Spatial–Temporal Distribution and Ecological Risk Assessment of Microplastic Pollution of Inland Fishing Ground in the Ubolratana Reservoir, Thailand
Abstract
:1. Introduction
2. Materials and Methods
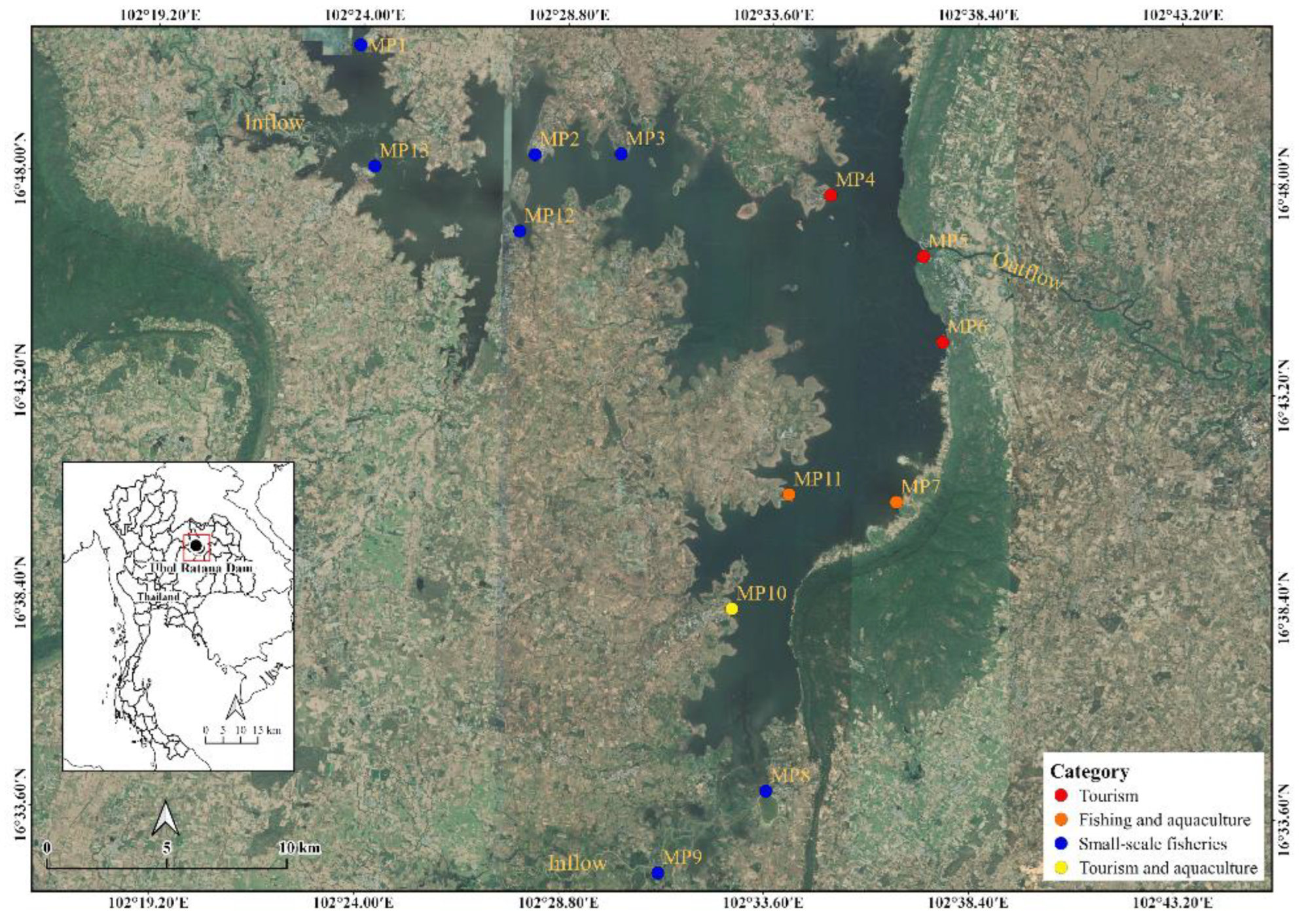
2.1. Sampling Sites
