Distribution of Heavy Metals in Water and Bottom Sediments in the Basin of Lake Gusinoe (Russia): Ecological Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Locations
2.2. Sample Collection and Preparation
2.3. BSs Quality Measurement
2.4. Analytical Technique and Accuracy Check
2.5. Ecological Assessment for Water and BSs
2.5.1. Hazard Index
2.5.2. Contamination Factor
2.5.3. Degree of Contamination
2.5.4. Pollution Load Index (PLI)
2.5.5. Geoaccumulation Index (Igeo)
2.5.6. Enrichment Factor (EF)
2.5.7. Ecological Risk Factor (Er)
2.5.8. Potential Ecological Risk Index (RI)
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Parameters of Water
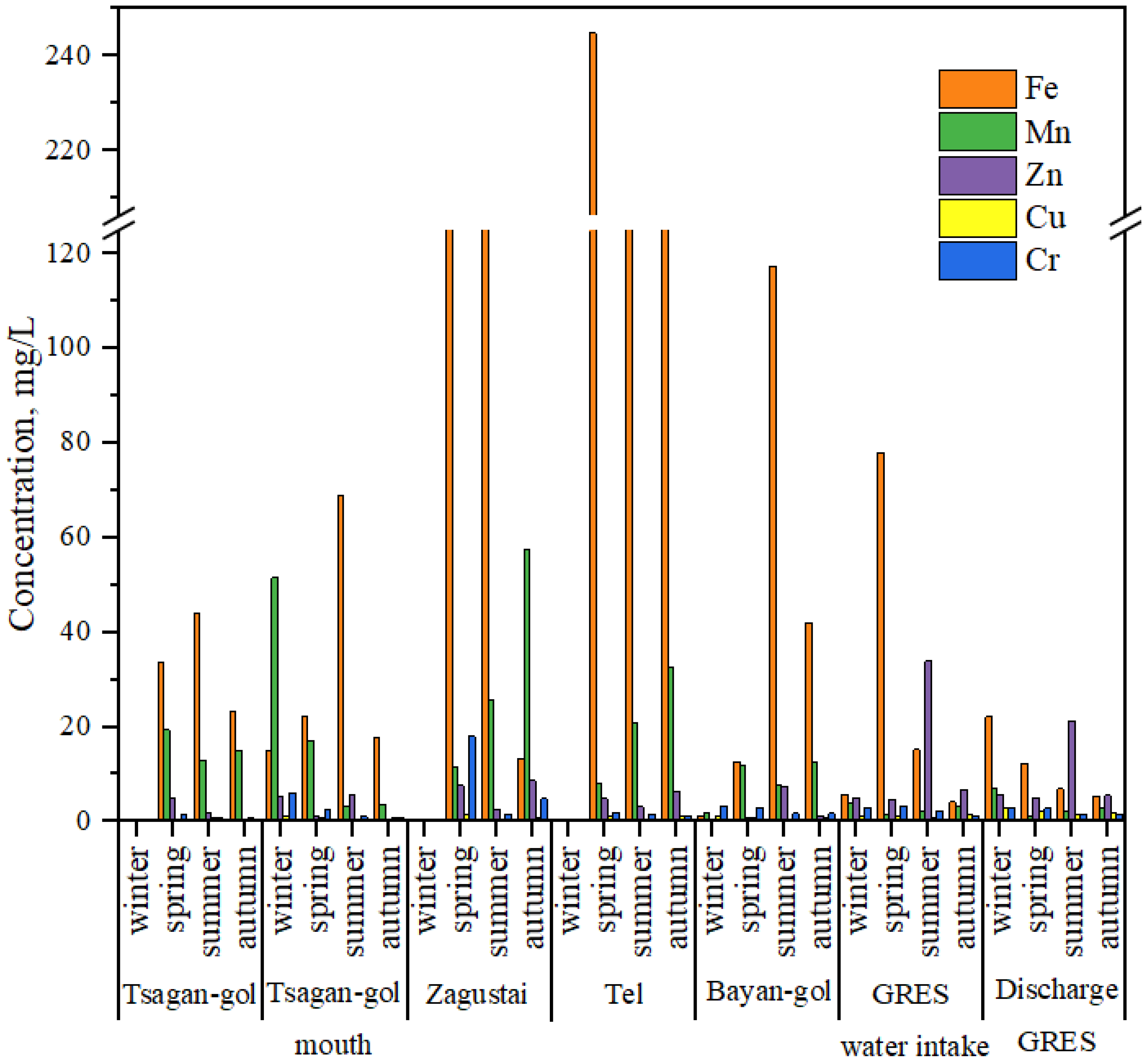
