Using Single-Species and Whole Community Stream Mesocosm Exposures for Identifying Major Ion Effects in Doses Mimicking Resource Extraction Wastewaters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Stream Mesocosm Set-Up
2.3. Colonizing the Mesocosm Biotic Communities
2.4. Background and Dosing Water Chemistries
2.5. Assessing Biotic Communities
2.6. Single-Species Tests
2.7. Data Analysis
3. Results
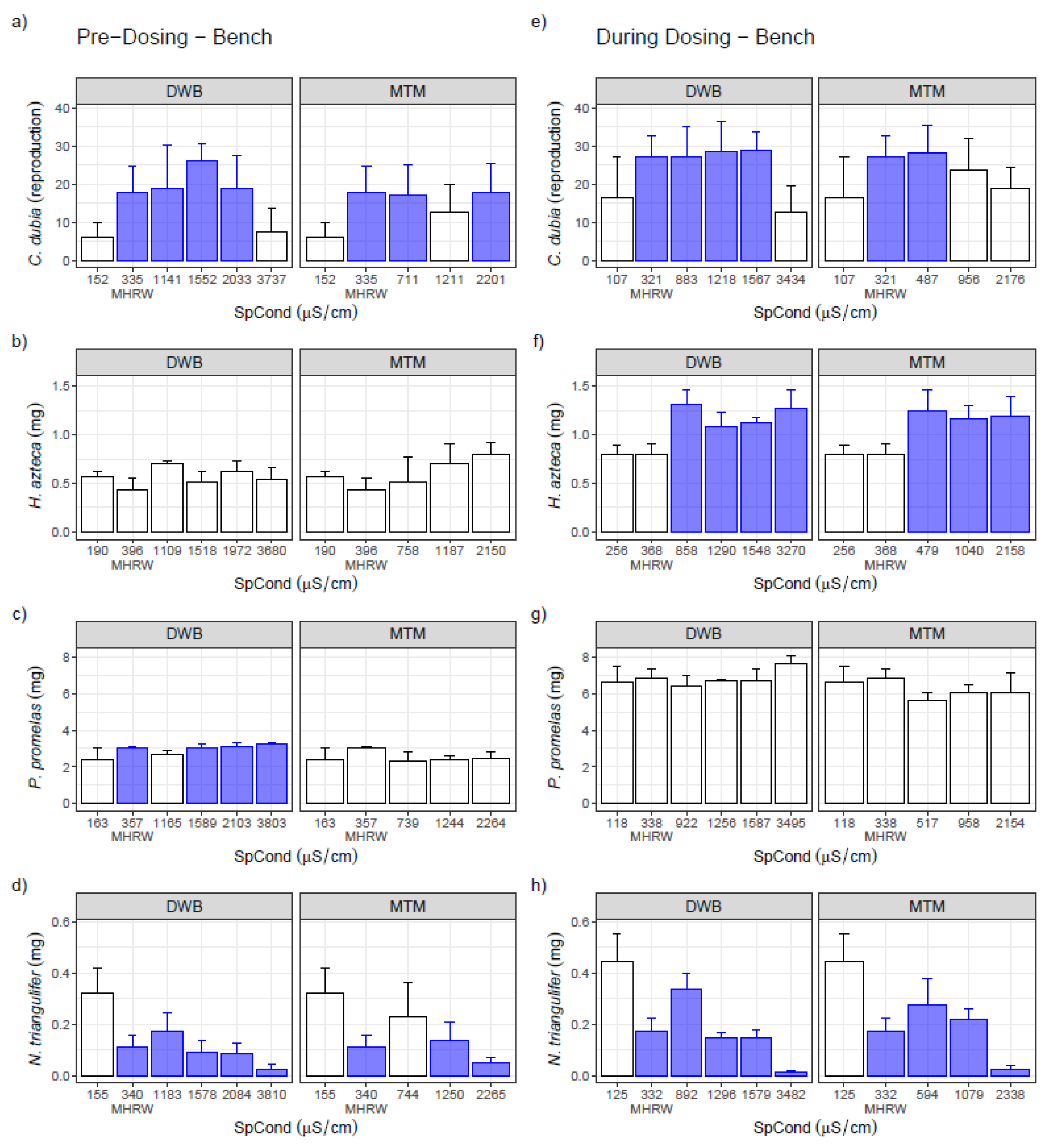
3.1. Single-Species Bench Tests
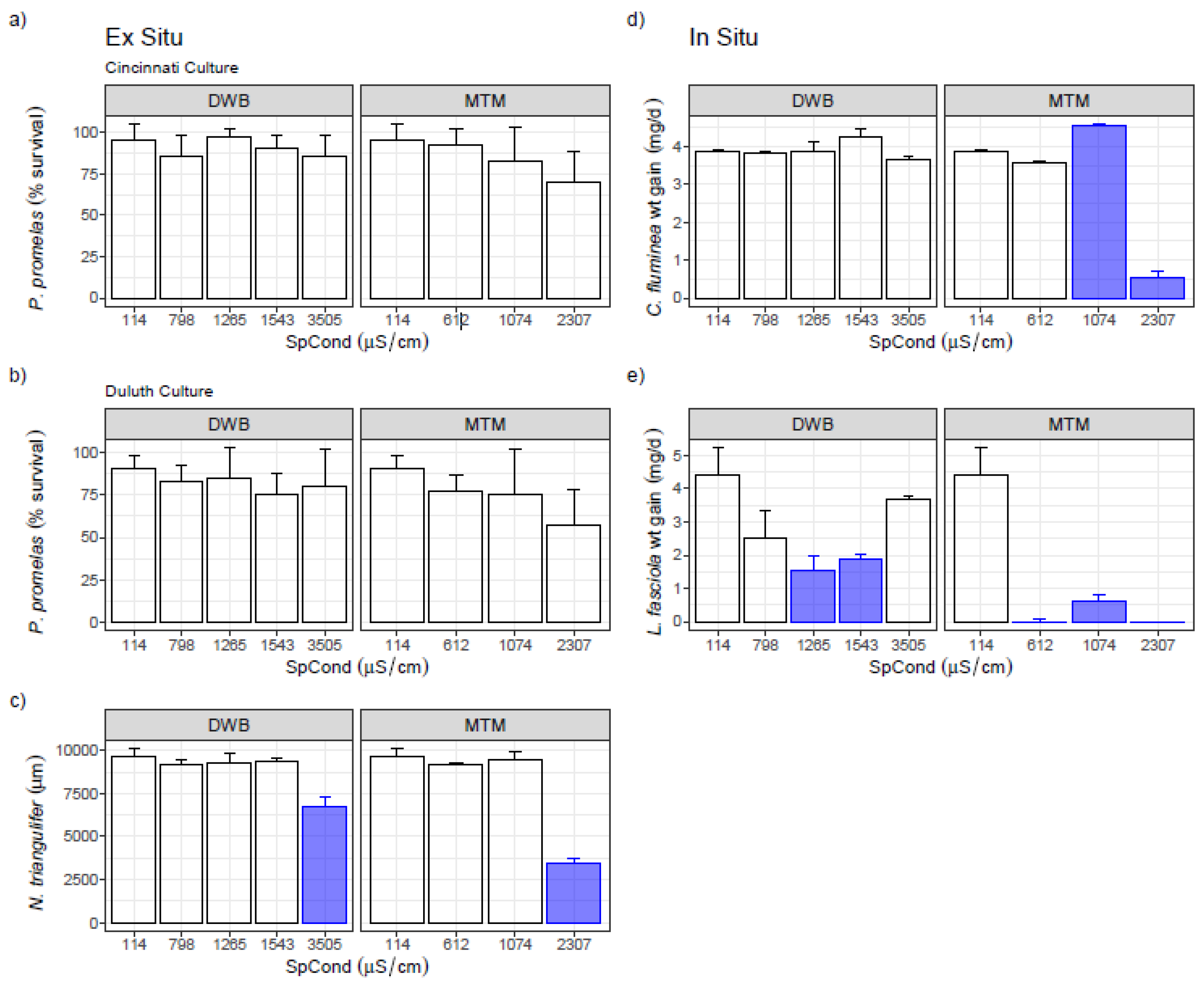
3.2. Single-Species Ex Situ/In Situ Tests
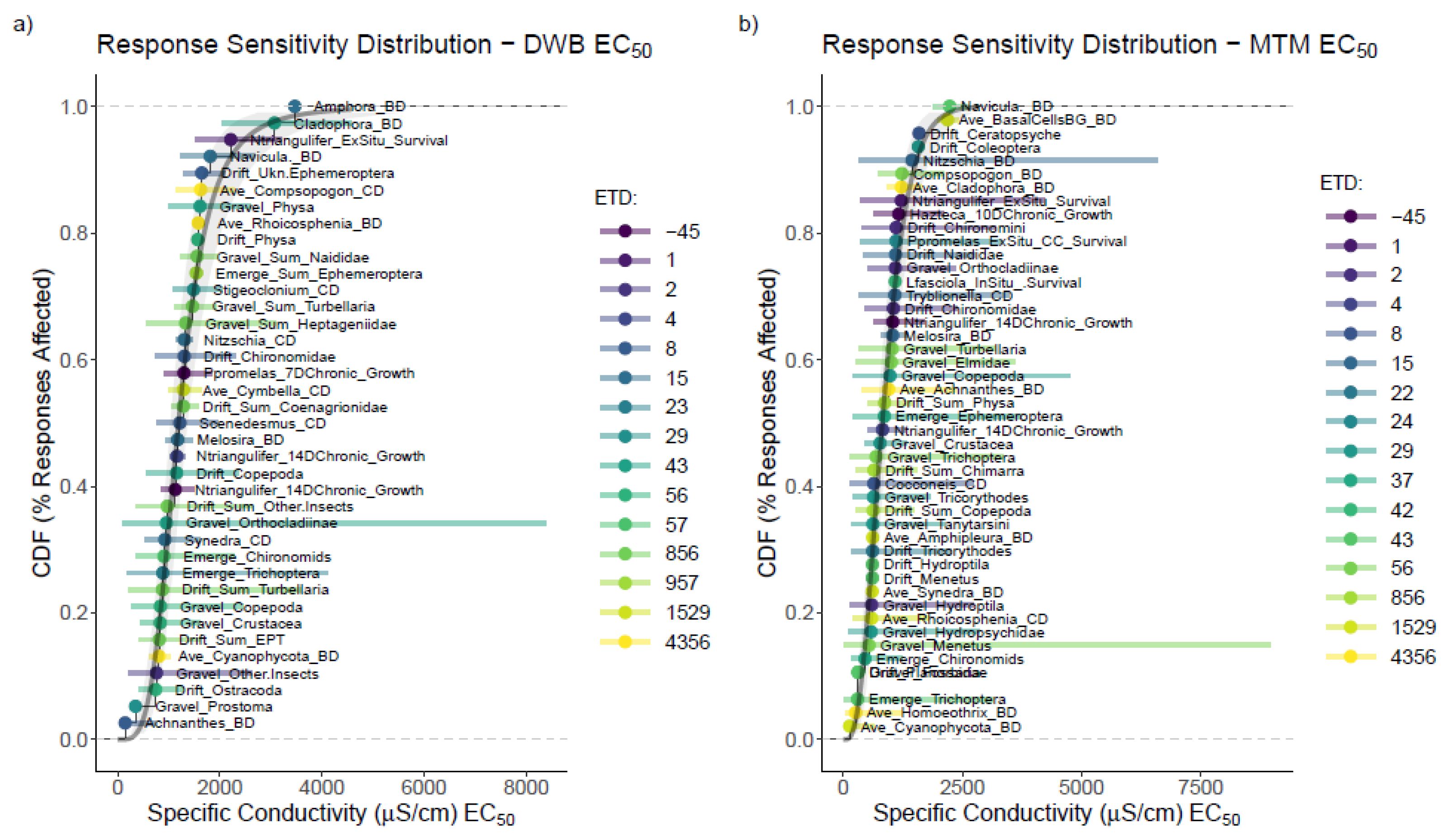
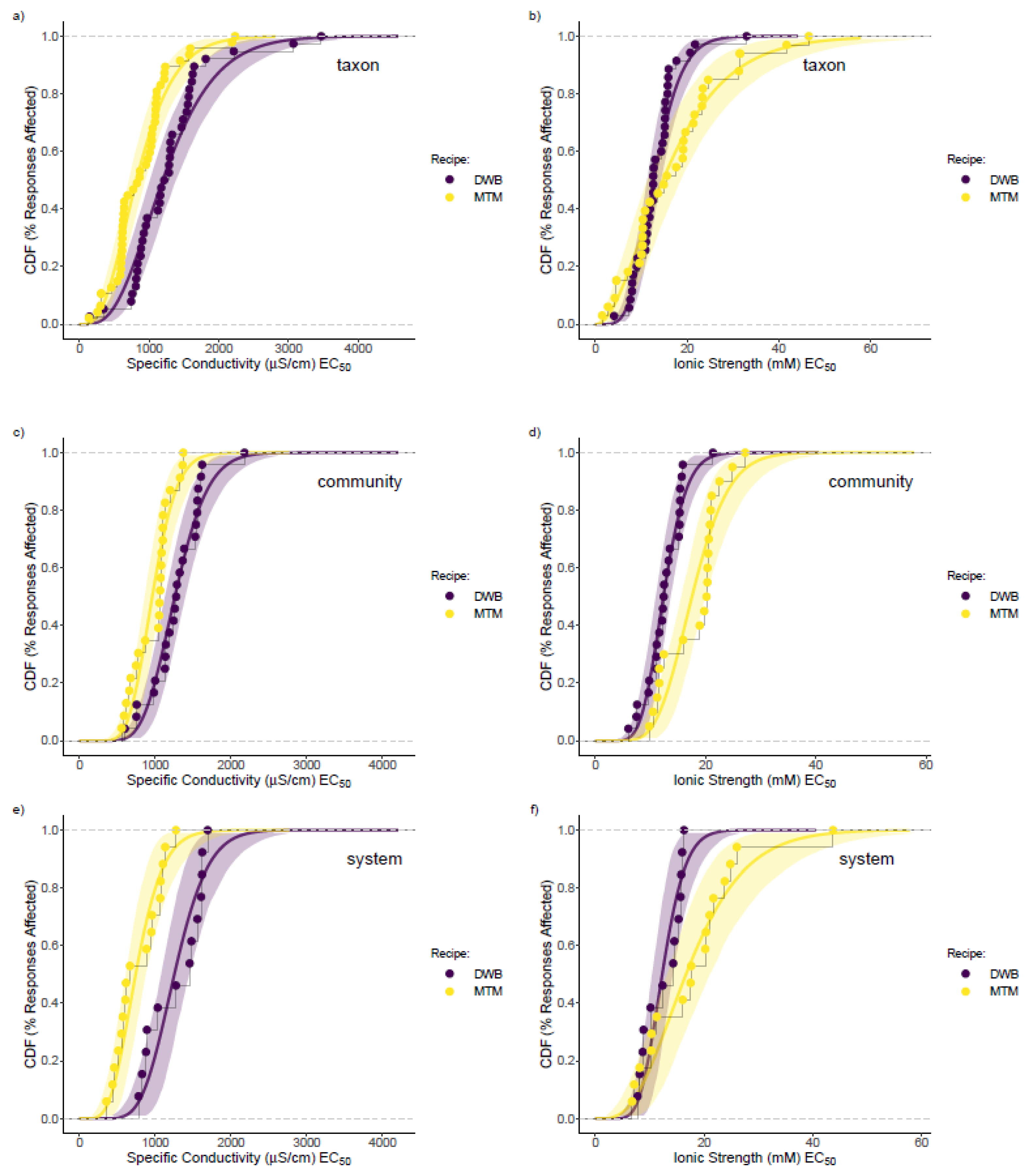
3.3. Mesocosm Taxa, Community, and System-Level Dose–Responses
3.4. Dosing Effects on Mesocosm Communities and Systems through Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodfellow, W.L.; Ausley, L.W.; Burton, D.T.; Denton, D.L.; Dorn, P.B.; Grothe, D.R.; Heber, M.A.; Norberg-King, T.J.; Rodgers, J.H. Major ion toxicity in effluents: A review with permitting recommendations. Environ. Toxicol. Chem. 2000, 19, 175–182. [Google Scholar] [CrossRef]
- Govenor, H.; Krometis, L.A.H.; Willis, L.; Angermeier, P.L.; Hession, W.C. Macroinvertebrate sensitivity thresholds for sediment in Virginia streams. Integr. Environ. Assess. Manag. 2019, 15, 77–92. [Google Scholar] [CrossRef]
- Cook, N.A.; Sarver, E.A.; Krometis, L.H.; Huang, J. Habitat and water quality as drivers of ecological system health in Central Appalachia. Ecol. Eng. 2015, 84, 180–189. [Google Scholar] [CrossRef]
- Timpano, A.J.; Schoenholtz, S.H.; Soucek, D.J.; Zipper, C.E. Benthic macroinvertebrate community response to salinization in headwater streams in Appalachia USA over multiple years. Ecol. Indic. 2018, 91, 645–656. [Google Scholar] [CrossRef]
- Clements, W.H.; Kotalik, C. Effects of major ions on natural benthic communities: An experimental assessment of the US Environmental Protection Agency aquatic life benchmark for conductivity. Freshw. Sci. 2016, 35, 126–138. [Google Scholar] [CrossRef]