2.2. Sample Collection
2.3. Microplastic Extraction
2.4. Identification of Microplastic Morphological Characteristics
2.5. Quality Assurance and Quality Control
2.6. Microplastic Ecological Risk Assessment
2.7. Statistical Analysis
3. Results and Discussion
3.1. Microplastic Abundance and Spatial–Temporal Distribution in Surface Water and Sediment
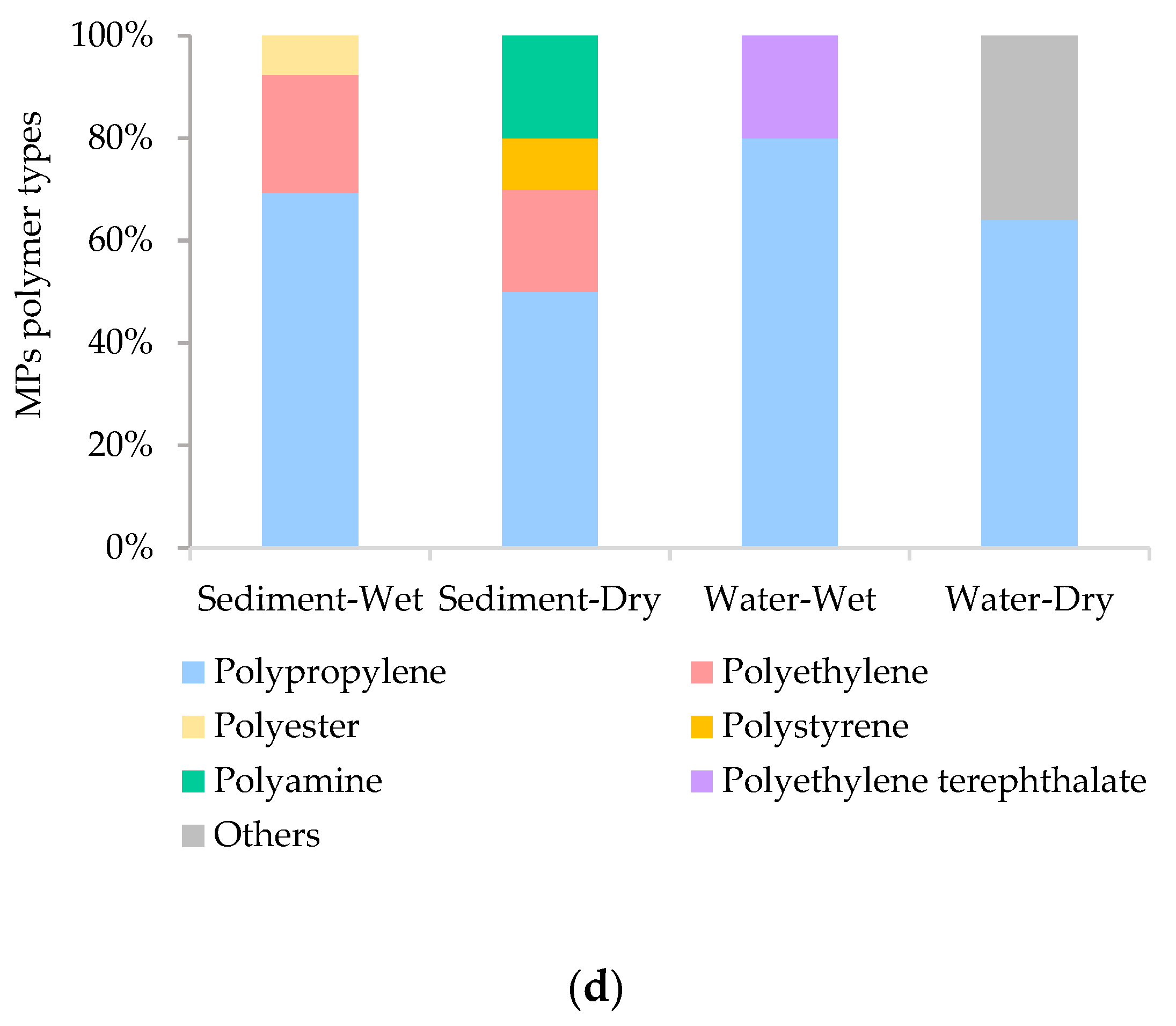
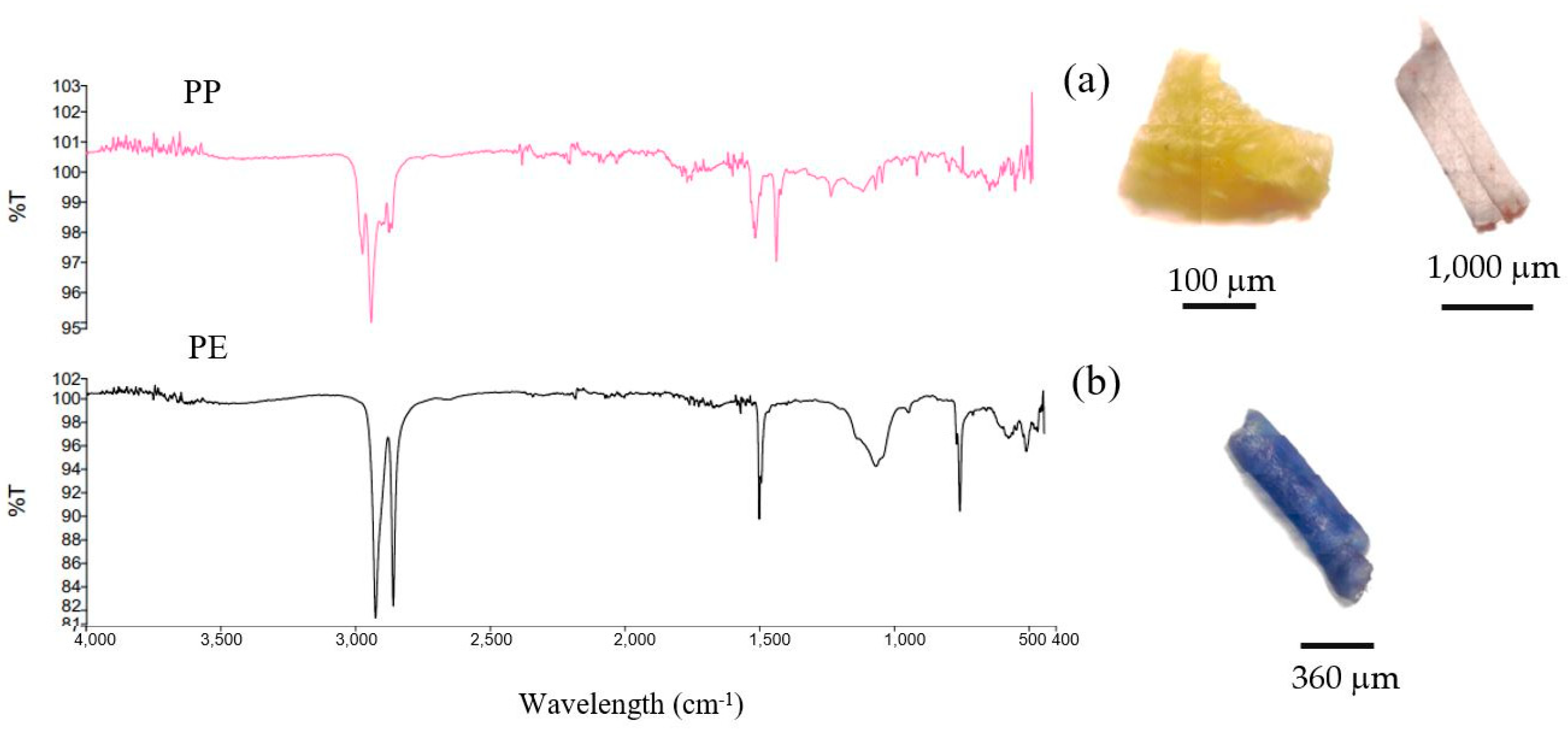
3.2. Microplastic Characteristics
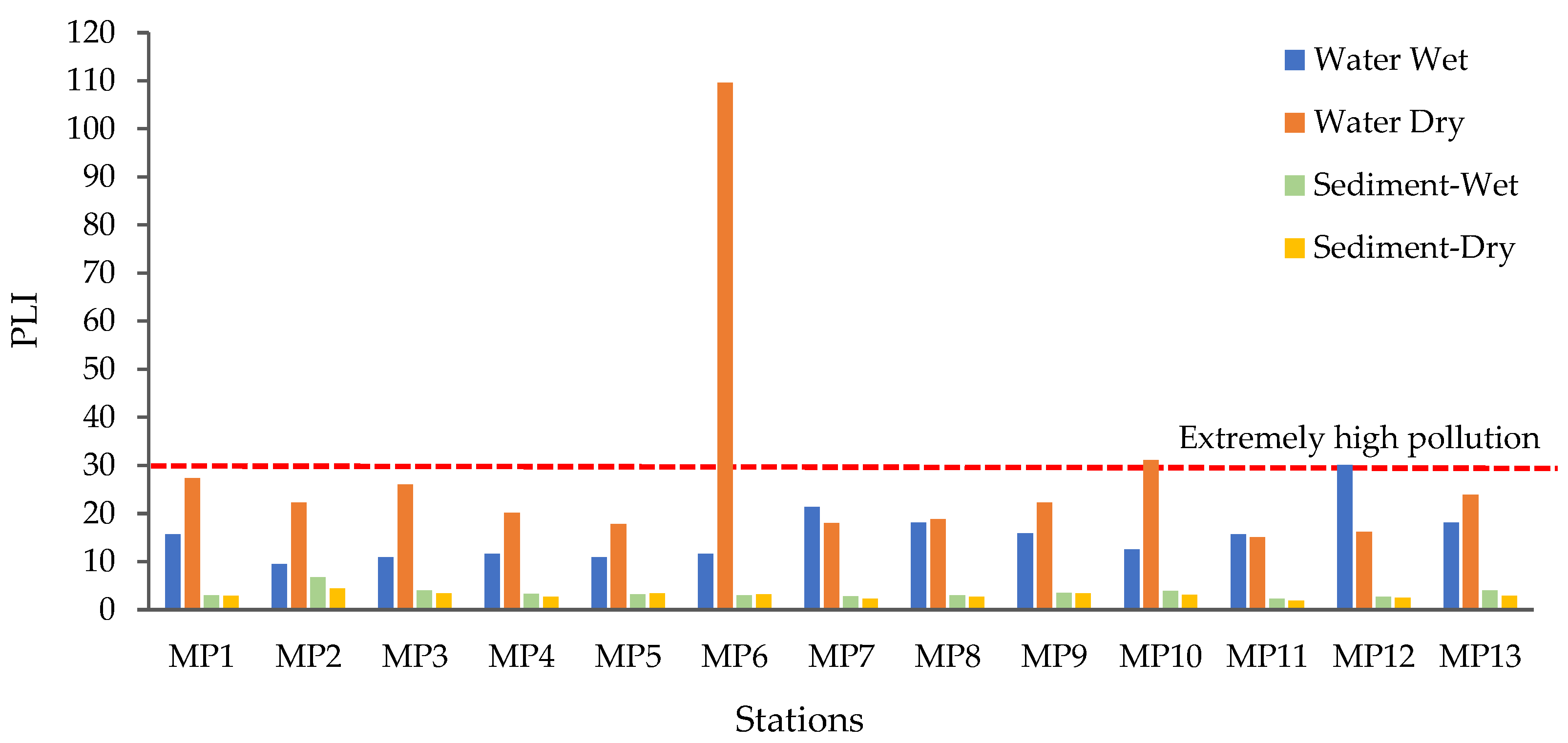
3.3. Microplastic Pollution Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PlasticsEurope. Plastics the fact 2021: Plastics-the fact 2021: An analysis of European plastics production, demand and waste data. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021 (accessed on 7 September 2022).
