Using Single-Species and Whole Community Stream Mesocosm Exposures for Identifying Major Ion Effects in Doses Mimicking Resource Extraction Wastewaters
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Stream Mesocosm Set-Up
2.3. Colonizing the Mesocosm Biotic Communities
2.4. Background and Dosing Water Chemistries
2.5. Assessing Biotic Communities
2.6. Single-Species Tests
2.7. Data Analysis
3. Results
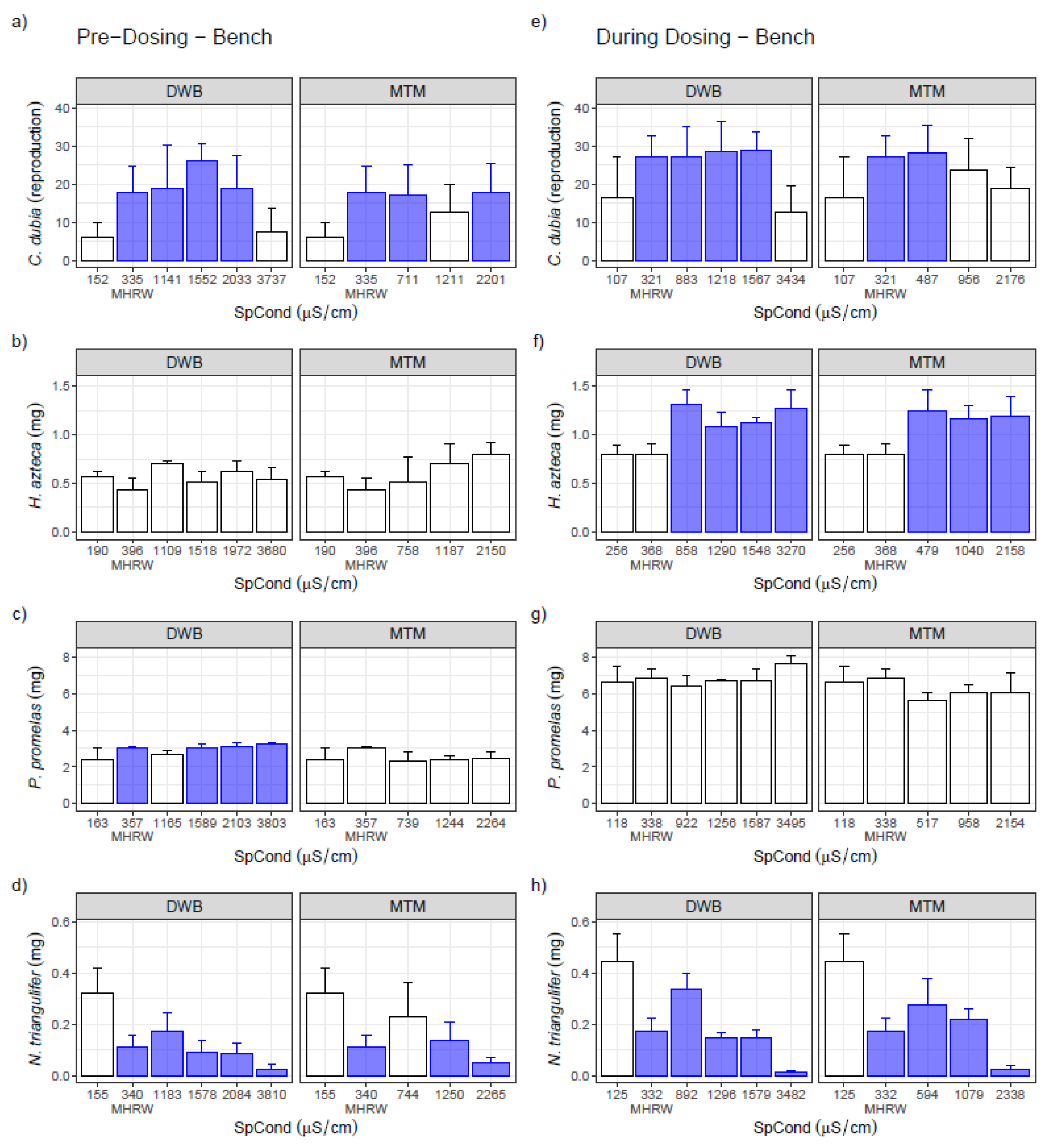
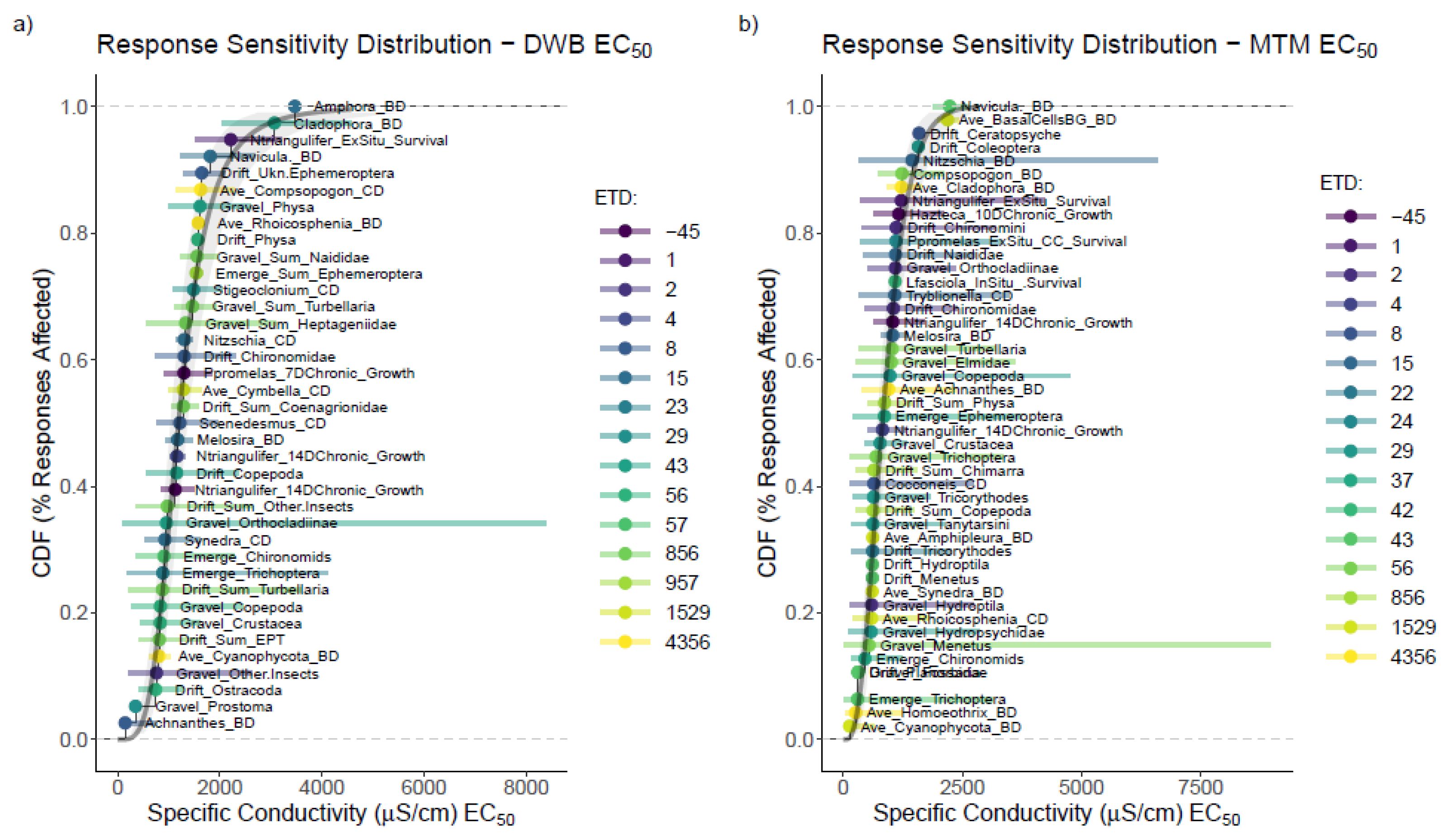
3.1. Single-Species Bench Tests
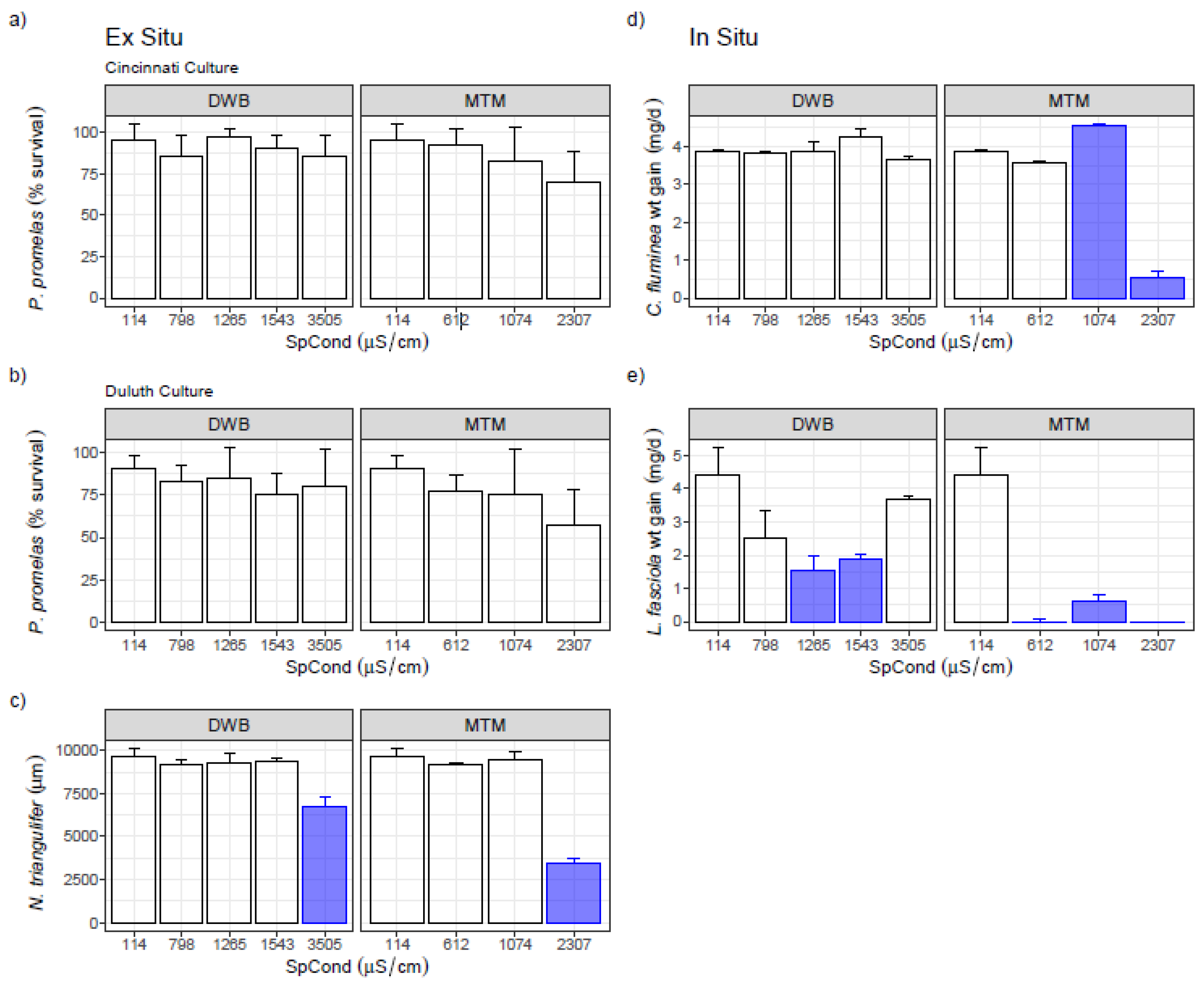
3.2. Single-Species Ex Situ/In Situ Tests
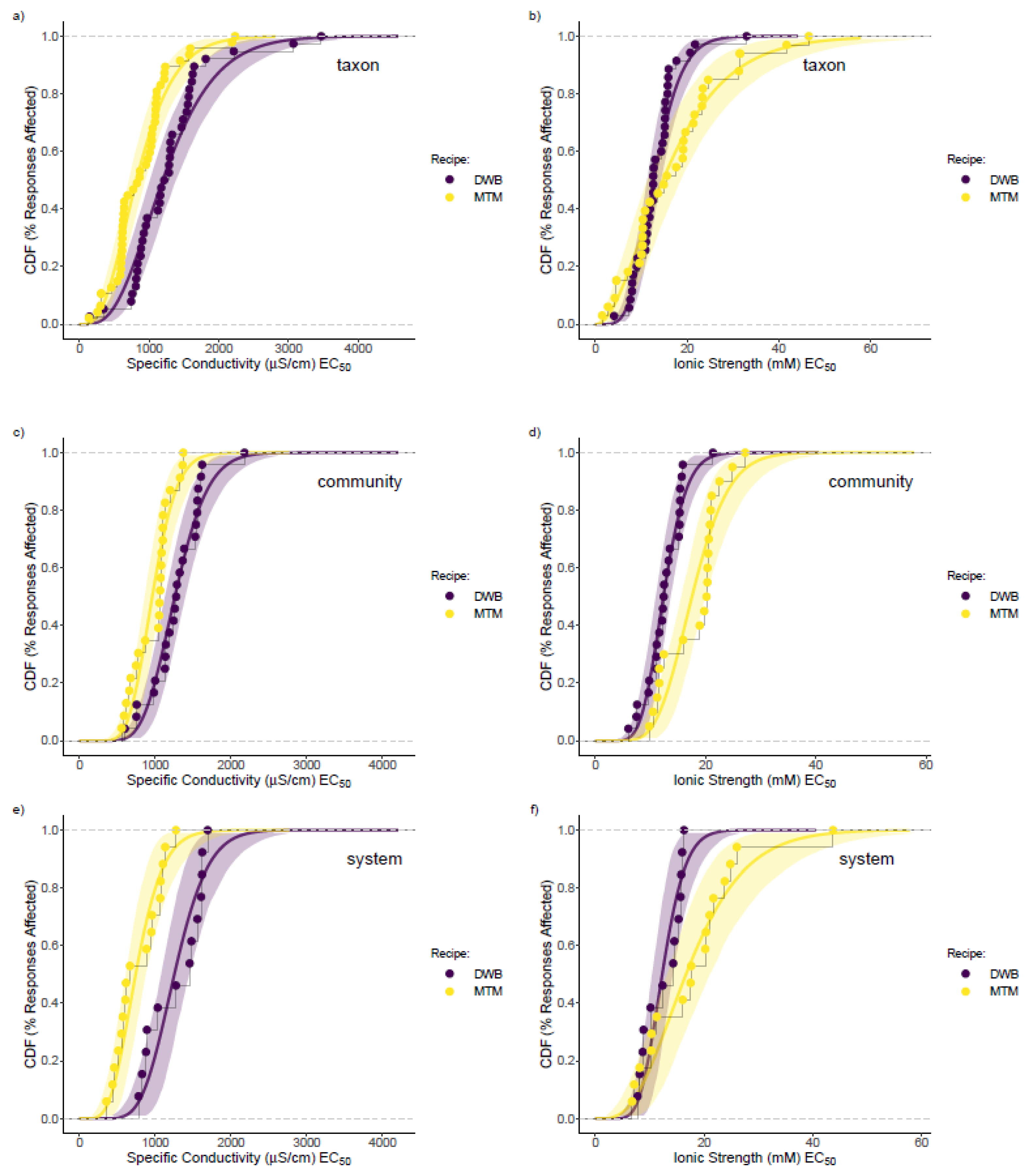
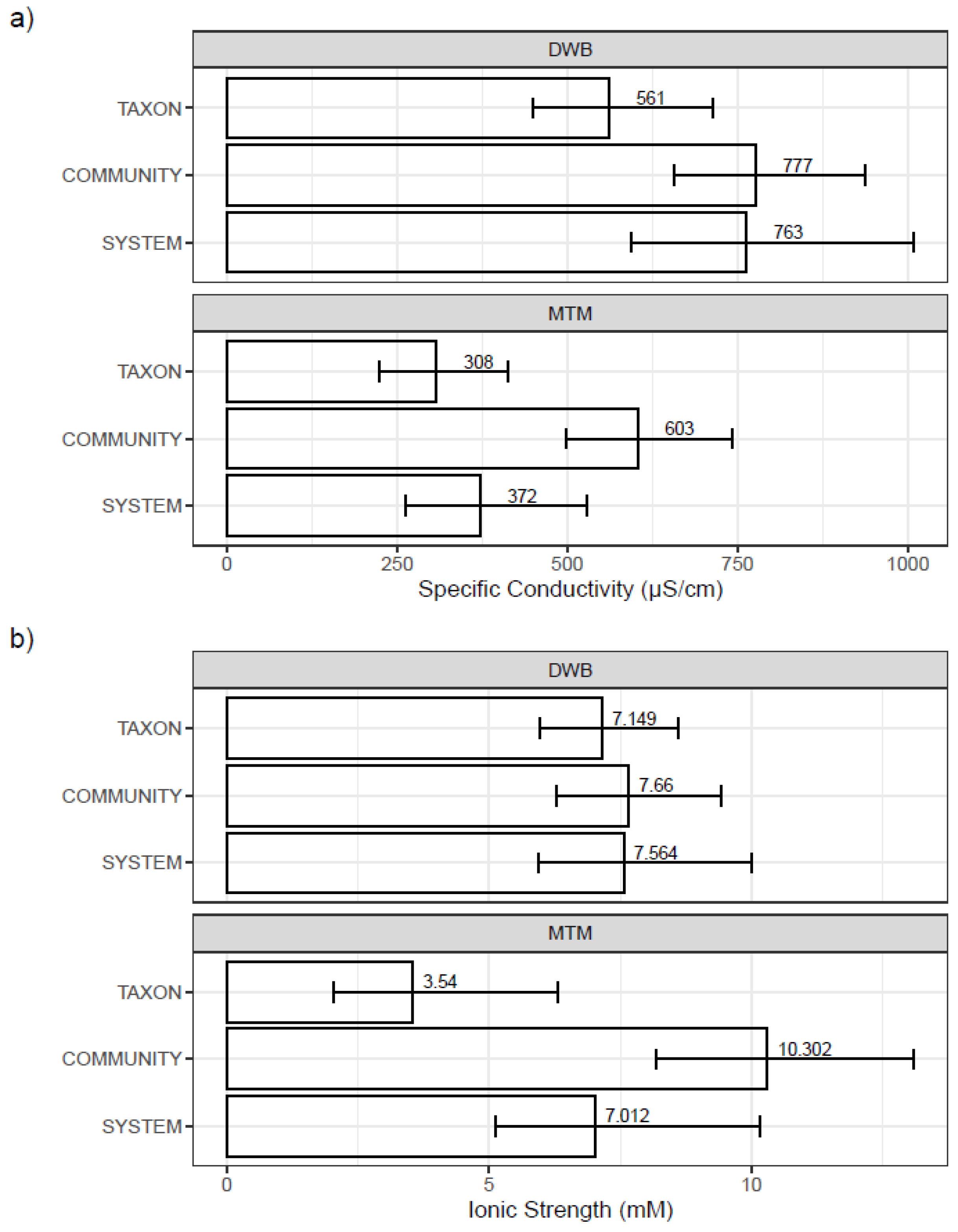
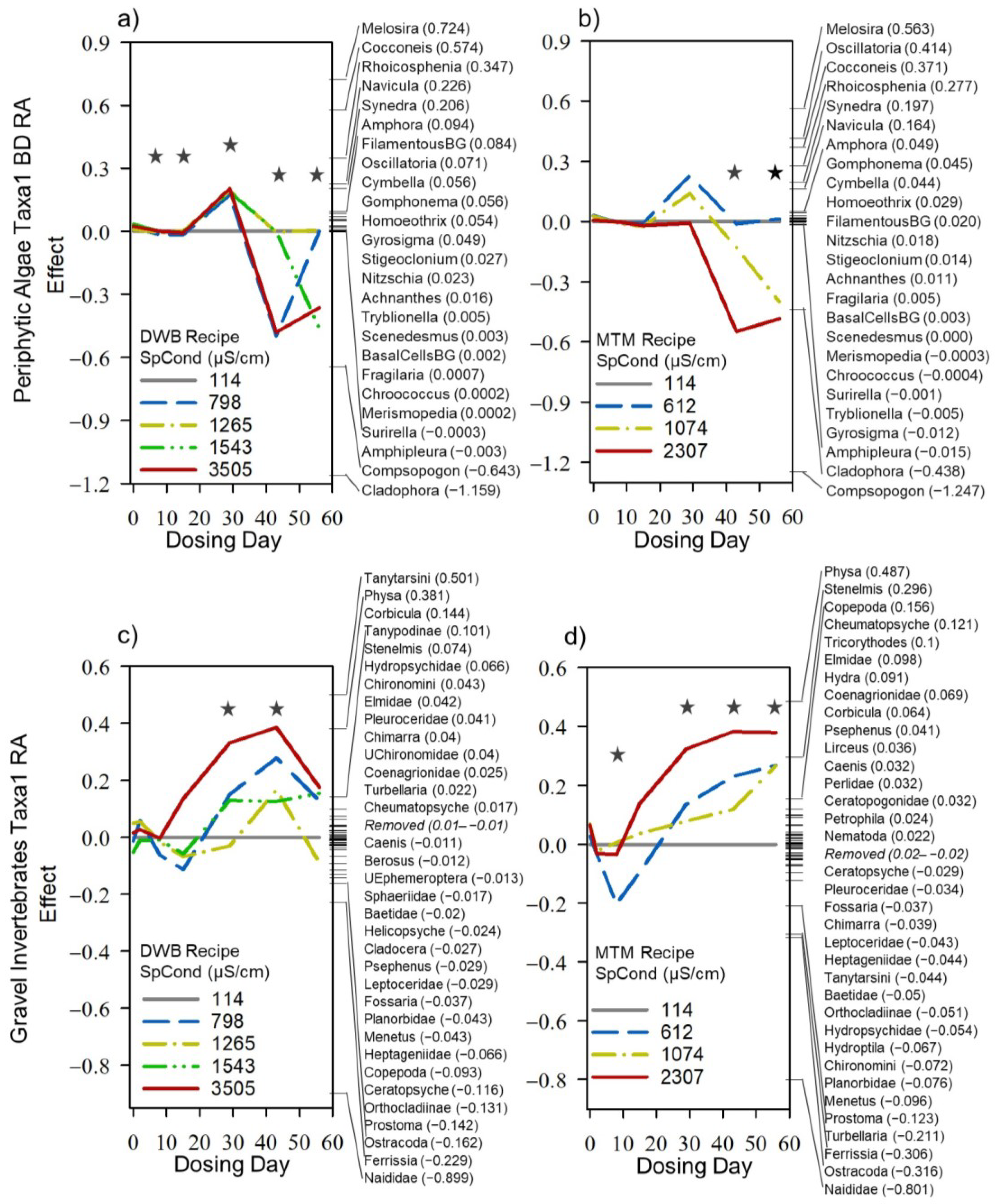
3.3. Mesocosm Taxa, Community, and System-Level Dose–Responses
3.4. Dosing Effects on Mesocosm Communities and Systems through Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodfellow, W.L.; Ausley, L.W.; Burton, D.T.; Denton, D.L.; Dorn, P.B.; Grothe, D.R.; Heber, M.A.; Norberg-King, T.J.; Rodgers, J.H. Major ion toxicity in effluents: A review with permitting recommendations. Environ. Toxicol. Chem. 2000, 19, 175–182. [Google Scholar] [CrossRef]
- Govenor, H.; Krometis, L.A.H.; Willis, L.; Angermeier, P.L.; Hession, W.C. Macroinvertebrate sensitivity thresholds for sediment in Virginia streams. Integr. Environ. Assess. Manag. 2019, 15, 77–92. [Google Scholar] [CrossRef]
- Cook, N.A.; Sarver, E.A.; Krometis, L.H.; Huang, J. Habitat and water quality as drivers of ecological system health in Central Appalachia. Ecol. Eng. 2015, 84, 180–189. [Google Scholar] [CrossRef]
- Timpano, A.J.; Schoenholtz, S.H.; Soucek, D.J.; Zipper, C.E. Benthic macroinvertebrate community response to salinization in headwater streams in Appalachia USA over multiple years. Ecol. Indic. 2018, 91, 645–656. [Google Scholar] [CrossRef]
- Clements, W.H.; Kotalik, C. Effects of major ions on natural benthic communities: An experimental assessment of the US Environmental Protection Agency aquatic life benchmark for conductivity. Freshw. Sci. 2016, 35, 126–138. [Google Scholar] [CrossRef]
- Bradley, T.J. Animal Osmoregulation; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Kefford, B.J. Why are mayflies (Ephemeroptera) lost following small increases in salinity? Three conceptual osmophysiological hypotheses. Philos. Trans. R. Soc. B 2019, 374, 20180021. [Google Scholar] [CrossRef]
- Kefford, B.J.; Hickey, G.L.; Gasith, A.; Ben-David, E.; Dunlop, J.E.; Palmer, C.G.; Allan, K.; Choy, S.C.; Piscart, C. Global Scale Variation in the Salinity Sensitivity of Riverine Macroinvertebrates: Eastern Australia, France, Israel and South Africa. PLoS ONE 2012, 7, e35224. [Google Scholar] [CrossRef]
