Efficient Removal of Cadmium (II) and Arsenic (V) from Water by the Composite of Iron Manganese Oxides Loaded Muscovite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorption Materials
2.3. Characterization Methods
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Screening Experiments of Cd(II) and As(V) Adsorption Materials
3.2. Adsorption Kinetics
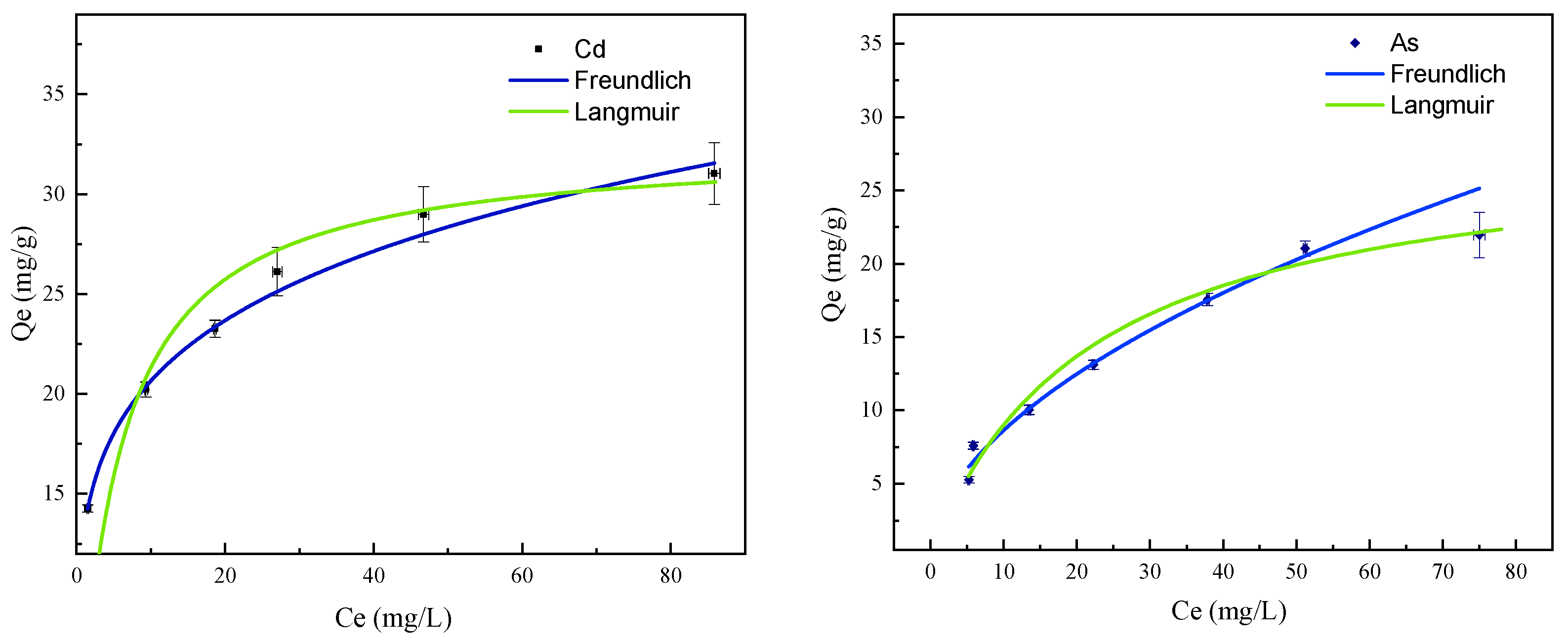
3.3. Adsorption Isotherms
| Adsorption Materials | Qmax-Cd(II) (mg/g) | Qmax-As(V) (mg/g) | Ref. |
|---|---|---|---|
| Hydrated iron oxide gel beads | 14.83 | [20] | |
| Iron manganese oxide gel beads | 26.65 | [19] | |
| Fe3O4-reduced graphite oxide–MnO2 nanocomposites | 12.22 | [23] | |
| Magnetite/non-oxidative graphene | 9.44 | [24] | |
| Calcium-based magnetic biochar (Ca-MBC) | 10.07 | [25] | |
| Zeolite-supported nanoscale zero-valent iron | 48.63 | [26] | |
| Mn-doped iron oxide | 35 | [27] | |
| Mn3O4/Fe3O4 | 18.8 | [28] | |
| Manganese modified wood | 28.1 | [29] | |
| Natural muscovite | 0.750 | 0.791 | [18] |
| FMM | 32.47 | 28.57 | This study |
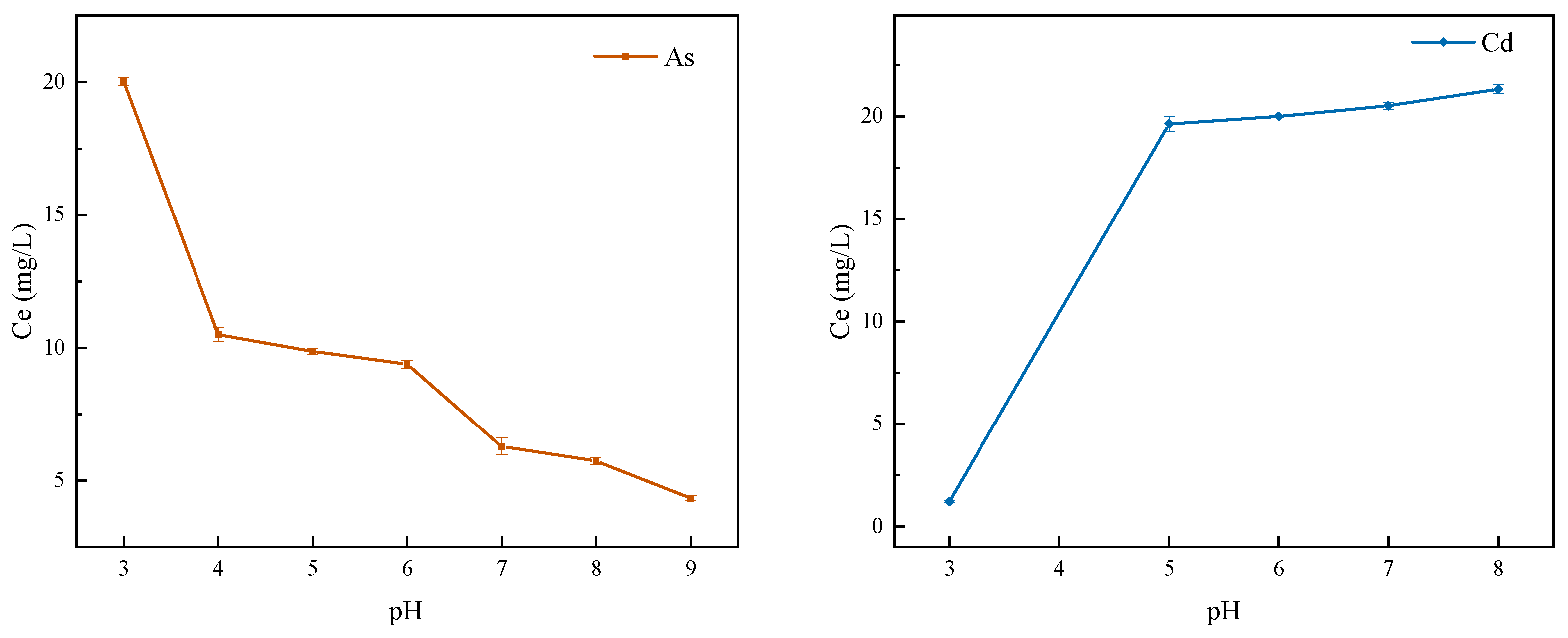
3.4. The Effect of Initial pH Value of Solution on Adsorption
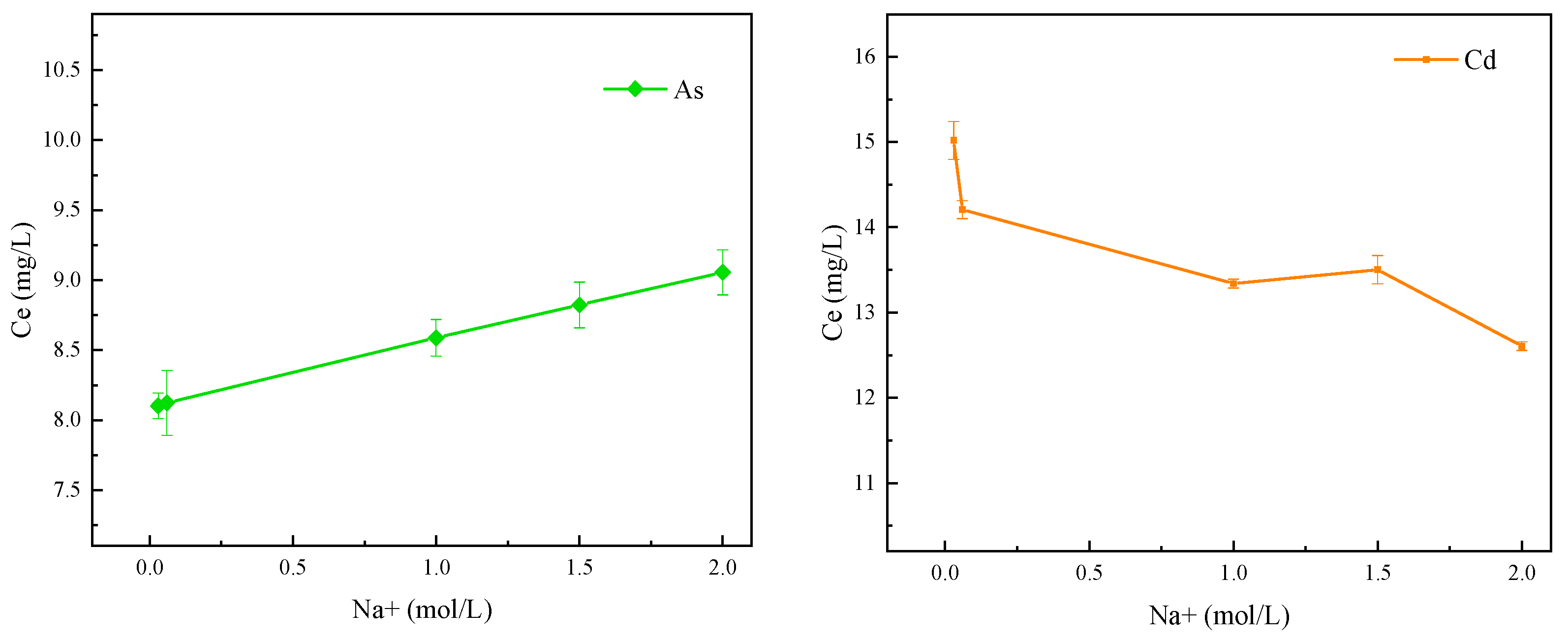
3.5. The Effect of Solution Ion Concentration on Adsorption
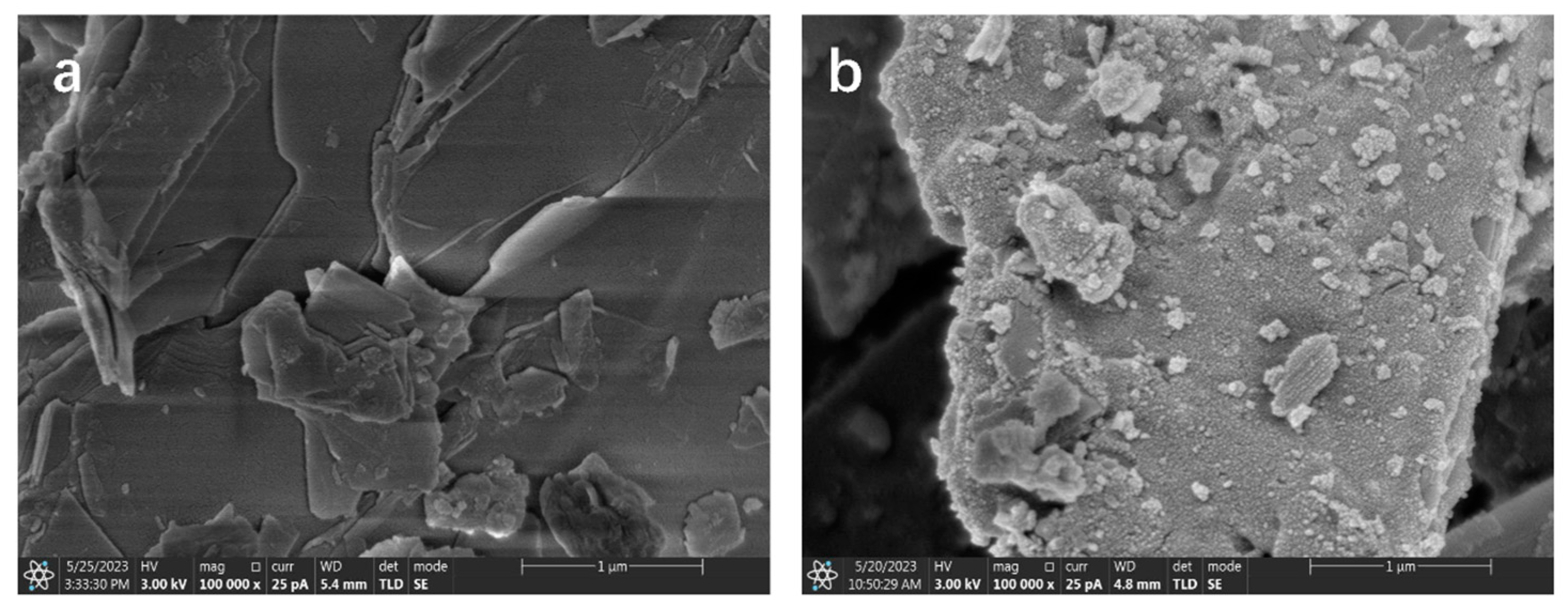
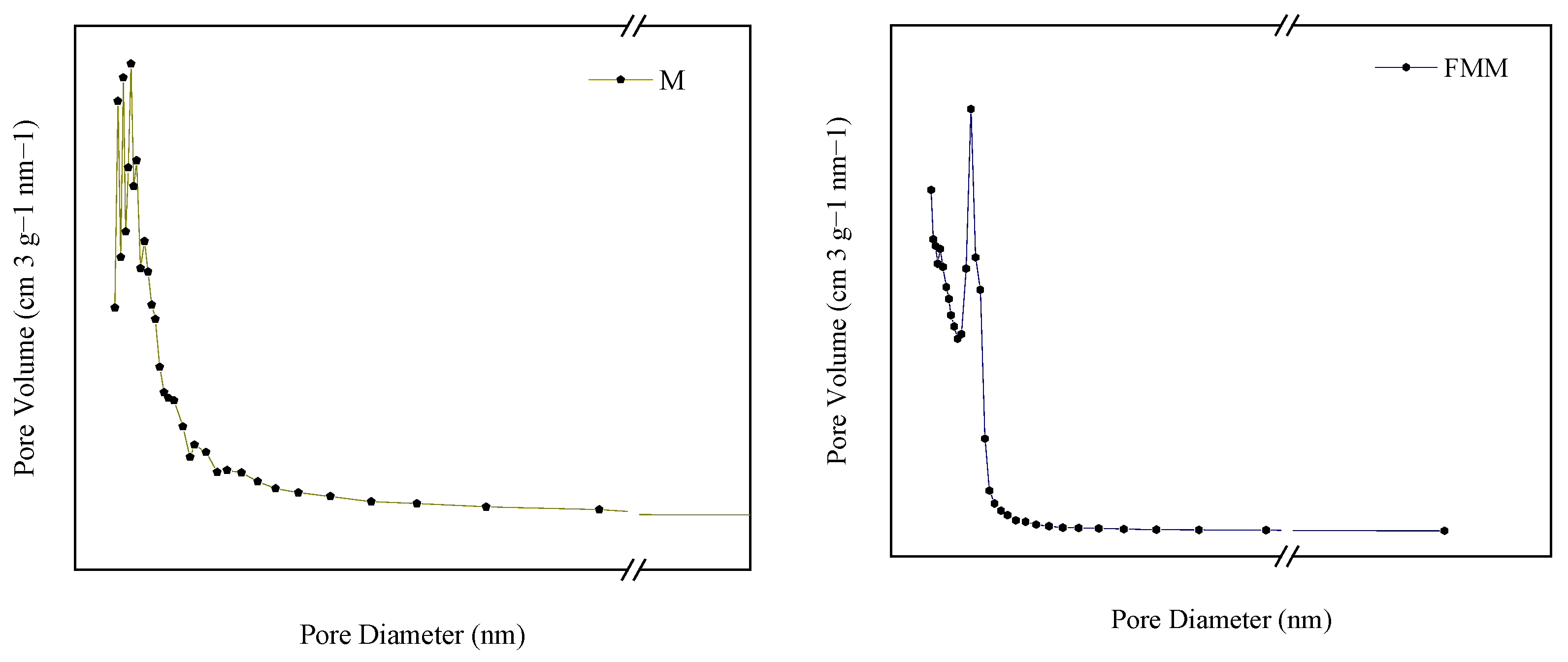
3.6. Surface Morphology
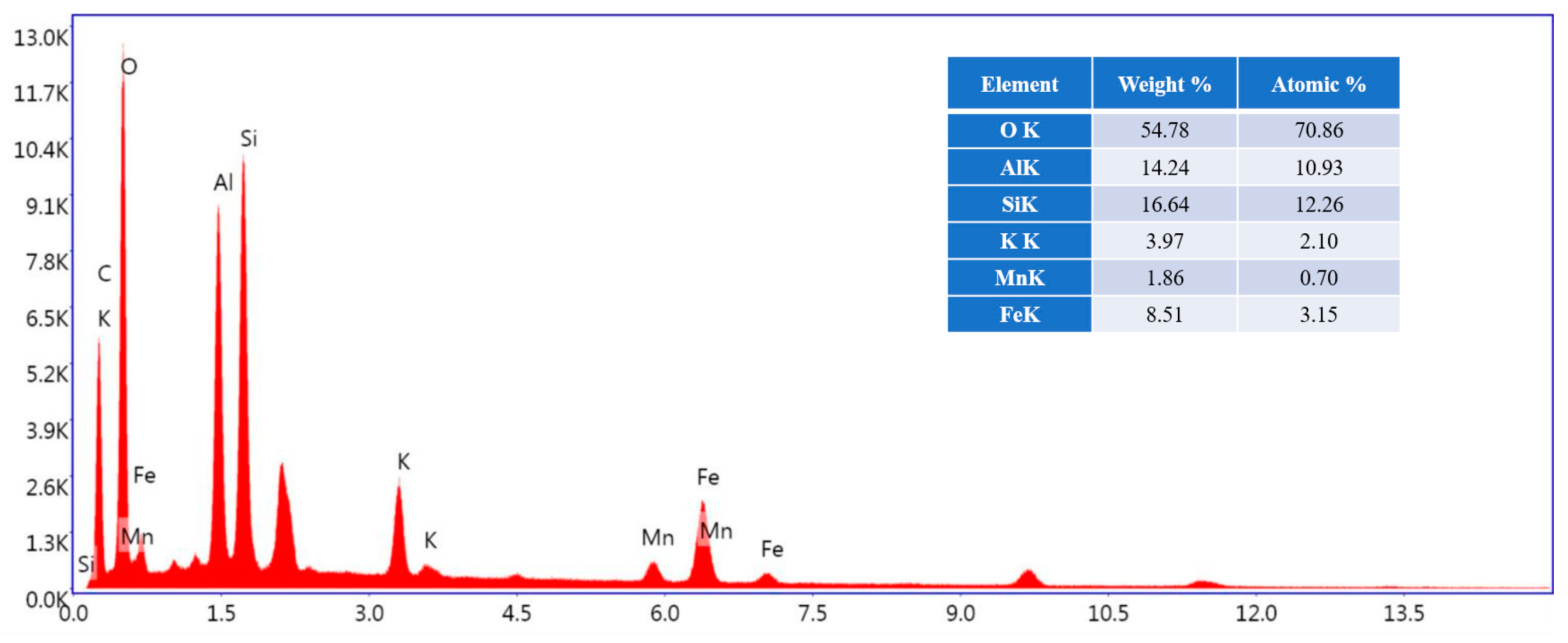
3.7. Structure Characteristics
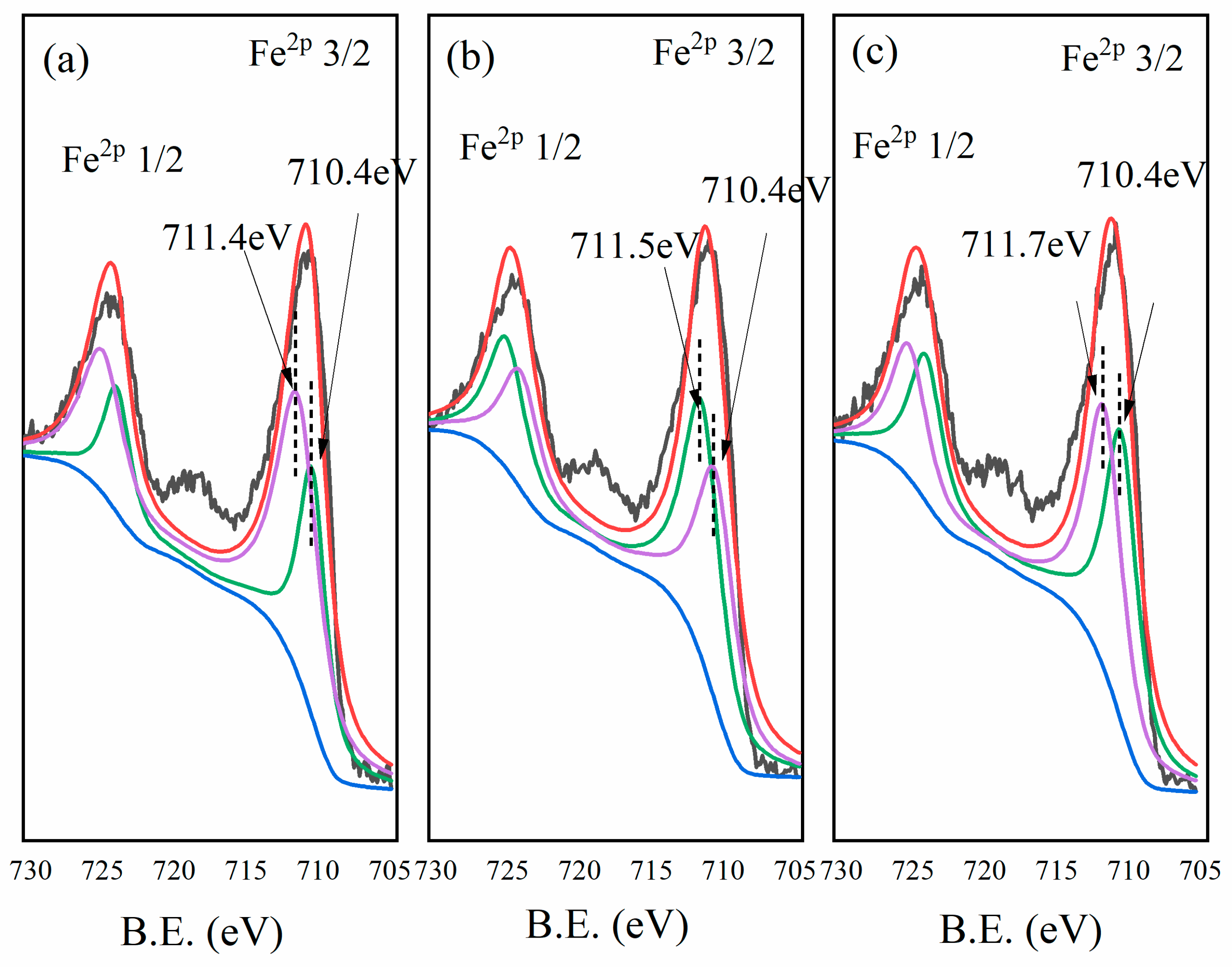
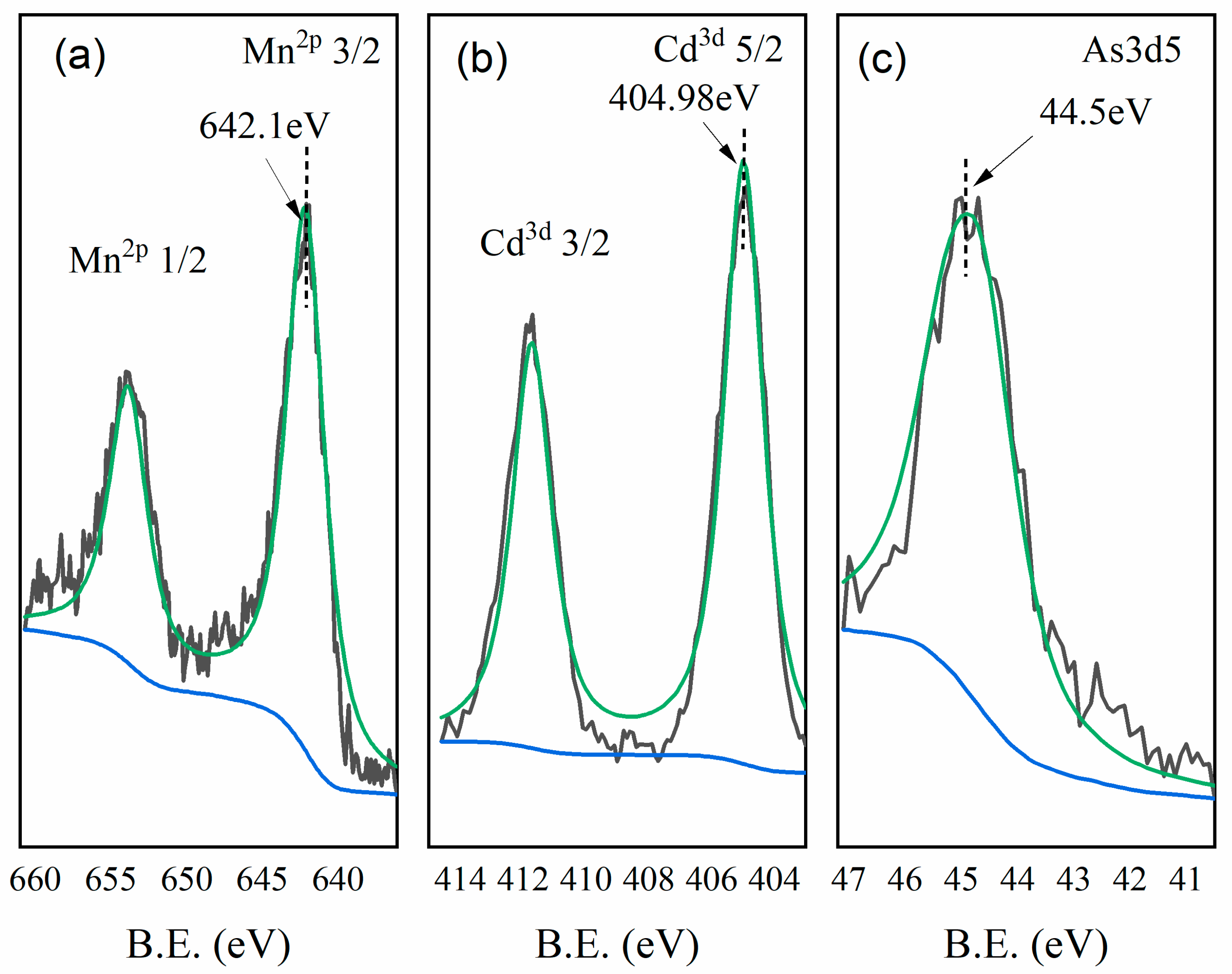