- Bradley, T.J. Animal Osmoregulation; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Kefford, B.J. Why are mayflies (Ephemeroptera) lost following small increases in salinity? Three conceptual osmophysiological hypotheses. Philos. Trans. R. Soc. B 2019, 374, 20180021. [Google Scholar] [CrossRef] [Green Version]
- Kefford, B.J.; Hickey, G.L.; Gasith, A.; Ben-David, E.; Dunlop, J.E.; Palmer, C.G.; Allan, K.; Choy, S.C.; Piscart, C. Global Scale Variation in the Salinity Sensitivity of Riverine Macroinvertebrates: Eastern Australia, France, Israel and South Africa. PLoS ONE 2012, 7, e35224. [Google Scholar] [CrossRef]
- Nowghani, F.; Chen, C.C.; Jonusaite, S.; Watson-Leung, T.; Kelly, S.P.; Donini, A. Impact of salt-contaminated freshwater on osmoregulation and tracheal gill function in nymphs of the mayfly Hexagenia rigida. Aquat. Toxicol. 2019, 211, 92–104. [Google Scholar] [CrossRef]
- Kefford, B.J.; Buchwalter, D.; Cañedo-Argüelles, M.; Davis, J.; Duncan, R.P.; Hoffmann, A.; Thompson, R. Salinized rivers: Degraded systems or new habitats for salt-tolerant faunas? Biol. Lett. 2016, 12, 20151072. [Google Scholar] [CrossRef] [Green Version]
- Miltner, R. Assessing the Impacts of Chloride and Sulfate Ions on Macroinvertebrate Communities in Ohio Streams. Water 2021, 13, 1815. [Google Scholar] [CrossRef]
- Burton, G.A.J.; Basu, N.; Ellis, B.R.; Kapo, K.E.; Entrekin, S.; Nadelhoffer, K. Hydraulic “Fracking”: Are Surface Water Impacts An Ecological Concern? Environ. Toxicol. Chem. 2014, 33, 1679–1689. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.B.; Webster, J.R. The Role of Macroinvertebrates in Stream Ecosystem Function. Annu. Rev. Entomol. 1996, 41, 115–139. [Google Scholar] [CrossRef]
- Fahrenfeld, N.L.; Delos Reyes, H.; Eramo, A.; Akob, D.M.; Mumford, A.C.; Cozzarelli, I.M. Shifts in microbial community structure and function in surface waters impacted by unconventional oil and gas wastewater revealed by metagenomics. Sci. Total Environ. 2017, 580, 1205–1213. [Google Scholar] [CrossRef]
