Recent Progress on Acid Mine Drainage Technological Trends in South Africa: Prevention, Treatment, and Resource Recovery
Abstract
1. Introduction
2. Past Impact of AMD on the Condition of Water in South Africa
| Provinces | Key Areas | Water Resources Impacted | References | |
|---|---|---|---|---|
| Gold | Northwest, Gauteng, Mpumalanga, Limpopo | Within Gauteng: Witwatersrand gold spans the Central and Eastern Basins Within the Witwatersrand Eastern Basin: Brakpan, Springs, Nigel Klerksdorp Kloof, Driefontein, Western Deep Levels | Tweelopiespruit, Hartbeespoort Dam, Crocodile River, Limpopo River, Vaal River, Klip River, Blesbokspruit, Barrage, Vaal Dam | [30,31,32] |
| Coal | Witbank, Delmas, Secunda | Boesmanspruit, Blesbokspruit, Vaal River | [33,34,35] |
3. Possible Future Impacts of AMD on the State of Water in South Africa
4. AMD Prevention
5. AMD Impact
6. Current Treatment Technologies and Resource Recovery
7. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baloyi, J.; Seadira, T.; Raphulu, M.; Ochieng, A. Preparation, Characterization and Growth Mechanism of Dandelion-like TiO2 Nanostructures and Their Application in Photocatalysis towards Reduction of Cr(VI). Mater. Today Proc. 2015, 2, 3973–3987. [Google Scholar] [CrossRef]
- Seadira, T.; Baloyi, J.; Raphulu, M.; Moutloali, R.; Ochieng, A. Acid Mine Drainage Treatment Using Constructed Wetland. In Proceedings of the International Conference on Chemical, Integrated Waste Management and Environmental Engineering, Johannesburg, South Africa, 15–16 April 2014. [Google Scholar]
- Ighalo, J.O.; Kurniawan, S.B.; Iwuozor, K.O.; Aniagor, C.O.; Ajala, O.J.; Oba, S.N.; Iwuchukwu, F.U.; Ahmadi, S.; Igwegbe, C.A. A Review of Treatment Technologies for the Mitigation of the Toxic Environmental Effects of Acid Mine Drainage (AMD). Process Saf. Environ. Prot. 2022, 157, 37–58. [Google Scholar] [CrossRef]
- Nepfumbada, C.; Tavengwa, N.T.; Masindi, V.; Foteinis, S.; Chatzisymeon, E. Recovery of Phosphate from Municipal Wastewater as Calcium Phosphate and Its Subsequent Application for the Treatment of Acid Mine Drainage. Resour. Conserv. Recycl. 2023, 190, 106779. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid Mine Drainage Remediation Options: A Review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Daraz, U.; Li, Y.; Ahmad, I.; Iqbal, R.; Ditta, A. Remediation Technologies for Acid Mine Drainage: Recent Trends and Future Perspectives. Chemosphere 2023, 311, 137089. [Google Scholar] [CrossRef]
- Larochelle, T.; Noble, A.; Ziemkiewicz, P.; Hoffman, D.; Constant, J. A Fundamental Economic Assessment of Recovering Rare Earth Elements and Critical Minerals from Acid Mine Drainage Using a Network Sourcing Strategy. Minerals 2021, 11, 1298. [Google Scholar] [CrossRef]
- Laker, M.C. Environmental Impacts of Gold Mining—With Special Reference to South Africa. Mining 2023, 3, 205–220. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, C.; Su, P.; Tang, Y.; Huang, Z.; Ma, T. A Review of Acid Mine Drainage: Formation Mechanism, Treatment Technology, Typical Engineering Cases and Resource Utilization. Process Saf. Environ. Prot. 2023, 170, 1240–1260. [Google Scholar] [CrossRef]
- Bai, S.J.; Li, J.; Yuan, J.Q.; Bi, Y.X.; Ding, Z.; Dai, H.X.; Wen, S.M. An Innovative Option for the Activation of Chalcopyrite Flotation Depressed in a High Alkali Solution with the Addition of Acid Mine Drainage. J. Cent. South Univ. 2023, 30, 811–822. [Google Scholar] [CrossRef]