3.8. XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Neelgund, G.M.; Jimenez, E.A.; Ray, R.L.; Kurkuri, M.D. Facilitated Adsorption of Mercury(II) and Chromium(VI) Ions over Functionalized Carbon Nanotubes. Toxics 2023, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, V.A.; Aralekallu, S.; Nemakal, M.; Palanna, M.; Prabhu, C.P.K.; Sannegowda, L.K. Nanomolar detection of lead using electrochemical methods based on a novel phthalocyanine. Inorg. Chim. Acta 2020, 506, 7. [Google Scholar] [CrossRef]
- Bastami, K.D.; Bagheri, H.; Kheirabadi, V.; Zaferani, G.G.; Teymori, M.B.; Hamzehpoor, A.; Soltani, F.; Haghparast, S.; Harami, S.R.M.; Ghorghani, N.F.; et al. Distribution and ecological risk assessment of heavy metals in surface sediments along southeast coast of the Caspian Sea. Mar. Pollut. Bull. 2014, 81, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Mlangeni, A.T. Health risk assessment of toxic metal(loids) (As, Cd, Pb, Cr, and Co) via consumption of medicinal herbs marketed in Malawi. Toxicol. Rep. 2023, 11, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Li, J.F.; Wang, J.L. Recovery of boric acid from the simulated radioactive wastewater by vacuum membrane distillation crystallization. Ann. Nucl. Energy 2017, 110, 1148–1155. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Yao, Y.; Li, X.Y.; Lu, J.; Zhou, J.; Huang, Z.L. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process. Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Bahsaine, K.; Mekhzoum, M.E.; Benzeid, H.; Qaiss, A.; Bouhfid, R. Recent progress in heavy metals extraction from phosphoric acid: A short review. J. Ind. Eng. Chem. 2022, 115, 120–134. [Google Scholar] [CrossRef]