- Olson, J.R.; Hawkins, C.P. Effects of total dissolved solids on growth and mortality predict distributions of stream macroinvertebrates. Freshw. Biol. 2017, 62, 779–791. [Google Scholar] [CrossRef]
- Potapova, M. Patterns of Diatom Distribution in Relation to Salinity. In The Diatom World; Seckbach, J., Kociolek, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 313–332. [Google Scholar]
- Griffith, M.B.; Zheng, L.; Cormier, S.M. Using extirpation to evaluate ionic tolerance of freshwater fish. Environ. Toxicol. Chem. 2018, 37, 871–883. [Google Scholar] [CrossRef]
- Griffith, M.B. Natural variation and current reference for specific conductivity and major ions in wadeable streams of the conterminous USA. Freshw. Sci. 2014, 33, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Cormier, S.M.; Zheng, L.; Flaherty, C. Field-based method for evaluating the annual maximum specific conductivity tolerated by freshwater invertebrates. Sci. Total Environ. 2018, 633, 1637–1646. [Google Scholar] [CrossRef]
- Mount, D.R.; Erickson, R.J.; Highland, T.L.; Hockett, J.R.; Hoff, D.J.; Jenson, C.T.; Norberg-King, T.J.; Peterson, K.N.; Polaske, Z.M.; Wisniewski, S. The acute toxicity of major ion salts to Ceriodaphnia dubia: I. influence of background water chemistry. Environ. Toxicol. Chem. 2016, 35, 3039–3057. [Google Scholar] [CrossRef]
- Cormier, S.M.; Zheng, L.; Flaherty, C.M. A field-based model of the relationship between extirpation of salt-intolerant benthic invertebrates and background conductivity. Sci. Total Environ. 2018, 633, 1629–1636. [Google Scholar] [CrossRef]
- Olmstead, S.M.; Muehlenbachs, L.A.; Shih, J.-S.; Chu, Z.; Krupnick, A.J. Shale gas development impacts on surface water quality in Pennsylvania. Proc. Natl. Acad. Sci. USA 2013, 110, 4962–4967. [Google Scholar] [CrossRef]
- PACode. Chapter 95. Wastewater Treatment Requirements. Available online: https://www.pacodeandbulletin.gov/Display/pacode?file=/secure/pacode/data/025/chapter95/chap95toc.html&d=reduce (accessed on 25 September 2022).