- Azapagic, A. Developing a Framework for Sustainable Development Indicators for the Mining and Minerals Industry. J. Clean. Prod. 2004, 12, 639–662. [Google Scholar] [CrossRef]
- Rezaie, B.; Anderson, A. Sustainable Resolutions for Environmental Threat of the Acid Mine Drainage. Sci. Total Environ. 2020, 717, 137211. [Google Scholar] [CrossRef]
- Iakovleva, E.; Mäkilä, E.; Salonen, J.; Sitarz, M.; Wang, S.; Sillanpää, M. Acid Mine Drainage (AMD) Treatment: Neutralization and Toxic Elements Removal with Unmodified and Modified Limestone. Ecol. Eng. 2015, 81, 30–40. [Google Scholar] [CrossRef]
- Vaziri Hassas, B.; Shekarian, Y.; Rezaee, M. Selective Precipitation of Rare Earth and Critical Elements from Acid Mine Drainage—Part I: Kinetics and Thermodynamics of Staged Precipitation Process. Resour. Conserv. Recycl. 2023, 188, 106654. [Google Scholar] [CrossRef]
- Al-Zoubi, H.; Rieger, A.; Steinberger, P.; Pelz, W.; Haseneder, R.; Härtel, G. Optimization Study for Treatment of Acid Mine Drainage Using Membrane Technology. Sep. Sci. Technol. 2010, 45, 2004–2016. [Google Scholar] [CrossRef]
- Felipe, E.C.B.; Batista, K.A.; Ladeira, A.C.Q. Recovery of Rare Earth Elements from Acid Mine Drainage by Ion Exchange. Environ. Technol. 2021, 42, 2721–2732. [Google Scholar] [CrossRef]
- van Rooyen, M.; van Staden, P.J.; du Preez, K.A. Sulphate Removal Technologies for the Treatment of Mine-Impacted Water. J. S. Afr. Inst. Min. Metall. 2021, 121, 523–530. [Google Scholar] [CrossRef]
- Mondaca, S.L.; Leiva, C.A.; Acuña, C.A.; Serey, E.A. Flow Enhancement of Mineral Pastes to Increase Water Recovery in Tailings: A Matlab-Based Imaging Processing Tool. Sci. Program. 2020, 2020, 5607242. [Google Scholar] [CrossRef]
- Chen, G.; Ye, Y.; Yao, N.; Hu, N.; Zhang, J.; Huang, Y. A Critical Review of Prevention, Treatment, Reuse, and Resource Recovery from Acid Mine Drainage. J. Clean. Prod. 2021, 329, 129666. [Google Scholar] [CrossRef]
- Hassas, B.V.; Rezaee, M.; Pisupati, S.V. Precipitation of Rare Earth Elements from Acid Mine Drainage by CO2 Mineralization Process. Chem. Eng. J. 2020, 399, 125716. [Google Scholar] [CrossRef]
- Cicek, Z. Selective Recovery of Rare Earth Elements from Acid Mine Selective Recovery of Rare Earth Elements from Acid Mine Drainage Treatment Byproduct Drainage Treatment Byproduct Recommended Citation Recommended Citation. Master’s Thesis, Statler College of Engineering and Mineral Resources, Morgantown, WV, USA, 2023. [Google Scholar]
- Elghali, A.; Benzaazoua, M.; Taha, Y.; Amar, H.; Ait-khouia, Y.; Bouzahzah, H.; Hakkou, R. Prediction of Acid Mine Drainage: Where We Are. Earth Sci. Rev. 2023, 241, 104421. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, G.; Zhou, C.; Zhou, H.; Wei, Y.; Liu, Y. The Influence of Gold Mining Wastes on the Migration-Transformation Behavior and Health Risks of Arsenic in the Surrounding Soil of Mined-Area. Front. Earth Sci. 2023, 10, 1068763. [Google Scholar] [CrossRef]
- Mukolu, N. The effect of waste management of oil drilling and gold mining extraction in the yakutia arctic (Russian). Am. J. Humanit. Soc. Sci. Res. (AJHSSR) 2023, 7, 59–72. [Google Scholar]
- Cacciuttolo, C.; Marinovic, A. Experiences of Underground Mine Backfilling Using Mine Tailings Developed in the Andean Region of Peru: A Green Mining Solution to Reduce Socio-Environmental Impacts. Sustainability 2023, 15, 12912. [Google Scholar] [CrossRef]