- Liu, X.J.; Wu, J.L.; Wang, J.L. Electro-adsorption of Sr(II) from aqueous solution by activated carbon cloth/nickel hexacyanoferrate composite electrode through capacitive deionization. J. Clean. Prod. 2022, 380, 10. [Google Scholar] [CrossRef]
- Badescu, I.S.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Valorisation possibilities of exhausted biosorbents loaded with metal ions—A review. J. Environ. Manag. 2018, 224, 288–297. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, F.B.; Xu, K.X.; Che, Y.J.; Qi, M.Y.; Song, C. Modified magnetic chitosan materials for heavy metal adsorption: A review. RSC Adv. 2023, 13, 6713–6736. [Google Scholar] [CrossRef]
- Yang, D.; Yang, S.Y.; Wang, L.; Xu, J.M.; Liu, X.M. Performance of biochar-supported nanoscale zero-valent iron for cadmium and arsenic co-contaminated soil remediation: Insights on availability, bioaccumulation and health risk. Environ. Pollut. 2021, 290, 9. [Google Scholar] [CrossRef]
- Sugawara, K.; Ichio, K.; Ichikawa, Y.; Ogawa, H.; Suzuki, S. Effects of Pyrolysis Temperature and Chemical Modification on the Adsorption of Cd and As(V) by Biochar Derived from Pteris vittata. Int. J. Environ. Res. Public Health 2022, 19, 16. [Google Scholar] [CrossRef]