- ORSANCO. Pollution Control Standards for Discharges to the Ohio River; Ohio River Valley Water Sanitation Commission: Cincinnati, OH, USA, 2011. [Google Scholar]
- Pond, G.J. Patterns of Ephemeroptera taxa loss in Appalachian headwater streams (Kentucky, USA). Hydrobiologia 2010, 641, 185–201. [Google Scholar] [CrossRef]
- Pond, G.J.; Passmore, M.E.; Pointon, N.D.; Felbinger, J.K.; Walker, C.A.; Krock, K.J.; Fulton, J.B.; Nash, W.L. Long-term impacts on macroinvertebrates downstream of reclaimed mountaintop mining valley fills in Central Appalachia. Environ. Manag. 2014, 54, 919–933. [Google Scholar] [CrossRef]
- U.S. EPA. A Field-Based Aquatic Life Benchmark for Conductivity in Central Appalachian Streams; U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- Cañedo-Argüelles, M.; Grantham, T.E.; Perrée, I.; Rieradevall, M.; Céspedes-Sánchez, R.; Prat, N. Response of stream invertebrates to short-term salinization: A mesocosm approach. Environ. Pollut. 2012, 166, 144–151. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M.; Bundschuh, M.; Gutiérrez-Cánovas, C.; Kefford, B.J.; Prat, N.; Trobajo, R.; Schäfer, R.B. Effects of repeated salt pulses on ecosystem structure and functions in a stream mesocosm. Sci. Total Environ. 2014, 476–477, 634–642. [Google Scholar] [CrossRef]
- Entrekin, S.A.; Maloney, K.O.; Kapo, K.E.; Walters, A.W.; Evans-White, M.A.; Klemow, K.M. Stream Vulnerability to Widespread and Emergent Stressors: A Focus on Unconventional Oil and Gas. PLoS ONE 2015, 10, e0137416. [Google Scholar] [CrossRef]
- Krupnick, A.J. Managing the Risks of Shale Gas: Identifiying a Pathway toward Reponsibile Development: A Review of Shale Gas Regulations by State; Center for Energy Economics and Policy: Washington, DC, USA, 2012. [Google Scholar]
- Posthuma, L.; Suter, G.W., II; Traas, T.P. Species Sensitivity Distributions in Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Stephan, C.E.; Mount, D.I.; Hansen, D.J.; Gentile, J.; Chapman, G.A.; Brungs, W.A. Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses; U.S. Environmental Protection Agency: Duluth, MN, USA, 1985.
- Soucek, D.J.; Linton, T.K.; Tarr, C.D.; Dickinson, A.; Wickramanayake, N.; Delos, C.G.; Cruz, L.A. Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates. Environ. Toxicol. Chem. 2011, 30, 930–938. [Google Scholar] [CrossRef]
- Erickson, R.J.; Mount, D.R.; Highland, T.L.; Hockett, J.R.; Hoff, D.J.; Jenson, C.T.; Norberg-King, T.J.; Forsman, B. Acute Toxicity of Major Geochemical Ions to Fathead Minnows (Pimephales promelas): Part A—Observed Relationships for Individual Salts and Salt Mixtures. Environ. Toxicol. Chem. 2022, 41, 2078–2094. [Google Scholar] [CrossRef]
- Cormier, S.M.; Suter, G.W.; Zheng, L.; Pond, G.J. Assessing causation of the extirpation of stream macroinvertebrates by a mixture of ions. Environ. Toxicol. Chem. 2013, 32, 277–287. [Google Scholar] [CrossRef]
- Kefford, B.J. Rapid Tests for Community-Level Risk Assessments in Ecotoxicology. In Encyclopedia of Aquatic Ecotoxicology; Férard, J.-F., Blaise, C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 957–966. [Google Scholar]
- Brent, R.N.; Kunkel, J.; Tomek, Z.; Buchardt, D.; DeLisle, P.F.; Sivers, S. A Novel Approach to Developing Thresholds for Total Dissolved Solids Using Standardized and Experimental Toxicity Test Methods. Environ. Toxicol. Chem. 2022, 41, 2782–2796. [Google Scholar] [CrossRef]
- Hintz, W.D.; Mattes, B.M.; Schuler, M.S.; Jones, D.K.; Stoler, A.B.; Lind, L.; Relyea, R.A. Salinization triggers a trophic cascade in experimental freshwater communities with varying food-chain length. Ecol. Appl. 2017, 27, 833–844. [Google Scholar] [CrossRef]
- Mooney, T.J.; McCullough, C.D.; Jansen, A.; Chandler, L.; Douglas, M.; Harford, A.J.; van Dam, R.; Humphrey, C. Elevated Magnesium Concentrations Altered Freshwater Assemblage Structures in a Mesocosm Experiment. Environ. Toxicol. Chem. 2020, 39, 1973–1987. [Google Scholar] [CrossRef]
- Haluszczak, L.O.; Rose, A.W.; Kump, L.R. Geochemical evaluation of flowback brine from Marcellus gas wells in Pennsylvania, USA. Appl. Geochem. 2013, 28, 55–61. [Google Scholar] [CrossRef]
- Sauer, T.C.; Costa, H.J.; Brown, J.S.; Ward, T.J. Toxicity identification evaluations of produced-water effluents. Environ. Toxicol. Chem. 1997, 16, 2020–2028. [Google Scholar] [CrossRef]
- Dresel, P.E.; Rose, A.W. Chemistry and Origin of Oil and Gas Well Brines in Western Pennsylvania; 4th Ser., Open-File Report OFOG 10-01.0; Pennsylvania Geological Survey: Harrisburg, PA, USA, 2010; 48p. [Google Scholar]
- Osborn, S.G.; McIntosh, J.C. Chemical and isotopic tracers of the contribution of microbial gas in Devonian organic-rich shales and reservoir sandstones, northern Appalachian Basin. Appl. Geochem. 2010, 25, 456–471. [Google Scholar] [CrossRef]
- Hayes, T. Sampling and Analysis of Water Streams Associated with the Development of Marcellus Shale Gas; Gas Technology Institute Report 2009; Marcellus Shale Coalition: Pittsburgh, PA, USA, 2009; 49p. [Google Scholar]
- Stout, W.; Lamborn, R.E.; Schaaf, D. Brines of Ohio (a Preliminary Report); 4th Series, Bulletin 37; Geological Survey of Ohio: Columbus, OH, USA, 1932; 35p. [Google Scholar]
- Breen, K.J.; Angelo, C.G.; Masters, R.W.; Sedam, A.C. Chemical and Isotopic Characteristics of Brines from Three Oil- and Gas-Producing Sandstones in Eastern Ohio, with Applications to the Geochemical Tracing of Brine Sources; 84-4314; US Department of the Interior, Geological Survey: Reston, VA, USA, 1985.