- Yuan, S.; Sui, W.; Han, G.; Duan, W. An Optimized Combination of Mine Water Control, Treatment, Utilization, and Reinjection for Environmentally Sustainable Mining: A Case Study. Mine Water Environ. 2022, 41, 828–839. [Google Scholar] [CrossRef]
- Gonah, T. Impact of Acid Mine Drainage on Water Resources in South Africa. In Management and Mitigation of Acid Mine Drainage in South Africa: Input for Mineral Beneficiation in Africa; Africa Institute of South Africa: Pretoria, South Africa, 2016; pp. 41–65. [Google Scholar] [CrossRef]
- Abiye, T.A.; Ali, K.A. Potential Role of Acid Mine Drainage Management towards Achieving Sustainable Development in the Johannesburg Region, South Africa. Groundw. Sustain. Dev. 2022, 19, 100839. [Google Scholar] [CrossRef]
- Windisch, J.; Gradwohl, A.; Gilbert, B.M.; Dos Santos, Q.M.; Wallner, G.; Avenant-Oldewage, A.; Jirsa, F. Toxic Elements in Sediment and Water of the Crocodile River (West) System, South Africa, Following Acid Mine Drainage. Appl. Sci. 2022, 12, 10531. [Google Scholar] [CrossRef]
- Minnaar, A. Water Pollution and Contamination from Gold Mines: Acid Mine Drainage in Gauteng Province, South Africa. In Water, Governance, and Crime Issues; Springer: Cham, Switzerland, 2020; pp. 193–219. [Google Scholar] [CrossRef]
- Nofal, A.P.; Dos Santos, Q.M.; Jirsa, F.; Avenant-Oldewage, A. Camallanid Nematodes from Clarias Gariepinus (Burchell, 1822) in the Crocodile River, Gauteng, South Africa: Exploring Diversity and Divergence in an Acid-Mine Drainage Impacted Environment. Int. J. Parasitol. Parasites Wildl. 2022, 19, 196–210. [Google Scholar] [CrossRef]
- Ouma, K.O.; Shane, A.; Syampungani, S. Aquatic Ecological Risk of Heavy-Metal Pollution Associated with Degraded Mining Landscapes of the Southern Africa River Basins: A Review. Minerals 2022, 12, 225. [Google Scholar] [CrossRef]
- Atangana, E. Evaluation of the Impact of Coal Mining on Surface Water in the Boesmanspruit, Mpumalanga, South Africa. 2023. Available online: https://doi.org/10.21203/RS.3.RS-3184680/V1 (accessed on 3 August 2023).
- Simpson, G.B.; Badenhorst, J.; Jewitt, G.P.W.; Berchner, M.; Davies, E. Competition for Land: The Water-Energy-Food Nexus and Coal Mining in Mpumalanga Province, South Africa. Front. Environ. Sci. 2019, 7, 422006. [Google Scholar] [CrossRef]
- Sakala, E.; Novhe, O.; Kumar Vadapalli, V.R. Application of Artificial Intelligence (AI) to Predict Mine Water Quality, a Case Study in South Africa. In Proceedings of the Mine Water Association Conference: Technological and Ecological Challenges, International Mine Water Association Annual Conference, Perm, Russia, 15–19 July 2019. [Google Scholar]
- McCarthy, T.S. The Impact of Acid Mine Drainage in South Africa. S. Afr. J. Sci. 2011, 107, 1–7. [Google Scholar] [CrossRef]
- Naidoo, S. Social Constructions of Water Quality in South Africa: A Case Study of the Blesbokspruit River in the Context of Acid Mine Drainage Treatment; Springer Nature: Berlin/Heidelberg, Germany, 2022; pp. 1–219. [Google Scholar] [CrossRef]
- Lourenco, M.; Curtis, C. The Influence of a High-Density Sludge Acid Mine Drainage (AMD) Chemical Treatment Plant on Water Quality along the Blesbokspruit Wetland, South Africa. Water SA 2021, 47, 35–44. [Google Scholar] [CrossRef]
