Cheng, R.; Song, X.; Song, W. A New Perspective: Revealing the Algicidal Properties of Bacillus subtilis to Alexandrium pacificum from Bacterial Communities and Toxins. Mar. Drugs 2022, 2010, 624. [Google Scholar] [CrossRef] [PubMed]
- Eckersley, E.; Berger, B.W. An engineered polysaccharide lyase to combat harmful algal blooms. Biochem. Eng. J. 2018, 132, 225–232. [Google Scholar] [CrossRef]
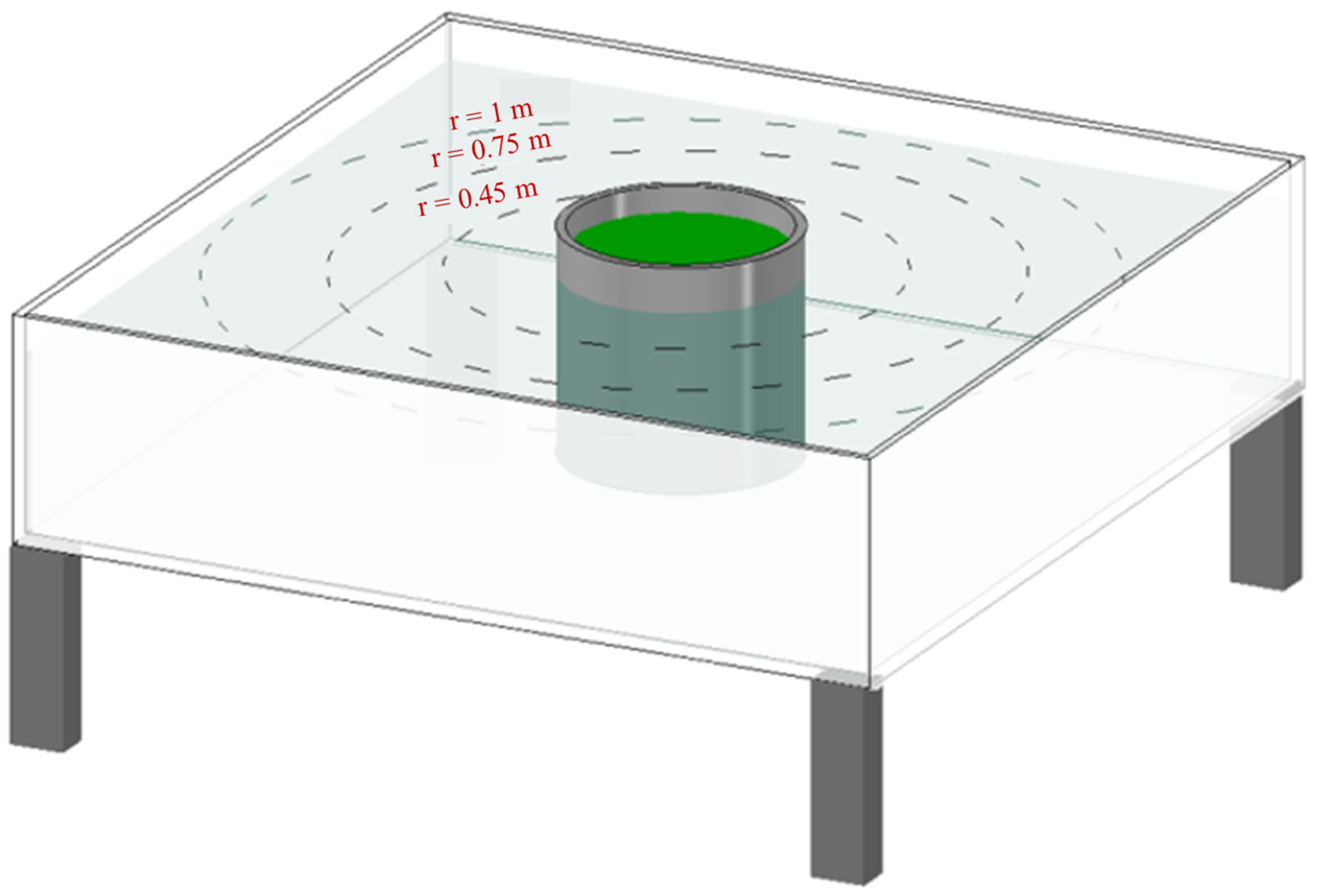

| Physicochemical Parameters | 5 September 2018 | 28 September 2018 | |
|---|---|---|---|
| Point 1 | Point 2 | Point 3 | |
| TN(mg/L) | 2.24 ± 0.18 | 2.51 ± 0.28 | 2.36 ± 0.25 |
| TP(mg/L) | 0.070 ± 0.008 | 0.073 ± 0.006 | 0.08 ± 0.004 |
| PO43−-P(mg/L) | 0.013 ± 0.002 | 0.017 ± 0.005 | 0.012 ± 0.004 |
| NH4+-N(mg/L) | 0.855 ± 0.012 | 1.042 ± 0.023 | 0.276 ± 0.013 |
| NO2−-N(mg/L) | 0.016 ± 0.0013 | 0.042 ± 0.0018 | 0.057 ± 0.0013 |
| NO3−-N(mg/L) | 1.462 ± 0.092 | 1.12 ± 0.083 | 1.374 ± 0.087 |
| DO(mg/L) | 7.82 ± 0.40 | 7.17 ± 0.33 | 8.32 ± 0.35 |
| Secchi depth/(cm) | 39.8 ± 6.4 | 24.7 ± 3.8 | 47.6 ± 8.3 |
| T/°C | 28.0 ± 1.0 | 28.0 ± 1.2 | 26.0 ± 1.3 |
| pH | 8.01 ± 0.03 | 7.98 ± 0.05 | 8.21 ± 0.05 |
| Chlorophyll a/(µg/L) | 15.46 ± 0.33 | 18.82 ± 0.48 | 16.42 ± 0.52 |
| Ultrasonic Power Density (W/L) | 1 min | 3 min | 5 min | 10 min | 15 min |
|---|---|---|---|---|---|
| 2.65 | 0.51 ± 0.07 | 0.43 ± 0.09 | 0.55 ± 0.06 | 0.48 ± 0.02 | 0.55 ± 0.05 |
| 10.62 | 0.34 ± 0.04 | 0.54 ± 0.03 | 0.82 ± 0.21 | 1.18 ± 0.19 | 1.36 ± 0.11 |
| 16 | 1.51 ± 0.42 | 1.21 ± 0.27 | 1.63 ± 0.18 | 2.12 ± 0.53 | 2.43 ± 0.17 |
| Dosage of CuSO4 (mg/L) | 1 d | 2 d | 4 d | 6 d | 8 d | |
|---|---|---|---|---|---|---|
| CuSO4 | 0.5 | 58.0 ± 8.7 | 25.81 ± 1.6 | 76.48 ± 4.5 | 18.13 ± 1.9 | 19.2 ± 3.9 |
| 2 | 56.28 ± 6.5 | 167.85 ± 22.1 | 59.02 ± 8.7 | 37.07 ± 4.6 | 24.14 ± 2.5 | |