- Soni, R.; Shukla, D.P. Synthesis of fly ash based zeolite-reduced graphene oxide composite and its evaluation as an adsorbent for arsenic removal. Chemosphere 2019, 219, 504–509. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Wen, N.; Wei, D.; Zhang, Y. Highly effective removal of lead and cadmium ions from wastewater by bifunctional magnetic mesoporous silica. Sep. Purif. Technol. 2021, 265, 118341. [Google Scholar] [CrossRef]
- Michalkova, Z.; Komarek, M.; Veselska, V.; Cihalova, S. Selected Fe and Mn (nano)oxides as perspective amendments for the stabilization of As in contaminated soils. Environ. Sci. Pollut. Res. 2016, 23, 10841–10854. [Google Scholar] [CrossRef]
- Zhou, Q.W.; Liao, B.H.; Lin, L.N.; Qiu, W.W.; Song, Z.G. Adsorption of Cu(II) and Cd(II) from aqueous solutions by ferromanganese binary oxide-biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Chakraborty, S.; Wolthers, M.; Chatterjee, D.; Charlet, L. Adsorption of arsenite and arsenate onto muscovite and biotite mica. J. Colloid Interface Sci. 2007, 309, 392–401. [Google Scholar] [CrossRef]
- Yang, J.S.; Lee, J.Y.; Park, Y.T.; Baek, K.; Choi, J. Adsorption of As(III), As(V), Cd(II), Cu(II), and Pb(II) from Aqueous Solutions by Natural Muscovite. Sep. Sci. Technol. 2010, 45, 814–823. [Google Scholar] [CrossRef]
- Salam, M.A.; Abukhadra, M.R.; Mostafa, M. Effective decontamination of As(V), Hg(II), and U(VI) toxic ions from water using novel muscovite/zeolite aluminosilicate composite: Adsorption behavior and mechanism. Environ. Sci. Pollut. Res. 2020, 27, 13247–13260. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Cai, L.K.; Wang, X.Y.; Chen, Z.H.; Yang, W.Z. Efficient adsorption of arsenic in groundwater by hydrated iron oxide and ferromanganese oxide chitosan gel beads. Sep. Purif. Technol. 2023, 315, 12. [Google Scholar] [CrossRef]