- Scott, R. Flooding of the Central and East Rand Gold Mines; WRC Report 486/1/95; Water Research Commission: Pretoria, South Africa, 1995. [Google Scholar]
- Addo-Bediako, A. Comparative Spatial Assessment of Trace Metal(Loid) Pollution in the Sediments of the Lower Olifants River Basin in South Africa. Front. Environ. Sci. 2022, 10, 882393. [Google Scholar] [CrossRef]
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.P.; Tekere, M.; Chatzisymeon, E. Challenges and Avenues for Acid Mine Drainage Treatment, Beneficiation, and Valorisation in Circular Economy: A Review. Ecol. Eng. 2022, 183, 106740. [Google Scholar] [CrossRef]
- Wolkersdorfer, C. Mine Water Treatment-Active and Passive Methods; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 3662657694. [Google Scholar]
- Marr, S.M.; Swemmer, A.M. Hydrological Characteristics of Extreme Floods in the Klaserie River, a Headwater Stream in Southern Africa. J. Limnol. 2023, 82. [Google Scholar] [CrossRef]
- Netshitungulwana, K.R.T.; Gauert, C.; Vermeulen, D.; Yibas, B.; Shai, M.; Lusunzi, R. Geochemical Characterisation of the Witbank Coalfield Geological Strata and Assessment of Potential Metal Impact on the Receiving Environment. In Proceedings of the International Mine Water Association 2022 Conference Reconnect, Christchurch, New Zealand, 6–10 November 2022. [Google Scholar]
- Obaid, A.; Adam, E.; Ali, K.A. Land Use and Land Cover Change in the Vaal Dam Catchment, South Africa: A Study Based on Remote Sensing and Time Series Analysis. Geomatics 2023, 3, 205–220. [Google Scholar] [CrossRef]
- Alexander, A.C.; Ndambuki, J.M. Impact of Mine Closure on Groundwater Resource: Experience from Westrand Basin-South Africa. Phys. Chem. Earth Parts A/B/C 2023, 131, 103432. [Google Scholar] [CrossRef]
- du Plessis, A. Progressive Deterioration of Water Quality Within South Africa. In South Africa’s Water Predicament: Freshwater’s Unceasing Decline; Springer: Berlin/Heidelberg, Germany, 2023; pp. 109–141. [Google Scholar]
- Wood, D.L.; Cole, K.A.; Herndon, E.M.; Singer, D.M. Lime Slurry Treatment of Soils Developing on Abandoned Coal Mine Spoil: Linking Contaminant Transport from the Micrometer to Pedon-Scale. Appl. Geochem. 2023, 151, 105617. [Google Scholar] [CrossRef]
- Bondarenko, V.I.; Kovalevska, I.A.; Podkopaiev, S.V.; Sheka, I.V.; Tsivka, Y.S. Substantiating Arched Support Made of Composite Materials (Carbon Fiber-Reinforced Plastic) for Mine Workings in Coal Mines. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1049, p. 012026. [Google Scholar]
- Camenzuli, D. Development of Orthophosphate and Silica Treatments for the Management of Environmental Contaminants at Wilkes Landfill, East Antarctica. Ph.D. Thesis, Macquarie University, Sydney, Australia, 2015. [Google Scholar]
- Mulopo, J. Active Physical Remediation of Acid Mine Drainage: Technologies Review and Perspectives. J. Ecol. Eng. 2022, 23, 148–163. [Google Scholar] [CrossRef]
- Alekseyev, V.A. Reasons for the Formation of Acidic Drainage Water in Dumps of Sulfide-Containing Rocks. Geochem. Int. 2022, 60, 78–91. [Google Scholar] [CrossRef]
- Markovic, R.; Bessho, M.; Masuda, N.; Stevanovic, Z.; Bozic, D.; Trujic, T.A.; Gardic, V. New Approach of Metals Removal from Acid Mine Drainage. Appl. Sci. 2020, 10, 5925. [Google Scholar] [CrossRef]