- Okoffo, E.D.; Donner, E.; McGrath, S.P.; Tscharke, B.J.; O’Brien, J.W.; O’Brien, S.; Ribero, F.; Burrows, S.D.; Toapanta, T.; Rauert, C.; et al. Plastics in biosolids from 1950 to 2016: A function of global plastic production and consumption. Water Res. 2021, 201, 117367. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Ylva, O.; Mitchell, R.P.; Anthony, D.; Rowland, S.J.; John, A.W.G.; Daniel, M.G.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Chen, C.; Bao, X.; Zhang, X.; Ling, X.; Lu, G. Microplastic pollution in an urbanized river affected by water diversion: Combining with active biomonitoring. J. Hazard. Mater. 2021, 417, 126058. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; et al. Current progress on plastic/microplastic degradation: Fact influences and mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Jiang, Z.; Liu, Y.; Zhao, X.; Liang, Y.; Lu, W.; Song, J. Microplastic contamination assessment in water and economic fishes in different trophic guilds from an urban water supply reservoir after flooding. J. Environ. Manage. 2021, 299, 113667. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Gao, B.; Wan, X.; Peng, W.; Zhang, B. Influence of catastrophic flood on microplastics organization in surface water of the Three Gorges Reservoir, China. Water Res. 2022, 211, 118018. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cao, L.; Wang, Y.; Chen, W.; Li, Y.; Zhao, X. Sources and distribution of microplastics in the east China sea under a three-dimensional numerical modelling. Environ. Pollut. 2022, 311, 119910. [Google Scholar] [CrossRef]
- Taha, Z.D.; Amin, R.M.; Anuar, S.T.; Nasser, A.A.A.; Sohaimi, E.S. Microplastics in seawater and zooplankton: A case study from Terengganu estuary and offshore waters, Malaysia. Sci. Total Environ. 2021, 786, 147466. [Google Scholar] [CrossRef]
- Yasaka, S.; Pitaksanurat, S.; Laohasiriwong, W.; Neeratanaphan, L.; Jungoth, R.; Donprajum, T.; Taweetanawanit, P. Bioaccumulation of microplastics in fish and snails in the Nam Pong River, Khon Kaen, Thailand. EnvironmentAsia 2022, 15, 81–93. [Google Scholar]
- Kasamesiri, P.; Thaimuangphol, W. Microplastics ingestion by freshwater fish in the Chi River, Thailand. Int. J. GEOMATE 2020, 18, 114–119. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Pan, Z.; Sun, D.; Zhou, A.; Xie, S.; Wang, J.; Zou, J. Microplastics in wild freshwater fish of different feeding habits from Beijiang and Pearl River Delta regions south China. Chemosphere 2020, 258, 127345. [Google Scholar] [CrossRef]
- Bertoli, M.; Pastorino, P.; Lesa, D.; Renzi, M.; Anselmi, S.; Prearo, M.; Pizzul, E. Microplastics accumulation in functional feeding guilds and functional habit groups of freshwater macrobenthic invertebrates: Novel insights in a riverine ecosystem. Sci. Total Environ. 2022, 804, 150207. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, X.; Li, M.; Cai, Z.; Gong, H.; Yan, M. Microplastics as an aquatic pollution affect gut microbiota with aquatic animals. J. Hazard. Mater. 2022, 423, 127094. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.; Chatel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Elizalde-Velazquez, G.A.; Gomez-Olivan, L.M. Microplastics in aquatic environments: A review on occurrence, distribution, toxic effects, and implications for human health. Sci. Total Environ. 2021, 780, 146551. [Google Scholar] [CrossRef]
- Kumar, A.; Upadhyay, P.; Prajapati, S.K. Impact of microplastics on riverine greenhouse gas emissions: A view point. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Guo, Z.; Boeing, W.J.; Xu, Y.; Borgomeo, E.; Mason, S.A.; Zhu, Y.G. Global meta-analysis of microplastic contamination in reservoirs with a novel framework. Water Res. 2021, 207, 117828. [Google Scholar] [CrossRef]
- Mao, R.; Hu, Y.; Zhang, S.; Wu, R.; Guo, X. Microplastics in the surface water of Wuliangsuhai Lake, Northern China. Sci. Total Environ. 2020, 723, 137820. [Google Scholar] [CrossRef]
- Department of Fisheries. Statistics of the Freshwater Animals Captured from Natural Sources in 2020. Available online: https://www4.fisheries.go.th/doffile/fkey/ref84142 (accessed on 13 January 2022).
