The Effect of Salinity on N2O Emissions during Domestic Wastewater Partial Nitrification Treatment in a Sequencing Batch Reactor
Abstract
:1. Introduction
2. Methods
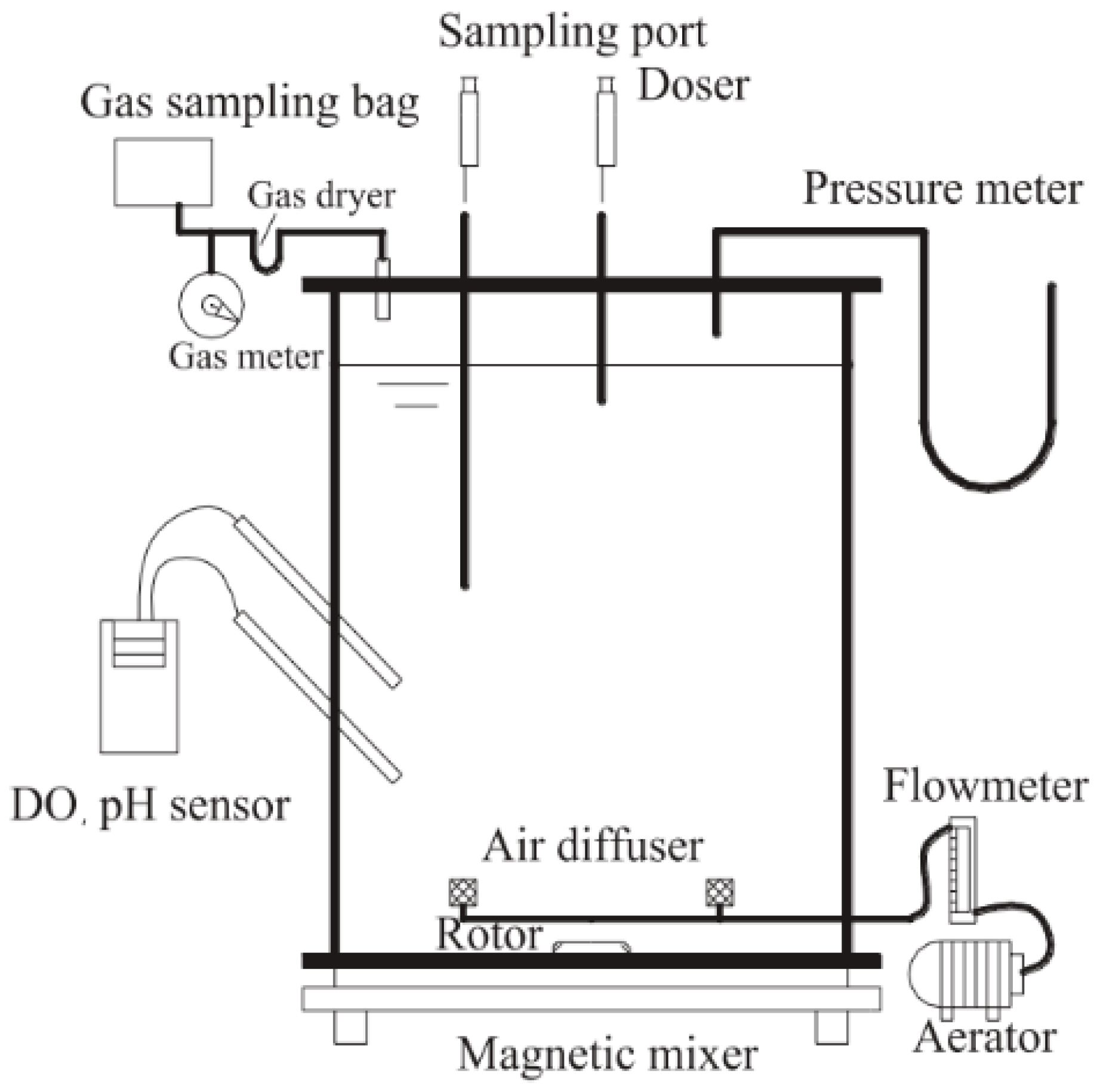
2.1. Sludge, Wastewater, and SBR (Sequencing Batch Reactor) Operations
2.2. Batch Test Rules
2.3. Detection Method
3. Results and Discussion
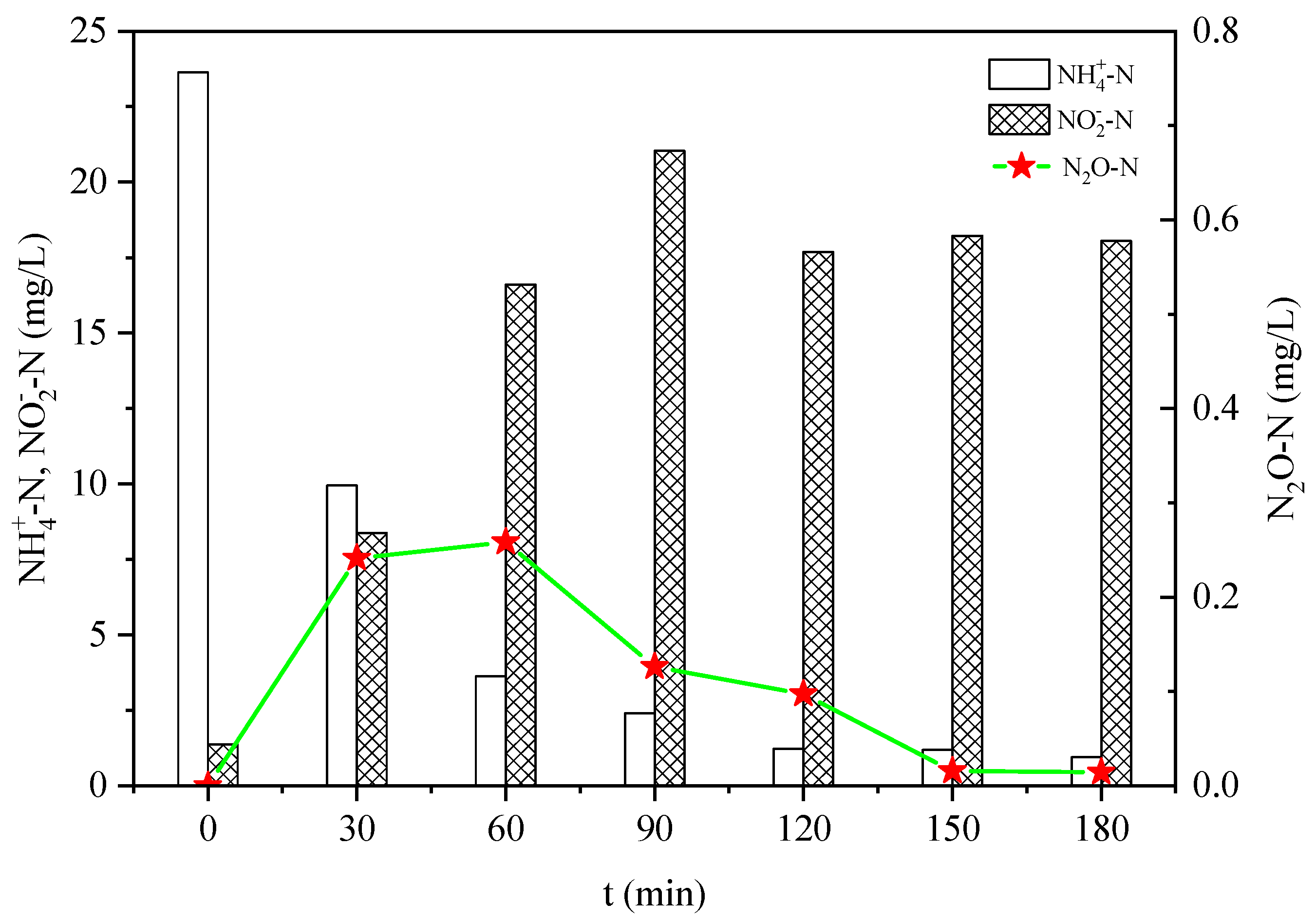
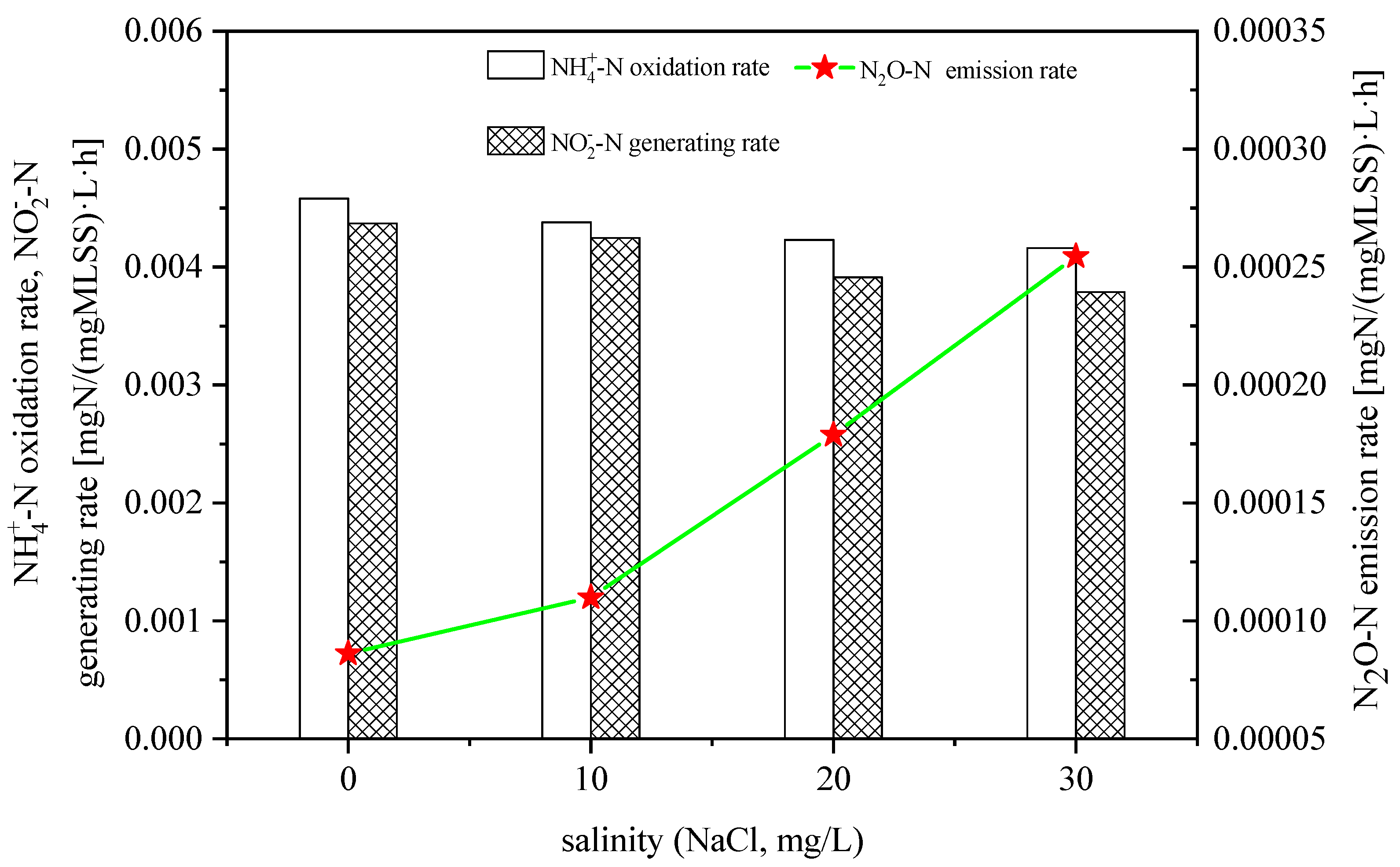
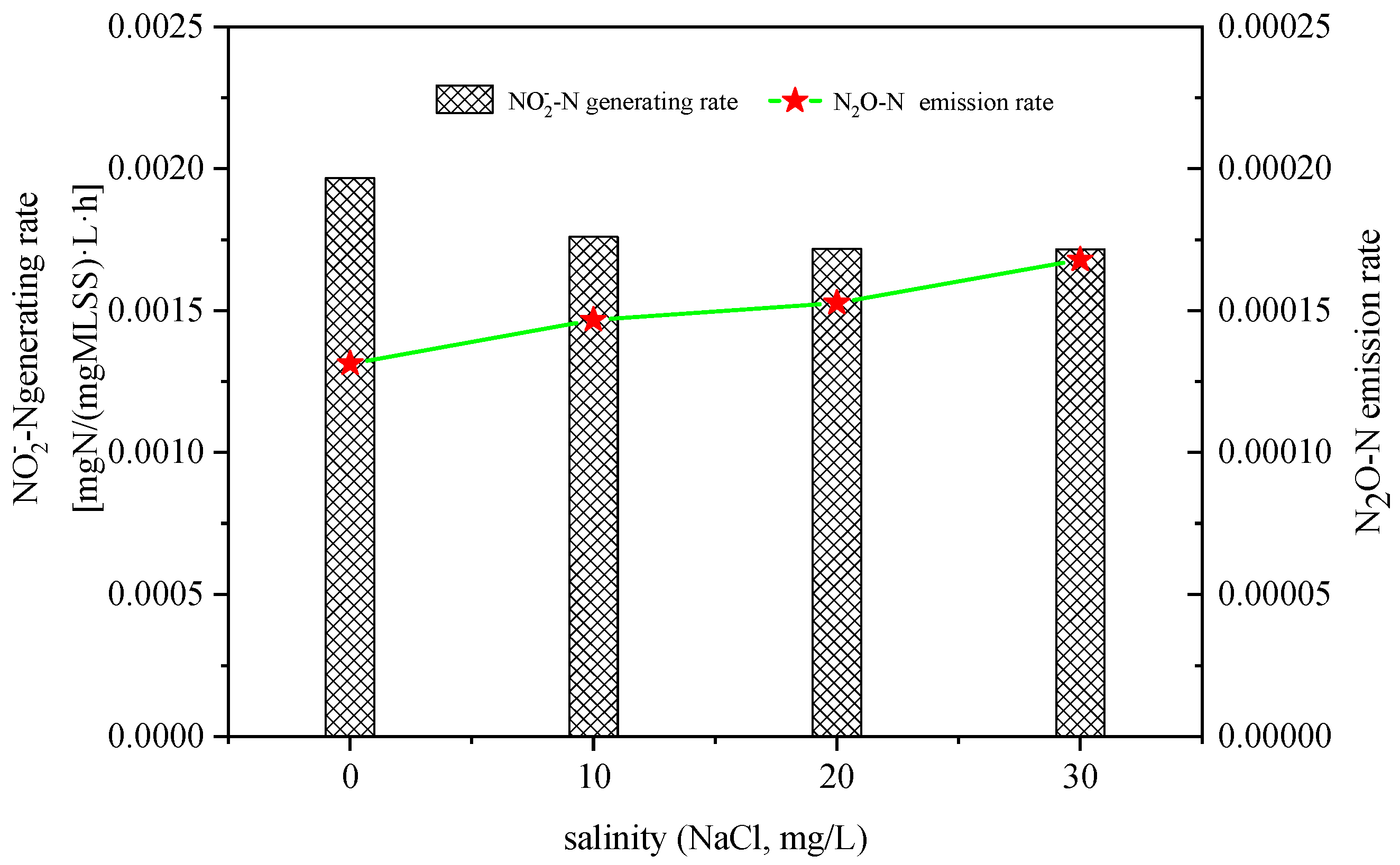
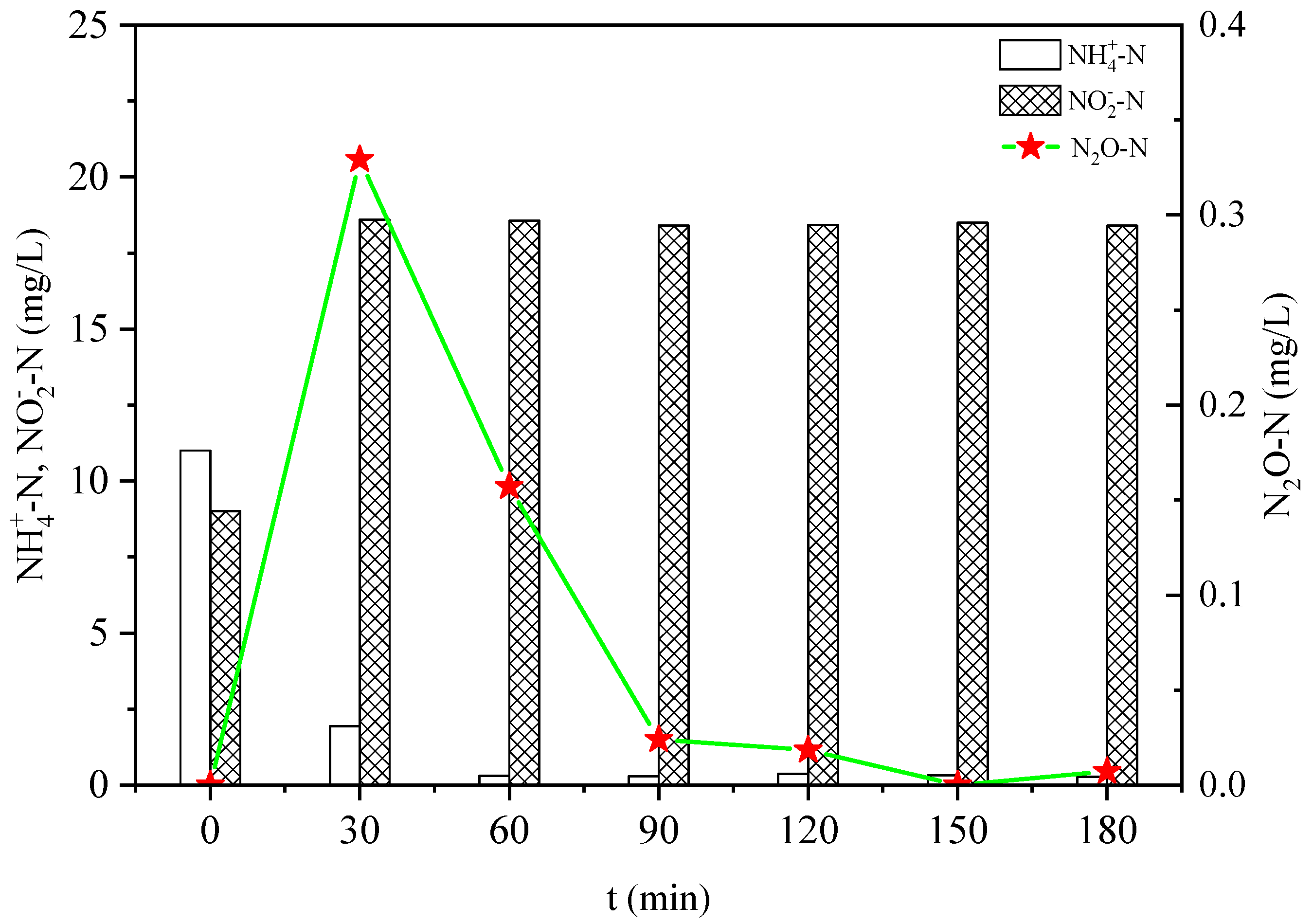
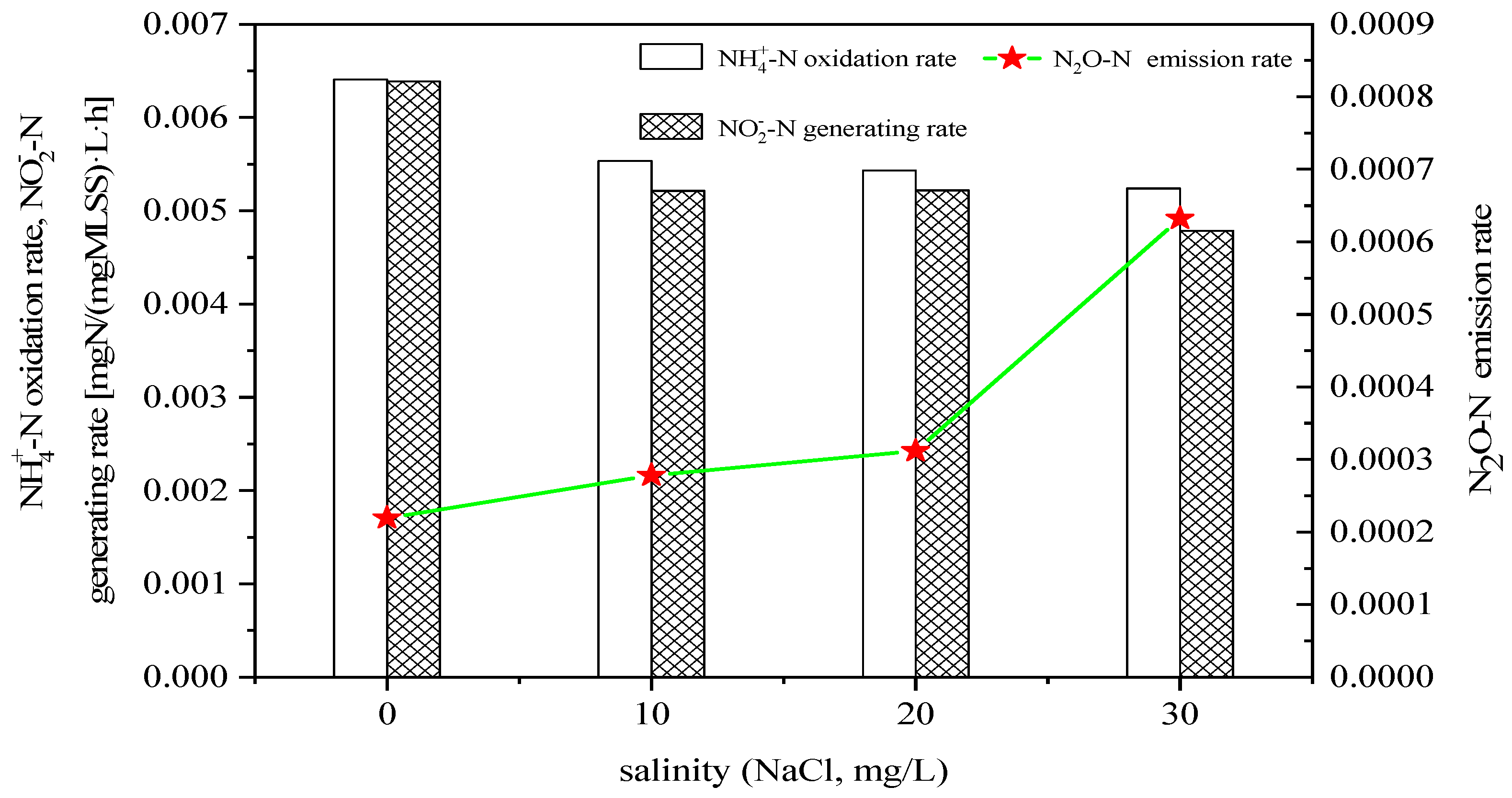
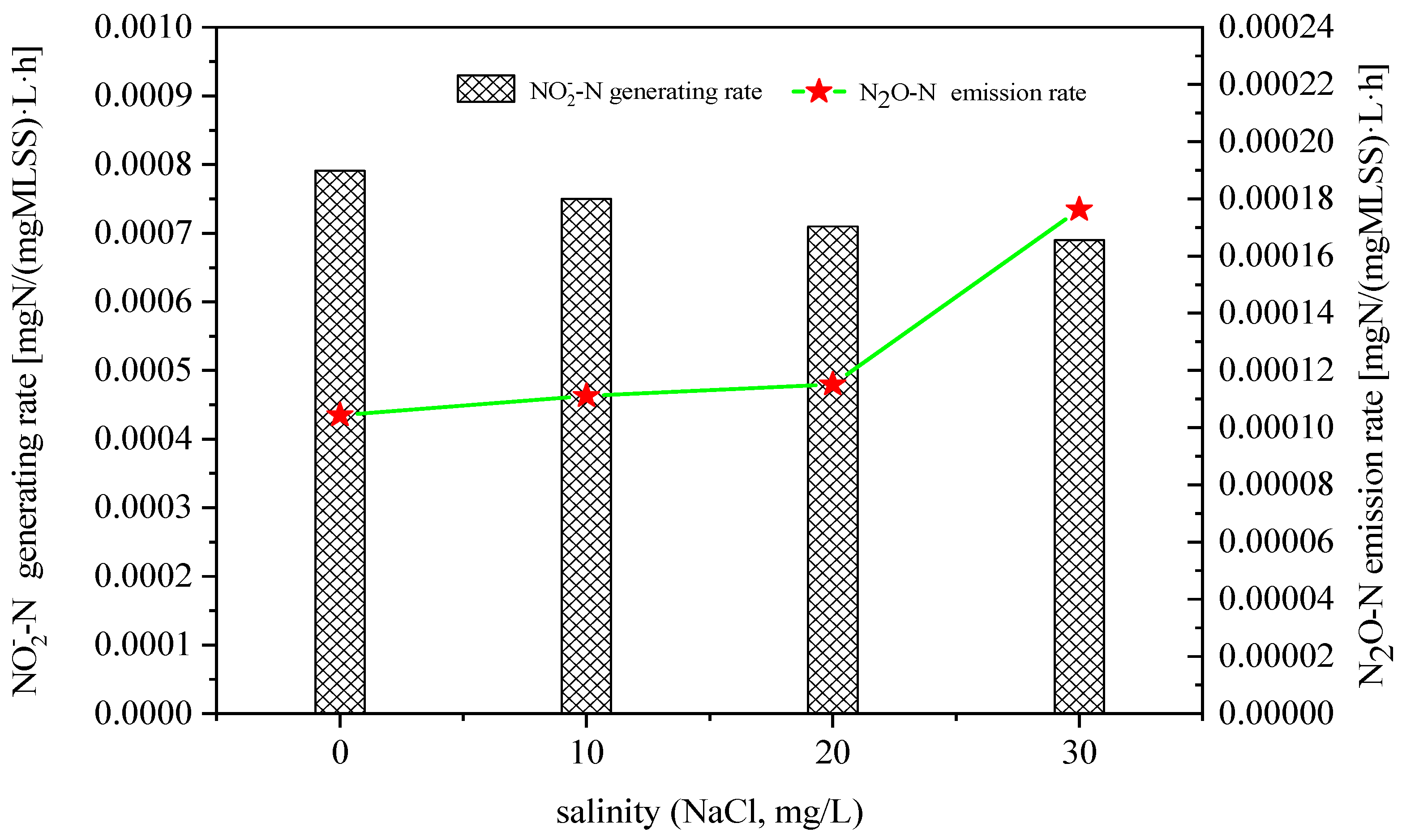
3.1. Effect of Salinity on N2O Emissions in Ammonia Nitrogen Oxidation Process
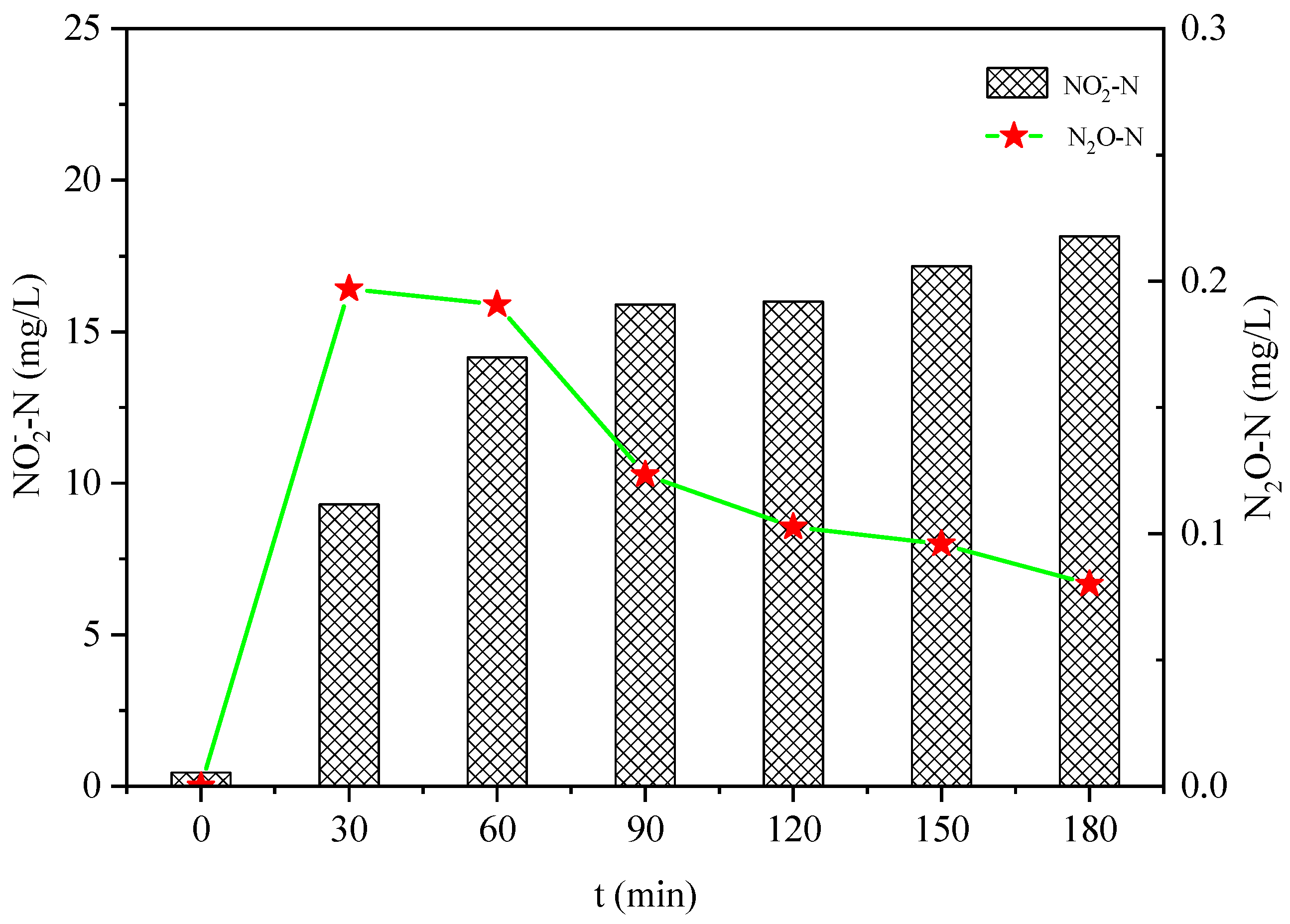
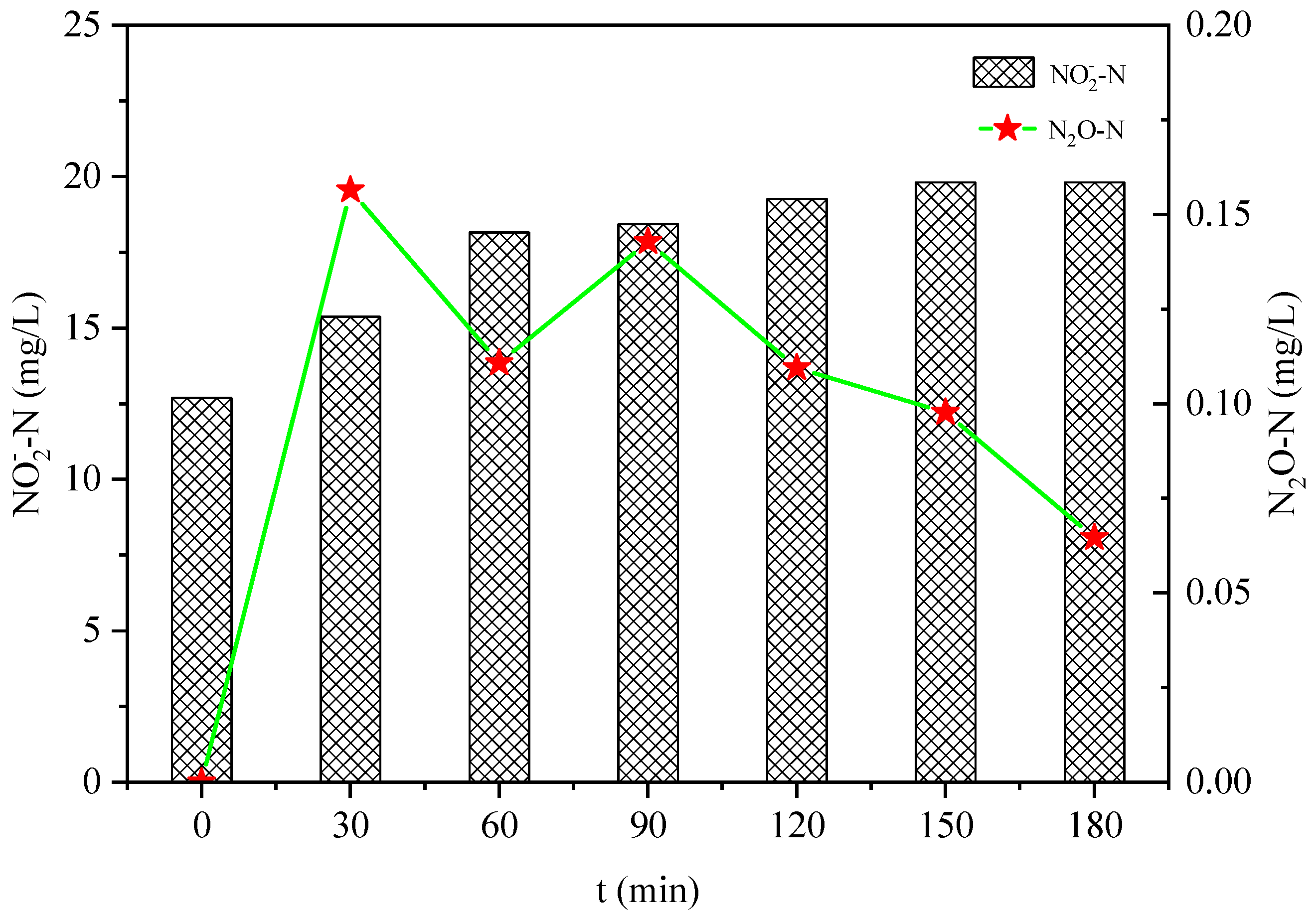
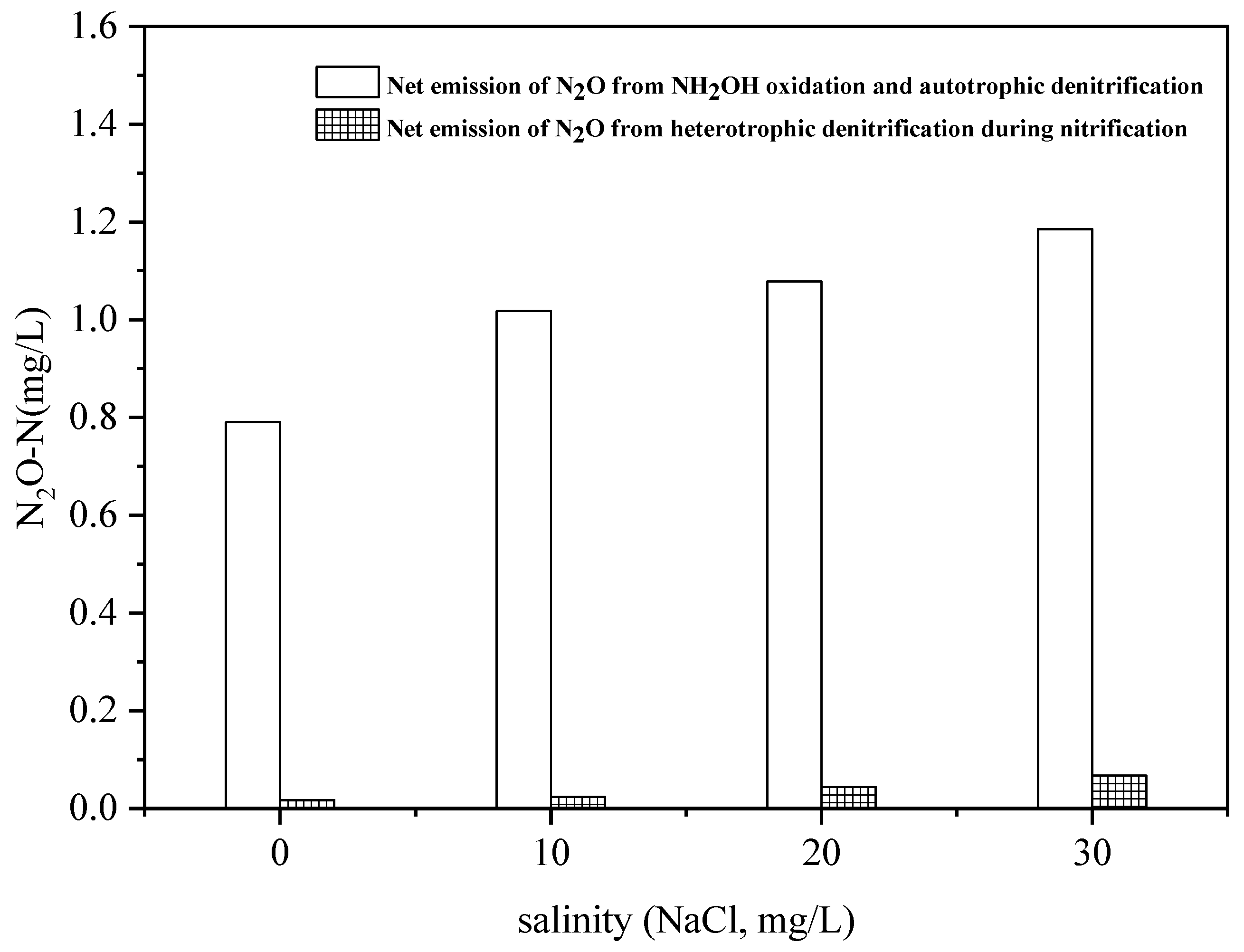
3.2. Effect of Salinity on N2O Emissions in the Hydroxylamine Oxidation Process
3.3. Effect of Salinity on N2O Emissions in the Autotrophic Denitrification Process
3.4. Effect of Salinity on N2O Emissions of Heterotrophic Denitrification during the Nitrification Process