| 4 | 102.11 ± 13.1 | 178.65 ± 16.7 | 87.67 ± 9.9 | 48.31 ± 2.8 | 40.92 ± 5.5 | |
| 8 | 95.98 ± 10.5 | 185.08 ± 13.8 | 74.59 ± 6.6 | 60.99 ± 7.7 | 43.02 ± 3.2 | |
| Dosage of BA (mg/L) | 1 d | 2 d | 4 d | 6 d | 8 d | |
|---|---|---|---|---|---|---|
| BA | 0.5 | −61.38 ± 3.2 | 13.2 ± 1.6 | −16.4 ± 3.2 | −32.12 ± 4.5 | −43.94 ± 8.1 |
| 1 | −94.61 ± 8.2 | 80.58 ± 6.2 | −0.77 ± 0.1 | −14.33 ± 2.6 | −32.18 ± 5.03 | |
| 2 | 99.44 ± 7.3 | 121.74 ± 10.1 | 6.24 ± 2.1 | −9.72 ± 2.9 | −20.14 ± 3.7 | |
| 4 | 133.62 ± 18.9 | 230.19 ± 28.8 | 17.7 ± 4.7 | 4.09 ± 0.56 | 6.99 ± 1.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Fang, W.; Ma, M.; Xu, W.; Ye, J. Changes in Extracellular Microcystins (MCs) Accompanying Algae/Cyanobacteria Removal during Three Representative Algae/Cyanobacteria Inactivation Processes and an MC Diffusion Model in Still Water. Water 2023, 15, 3591. https://doi.org/10.3390/w15203591
Shi C, Fang W, Ma M, Xu W, Ye J. Changes in Extracellular Microcystins (MCs) Accompanying Algae/Cyanobacteria Removal during Three Representative Algae/Cyanobacteria Inactivation Processes and an MC Diffusion Model in Still Water. Water. 2023; 15(20):3591. https://doi.org/10.3390/w15203591
Chicago/Turabian StyleShi, Chengcheng, Weijian Fang, Mengru Ma, Wei Xu, and Jingjing Ye. 2023. "Changes in Extracellular Microcystins (MCs) Accompanying Algae/Cyanobacteria Removal during Three Representative Algae/Cyanobacteria Inactivation Processes and an MC Diffusion Model in Still Water" Water 15, no. 20: 3591. https://doi.org/10.3390/w15203591
APA StyleShi, C., Fang, W., Ma, M., Xu, W., & Ye, J. (2023). Changes in Extracellular Microcystins (MCs) Accompanying Algae/Cyanobacteria Removal during Three Representative Algae/Cyanobacteria Inactivation Processes and an MC Diffusion Model in Still Water. Water, 15(20), 3591. https://doi.org/10.3390/w15203591