- Humphries, M.S.; McCarthy, T.S.; Pillay, L. Attenuation of Pollution Arising from Acid Mine Drainage by a Natural Wetland on the Witwatersrand. S. Afr. J. Sci. 2017, 113, 9. [Google Scholar] [CrossRef]
- Seervi, V.; Yadav, H.L.; Srivastav, S.K.; Jamal, A. Overview of Active and Passive Systems for Treating Acid Mine Drainage. IARJSET 2017, 4, 131–137. [Google Scholar] [CrossRef]
- Porter, C.M.; Nairn, R.W. Ecosystem Functions within a Mine Drainage Passive Treatment System. Ecol. Eng. 2008, 32, 337–346. [Google Scholar] [CrossRef]
- Ramla, B.; Sheridan, C. The Potential Utilisation of Indigenous South African Grasses for Acid Mine Drainage Remediation. Water SA 2015, 41, 247. [Google Scholar] [CrossRef]
- Qian, G.; Li, Y. Acid and Metalliferous Drainage–A Global Environmental Issue. J. Min. Mech. Eng. 2019, 1, 1–4. [Google Scholar] [CrossRef]
- Dama-Fakir, P.; Sithole, Z.; van Niekerk, A.M.; Dateling, J.; Maree, J.P.; Rukuni, T.; Mthombeni, T.; Ruto, S.; Zikalala, N.; Hughes, C.; et al. Mine Water Treatment Technology Selection Tool: Users’ Guide (TT 711/17); Water Research Commission: Pretoria, South Africa, 2017. [Google Scholar]
- Bwapwa, J.K. A Review of Acid Mine Drainage in a Water-Scarce Country: Case of South Africa. Environ. Manag. Sustain. Dev. 2017, 7, 1. [Google Scholar] [CrossRef]
- Trumm, D. Selection of Active and Passive Treatment Systems for AMDflow Charts for New Zealand Conditions. N. Z. J. Geol. Geophys. 2010, 53, 195–210. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Sarkar, D.; Datta, R. Remediation of Acid Mine Drainage-Impacted Water. Curr. Pollut. Rep. 2015, 1, 131–141. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, Treatment and Case Studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Nleya, Y.; Simate, G.S.; Ndlovu, S. Sustainability Assessment of the Recovery and Utilisation of Acid from Acid Mine Drainage. J. Clean. Prod. 2016, 113, 17–27. [Google Scholar] [CrossRef]
- Yuan, J.; Ding, Z.; Bi, Y.; Li, J.; Wen, S.; Bai, S. Resource Utilization of Acid Mine Drainage (AMD): A Review. Water 2022, 14, 2385. [Google Scholar] [CrossRef]
- Brewster, E.T.; Freguia, S.; Edraki, M.; Berry, L.; Ledezma, P. Staged Electrochemical Treatment Guided by Modelling Allows for Targeted Recovery of Metals and Rare Earth Elements from Acid Mine Drainage. J. Environ. Manag. 2020, 275, 111266. [Google Scholar] [CrossRef]
- Kaur, G.; Couperthwaite, S.J.; Hatton-Jones, B.W.; Millar, G.J. Alternative Neutralisation Materials for Acid Mine Drainage Treatment. J. Water Process Eng. 2018, 22, 46–58. [Google Scholar] [CrossRef]
- Acharya, B.S.; Kharel, G. Acid Mine Drainage from Coal Mining in the United States—An Overview. J. Hydrol. 2020, 588, 125061. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid Mine Drainage: Prevention, Treatment Options, and Resource Recovery: A Review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil. Pollut. 2020, 231, 1–17. [Google Scholar] [CrossRef]
- Saleem, J.; Bin Shahid, U.; Hijab, M.; Mackey, H.; McKay, G. Production and Applications of Activated Carbons as Adsorbents from Olive Stones. Biomass Convers. Biorefinery 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Simate, G.S.; Ndlovu, S. Acid Mine Drainage: Challenges and Opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Ruihua, L.; Lin, Z.; Tao, T.; Bo, L. Phosphorus Removal Performance of Acid Mine Drainage from Wastewater. J. Hazard. Mater. 2011, 190, 669–676. [Google Scholar] [CrossRef]