- Lilhare, S.; Mathew, S.B.; Singh, A.K.; Carabineiro, S.A.C. Calcium Alginate Beads with Entrapped Iron Oxide Magnetic Nanoparticles Functionalized with Methionine-A Versatile Adsorbent for Arsenic Removal. Nanomaterials 2021, 11, 21. [Google Scholar] [CrossRef]
- Gupta, K.; Maity, A.; Ghosh, U.C. Manganese associated nanoparticles agglomerate of iron(III) oxide: Synthesis, characterization and arsenic(III) sorption behavior with mechanism. J. Hazard. Mater. 2010, 184, 832–842. [Google Scholar] [CrossRef]
- Luo, X.B.; Wang, C.C.; Luo, S.L.; Dong, R.Z.; Tu, X.M.; Zeng, G.S. Adsorption of As (III) and As(V) from water using magnetite Fe3O4-reduced graphite oxide-MnO2 nanocomposites. Chem. Eng. J. 2012, 187, 45–52. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Zheng, M.L.; Ahn, Y.T.; Park, W.K.; Yang, W.S.; Kang, J.W. Synthesis of magnetite/non-oxidative graphene composites and their application for arsenic removal. Sep. Purif. Technol. 2017, 178, 40–48. [Google Scholar] [CrossRef]
- Wu, J.Z.; Huang, D.; Liu, X.M.; Meng, J.; Tang, C.X.; Xu, J.M. Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef]
- Li, Z.T.; Wang, L.; Meng, J.; Liu, X.M.; Xu, J.M.; Wang, F.; Brookes, P. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Warner, C.L.; Chouyyok, W.; Mackie, K.E.; Neiner, D.; Saraf, L.V.; Droubay, T.C.; Warner, M.G.; Addleman, R.S. Manganese Doping of Magnetic Iron Oxide Nanoparticles: Tailoring Surface Reactivity for a Regenerable Heavy Metal Sorbent. Langmuir 2012, 28, 3931–3937. [Google Scholar] [CrossRef]