- Chaleeraktrakoon, C.; Chinsomboon, Y. Dynamic rule curves for flood control of a multipurpose dam. J. Hydro-Environ. Res. 2015, 9, 133–144. [Google Scholar] [CrossRef]
- Thaiwater. Available online: https://tiwrm.hii.or.th (accessed on 13 January 2022).
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresum 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Di, M.; Wang, J. Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 616–617, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Di, M.; Liu, X.; Wang, W.; Wang, J. Pollution in drinking water source area: Microplastics in the Danjiangkou Reservoir, China. Environ. Toxicol. Pharmacol. 2019, 65, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Pan, X.; Zhang, S.; Li, D.; Zhai, W.; Wang, Z.; Tao, J.; Mi, C.; Li, Q.; Crittenden, J.C. Distribution and source of microplastics in China’s second largest reservoir-Danjiangkou Reservoir. J. Environ. Sci. 2021, 102, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Tang, Y.; Dang, M.; Wang, S.; Jin, H.; Liu, Y.; Jing, H.; Zheng, C.; Yi, S.; Cai, Z. Spatial-temporal distribution of microplastics in surface water and sediments of Maozhou River within Guangong-Hong Kong-Macao Greater Bay Area. Sci. total Environ. 2020, 717, 135187. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gan, Y.; Dong, J.; Fang, J.; Chen, H.; Quan, Q.; Liu, J. Impact of microplastics on microbial community in sediments of the Huangjinxia Reservoir-water source of a water diversion project in Western China. Chemosphere 2020, 253, 126740. [Google Scholar] [CrossRef]
- Jian, M.; Zhang, Y.; Yang, W.; Zhou, L.; Lui, S.; Xu, E.G. Occurrence and distribution of microplastics in China’s largest freshwater lake system. Chemosphere 2020, 261, 128186. [Google Scholar] [CrossRef]
- Kasamesiri, P.; Meksumpun, C.; Meksumpun, S.; Ruengsorn, C. Assessment on microplastics contamination in freshwater fish: A case study of the Ubolratana Reservoir, Thailand. Int. J. GEOMATE 2021, 20, 62–68. [Google Scholar] [CrossRef]
- Saliu, F.; Veronelli, M.; Raguso, C.; Barana, D.; Galli, P. The release process of microfibers: From surgical face masks into the marine environment. Environ. Adv. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Ray, S.S.; Lee, H.K.; Huyen, D.T.T.; Chen, S.S.; Kwon, Y.N. Microplastics waste in environment: A perspective on recycling issues from PPE kits and face masks during the COVID-19 pandemic. Environ. Technol. Innov. 2022, 26, 102290. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Lee, S.H.; Lee, M.S.; Lee, J.K.; Lee, S.H.; Zoh, K.D. Occurrence of microplastics in the Han River and riverine fish in South Korea. Sci. Total Environ. 2020, 708, 134535. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO Fisheries and Aquaculture Technical Paper 615; FAO: Roma, Italy, 2017; 126p.