- Kumari, M.; Bhattacharya, T. A Review on Bioaccessibility and the Associated Health Risks Due to Heavy Metal Pollution in Coal Mines: Content and Trend Analysis. Environ. Dev. 2023, 46, 100859. [Google Scholar] [CrossRef]
- Orlović-Leko, P.; Farkaš, B.; Galić, I. A Short Review of Environmental and Health Impacts of Gold Mining. Reliab. Theory Appl. 2022, 4, 242–248. [Google Scholar]
- Li, S.; Yu, L.; Jiang, W.; Yu, H.; Wang, X. The Recent Progress China Has Made in Green Mine Construction, Part I: Mining Groundwater Pollution and Sustainable Mining. Int. J. Environ. Res. Public. Health 2022, 19, 5673. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, B.; Liu, G. Groundwater Risk Assessment of Abandoned Mines Based on Pressure-State-Response—The Example of an Abandoned Mine in Southwest China. Energy Rep. 2022, 8, 10728–10740. [Google Scholar] [CrossRef]
- Weinberg, R.; Coyte, R.; Wang, Z.; Das, D.; Vengosh, A. Water Quality Implications of the Neutralization of Acid Mine Drainage with Coal Fly Ash from India and the United States. Fuel 2022, 330, 125675. [Google Scholar] [CrossRef]
- Li, Q.; Ji, B.; Honaker, R.; Noble, A.; Zhang, W. Partitioning Behavior and Mechanisms of Rare Earth Elements during Precipitation in Acid Mine Drainage. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128563. [Google Scholar] [CrossRef]
- Marove, C.A.; Sotozono, R.; Tangviroon, P.; Tabelin, C.B.; Igarashi, T. Assessment of Soil, Sediment and Water Contaminations around Open-Pit Coal Mines in Moatize, Tete Province, Mozambique. Environ. Adv. 2022, 8, 100215. [Google Scholar] [CrossRef]
- Thisani, S.K.; Von Kallon, D.V.; Byrne, P. A Fixed Bed Pervious Concrete Anaerobic Bioreactor for Biological Sulphate Remediation of Acid Mine Drainage Using Simple Organic Matter. Sustainability 2021, 13, 6529. [Google Scholar] [CrossRef]
- Lozano, A.; Ayora, C.; Fernández-Martínez, A. Sorption of Rare Earth Elements on Schwertmannite and Their Mobility in Acid Mine Drainage Treatments. Appl. Geochem. 2020, 113, 104499. [Google Scholar] [CrossRef]
- Song, G.; Wang, X.; Romero, C.; Chen, H.; Yao, Z.; Kaziunas, A.; Schlake, R.; Anand, M.; Lowe, T.; Driscoll, G. Extraction of Selected Rare Earth Elements from Anthracite Acid Mine Drainage Using Supercritical CO2 via Coagulation and Complexation. J. Rare Earths 2021, 39, 83–89. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.; Fang, X.; Shi, T.; Tan, K. Recovery of Bio-sulfur and Metal Resources from Mine Wastewater by Sulfide Biological Oxidation-Alkali Flocculation: A Pilot-Scale Study. Sci. Total Environ. 2023, 876, 162546. [Google Scholar] [CrossRef]
- Vo, T.D.H.; Nguyen, B.S.; Vu, C.T.; Shih, Y.J.; Huang, Y.H. Recovery of Iron (II) and Aluminum (III) from Acid Mine Drainage by Sequential Selective Precipitation and Fluidized Bed Homogeneous Crystallization (FBHC). J. Taiwan Inst. Chem. Eng. 2020, 115, 135–143. [Google Scholar]
- Maroufi, N.; Hajilary, N. Nanofiltration Membranes Types and Application in Water Treatment: A Review. Sustain. Water Resour. Manag. 2023, 9, 142. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Y.; Wu, G.; Li, J.; Ren, Y.; Duan, X. Investigation on Nanofiltration Membrane Fouling Behaviour of Cation-Induced Apam in Strontium-Bearing Mine Water. J. Environ. Chem. Eng. 2023, 11, 110940. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Ahmad, N.N.R.; Teow, Y.H. Role of Nanofiltration Process for Sustainability in Industries: Reuse, Recycle, and Resource Recovery. In Nanofiltration for Sustainability; CRC Press: Boca Raton, FL, USA, 2023; pp. 1–13. [Google Scholar]