- Gong, J.L.; Chen, L.; Zeng, G.M.; Long, F.; Deng, J.H.; Niu, Q.Y.; He, X. Shellac-coated iron oxide nanoparticles for removal of cadmium(II) ions from aqueous solution. J. Environ. Sci. 2012, 24, 1165–1173. [Google Scholar] [CrossRef]
- Wang, H.Y.; Gao, B.; Wang, S.S.; Fang, J.; Xue, Y.W.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef]
- Raju, N.J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies. Environ. Res. 2022, 203, 15. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.M.; Yu, Z.G.; Zeng, G.M.; Luo, Y.; Jiang, L.B.; Yang, Z.X.; Qian, Y.Y.; Wu, H.P. Amorphous MnO2 Modified Biochar Derived from Aerobically Composted Swine Manure for Adsorption of Pb(II) and Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 5049–5058. [Google Scholar] [CrossRef]
- Su, Y.M.; Adeleye, A.S.; Huang, Y.X.; Sun, X.Y.; Dai, C.M.; Zhou, X.F.; Zhang, Y.L.; Keller, A.A. Simultaneous removal of cadmium and nitrate in aqueous media by nanoscale zerovalent iron (nZVI) and Au doped nZVI particles. Water Res. 2014, 63, 102–111. [Google Scholar] [CrossRef]
- Ben Issa, N.; Rajakovic-Ognjanovic, V.N.; Jovanovic, B.M.; Rajakovic, L.V. Determination of inorganic arsenic species in natural waters-Benefits of separation and preconcentration on ion exchange and hybrid resins. Anal. Chim. Acta 2010, 673, 185–193. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Yin, D.Q.; Zhu, S.J.; Hu, X.L. Adsorption of cadmium(II) on humic acid coated titanium dioxide. J. Colloid Interface Sci. 2012, 367, 241–248. [Google Scholar] [CrossRef]