- USEPA. Proceedings of the Technical Workshops for the Hydrualic Fracturing Study: Water Resources Management; EPA/600/R-11/048; U.S. Environmental Protection Agency, Office of Research and Development: Washington, DC, USA, 2011; 125p.
- USEPA. The Effects of Moutaintop Mines and Valley Fills on Aquatic Ecosystems of the Central Appalachian Coalfields; EPA/600/R-09/138F; U.S. Environmental Protection Agency: Washington, DC, USA, 2011; 153p.
- Pond, G.J.; Passmore, M.E.; Borsuk, F.A.; Reynolds, L.; Rose, C.J. Downstream effects of mountaintop coal mining: Comparing biological conditions using family- and genus-level macroinvertebrate bioassessment tools. J. North Am. Benthol. Soc. 2008, 27, 717–737. [Google Scholar] [CrossRef]
- Fritz, K.M.; Fulton, S.; Johnson, B.R.; Barton, C.D.; Jack, J.D.; Word, D.A.; Burke, R.A. Structural and functional characteristics of natural and constructed channels draining a reclaimed mountaintop removal and valley fill coal mine. J. North Am. Benthol. Soc. 2010, 29, 673–689. [Google Scholar] [CrossRef] [Green Version]
- Hartman, K.J.; Kaller, M.D.; Howell, J.W.; Sweka, J.A. How much do valley fills influence headwater streams? Hydrobiologia 2005, 532, 91. [Google Scholar] [CrossRef]
- Bryant, L.D.; McPhilliamy, S.; Childers, H. Draft Programmatic Environmental Impact Statement on Mountaintop-mining/Valley Fills in Appalachia—2003: A Survey of the Water Quality of Streams in the Primary Region of Mountaintop/Valley Fill Coal Mining, October 1999 to January 2001; U.S. Environmental Protection Agency, Region 3: Philadelphia, PA, USA, 2002.
- Griffith, M.B.; Norton, S.B.; Alexander, L.C.; Pollard, A.I.; LeDuc, S.D. The effects of mountaintop mines and valley fills on the physicochemical quality of stream ecosystems in the central Appalachians: A review. Sci. Total Environ. 2012, 417, 1–12. [Google Scholar] [CrossRef]
- Kunz, J.L.; Conley, J.M.; Buchwalter, D.B.; Norberg-King, T.J.; Kemble, N.E.; Wang, N.; Ingersoll, C.G. Use of reconstituted waters to evaluate effects of elevated major ions associated with mountaintop coal mining on freshwater invertebrates. Environ. Toxicol. Chem. 2013, 32, 2826–2835. [Google Scholar] [CrossRef]
- U.S. EPA. Environmental Measurements and Modeling: Collection of Methods. Available online: https://www.epa.gov/cwa-methods (accessed on 10 January 2014).
- U.S. EPA. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, 4th ed.; EPA-821-R-02-013; U.S. EPA Office of Water: Washington, DC, USA, 2002.
- Van den Brink, P.J.; Braak, C.J.F.T. Principal response curves: Analysis of time-dependent multivariate responses of biological community to stress. Environ. Toxicol. Chem. 1999, 18, 138–148. [Google Scholar] [CrossRef]
- Rao, C.R. The Use and Interpretation of Principal Component Analysis in Applied Research. Sankhyā: The Indian J. Stat. 1964, 26, 329–358. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.B.; Simpson, G.; Solymos, P.; et al. R Package ‘Vegan’: Community Ecology Package, version 2.5—7, 28 November 2020. 2020. Available online: https://cran.r-project.org; https://github.com/vegandevs/vegan (accessed on 22 October 2022).
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2016, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
- U.S. EPA. Study of Oil and Gas Extraction Wastewater Management Under the Clean Water Act; EPA-821-R19-001; U.S. EPA Office of Water: Washington, DC, USA, 2019.
- Suter, G.W.; Cormier, S.M. A method for assessing the potential for confounding applied to ionic strength in central Appalachian streams. Environ. Toxicol. Chem. 2013, 32, 288–295. [Google Scholar] [CrossRef]
- Farrar, D.; Alexander, L.C.; Yuan, L.L.; Gerritsen, J. Regional Observational Studies: Addressing Confounding. In Ecological Causal Assessment; Norton, S.B., Cormier, S.M., Suter, G.W., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2014; p. 15. [Google Scholar]
- Ohio EPA. Biological and Water Quality Study of the East Fork Little Miami River and Select Tributaries 2012; Ohio Environmental Protection Agency: Columbus, OH, USA, 2014; p. 126.
- Erickson, R.J.; Mount, D.R.; Highland, T.L.; Hockett, J.R.; Hoff, D.J.; Jenson, C.T.; Norberg-King, T.J.; Peterson, K.N. The acute toxicity of major ion salts to Ceriodaphnia dubia. II. Empirical relationships in binary salt mixtures. Environ. Toxicol. Chem. 2017, 36, 1525–1537. [Google Scholar] [CrossRef]
- Mount, D.R.; Erickson, R.J.; Highland, T.L.; Hockett, J.R.; Hoff, D.J.; Jenson, C.T.; Norberg-King, T.J.; Forsman, B. Acute toxicity of major ions to Amphipod Hyalella azteca. Environ. Toxicol. Chem. in revision.