- Nowghani, F.; Chen, C.C.; Jonusaite, S.; Watson-Leung, T.; Kelly, S.P.; Donini, A. Impact of salt-contaminated freshwater on osmoregulation and tracheal gill function in nymphs of the mayfly Hexagenia rigida. Aquat. Toxicol. 2019, 211, 92–104. [Google Scholar] [CrossRef]
- Kefford, B.J.; Buchwalter, D.; Cañedo-Argüelles, M.; Davis, J.; Duncan, R.P.; Hoffmann, A.; Thompson, R. Salinized rivers: Degraded systems or new habitats for salt-tolerant faunas? Biol. Lett. 2016, 12, 20151072. [Google Scholar] [CrossRef]
- Miltner, R. Assessing the Impacts of Chloride and Sulfate Ions on Macroinvertebrate Communities in Ohio Streams. Water 2021, 13, 1815. [Google Scholar] [CrossRef]
- Burton, G.A.J.; Basu, N.; Ellis, B.R.; Kapo, K.E.; Entrekin, S.; Nadelhoffer, K. Hydraulic “Fracking”: Are Surface Water Impacts An Ecological Concern? Environ. Toxicol. Chem. 2014, 33, 1679–1689. [Google Scholar] [CrossRef]
- Wallace, J.B.; Webster, J.R. The Role of Macroinvertebrates in Stream Ecosystem Function. Annu. Rev. Entomol. 1996, 41, 115–139. [Google Scholar] [CrossRef]
- Fahrenfeld, N.L.; Delos Reyes, H.; Eramo, A.; Akob, D.M.; Mumford, A.C.; Cozzarelli, I.M. Shifts in microbial community structure and function in surface waters impacted by unconventional oil and gas wastewater revealed by metagenomics. Sci. Total Environ. 2017, 580, 1205–1213. [Google Scholar] [CrossRef]
- Olson, J.R.; Hawkins, C.P. Effects of total dissolved solids on growth and mortality predict distributions of stream macroinvertebrates. Freshw. Biol. 2017, 62, 779–791. [Google Scholar] [CrossRef]
- Potapova, M. Patterns of Diatom Distribution in Relation to Salinity. In The Diatom World; Seckbach, J., Kociolek, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 313–332. [Google Scholar]
- Griffith, M.B.; Zheng, L.; Cormier, S.M. Using extirpation to evaluate ionic tolerance of freshwater fish. Environ. Toxicol. Chem. 2018, 37, 871–883. [Google Scholar] [CrossRef]
- Griffith, M.B. Natural variation and current reference for specific conductivity and major ions in wadeable streams of the conterminous USA. Freshw. Sci. 2014, 33, 1–17. [Google Scholar] [CrossRef]
- Cormier, S.M.; Zheng, L.; Flaherty, C. Field-based method for evaluating the annual maximum specific conductivity tolerated by freshwater invertebrates. Sci. Total Environ. 2018, 633, 1637–1646. [Google Scholar] [CrossRef]