- Park, C.M.; Han, J.H.; Chu, K.H.; Al-Hamadani, Y.A.J.; Her, N.; Heo, J.Y.; Yoon, Y.M. Influence of solution pH, ionic strength, and humic acid on cadmium adsorption onto activated biochar: Experiment and modeling. J. Ind. Eng. Chem. 2017, 48, 186–193. [Google Scholar] [CrossRef]
- Taylor, J.A. An XPS study of the oxidation of ALAs thin-films grown by MBE. J. Vac. Sci. Technol. 1982, 20, 751–755. [Google Scholar] [CrossRef]
- Dang, T.A.; Chau, C.N. Electron spectroscopy for chemical analysis of cool white phosphors coated with SiO2 thin film. J. Electrochem. Soc. 1996, 143, 302–305. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1992; Volume 40, p. 221. [Google Scholar]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. XPS studies of solvated metal atom dispersed catalysts—Evidence for layered cobalt manganese particles on alumina and silica. J. Am. Chem. Soc. 1991, 113, 855–861. [Google Scholar] [CrossRef]
- Zhang, S.J.; Li, X.Y.; Chen, J.P. An XPS study for mechanisms of arsenate adsorption onto a magnetite-doped activated carbon fiber. J. Colloid Interface Sci. 2010, 343, 232–238. [Google Scholar] [CrossRef]
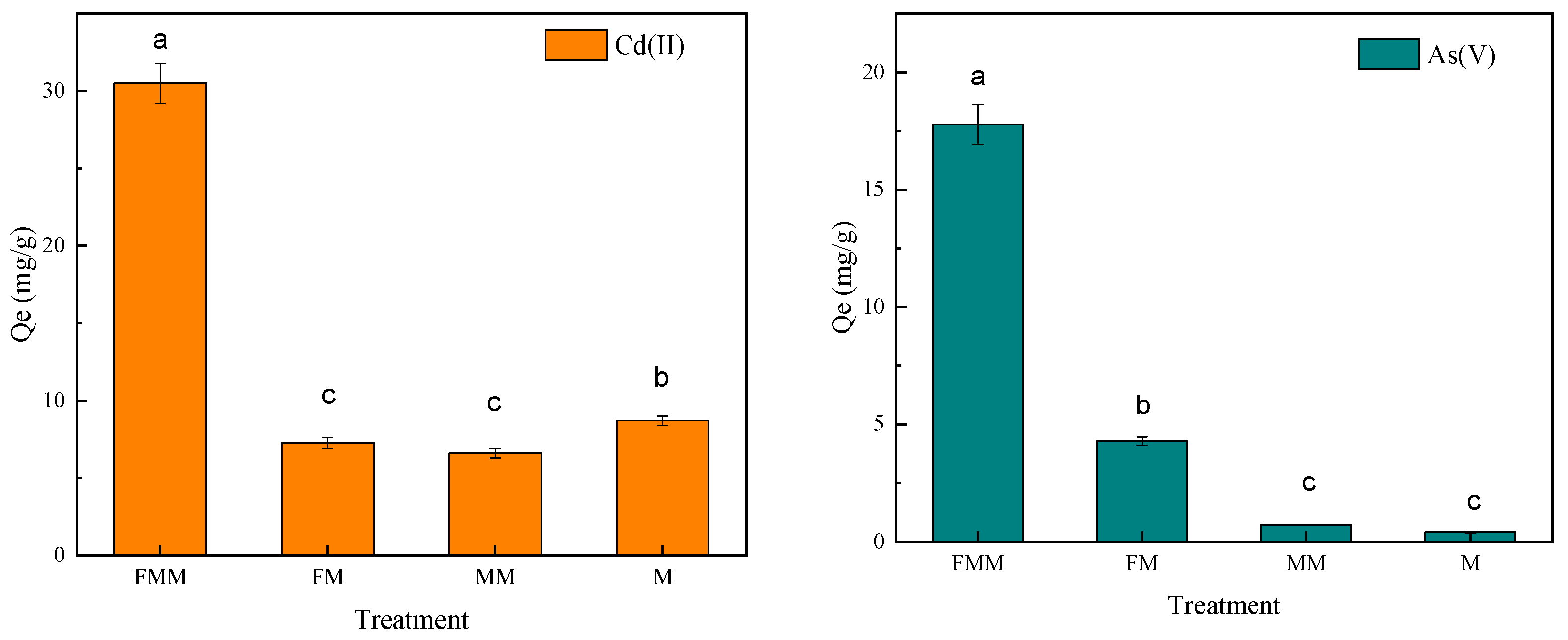
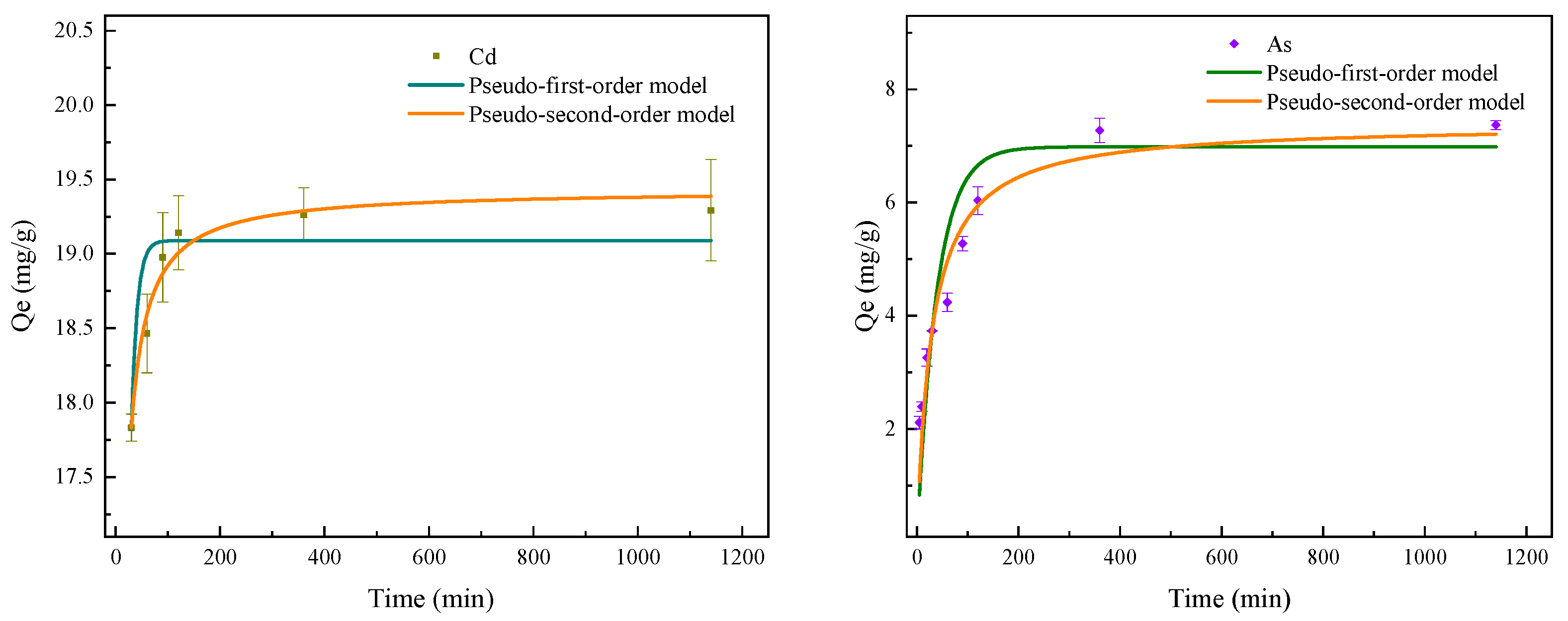

| Pseudo-First-Order Model | Pseudo-Second-Order Model | Exp. | |||||
|---|---|---|---|---|---|---|---|
| Qe (mg/g) | k1 (1/min) | R2 | Qe (mg/g) | k2 (g/(mg min)) | R2 | Qe (mg/g) | |
| Cd(II) | 19.09 | 0.090 | 0.912 | 19.43 | 0.019 | 0.988 | 19.29 |