- Wang, N.; Ingersoll, C.G.; Kunz, J.L.; Brumbaugh, W.G.; Kane, C.M.; Evans, R.B.; Alexander, S.; Walker, C.; Bakaletz, S. Toxicity of sediments potentially contaminated by coal mining and natural gas extraction to unionid mussels and commonly tested benthic invertebrates. Environ. Toxicol. Chem. 2013, 32, 207–221. [Google Scholar] [CrossRef]
- Wang, N.; Dorman, R.A.; Ingersoll, C.G.; Hardesty, D.K.; Brumbaugh, W.G.; Hammer, E.J.; Bauer, C.R.; Mount, D.R. Acute and chronic toxicity of sodium sulfate to four freshwater organisms in water-only exposures. Environ. Toxicol. Chem. 2016, 35, 115–127. [Google Scholar] [CrossRef]
- Suter, I.; Traas, T.; Posthuma, L. Issues and practices in the derivation and use of species sensitivity distributions. In Species Sensitivity Distributions in Ecotoxicology; Posthuma, L., Suter, G.W.I., Traas, T., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 437–474. [Google Scholar]
- Buchwalter, D.; Scheibener, S.; Chou, H.; Soucek, D.; Elphick, J. Are sulfate effects in the mayfly Neocloeon triangulifer driven by the cost of ion regulation? Phil. Trans. R. Soc. 2019, 374, 20180013. [Google Scholar] [CrossRef] [Green Version]
- Griffith, M.B. Toxicological perspective on the osmoregulation and ionoregulation physiology of major ions by freshwater animals: Teleost fish, crustacea, aquatic insects, and Mollusca. Environ. Toxicol. Chem. 2017, 36, 576–600. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. National Recommended Water Quality Criteria—Aquatic Life Criteria Table. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 7 July 2022).
- Hem, D. Study and Interpretation the Chemical of Natural of Characteristics Natural Water, 3rd ed.; USGS Water Supply Paper 2254 66-69; U.S. Government Publishing Office: Washington, DC, USA, 1985.
- Rusydi, A.F. Correlation between conductivity and total dissolved solid in various type of water: A review. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012019. [Google Scholar] [CrossRef]
- Miller, R.L.; Bradford, W.L.; Peters, N.E. Specific Conductance: Theoretical Considerations and Application to Analytical Quality Control; Water-Supply Paper 2311; U.S. Geological Survey: Washington, DC, USA, 1986.

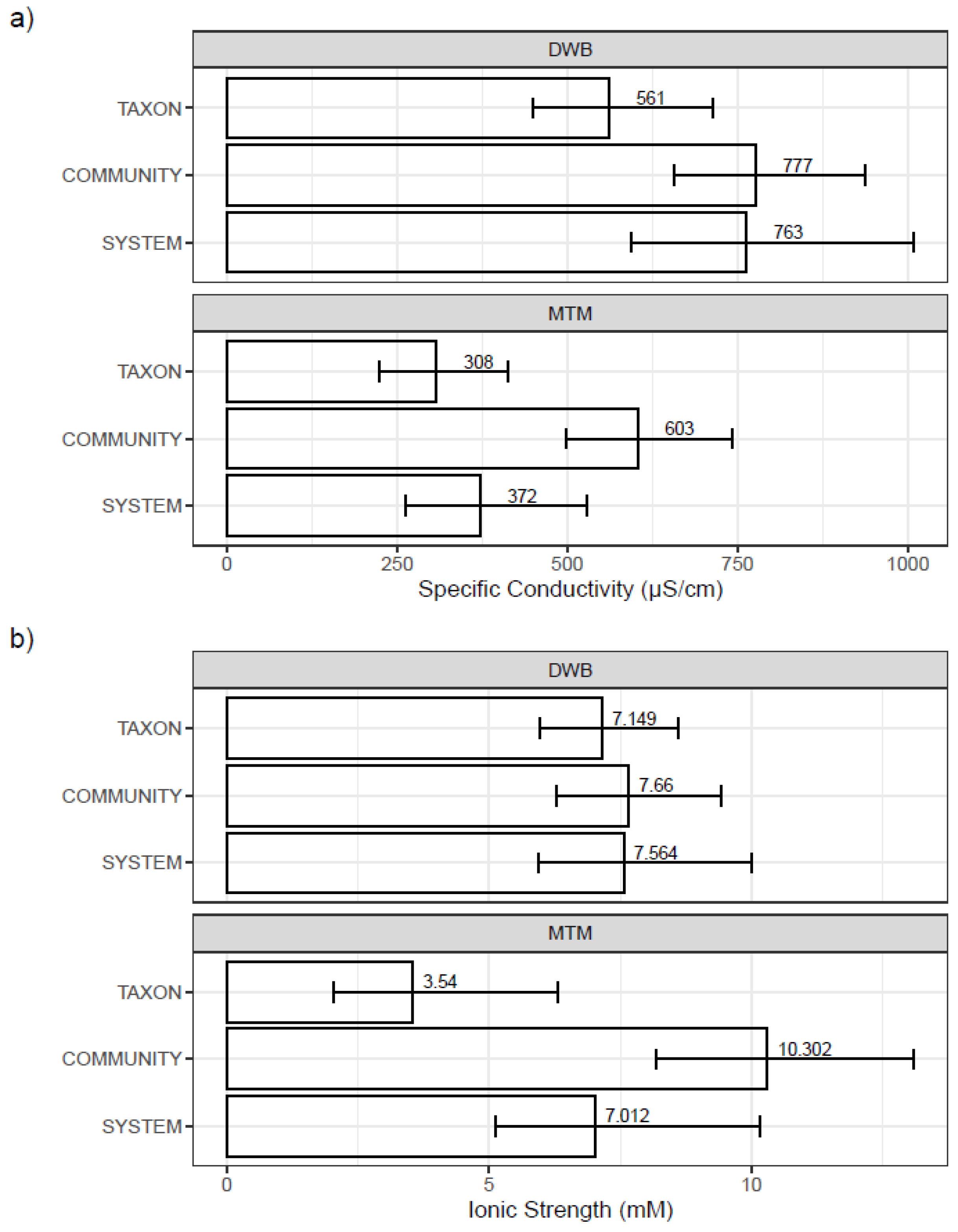
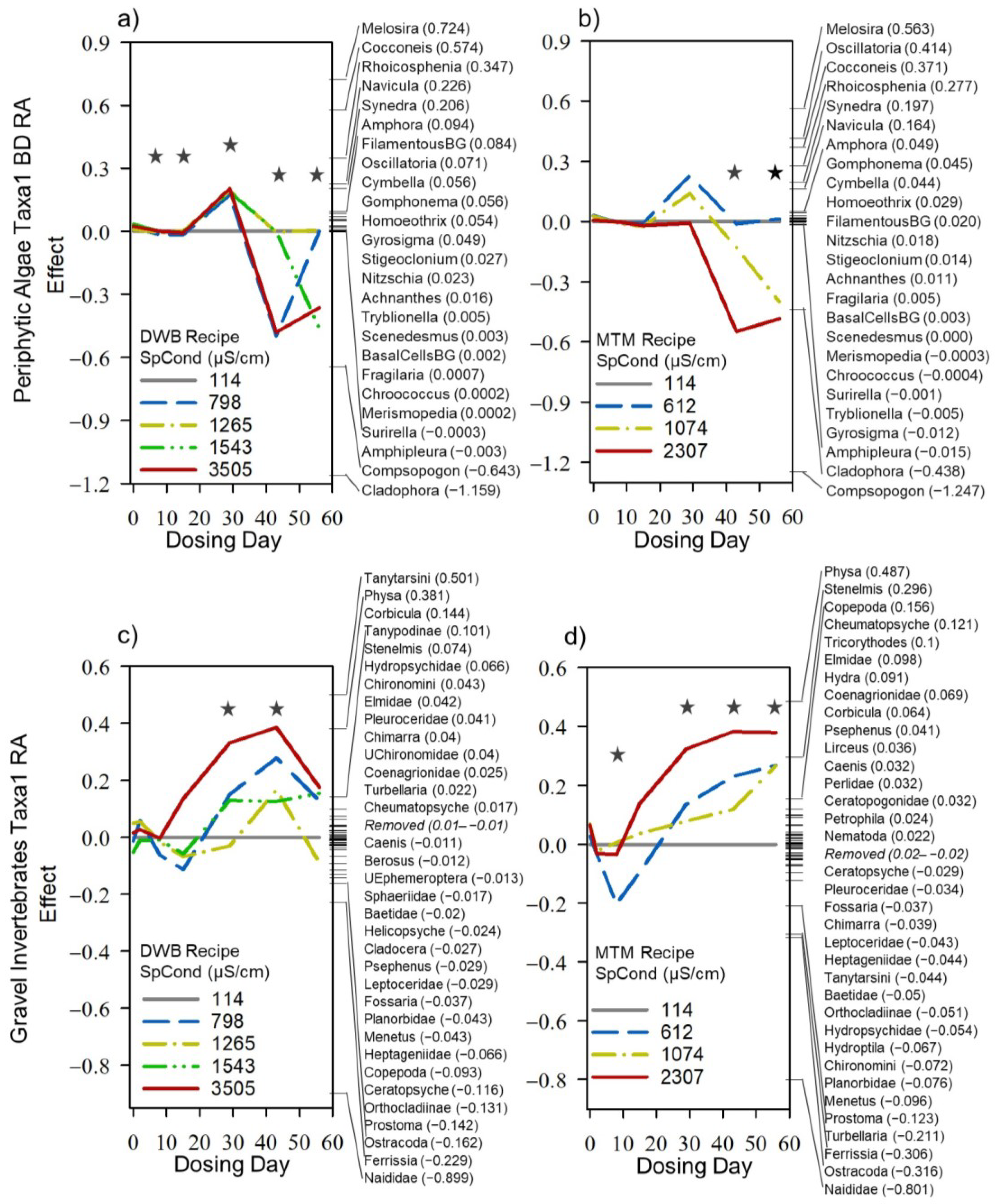

| Period | Mesocosm ID | Recipe | Nominal TDS Target (mg/L) | Observed TDS (mg/L) | Specific Conductivity (µS/cm) | Ionic Strength (mM) | Osmolarity (mOsM) | Alkalinity (mg/L) | Hardness (mg/L) | pH | Temp (°C) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colonization | E06.1, 2 | NA | 100 | 87 | 118 | 1.23 | 1.38 | 30.4 | 35.0 | 7.7 | 22.8 | ||
| E04.1, 2 | NA | 100 | 73 | 130 | 1.38 | 1.59 | 37.1 | 43.0 | 7.6 | 22.6 | |||