- Mount, D.R.; Erickson, R.J.; Highland, T.L.; Hockett, J.R.; Hoff, D.J.; Jenson, C.T.; Norberg-King, T.J.; Peterson, K.N.; Polaske, Z.M.; Wisniewski, S. The acute toxicity of major ion salts to Ceriodaphnia dubia: I. influence of background water chemistry. Environ. Toxicol. Chem. 2016, 35, 3039–3057. [Google Scholar] [CrossRef]
- Cormier, S.M.; Zheng, L.; Flaherty, C.M. A field-based model of the relationship between extirpation of salt-intolerant benthic invertebrates and background conductivity. Sci. Total Environ. 2018, 633, 1629–1636. [Google Scholar] [CrossRef]
- Olmstead, S.M.; Muehlenbachs, L.A.; Shih, J.-S.; Chu, Z.; Krupnick, A.J. Shale gas development impacts on surface water quality in Pennsylvania. Proc. Natl. Acad. Sci. USA 2013, 110, 4962–4967. [Google Scholar] [CrossRef]
- PACode. Chapter 95. Wastewater Treatment Requirements. Available online: https://www.pacodeandbulletin.gov/Display/pacode?file=/secure/pacode/data/025/chapter95/chap95toc.html&d=reduce (accessed on 25 September 2022).
- ORSANCO. Pollution Control Standards for Discharges to the Ohio River; Ohio River Valley Water Sanitation Commission: Cincinnati, OH, USA, 2011. [Google Scholar]
- Pond, G.J. Patterns of Ephemeroptera taxa loss in Appalachian headwater streams (Kentucky, USA). Hydrobiologia 2010, 641, 185–201. [Google Scholar] [CrossRef]
- Pond, G.J.; Passmore, M.E.; Pointon, N.D.; Felbinger, J.K.; Walker, C.A.; Krock, K.J.; Fulton, J.B.; Nash, W.L. Long-term impacts on macroinvertebrates downstream of reclaimed mountaintop mining valley fills in Central Appalachia. Environ. Manag. 2014, 54, 919–933. [Google Scholar] [CrossRef]
- U.S. EPA. A Field-Based Aquatic Life Benchmark for Conductivity in Central Appalachian Streams; U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- Cañedo-Argüelles, M.; Grantham, T.E.; Perrée, I.; Rieradevall, M.; Céspedes-Sánchez, R.; Prat, N. Response of stream invertebrates to short-term salinization: A mesocosm approach. Environ. Pollut. 2012, 166, 144–151. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M.; Bundschuh, M.; Gutiérrez-Cánovas, C.; Kefford, B.J.; Prat, N.; Trobajo, R.; Schäfer, R.B. Effects of repeated salt pulses on ecosystem structure and functions in a stream mesocosm. Sci. Total Environ. 2014, 476–477, 634–642. [Google Scholar] [CrossRef]
- Entrekin, S.A.; Maloney, K.O.; Kapo, K.E.; Walters, A.W.; Evans-White, M.A.; Klemow, K.M. Stream Vulnerability to Widespread and Emergent Stressors: A Focus on Unconventional Oil and Gas. PLoS ONE 2015, 10, e0137416. [Google Scholar] [CrossRef]
- Krupnick, A.J. Managing the Risks of Shale Gas: Identifiying a Pathway toward Reponsibile Development: A Review of Shale Gas Regulations by State; Center for Energy Economics and Policy: Washington, DC, USA, 2012. [Google Scholar]