4. Conclusions
- (1)
- The increase in salinity decreased the oxidation rate of NH4+ and the formation rate of NO2− during the reaction.
- (2)
- The increase in salinity increased the N2O emissions during the oxidation of NH4+ and NH2OH, and decreased the production rate of NO2−-N during the oxidation of hydroxylamine.
- (3)
- The total amount of N2O emissions in the hydroxylamine oxidation process was less than that during ammonia nitrogen oxidation, and more NO2− might have been reduced to N2 instead of N2O during hydroxylamine oxidation.
- (4)
- During partial nitrification with the available organic matter, the N2O emissions due to heterotrophic denitrification by heterotrophic bacteria could not be ignored, and the increase in salinity could increase the amount of N2O emissions due to heterotrophic denitrification.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Zhang, G. The practice of flushing toilets with seawater in Hong Kong. Water Supply Drain. China 2000, 16, 49–50. [Google Scholar]
- Zhang, Z.; Sato, Y.; Dai, J.; Chui, H.K.; Daigger, G.; Van Loosdrecht, M.C.M.; Chen, G. Flushing Toilets and Cooling Spaces with Seawater Improve Water-Energy Securities and Achieve Carbon Mitigations in Coastal Cities. Environ. Sci. Technol. 2023, 57, 5068–5078. [Google Scholar] [CrossRef] [PubMed]
- Cantelon, J.A.; Guimond, J.A.; Robinson, C.E.; Michael, M.A.; Kurylyk, B.L. Vertical Saltwater Intrusion in Coastal Aquifers Driven by Episodic Flooding: A Review. Water Resour. Res. 2022, 58, 43–1397. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Scientific Basis; Cambridge University Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.; van Loosdrecht, M.C. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Tallec, G.; Gamier, J.; Billen, G.; Gousailles, M. Nitrous oxide emissions from secondary activated sludge in nitrifying conditions of urban wastewater treatment plants: Effect of oxygenation level. Water Res. 2006, 40, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Arp, D.J.; Stein, L.Y. Metabolism of inorganic N compounds by ammonia-oxidizing bacteria. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 471–495. [Google Scholar] [CrossRef] [PubMed]