3.2. Concentration of HMs in Water
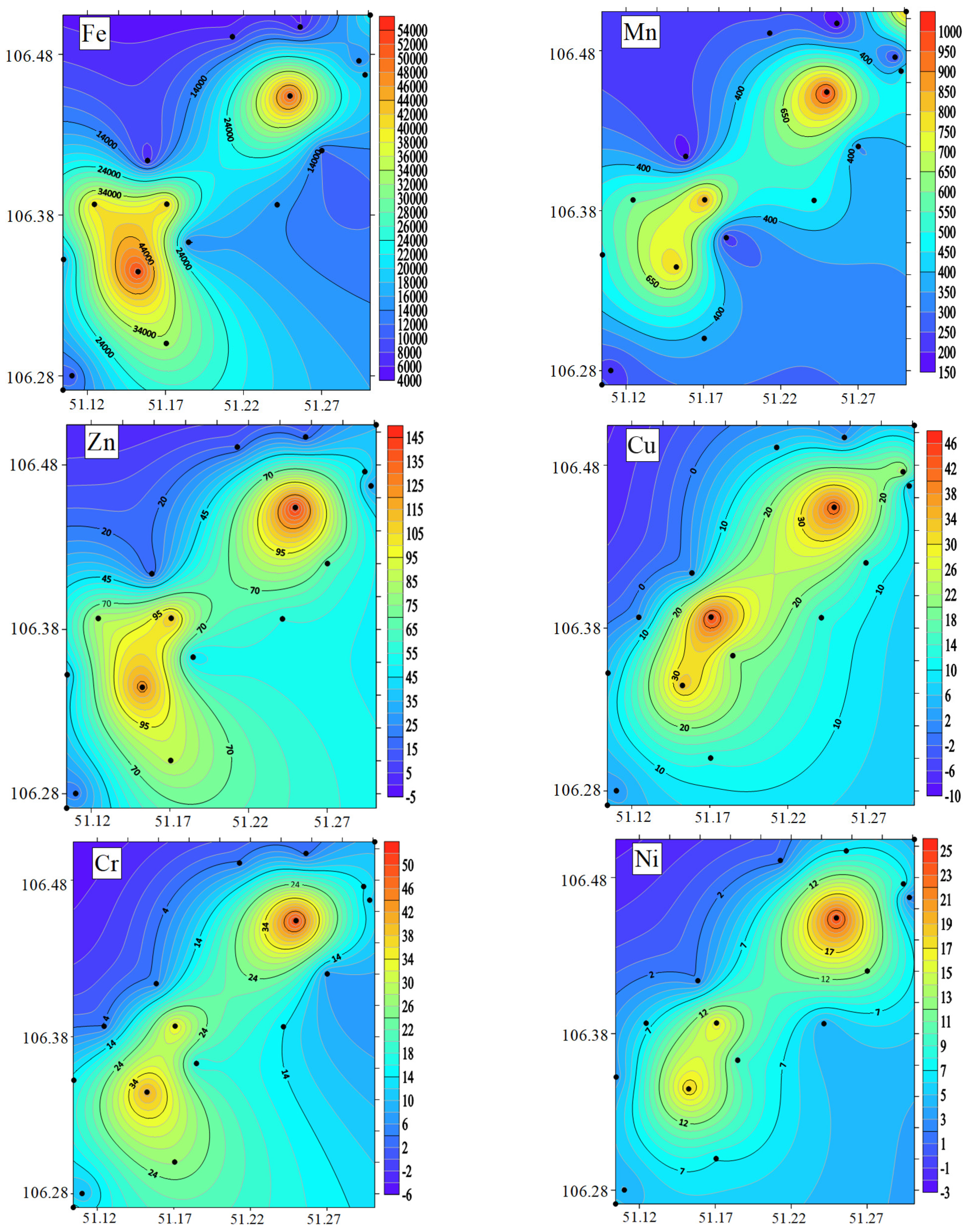
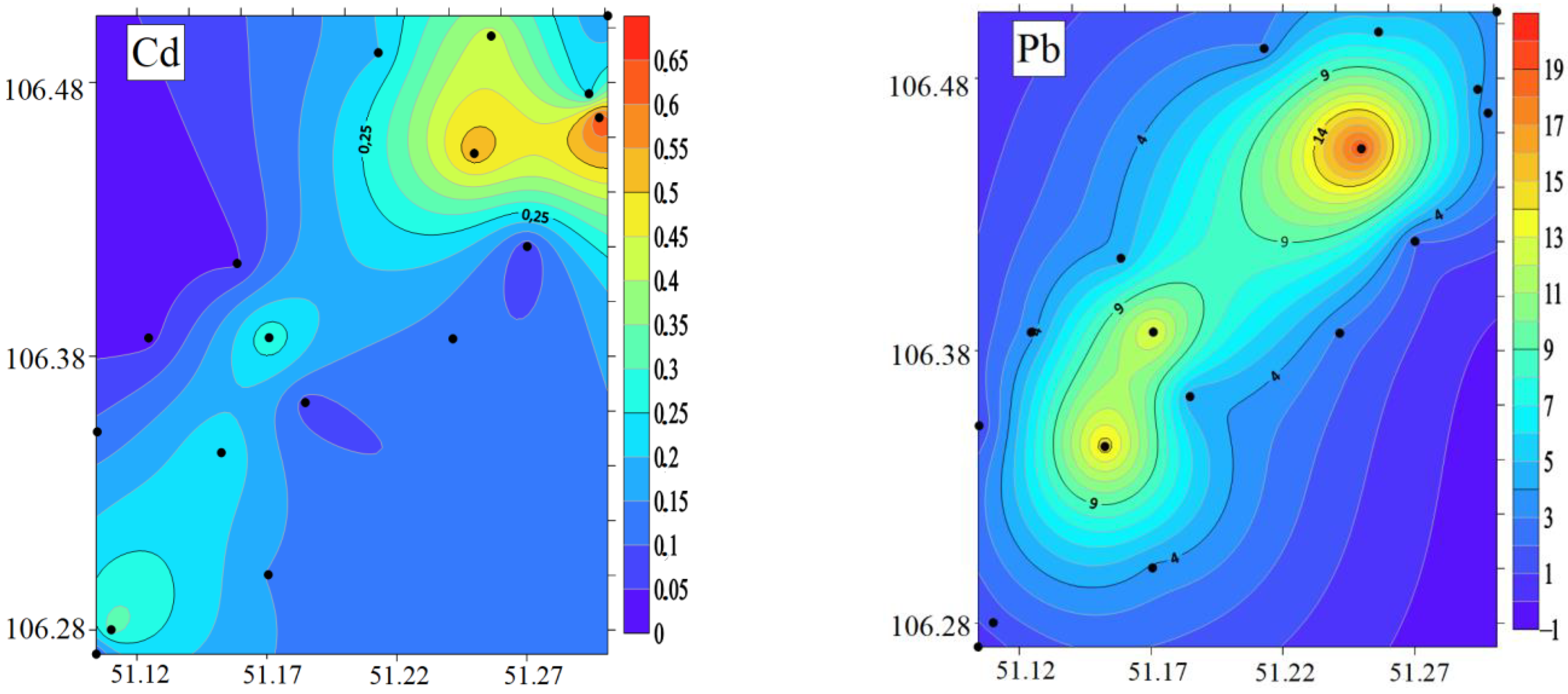
3.3. Concentration of HMs in the BSs
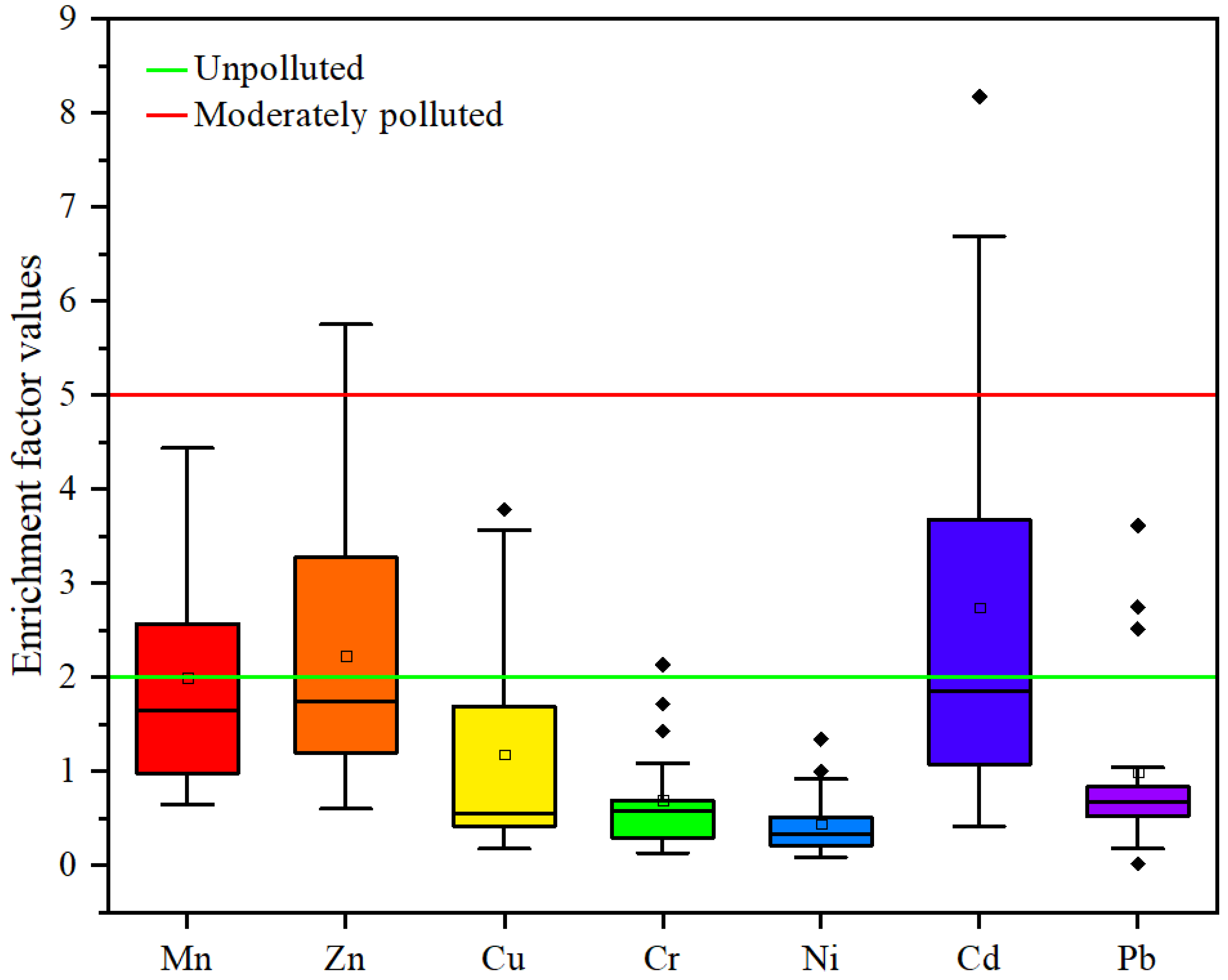
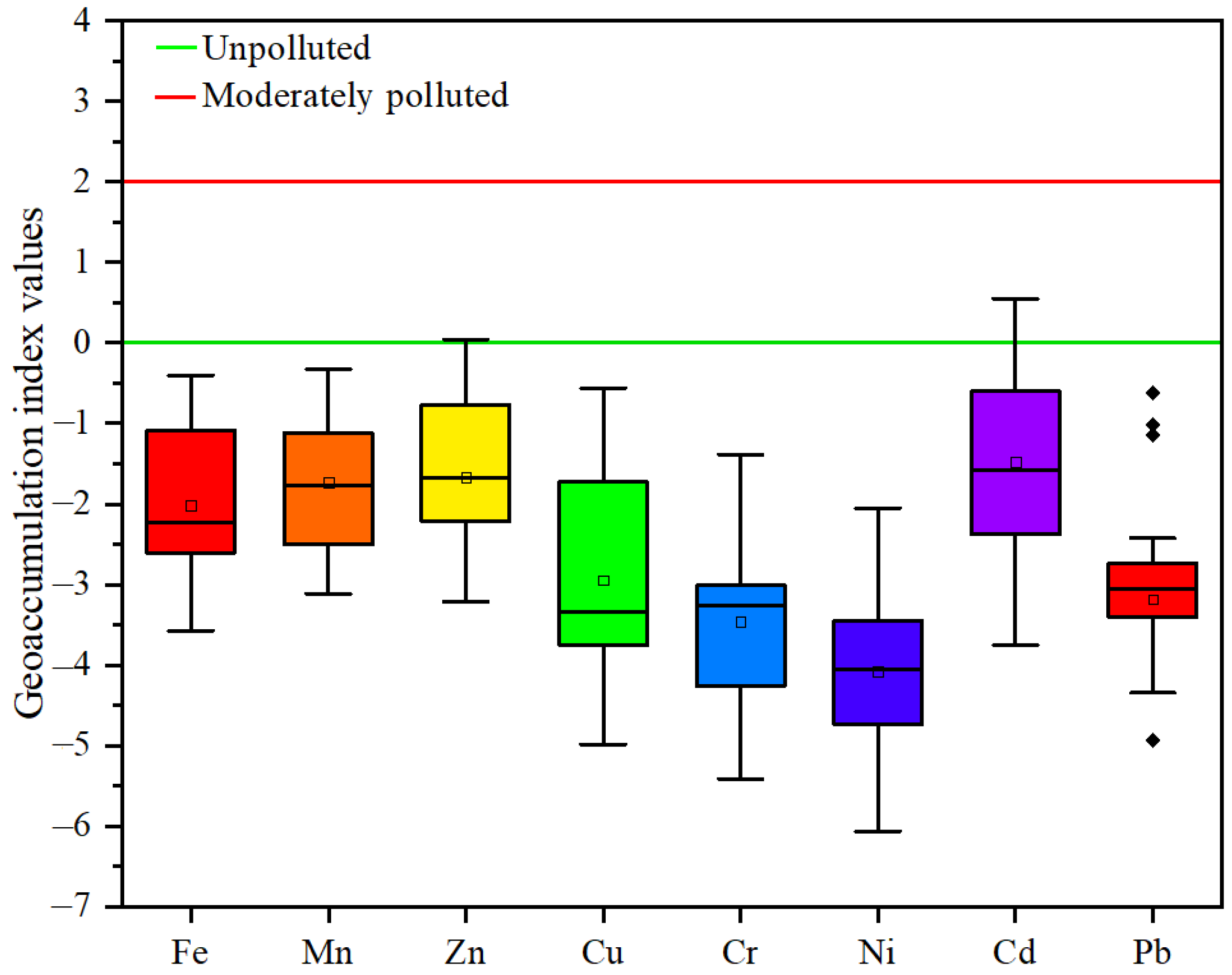