- Posthuma, L.; Suter, G.W., II; Traas, T.P. Species Sensitivity Distributions in Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Stephan, C.E.; Mount, D.I.; Hansen, D.J.; Gentile, J.; Chapman, G.A.; Brungs, W.A. Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses; U.S. Environmental Protection Agency: Duluth, MN, USA, 1985.
- Soucek, D.J.; Linton, T.K.; Tarr, C.D.; Dickinson, A.; Wickramanayake, N.; Delos, C.G.; Cruz, L.A. Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates. Environ. Toxicol. Chem. 2011, 30, 930–938. [Google Scholar] [CrossRef]
- Erickson, R.J.; Mount, D.R.; Highland, T.L.; Hockett, J.R.; Hoff, D.J.; Jenson, C.T.; Norberg-King, T.J.; Forsman, B. Acute Toxicity of Major Geochemical Ions to Fathead Minnows (Pimephales promelas): Part A—Observed Relationships for Individual Salts and Salt Mixtures. Environ. Toxicol. Chem. 2022, 41, 2078–2094. [Google Scholar] [CrossRef]
- Cormier, S.M.; Suter, G.W.; Zheng, L.; Pond, G.J. Assessing causation of the extirpation of stream macroinvertebrates by a mixture of ions. Environ. Toxicol. Chem. 2013, 32, 277–287. [Google Scholar] [CrossRef]
- Kefford, B.J. Rapid Tests for Community-Level Risk Assessments in Ecotoxicology. In Encyclopedia of Aquatic Ecotoxicology; Férard, J.-F., Blaise, C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 957–966. [Google Scholar]
- Brent, R.N.; Kunkel, J.; Tomek, Z.; Buchardt, D.; DeLisle, P.F.; Sivers, S. A Novel Approach to Developing Thresholds for Total Dissolved Solids Using Standardized and Experimental Toxicity Test Methods. Environ. Toxicol. Chem. 2022, 41, 2782–2796. [Google Scholar] [CrossRef]
- Hintz, W.D.; Mattes, B.M.; Schuler, M.S.; Jones, D.K.; Stoler, A.B.; Lind, L.; Relyea, R.A. Salinization triggers a trophic cascade in experimental freshwater communities with varying food-chain length. Ecol. Appl. 2017, 27, 833–844. [Google Scholar] [CrossRef]
- Mooney, T.J.; McCullough, C.D.; Jansen, A.; Chandler, L.; Douglas, M.; Harford, A.J.; van Dam, R.; Humphrey, C. Elevated Magnesium Concentrations Altered Freshwater Assemblage Structures in a Mesocosm Experiment. Environ. Toxicol. Chem. 2020, 39, 1973–1987. [Google Scholar] [CrossRef]
- Haluszczak, L.O.; Rose, A.W.; Kump, L.R. Geochemical evaluation of flowback brine from Marcellus gas wells in Pennsylvania, USA. Appl. Geochem. 2013, 28, 55–61. [Google Scholar] [CrossRef]
- Sauer, T.C.; Costa, H.J.; Brown, J.S.; Ward, T.J. Toxicity identification evaluations of produced-water effluents. Environ. Toxicol. Chem. 1997, 16, 2020–2028. [Google Scholar] [CrossRef]