- Singh, R.; Kumar, N.; Mehrotra, T.; Bisaria, K.; Sinha, S. Environmental hazards and biodegradation of plastic waste: Challenges and future prospects. In Bioremediation for Environmental Sustainability: Toxicity, Mechanisms of Contaminants Degradation, Detoxification and Challenges; Saxena, G., Kumer, V., Shah, M.P., Eds.; Elsevier Inc.: New York, NY, USA, 2020; pp. 193–214. [Google Scholar] [CrossRef]
- Fadare, O.O.; Okoffo, E.D. COVID-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef] [PubMed]
- Leiser, R.; Wu, G.M.; Neu, T.R.; Wedt-Potthoff, K. Biofouling, metal sorption and aggregation are related to sinking of microplastics in a stratified reservoir. Water Res. 2020, 176, 115748. [Google Scholar] [CrossRef]
- Xu, P.; Peng, G.; Su, L.; Gao, Y.; Gao, L.; Li, D. Microplastic risk assessment in surface waters: A case study in the Changjiang Estuary, China. Mar. Pollut. Bull. 2018, 133, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Pico, Y.; Soursou, V.; Alfarhan, A.H.; El-Sheikh, M.A.; Barcelo, D. First evidence of microplastics occurrence in mixed surface and treated wastewater from two major Saudi Arabian cities and assessment of their ecological risk. J. Hazard. Mater. 2021, 416, 125747. [Google Scholar] [CrossRef] [PubMed]
- Bian, P.; Liu, Y.; Zhao, K.; Hu, Y.; Zhang, J.; Kang, L.; Shen, W. Sptial variability of microplastic pollution on surface of rivers in a mountain-plain transitional area: A case study in the Chin Ling-Wei River Plain, China. Ecotoxicol. Environ. Saf. 2022, 232, 113298. [Google Scholar] [CrossRef]
- Ranjani, M.; Veerasingam, S.; Venkatachalapathy, R.; Mugilarasan, M.; Bagaev, A.; Mukhanov, V.; Vethamony, P. Assessment of potential ecological risk of microplastics in the coastal sediments of India: A meta-analysis. Mar. Pollut. Bull. 2021, 163, 111969. [Google Scholar] [CrossRef]


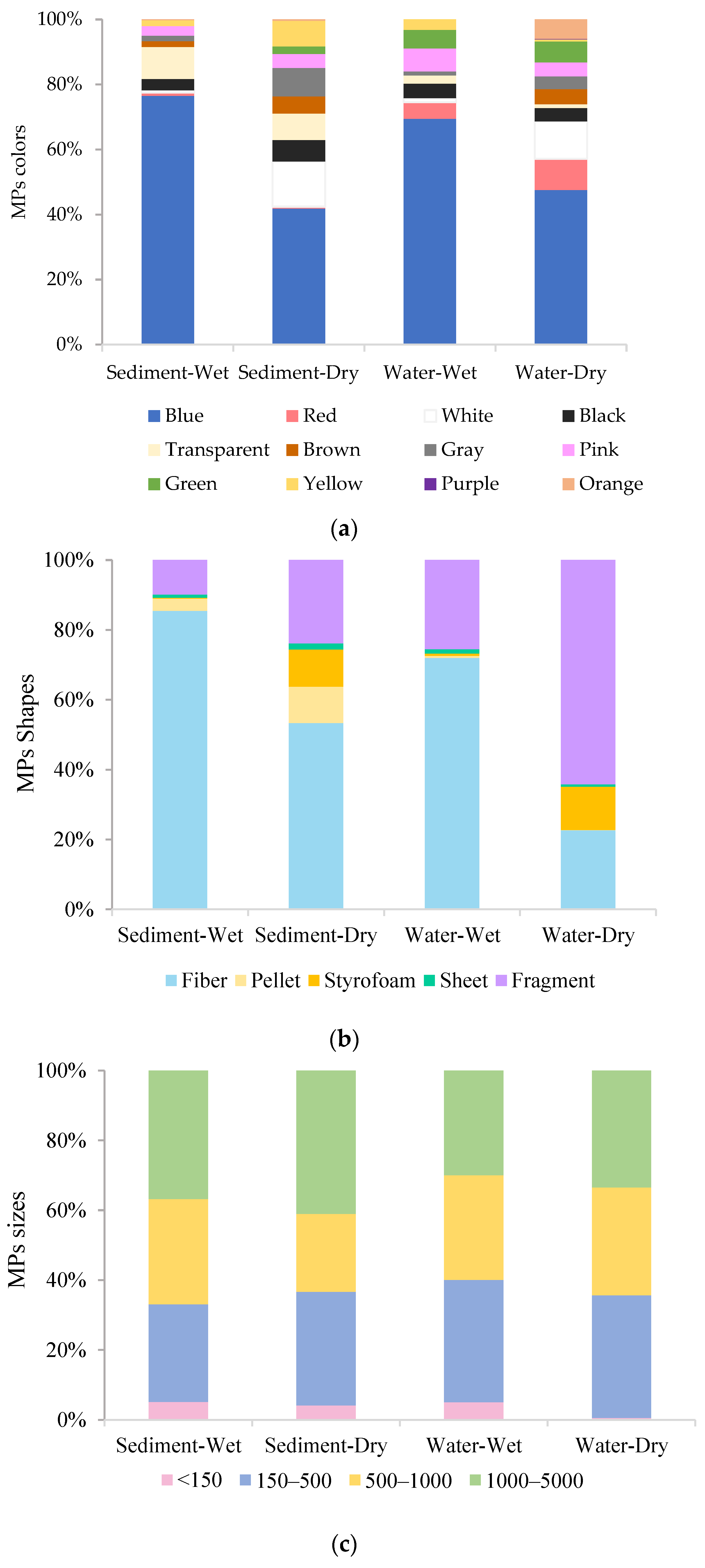