- Andalaft, J.; Schwarz, A.; Pino, L.; Fuentes, P.; Bόrquez, R.; Aybar, M. Assessment and Modeling of Nanofiltration of Acid Mine Drainage. Ind. Eng. Chem. Res. 2018, 57, 14727–14739. [Google Scholar] [CrossRef]
- Wadekar, S.S.; Vidic, R.D. Comparison of Ceramic and Polymeric Nanofiltration Membranes for Treatment of Abandoned Coal Mine Drainage. Desalination 2018, 440, 135–145. [Google Scholar] [CrossRef]
- Masindi, V. Recovery of Drinking Water and Valuable Minerals from Acid Mine Drainage Using an Integration of Magnesite, Lime, Soda Ash, CO2 and Reverse Osmosis Treatment Processes. J. Environ. Chem. Eng. 2017, 5, 3136–3142. [Google Scholar] [CrossRef]
- León-Venegas, E.; Vilches-Arenas, L.F.; Fernández-Baco, C.; Arroyo-Torralvo, F. Potential for Water and Metal Recovery from Acid Mine Drainage by Combining Hybrid Membrane Processes with Selective Metal Precipitation. Resour. Conserv. Recycl. 2023, 188, 106629. [Google Scholar] [CrossRef]
- Asif, M.B.; Price, W.E.; Fida, Z.; Tufail, A.; Ren, T.; Hai, F.I. Acid Mine Drainage and Sewage Impacted Groundwater Treatment by Membrane Distillation: Organic Micropollutant and Metal Removal and Membrane Fouling. J. Environ. Manag. 2021, 291, 112708. [Google Scholar] [CrossRef]
- Mulopo, J.; Zvimba, J.N.; Swanepoel, H.; Bologo, L.T.; Maree, J. Regeneration of Barium Carbonate from Barium Sulphide in a Pilot-Scale Bubbling Column Reactor and Utilization for Acid Mine Drainage. Water Sci. Technol. 2012, 65, 324–331. [Google Scholar] [CrossRef]
- Motaung, S.; Maree, J.; De Beer, M.; Bologo, L.; Theron, D.; Baloyi, J. Recovery of Drinking Water and By-Products from Gold Mine Effluents. Int. J. Water Resour. Dev. 2008, 24, 433–450. [Google Scholar] [CrossRef]
- Swanepoel, H.; de Beer, M.; Liebenberg, L. Complete Sulphate Removal from Neutralised Acidic Mine Drainage with Barium Carbonate. Water Pract. Technol. 2012, 7, wpt2012003. [Google Scholar] [CrossRef]
- De Beer, M.; Maree, J.P.; Wilsenach, J.; Motaung, S.; Bologo, L.; Radebe, V. Acid Mine Water Reclamation Using the ABC Process. In Proceedings of the International Mine Water Association Symposium, Sydney, NS, Canada, 4–9 September 2010. [Google Scholar]
- Bologo, V.; Maree, J.P.; Carlsson, F. Application of Magnesium Hydroxide and Barium Hydroxide for the Removal of Metals and Sulphate from Mine Water. Water SA 2012, 38, 23–28. [Google Scholar] [CrossRef]
- Masindi, V.; Chatzisymeon, E.; Kortidis, I.; Foteinis, S. Assessing the Sustainability of Acid Mine Drainage (AMD) Treatment in South Africa. Sci. Total Environ. 2018, 635, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Masindi, V.; Osman, M.S.; Shingwenyana, R. Valorization of Acid Mine Drainage (AMD): A Simplified Approach to Reclaim Drinking Water and Synthesize Valuable Minerals—Pilot Study. J. Environ. Chem. Eng. 2019, 7, 103082. [Google Scholar] [CrossRef]
- Petterson, D. Addressing Legacy Challenges. Inside Min. 2018, 11, 22–23. [Google Scholar]
- van Rooyen, M.; van Staden, P.J. Deriving Value from Acid Mine Drainage. In Recovery of Byproducts from Acid Mine Drainage Treatment; Scrivener Publishing: Beverly, MA, USA, 2020; pp. 235–261. [Google Scholar] [CrossRef]