- Chandran, K.; Stein, L.Y.; Klotz, M.G.; van Loosdrecht, M.C.M. Nitrous oxide production by lithotrophic ammonia-oxidizing bacteria and implications for engineered nitrogen-removal systems. Biochem. Soc. Trans. 2011, 39, 1832–1837. [Google Scholar] [CrossRef]
- Law, Y.; Ni, B.J.; Lant, P.; Yuan, Z. N2O production rate of an enriched ammonia oxidizing bateria culture exponentially correlates to its ammonia oxidation rate. Water Res. 2012, 46, 3409–3419. [Google Scholar] [CrossRef]
- Stein, L.Y. Surveying N2O-producing pathways in bacteria. In Methods Enzymology: Research on Nitrification and Related Processes; Klotz, M.G., Ed.; Academic Press: Edmonton, AB, Canada, 2010; pp. 131–152. [Google Scholar]
- Kim, S.; Miyahara, M.; Fushinobu, S.; Wakagi, T.; Shoun, H. Nitrous oxide emission from nitrifying activated sludge dependent on denitrification by ammonia-oxidizing bacteria. Bioresour. Technol. 2010, 101, 3958–3963. [Google Scholar] [CrossRef] [PubMed]
- Taseli, B.K. Greenhouse gas emissions from a horizontal subsurface-flow constructed wetland for wastewater treatment in small villages. Glob. Nest J. 2020, 22, 192–196. [Google Scholar]
- Chen, X.; Zhang, S.; Liu, J.; Wang, J.; Xin, Y.; Sun, X.; Xia, X. Tracing Microbial Production and Consumption Sources of N2O in Rivers on the Qinghai-Tibet Plateau via Isotopocule and Functional Microbe Analyses. Environ. Sci. Technol. 2023, 57, 1520–5851. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Jiang, G.; Ye, L.; Pijuan, M.; Yuan, Z. Heterotrophic denitrification plays an important role in N2O production from nitritation reactors treating anaerobic sludge digestion liquor. Water Res. 2014, 62, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Eldyasti, A. Ammonia-Oxidizing Bacteria (AOB): Opportunities and applications-a review. Rev. Environ. Sci. Biotechnol. 2018, 17, 285–321. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kwan, T.; Chandran, K. Comparison of Partial and Full Nitrification Processes Applied for Treating High-Strength Nitrogen Wastewaters: Microbial Ecology through Nitrous Oxide Production. Environ. Sci. Technol. 