- Dresel, P.E.; Rose, A.W. Chemistry and Origin of Oil and Gas Well Brines in Western Pennsylvania; 4th Ser., Open-File Report OFOG 10-01.0; Pennsylvania Geological Survey: Harrisburg, PA, USA, 2010; 48p. [Google Scholar]
- Osborn, S.G.; McIntosh, J.C. Chemical and isotopic tracers of the contribution of microbial gas in Devonian organic-rich shales and reservoir sandstones, northern Appalachian Basin. Appl. Geochem. 2010, 25, 456–471. [Google Scholar] [CrossRef]
- Hayes, T. Sampling and Analysis of Water Streams Associated with the Development of Marcellus Shale Gas; Gas Technology Institute Report 2009; Marcellus Shale Coalition: Pittsburgh, PA, USA, 2009; 49p. [Google Scholar]
- Stout, W.; Lamborn, R.E.; Schaaf, D. Brines of Ohio (a Preliminary Report); 4th Series, Bulletin 37; Geological Survey of Ohio: Columbus, OH, USA, 1932; 35p. [Google Scholar]
- Breen, K.J.; Angelo, C.G.; Masters, R.W.; Sedam, A.C. Chemical and Isotopic Characteristics of Brines from Three Oil- and Gas-Producing Sandstones in Eastern Ohio, with Applications to the Geochemical Tracing of Brine Sources; 84-4314; US Department of the Interior, Geological Survey: Reston, VA, USA, 1985.
- USEPA. Proceedings of the Technical Workshops for the Hydrualic Fracturing Study: Water Resources Management; EPA/600/R-11/048; U.S. Environmental Protection Agency, Office of Research and Development: Washington, DC, USA, 2011; 125p.
- USEPA. The Effects of Moutaintop Mines and Valley Fills on Aquatic Ecosystems of the Central Appalachian Coalfields; EPA/600/R-09/138F; U.S. Environmental Protection Agency: Washington, DC, USA, 2011; 153p.
- Pond, G.J.; Passmore, M.E.; Borsuk, F.A.; Reynolds, L.; Rose, C.J. Downstream effects of mountaintop coal mining: Comparing biological conditions using family- and genus-level macroinvertebrate bioassessment tools. J. North Am. Benthol. Soc. 2008, 27, 717–737. [Google Scholar] [CrossRef]
- Fritz, K.M.; Fulton, S.; Johnson, B.R.; Barton, C.D.; Jack, J.D.; Word, D.A.; Burke, R.A. Structural and functional characteristics of natural and constructed channels draining a reclaimed mountaintop removal and valley fill coal mine. J. North Am. Benthol. Soc. 2010, 29, 673–689. [Google Scholar] [CrossRef]
- Hartman, K.J.; Kaller, M.D.; Howell, J.W.; Sweka, J.A. How much do valley fills influence headwater streams? Hydrobiologia 2005, 532, 91. [Google Scholar] [CrossRef]
- Bryant, L.D.; McPhilliamy, S.; Childers, H. Draft Programmatic Environmental Impact Statement on Mountaintop-mining/Valley Fills in Appalachia—2003: A Survey of the Water Quality of Streams in the Primary Region of Mountaintop/Valley Fill Coal Mining, October 1999 to January 2001; U.S. Environmental Protection Agency, Region 3: Philadelphia, PA, USA, 2002.