- Robertson, A.M.; Everett, D.J.; Du Plessis, N.J. Sulfates Removal by the GYP-CIX Process Following Lime Treatment. In Proceedings of the Superfund XIV Conference and Exhibition, Washington, DC, USA, 30 November–2 December 1993. [Google Scholar]
- Fernando, W.A.M.; Ilankoon, I.M.S.K.; Syed, T.H.; Yellishetty, M. Challenges and Opportunities in the Removal of Sulphate Ions in Contaminated Mine Water: A Review. Miner. Eng. 2018, 117, 74–90. [Google Scholar] [CrossRef]
- Sullivan, D.; Arena, B.; de Vegt, A.; Buisman, C.; Jannsen, A. Converting Sulfide Biologically. In Proceedings of the PETSOC Annual Technical Meeting, Calgary, AB, Canada, 8–11 June 1997. [Google Scholar]
- Dhir, B. Biotechnological Tools for Remediation of Acid Mine Drainage (Removal of Metals from Wastewater and Leachate). In Bio-Geotechnologies for Mine Site Rehabilitation; Elsevier: London, UK, 2018; pp. 67–82. [Google Scholar] [CrossRef]
- Rose, P. Review: Long-Term Sustainability in the Management of Acid Mine Drainage Wastewaters Development of the Rhodes BioSURE Process. Water SA 2013, 39, 582. [Google Scholar] [CrossRef][Green Version]
- Rose, P.; Corbett, C.; Neba, A. Sewage Sludge as an Electron Donor in Biological Mine Wastewater Treatment: Development of the Rhodes BioSURE Process®. In Proceedings of the Mine Water 2004–Proceedings International Mine Water Association Symposium, Newcastle upon Tyne, UK, 20–25 September 2004; pp. 111–118. [Google Scholar]
- Corbett, C.J. The Rhodes BioSURE Process in the Treatment of Acid Mine Drainage Wastewaters. Ph.D. Thesis, Rhodes University, Makhanda, South Africa, 2001. [Google Scholar]
- Hutton, B.; Kahan, I.; Naidu, T.; Gunther, P. Operating and Maintenance Experience at the Emalahleni Water Reclamation Plant. In Proceedings of the International Mine Water Conference, Pretoria, South Africa, 19–23 October 2009. [Google Scholar]
- Luo, H.; Liu, G.; Zhang, R.; Bai, Y.; Fu, S.; Hou, Y. Heavy Metal Recovery Combined with H2 Production from Artificial Acid Mine Drainage Using the Microbial Electrolysis Cell. J. Hazard. Mater. 2014, 270, 153–159. [Google Scholar] [CrossRef]
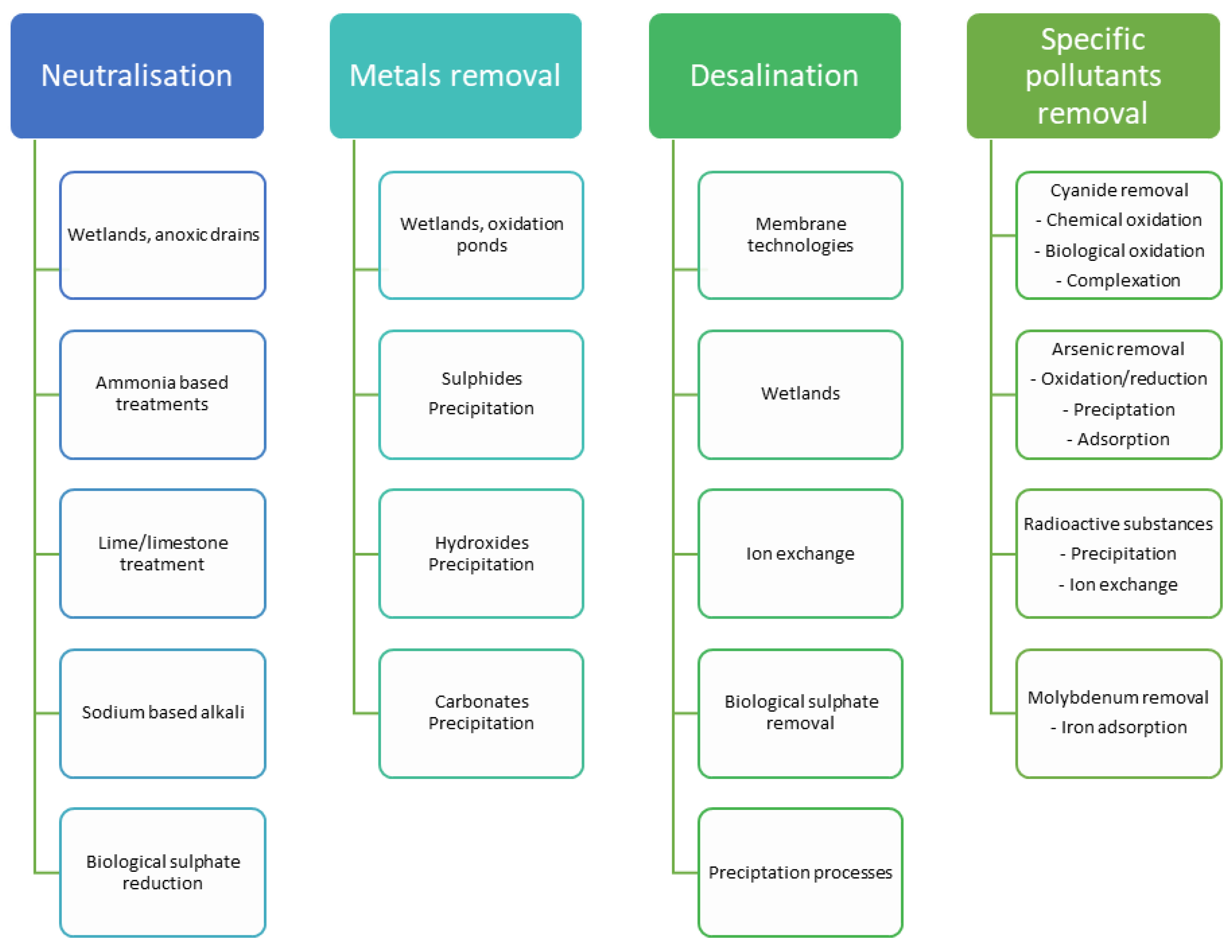
| Active/Passive | Biotic/Abiotic | Treatment Methods | Advantages |
|---|---|---|---|
| Passive | Abiotic |
|
|
| Biotic |
|
| |
| Active | Abiotic |
|
|
| Biotic |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baloyi, J.; Ramdhani, N.; Mbhele, R.; Ramutshatsha-Makhwedzha, D. Recent Progress on Acid Mine Drainage Technological Trends in South Africa: Prevention, Treatment, and Resource Recovery. Water 2023, 15, 3453. https://doi.org/10.3390/w15193453
Baloyi J, Ramdhani N, Mbhele R, Ramutshatsha-Makhwedzha D. Recent Progress on Acid Mine Drainage Technological Trends in South Africa: Prevention, Treatment, and Resource Recovery. Water. 2023; 15(19):3453. https://doi.org/10.3390/w15193453
Chicago/Turabian StyleBaloyi, Jeffrey, Nishani Ramdhani, Ryneth Mbhele, and Denga Ramutshatsha-Makhwedzha. 2023. "Recent Progress on Acid Mine Drainage Technological Trends in South Africa: Prevention, Treatment, and Resource Recovery" Water 15, no. 19: 3453. https://doi.org/10.3390/w15193453
APA StyleBaloyi, J., Ramdhani, N., Mbhele, R., & Ramutshatsha-Makhwedzha, D. (2023). Recent Progress on Acid Mine Drainage Technological Trends in South Africa: Prevention, Treatment, and Resource Recovery. Water, 15(19), 3453. https://doi.org/10.3390/w15193453