2011, 45, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; Port City Press: Baltimore, MA, USA, 1998. [Google Scholar]
- Lin, W.; Ding, J.J.; Li, Y.J.; Zheng, Q.; Zhuang, S.; Zhang, D.; Zhou, W.; Qi, Z.; Li, Y. Determination of N2O reduction to N-2 from manure-amended soil based on isotopocule mapping and acetylene inhibition. Atmos. Environ. 2021, 224, 1352–2310. [Google Scholar] [CrossRef]
- Wunderlin, P.; Mohn, J.; Joss, A.; Emmenegger, L.; Siegrist, H. Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res. 2012, 46, 1027–1037. [Google Scholar] [CrossRef]
| COD (mg/L) | NH4+-N (mg/L) | NO2−-N (mg/L) | NO3−-N (mg/L) | TN (mg/L) | pH | Alkalinity | |
|---|---|---|---|---|---|---|---|
| Minimum | 88 | 39.6 | 0 | 0 | 56.4 | 6.9 | 262 |
| Maximum | 276 | 91.2 | 2.8 | 1.2 | 98.5 | 7.7 | 343 |
| Average | 182 | 65.4 | 1.4 | 0.6 | 77.4 | 7.3 | 303 |
| Batch Test Number | Salinity (mg/L NaCl) | Sludge-Water Mixture Type | Initial pH | ATU (mg/L) | NH4+-N (mg/L) | NH2OH-N (mg/L) | NO2−-N (mg/L) | DO (mg/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | Partial nitrification sludge/effluent | 7.5 | 20 | 0.5 | |||
| 10 | 7.5 | 20 | 0.5 | |||||
| 20 | 7.5 | 20 | 0.5 | |||||
| 30 | 7.5 | 20 | 0.5 | |||||
| 2 | 0 | Partial nitrification sludge/effluent | 7.5 | 20 | 0.5 | |||
| 10 | 7.5 | 20 | 0.5 | |||||
| 20 | 7.5 | 20 | 0.5 | |||||
| 30 | 7.5 | 20 | 0.5 | |||||
| 3 | 0 | Partial nitrification sludge/effluent | 7.5 | 10 | 10 | 0.5 | ||
| 10 | 7.5 | 10 | 10 | 0.5 | ||||
| 20 | 7.5 | 10 | 10 | 0.5 | ||||
| 30 | 7.5 | 10 | 10 | 0.5 | ||||
| 4 | 0 | Partial nitrification sludge/effluent | 7.5 | 10 | 10 | 0.5 | ||
| 10 | 7.5 | 10 | 10 | 0.5 | ||||
| 20 | 7.5 | 10 | 10 | 0.5 | ||||
| 30 | 7.5 | 10 | 10 | 0.5 | ||||
| 5 | 0 | Partial nitrification sludge/inflow | 7.5 | 20 | 20 | 0.5 | ||
| 10 | 7.5 | 20 | 20 | 0.5 | ||||
| 20 | 7.5 | 20 | 20 | 0.5 | ||||
| 30 | 7.5 | 20 | 20 | 0.5 | ||||
| 6 | 0 | Partial nitrification sludge/effluent | 7.5 | 20 | 20 | 0.5 | ||
| 10 | 7.5 | 20 | 20 | 0.5 | ||||
| 20 | 7.5 | 20 | 20 | 0.5 | ||||