- Griffith, M.B.; Norton, S.B.; Alexander, L.C.; Pollard, A.I.; LeDuc, S.D. The effects of mountaintop mines and valley fills on the physicochemical quality of stream ecosystems in the central Appalachians: A review. Sci. Total Environ. 2012, 417, 1–12. [Google Scholar] [CrossRef]
- Kunz, J.L.; Conley, J.M.; Buchwalter, D.B.; Norberg-King, T.J.; Kemble, N.E.; Wang, N.; Ingersoll, C.G. Use of reconstituted waters to evaluate effects of elevated major ions associated with mountaintop coal mining on freshwater invertebrates. Environ. Toxicol. Chem. 2013, 32, 2826–2835. [Google Scholar] [CrossRef]
- U.S. EPA. Environmental Measurements and Modeling: Collection of Methods. Available online: https://www.epa.gov/cwa-methods (accessed on 10 January 2014).
- U.S. EPA. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, 4th ed.; EPA-821-R-02-013; U.S. EPA Office of Water: Washington, DC, USA, 2002.
- Van den Brink, P.J.; Braak, C.J.F.T. Principal response curves: Analysis of time-dependent multivariate responses of biological community to stress. Environ. Toxicol. Chem. 1999, 18, 138–148. [Google Scholar] [CrossRef]
- Rao, C.R. The Use and Interpretation of Principal Component Analysis in Applied Research. Sankhyā: The Indian J. Stat. 1964, 26, 329–358. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.B.; Simpson, G.; Solymos, P.; et al. R Package ‘Vegan’: Community Ecology Package, version 2.5—7, 28 November 2020. 2020. Available online: https://cran.r-project.org; https://github.com/vegandevs/vegan (accessed on 22 October 2022).
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2016, 10, e0146021. [Google Scholar] [CrossRef]
- U.S. EPA. Study of Oil and Gas Extraction Wastewater Management Under the Clean Water Act; EPA-821-R19-001; U.S. EPA Office of Water: Washington, DC, USA, 2019.
- Suter, G.W.; Cormier, S.M. A method for assessing the potential for confounding applied to ionic strength in central Appalachian streams. Environ. Toxicol. Chem. 2013, 32, 288–295. [Google Scholar] [CrossRef]
- Farrar, D.; Alexander, L.C.; Yuan, L.L.; Gerritsen, J. Regional Observational Studies: Addressing Confounding. In Ecological Causal Assessment; Norton, S.B., Cormier, S.M., Suter, G.W., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2014; p. 15. [Google Scholar]
- Ohio EPA. Biological and Water Quality Study of the East Fork Little Miami River and Select Tributaries 2012; Ohio Environmental Protection Agency: Columbus, OH, USA, 2014; p. 126.