| As(V) | 6.98 | 0.026 | 0.857 | 7.39 | 0.005 | 0.940 | 7.37 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| Qe (mg/g) | KL (L/mg) | R2 | n | KF (mg/g) | R2 | |
| Cd | 32.47 | 0.191 | 0.996 | 5.07 | 13.11 | 0.997 |
| As | 28.57 | 0.046 | 0.978 | 1.89 | 2.55 | 0.966 |
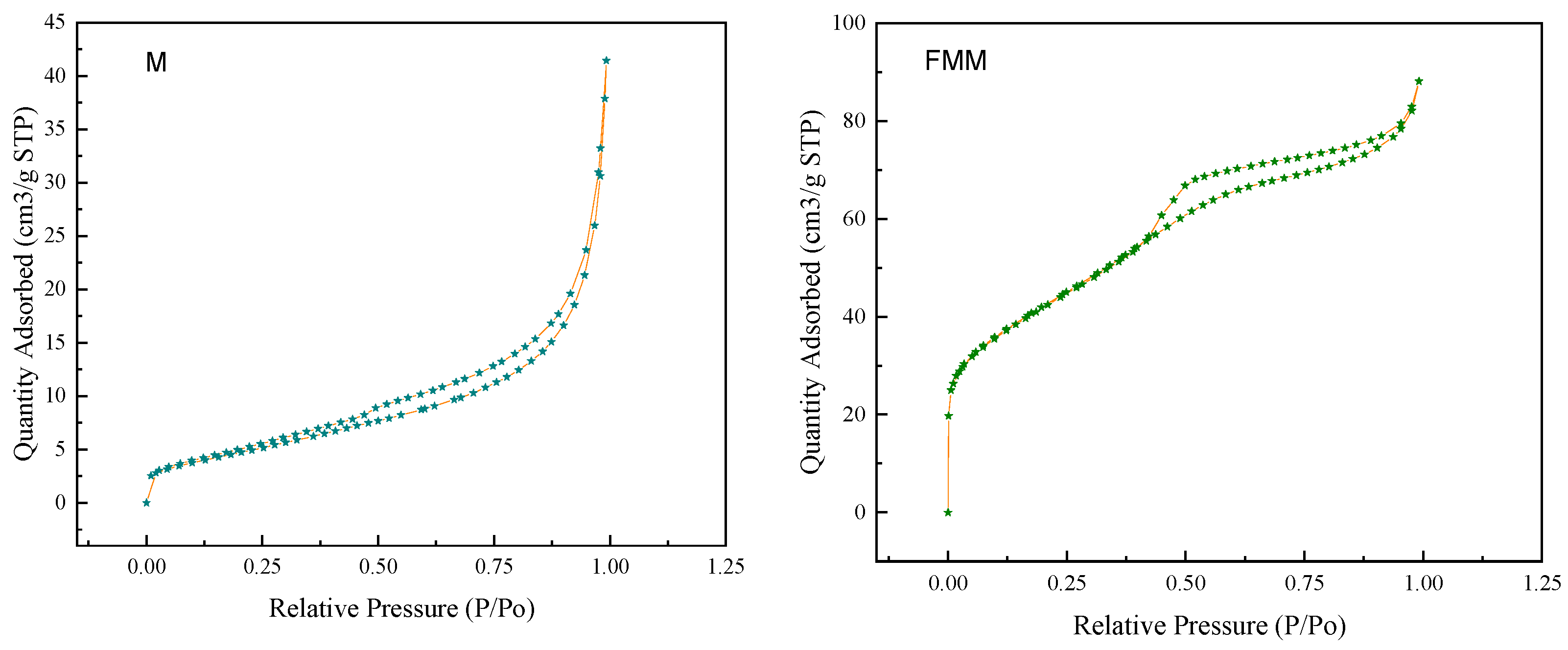
| Surface Area of BET(m2/g) | Average Pore Size (nm) | Vtotal (m3/g) | |
|---|---|---|---|
| M | 18.12 | 13.20 | 0.060 |
| FMM | 150.82 | 3.60 | 0.136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhao, Y.; Xu, Z.; Wang, R.; Zhang, H.; Feng, S.; Guo, J. Efficient Removal of Cadmium (II) and Arsenic (V) from Water by the Composite of Iron Manganese Oxides Loaded Muscovite. Water 2023, 15, 3579. https://doi.org/10.3390/w15203579
Wu Y, Zhao Y, Xu Z, Wang R, Zhang H, Feng S, Guo J. Efficient Removal of Cadmium (II) and Arsenic (V) from Water by the Composite of Iron Manganese Oxides Loaded Muscovite. Water. 2023; 15(20):3579. https://doi.org/10.3390/w15203579
Chicago/Turabian StyleWu, Yan, Yue Zhao, Zhuben Xu, Rui Wang, Han Zhang, Shuaitao Feng, and Jianhua Guo. 2023. "Efficient Removal of Cadmium (II) and Arsenic (V) from Water by the Composite of Iron Manganese Oxides Loaded Muscovite" Water 15, no. 20: 3579. https://doi.org/10.3390/w15203579
APA StyleWu, Y., Zhao, Y., Xu, Z., Wang, R., Zhang, H., Feng, S., & Guo, J. (2023). Efficient Removal of Cadmium (II) and Arsenic (V) from Water by the Composite of Iron Manganese Oxides Loaded Muscovite. Water, 15(20), 3579. https://doi.org/10.3390/w15203579