| 30 | 7.5 | 20 | 20 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wang, Y.; Liu, Y.; Wang, S.; Peng, Y. The Effect of Salinity on N2O Emissions during Domestic Wastewater Partial Nitrification Treatment in a Sequencing Batch Reactor. Water 2023, 15, 3502. https://doi.org/10.3390/w15193502
Li P, Wang Y, Liu Y, Wang S, Peng Y. The Effect of Salinity on N2O Emissions during Domestic Wastewater Partial Nitrification Treatment in a Sequencing Batch Reactor. Water. 2023; 15(19):3502. https://doi.org/10.3390/w15193502
Chicago/Turabian StyleLi, Pengzhang, Yun Wang, Yue Liu, Shuying Wang, and Yongzhen Peng. 2023. "The Effect of Salinity on N2O Emissions during Domestic Wastewater Partial Nitrification Treatment in a Sequencing Batch Reactor" Water 15, no. 19: 3502. https://doi.org/10.3390/w15193502
APA StyleLi, P., Wang, Y., Liu, Y., Wang, S., & Peng, Y. (2023). The Effect of Salinity on N2O Emissions during Domestic Wastewater Partial Nitrification Treatment in a Sequencing Batch Reactor. Water, 15(19), 3502. https://doi.org/10.3390/w15193502