- Erickson, R.J.; Mount, D.R.; Highland, T.L.; Hockett, J.R.; Hoff, D.J.; Jenson, C.T.; Norberg-King, T.J.; Peterson, K.N. The acute toxicity of major ion salts to Ceriodaphnia dubia. II. Empirical relationships in binary salt mixtures. Environ. Toxicol. Chem. 2017, 36, 1525–1537. [Google Scholar] [CrossRef]
- Mount, D.R.; Erickson, R.J.; Highland, T.L.; Hockett, J.R.; Hoff, D.J.; Jenson, C.T.; Norberg-King, T.J.; Forsman, B. Acute toxicity of major ions to Amphipod Hyalella azteca. Environ. Toxicol. Chem. in revision.
- Wang, N.; Ingersoll, C.G.; Kunz, J.L.; Brumbaugh, W.G.; Kane, C.M.; Evans, R.B.; Alexander, S.; Walker, C.; Bakaletz, S. Toxicity of sediments potentially contaminated by coal mining and natural gas extraction to unionid mussels and commonly tested benthic invertebrates. Environ. Toxicol. Chem. 2013, 32, 207–221. [Google Scholar] [CrossRef]
- Wang, N.; Dorman, R.A.; Ingersoll, C.G.; Hardesty, D.K.; Brumbaugh, W.G.; Hammer, E.J.; Bauer, C.R.; Mount, D.R. Acute and chronic toxicity of sodium sulfate to four freshwater organisms in water-only exposures. Environ. Toxicol. Chem. 2016, 35, 115–127. [Google Scholar] [CrossRef]
- Suter, I.; Traas, T.; Posthuma, L. Issues and practices in the derivation and use of species sensitivity distributions. In Species Sensitivity Distributions in Ecotoxicology; Posthuma, L., Suter, G.W.I., Traas, T., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 437–474. [Google Scholar]
- Buchwalter, D.; Scheibener, S.; Chou, H.; Soucek, D.; Elphick, J. Are sulfate effects in the mayfly Neocloeon triangulifer driven by the cost of ion regulation? Phil. Trans. R. Soc. 2019, 374, 20180013. [Google Scholar] [CrossRef]
- Griffith, M.B. Toxicological perspective on the osmoregulation and ionoregulation physiology of major ions by freshwater animals: Teleost fish, crustacea, aquatic insects, and Mollusca. Environ. Toxicol. Chem. 2017, 36, 576–600. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. National Recommended Water Quality Criteria—Aquatic Life Criteria Table. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 7 July 2022).
- Hem, D. Study and Interpretation the Chemical of Natural of Characteristics Natural Water, 3rd ed.; USGS Water Supply Paper 2254 66-69; U.S. Government Publishing Office: Washington, DC, USA, 1985.
- Rusydi, A.F. Correlation between conductivity and total dissolved solid in various type of water: A review. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012019. [Google Scholar] [CrossRef]
- Miller, R.L.; Bradford, W.L.; Peters, N.E. Specific Conductance: Theoretical Considerations and Application to Analytical Quality Control; Water-Supply Paper 2311; U.S. Geological Survey: Washington, DC, USA, 1986.


| Period | Mesocosm ID | Recipe | Nominal TDS Target (mg/L) | Observed TDS (mg/L) | Specific Conductivity (µS/cm) | Ionic Strength (mM) | Osmolarity (mOsM) | Alkalinity (mg/L) | Hardness (mg/L) | pH | Temp (°C) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colonization | E06.1, 2 | NA | 100 | 87 | 118 | 1.23 | 1.38 | 30.4 | 35.0 | 7.7 | 22.8 | ||
| E04.1, 2 | NA | 100 | 73 | 130 | 1.38 | 1.59 | 37.1 | 43.0 | 7.6 | 22.6 | |||
| E05.1, 2 | NA | 100 | 106 | 167 | 1.71 | 2.08 | 45.6 | 55.0 | 7.2 | 22.8 | |||
| E07.1, 2 | NA | 100 | 116 | 167 | 1.97 | 2.26 | 52.3 | 63.0 | 7.6 | 22.8 | |||
| E08.1, 2 | NA | 100 | 141 | 198 | 2.22 | 2.56 | 58.0 | 74.0 | 7.5 | 22.4 | |||
| E01.1, 2 | NA | 100 | 68 | 136 | 1.58 | 1.71 | 39.6 | 49.0 | 7.7 | 23.2 | |||
| E02.1, 2 | NA | 100 | 109 | 177 | 2.03 | 2.28 | 52.8 | 61.0 | 7.6 | 23.1 | |||
| E03.1, 2 | NA | 100 | 113 | 195 | 2.30 | 2.65 | 60.5 | 69.0 | 7.5 | 22.9 | |||
| Dosing | E06.1, 2 | Control | 100 | 73 | 114 | 1.35 | 1.50 | 33.4 | 41.7 | 7.5 | 22.3 | ||
| E04.1, 2 | DWB | 500 | 540 | 798 | 7.97 | 11.80 | 39.1 | 144.3 | 7.4 | 22.3 | |||
| E05.1, 2 | DWB | 750 | 855 | 1265 | 11.96 | 17.57 | 47.4 | 209.4 | 6.6 | 22.2 | |||
| E07.1, 2 | DWB | 1000 | 1063 | 1543 | 15.87 | 23.47 | 53.4 | 297.1 | 6.9 | 22.2 | |||
| E08.1, 2 | DWB | 2000 | 2405 | 3505 | 35.15 | 52.40 | 58.3 | 561.7 | 7.4 | 21.8 | |||
| E01.1, 2 | MTM | 500 | 458 | 612 | 10.17 | 7.06 | 59.1 | 252.0 | 7.9 | 22.8 | |||
| E02.1, 2 | MTM | 1000 | 799 | 1074 | 20.69 | 13.62 | 91.1 | 468.6 | 7.9 | 22.8 | |||
| E03.1, 2 | MTM | 2000 | 1677 | 2307 | 51.50 | 31.52 | 138.4 | 1188.0 | 7.6 | 22.5 | |||
| Bench Toxicity Assay | E06.1, 2 | Control | 100 | 72 | 136 | 1.82 | 2.03 | 41.0 | 50.0 | 7.2 | 22.6 | ||
| E04.1, 2 | DWB | 500 | 754 | 1136 | 10.94 | 16.05 | 70.7 | 202.0 | 7.8 | 22.9 | |||