| E05.1, 2 | NA | 100 | 106 | 167 | 1.71 | 2.08 | 45.6 | 55.0 | 7.2 | 22.8 | |||
| E07.1, 2 | NA | 100 | 116 | 167 | 1.97 | 2.26 | 52.3 | 63.0 | 7.6 | 22.8 | |||
| E08.1, 2 | NA | 100 | 141 | 198 | 2.22 | 2.56 | 58.0 | 74.0 | 7.5 | 22.4 | |||
| E01.1, 2 | NA | 100 | 68 | 136 | 1.58 | 1.71 | 39.6 | 49.0 | 7.7 | 23.2 | |||
| E02.1, 2 | NA | 100 | 109 | 177 | 2.03 | 2.28 | 52.8 | 61.0 | 7.6 | 23.1 | |||
| E03.1, 2 | NA | 100 | 113 | 195 | 2.30 | 2.65 | 60.5 | 69.0 | 7.5 | 22.9 | |||
| Dosing | E06.1, 2 | Control | 100 | 73 | 114 | 1.35 | 1.50 | 33.4 | 41.7 | 7.5 | 22.3 | ||
| E04.1, 2 | DWB | 500 | 540 | 798 | 7.97 | 11.80 | 39.1 | 144.3 | 7.4 | 22.3 | |||
| E05.1, 2 | DWB | 750 | 855 | 1265 | 11.96 | 17.57 | 47.4 | 209.4 | 6.6 | 22.2 | |||
| E07.1, 2 | DWB | 1000 | 1063 | 1543 | 15.87 | 23.47 | 53.4 | 297.1 | 6.9 | 22.2 | |||
| E08.1, 2 | DWB | 2000 | 2405 | 3505 | 35.15 | 52.40 | 58.3 | 561.7 | 7.4 | 21.8 | |||
| E01.1, 2 | MTM | 500 | 458 | 612 | 10.17 | 7.06 | 59.1 | 252.0 | 7.9 | 22.8 | |||
| E02.1, 2 | MTM | 1000 | 799 | 1074 | 20.69 | 13.62 | 91.1 | 468.6 | 7.9 | 22.8 | |||
| E03.1, 2 | MTM | 2000 | 1677 | 2307 | 51.50 | 31.52 | 138.4 | 1188.0 | 7.6 | 22.5 | |||
| Bench Toxicity Assay | E06.1, 2 | Control | 100 | 72 | 136 | 1.82 | 2.03 | 41.0 | 50.0 | 7.2 | 22.6 | ||
| E04.1, 2 | DWB | 500 | 754 | 1136 | 10.94 | 16.05 | 70.7 | 202.0 | 7.8 | 22.9 | |||
| E05.1, 2 | DWB | 750 | 1102 | 1562 | 15.56 | 23.61 | 70.9 | 264.0 | 7.7 | 22.2 | |||
| E07.1, 2 | DWB | 1000 | 1491 | 2080 | 19.84 | 30.53 | 70.7 | 330.0 | 7.7 | 22.1 | |||
| E08.1, 2 | DWB | 2000 | 2869 | 3800 | 36.43 | 56.77 | 69.8 | 570.0 | 7.7 | 22.5 | |||
| E01.1, 2 | MTM | 500 | 506 | 742 | 13.14 | 8.69 | 74.5 | 342.0 | 6.7 | 22.8 | |||
| E02.1, 2 | MTM | 1000 | 937 | 1241 | 24.63 | 15.64 | 131.6 | 616.0 | 6.7 | 22.9 | |||
| E03.1, 2 | MTM | 2000 | 1853 | 2240 | 53.12 | 31.28 | 209.2 | 1246.0 | 6.5 | 23.0 | |||
| Period | Cl− (mg/L) | SO42− (mg/L) | HCO3− (mg/L) | Br− (mg/L) | N_NO2−3− (mg/L) | P_PO43− (mg/L) | Na+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Sr2+ (mg/L) | K+ (mg/L) | Ba2+ (mg/L) | NH4+ (mg/L) |
| Colonization | 6.3 | 6.8 | 39.0 | 0.005 | 0.218 | 0.035 | 3.8 | 7.4 | 2.5 | 1.4 | 0.007 | 0.019 | |
| 7.0 | 7.4 | 48.8 | 0.009 | 0.232 | 0.037 | 4.1 | 7.8 | 2.7 | 1.5 | 0.008 | 0.016 | ||
| 9.6 | 9.4 | 56.1 | 0.319 | 0.049 | 5.3 | 9.4 | 3.6 | 2.0 | 0.011 | 0.018 | |||
| 10.0 | 10.4 | 70.6 | 0.012 | 0.346 | 0.055 | 5.9 | 10.8 | 4.0 | 2.3 | 0.012 | 0.018 | ||
| 11.6 | 11.6 | 75.4 | 0.014 | 0.382 | 0.059 | 6.6 | 12.8 | 4.5 | 2.5 | 0.014 | 0.020 | ||
| 7.3 | 7.6 | 58.5 | 0.009 | 0.247 | 0.038 | 4.3 | 9.8 | 2.9 | 1.6 | 0.008 | 0.016 | ||
| 9.6 | 10.1 | 68.3 | 0.014 | 0.332 | 0.052 | 5.8 | 12.7 | 4.0 | 2.2 | 0.012 | 0.015 | ||
| 11.6 | 11.4 | 78.0 | 0.016 | 0.382 | 0.061 | 6.5 | 14.2 | 4.5 | 2.5 | 0.014 | 0.018 | ||