| Sample Location | Environmental Media | Abundance Range | Reference |
|---|---|---|---|
| Dafangying Reservoir, China | Water | 8800–32,050 particles/m3 | [7] |
| Three Gorges Reservoir, China | Water | 974–21,132 particles/m3 | [8] |
| Wuliangsuhai Lake, Mongolia | Water | 3120–11,250 particles/m3 | [20] |
| Three Gorges Reservoir, China | Water | 1597–12,611 particles/m3 | [26] |
| Sediment | 25–300 particles/kg | ||
| Danjiangkou Reservoir, China | Water | 467–15,017 particles/m3 | [27] |
| Sediment | 15–40 particles/kg | ||
| Danjiangkou Reservoir, China | Water | 530–24,798 particles/m3 | [28] |
| Sediment | 708–3237 particles/kg | ||
| Ubolratana Reservoir, Thailand | Water | 25–3363 particles/m3 | This study |
| Sediment | 6–81 particles/kg |
| Polymer Hazard Index | Risk Level Criteria | PLI | Risk Level Criteria | |
|---|---|---|---|---|
| Sediment-Wet | 322 | III | 3 | I |
| Sediment-Dry | 1510 | IV | 3 | I |
| Water-Wet | 160 | III | 16 | II |
| Water-Dry | 64 | II | 28 | III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasamesiri, P.; Panchan, R.; Thaimuangphol, W. Spatial–Temporal Distribution and Ecological Risk Assessment of Microplastic Pollution of Inland Fishing Ground in the Ubolratana Reservoir, Thailand. Water 2023, 15, 330. https://doi.org/10.3390/w15020330
Kasamesiri P, Panchan R, Thaimuangphol W. Spatial–Temporal Distribution and Ecological Risk Assessment of Microplastic Pollution of Inland Fishing Ground in the Ubolratana Reservoir, Thailand. Water. 2023; 15(2):330. https://doi.org/10.3390/w15020330
Chicago/Turabian StyleKasamesiri, Pattira, Ruamruedee Panchan, and Wipavee Thaimuangphol. 2023. "Spatial–Temporal Distribution and Ecological Risk Assessment of Microplastic Pollution of Inland Fishing Ground in the Ubolratana Reservoir, Thailand" Water 15, no. 2: 330. https://doi.org/10.3390/w15020330