| E05.1, 2 | DWB | 750 | 1102 | 1562 | 15.56 | 23.61 | 70.9 | 264.0 | 7.7 | 22.2 | |||
| E07.1, 2 | DWB | 1000 | 1491 | 2080 | 19.84 | 30.53 | 70.7 | 330.0 | 7.7 | 22.1 | |||
| E08.1, 2 | DWB | 2000 | 2869 | 3800 | 36.43 | 56.77 | 69.8 | 570.0 | 7.7 | 22.5 | |||
| E01.1, 2 | MTM | 500 | 506 | 742 | 13.14 | 8.69 | 74.5 | 342.0 | 6.7 | 22.8 | |||
| E02.1, 2 | MTM | 1000 | 937 | 1241 | 24.63 | 15.64 | 131.6 | 616.0 | 6.7 | 22.9 | |||
| E03.1, 2 | MTM | 2000 | 1853 | 2240 | 53.12 | 31.28 | 209.2 | 1246.0 | 6.5 | 23.0 | |||
| Period | Cl− (mg/L) | SO42− (mg/L) | HCO3− (mg/L) | Br− (mg/L) | N_NO2−3− (mg/L) | P_PO43− (mg/L) | Na+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Sr2+ (mg/L) | K+ (mg/L) | Ba2+ (mg/L) | NH4+ (mg/L) |
| Colonization | 6.3 | 6.8 | 39.0 | 0.005 | 0.218 | 0.035 | 3.8 | 7.4 | 2.5 | 1.4 | 0.007 | 0.019 | |
| 7.0 | 7.4 | 48.8 | 0.009 | 0.232 | 0.037 | 4.1 | 7.8 | 2.7 | 1.5 | 0.008 | 0.016 | ||
| 9.6 | 9.4 | 56.1 | 0.319 | 0.049 | 5.3 | 9.4 | 3.6 | 2.0 | 0.011 | 0.018 | |||
| 10.0 | 10.4 | 70.6 | 0.012 | 0.346 | 0.055 | 5.9 | 10.8 | 4.0 | 2.3 | 0.012 | 0.018 | ||
| 11.6 | 11.6 | 75.4 | 0.014 | 0.382 | 0.059 | 6.6 | 12.8 | 4.5 | 2.5 | 0.014 | 0.020 | ||
| 7.3 | 7.6 | 58.5 | 0.009 | 0.247 | 0.038 | 4.3 | 9.8 | 2.9 | 1.6 | 0.008 | 0.016 | ||
| 9.6 | 10.1 | 68.3 | 0.014 | 0.332 | 0.052 | 5.8 | 12.7 | 4.0 | 2.2 | 0.012 | 0.015 | ||
| 11.6 | 11.4 | 78.0 | 0.016 | 0.382 | 0.061 | 6.5 | 14.2 | 4.5 | 2.5 | 0.014 | 0.018 | ||
| Dosing | 5.4 | 4.7 | 40.6 | 0.429 | 0.051 | 3.4 | 10.4 | 2.6 | 1.7 | 0.004 | 0.022 | ||
| 204.3 | 6.7 | 47.6 | 2.220 | 0.515 | 0.053 | 82.4 | 37.9 | 6.8 | 6.714 | 7.3 | 3.044 | 0.009 | |
| 322.5 | 7.9 | 57.8 | 3.533 | 0.713 | 0.073 | 105.4 | 64.8 | 10.0 | 10.360 | 7.4 | 4.748 | 0.016 | |
| 415.8 | 8.9 | 64.7 | 4.386 | 0.776 | 0.082 | 169.1 | 83.6 | 10.5 | 14.140 | 10.0 | 5.611 | 0.012 | |
| 1077.0 | 10.4 | 71.1 | 11.377 | 0.834 | 0.077 | 336.3 | 179.1 | 20.5 | 30.622 | 22.0 | 11.117 | 0.014 | |
| 27.5 | 186.5 | 71.6 | 0.333 | 0.444 | 0.059 | 17.0 | 27.8 | 41.1 | 0.131 | 12.2 | 0.009 | 0.009 | |
| 48.5 | 397.7 | 110.1 | 0.517 | 0.708 | 0.080 | 37.9 | 37.2 | 94.6 | 0.202 | 26.8 | 0.009 | 0.009 | |
| 103.2 | 1016.1 | 168.1 | 1.303 | 0.594 | 0.075 | 94.8 | 53.0 | 264.5 | 0.356 | 79.5 | 0.011 | 0.008 | |
| Bench Toxicity Assay | 7.8 | 7.8 | 49.9 | 0.700 | 0.075 | 5.0 | 15.1 | 3.1 | 0.1 | 1.5 | 0.011 | 0.007 | |
| 264.7 | 12.2 | 85.8 | 2.394 | 1.207 | 0.124 | 115.3 | 55.5 | 8.0 | 6.7 | 5.5 | 3.996 | 0.012 | |
| 413.8 | 12.3 | 86.1 | 3.802 | 1.268 | 0.123 | 179.2 | 72.6 | 9.6 | 10.4 | 7.1 | 6.269 | 0.013 | |
| 545.2 | 11.5 | 85.9 | 5.085 | 1.184 | 0.120 | 241.0 | 88.8 | 11.3 | 14.1 | 9.0 | 8.755 | 0.012 | |
| 1084.9 | 12.9 | 84.7 | 10.029 | 1.177 | 0.121 | 446.7 | 157.6 | 18.8 | 28.4 | 14.1 | 9.215 | 0.012 | |
| 13.9 | 269.0 | 90.9 | 0.079 | 0.771 | 0.083 | 10.9 | 38.1 | 52.4 | 0.1 | 10.8 | 0.014 | 0.008 | |
| 22.4 | 520.8 | 160.5 | 1.036 | 0.108 | 23.4 | 77.3 | 91.4 | 0.2 | 19.4 | 0.016 | 0.010 | ||
| 38.4 | 1116.9 | 255.2 | 1.071 | 0.109 | 44.5 | 152.6 | 220.4 | 0.3 | 39.2 | 0.022 | 0.011 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nietch, C.T.; Smucker, N.J.; Gains-Germain, L.; Peck, C.P.; Guglielmi, S.; DeCelles, S.; Lazorchak, J.; Johnson, B.; Weaver, P. Using Single-Species and Whole Community Stream Mesocosm Exposures for Identifying Major Ion Effects in Doses Mimicking Resource Extraction Wastewaters. Water 2023, 15, 249. https://doi.org/10.3390/w15020249
Nietch CT, Smucker NJ, Gains-Germain L, Peck CP, Guglielmi S, DeCelles S, Lazorchak J, Johnson B, Weaver P. Using Single-Species and Whole Community Stream Mesocosm Exposures for Identifying Major Ion Effects in Doses Mimicking Resource Extraction Wastewaters. Water. 2023; 15(2):249. https://doi.org/10.3390/w15020249
Chicago/Turabian StyleNietch, Christopher T., Nathan J. Smucker, Leslie Gains-Germain, Christopher P. Peck, Stefania Guglielmi, Susanna DeCelles, James Lazorchak, Brent Johnson, and Paul Weaver. 2023. "Using Single-Species and Whole Community Stream Mesocosm Exposures for Identifying Major Ion Effects in Doses Mimicking Resource Extraction Wastewaters" Water 15, no. 2: 249. https://doi.org/10.3390/w15020249
APA StyleNietch, C. T., Smucker, N. J., Gains-Germain, L., Peck, C. P., Guglielmi, S., DeCelles, S., Lazorchak, J., Johnson, B., & Weaver, P. (2023). Using Single-Species and Whole Community Stream Mesocosm Exposures for Identifying Major Ion Effects in Doses Mimicking Resource Extraction Wastewaters. Water, 15(2), 249. https://doi.org/10.3390/w15020249