| Dosing | 5.4 | 4.7 | 40.6 | 0.429 | 0.051 | 3.4 | 10.4 | 2.6 | 1.7 | 0.004 | 0.022 | ||
| 204.3 | 6.7 | 47.6 | 2.220 | 0.515 | 0.053 | 82.4 | 37.9 | 6.8 | 6.714 | 7.3 | 3.044 | 0.009 | |
| 322.5 | 7.9 | 57.8 | 3.533 | 0.713 | 0.073 | 105.4 | 64.8 | 10.0 | 10.360 | 7.4 | 4.748 | 0.016 | |
| 415.8 | 8.9 | 64.7 | 4.386 | 0.776 | 0.082 | 169.1 | 83.6 | 10.5 | 14.140 | 10.0 | 5.611 | 0.012 | |
| 1077.0 | 10.4 | 71.1 | 11.377 | 0.834 | 0.077 | 336.3 | 179.1 | 20.5 | 30.622 | 22.0 | 11.117 | 0.014 | |
| 27.5 | 186.5 | 71.6 | 0.333 | 0.444 | 0.059 | 17.0 | 27.8 | 41.1 | 0.131 | 12.2 | 0.009 | 0.009 | |
| 48.5 | 397.7 | 110.1 | 0.517 | 0.708 | 0.080 | 37.9 | 37.2 | 94.6 | 0.202 | 26.8 | 0.009 | 0.009 | |
| 103.2 | 1016.1 | 168.1 | 1.303 | 0.594 | 0.075 | 94.8 | 53.0 | 264.5 | 0.356 | 79.5 | 0.011 | 0.008 | |
| Bench Toxicity Assay | 7.8 | 7.8 | 49.9 | 0.700 | 0.075 | 5.0 | 15.1 | 3.1 | 0.1 | 1.5 | 0.011 | 0.007 | |
| 264.7 | 12.2 | 85.8 | 2.394 | 1.207 | 0.124 | 115.3 | 55.5 | 8.0 | 6.7 | 5.5 | 3.996 | 0.012 | |
| 413.8 | 12.3 | 86.1 | 3.802 | 1.268 | 0.123 | 179.2 | 72.6 | 9.6 | 10.4 | 7.1 | 6.269 | 0.013 | |
| 545.2 | 11.5 | 85.9 | 5.085 | 1.184 | 0.120 | 241.0 | 88.8 | 11.3 | 14.1 | 9.0 | 8.755 | 0.012 | |
| 1084.9 | 12.9 | 84.7 | 10.029 | 1.177 | 0.121 | 446.7 | 157.6 | 18.8 | 28.4 | 14.1 | 9.215 | 0.012 | |
| 13.9 | 269.0 | 90.9 | 0.079 | 0.771 | 0.083 | 10.9 | 38.1 | 52.4 | 0.1 | 10.8 | 0.014 | 0.008 | |
| 22.4 | 520.8 | 160.5 | 1.036 | 0.108 | 23.4 | 77.3 | 91.4 | 0.2 | 19.4 | 0.016 | 0.010 | ||
| 38.4 | 1116.9 | 255.2 | 1.071 | 0.109 | 44.5 | 152.6 | 220.4 | 0.3 | 39.2 | 0.022 | 0.011 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nietch, C.T.; Smucker, N.J.; Gains-Germain, L.; Peck, C.P.; Guglielmi, S.; DeCelles, S.; Lazorchak, J.; Johnson, B.; Weaver, P. Using Single-Species and Whole Community Stream Mesocosm Exposures for Identifying Major Ion Effects in Doses Mimicking Resource Extraction Wastewaters. Water 2023, 15, 249. https://doi.org/10.3390/w15020249
Nietch CT, Smucker NJ, Gains-Germain L, Peck CP, Guglielmi S, DeCelles S, Lazorchak J, Johnson B, Weaver P. Using Single-Species and Whole Community Stream Mesocosm Exposures for Identifying Major Ion Effects in Doses Mimicking Resource Extraction Wastewaters. Water. 2023; 15(2):249. https://doi.org/10.3390/w15020249
Chicago/Turabian StyleNietch, Christopher T., Nathan J. Smucker, Leslie Gains-Germain, Christopher P. Peck, Stefania Guglielmi, Susanna DeCelles, James Lazorchak, Brent Johnson, and Paul Weaver. 2023. "Using Single-Species and Whole Community Stream Mesocosm Exposures for Identifying Major Ion Effects in Doses Mimicking Resource Extraction Wastewaters" Water 15, no. 2: 249. https://doi.org/10.3390/w15020249