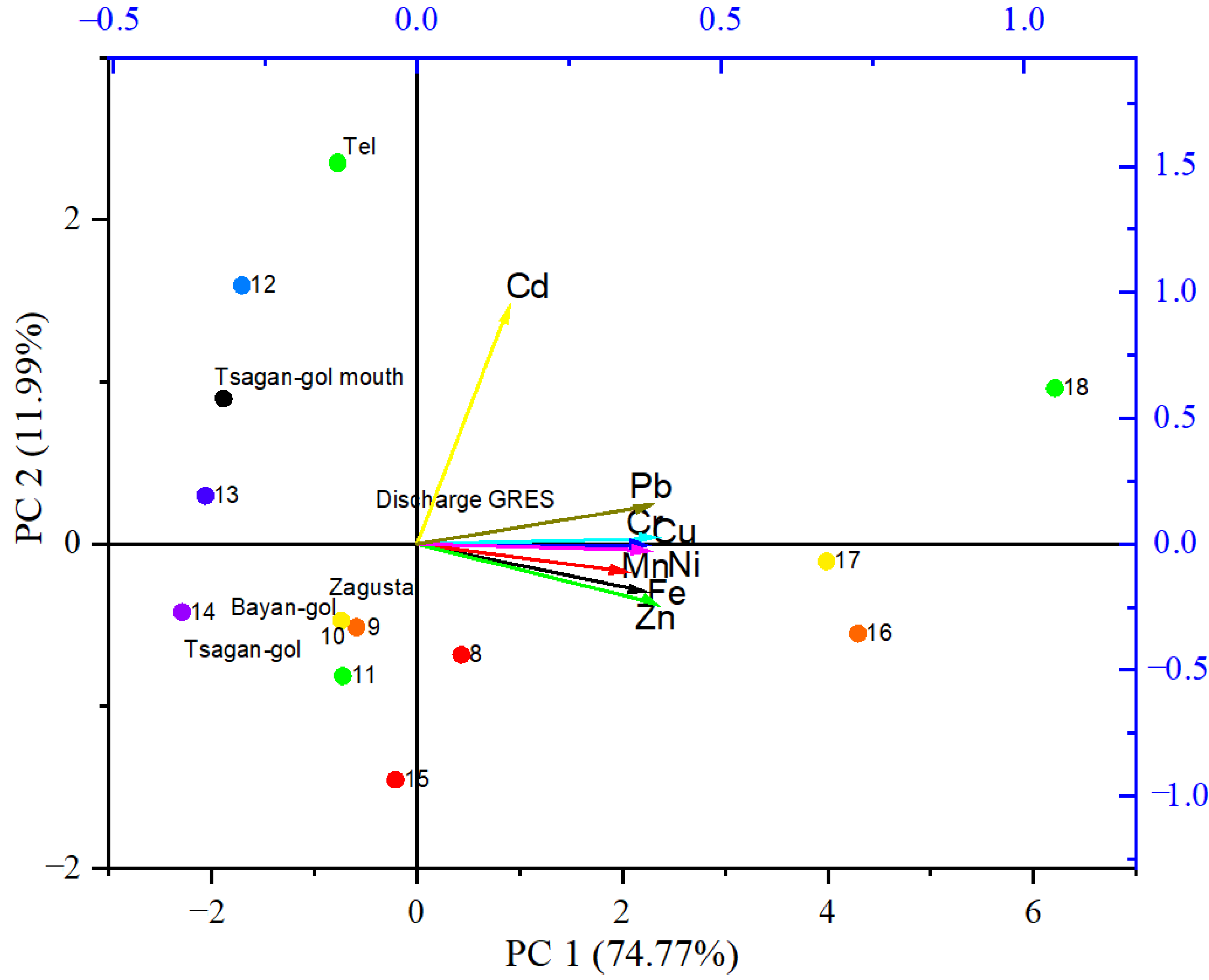
3.4. Pollution Assessment
4. Discussion
4.1. Physicochemical Parameters of Water
4.2. HMs in Water
4.3. Heavy Metals in BSs
4.4. Pollution Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Sibagariang, Y.P.; Napitupulu, F.H.; Kawai, H.; Ambarita, H. Study on the effect of oil palm shells on fresh water productivity of double slope solar still. J. Energy Storage 2023, 70, 108000. [Google Scholar] [CrossRef]
- Tang, W.; Pei, Y.; Zheng, H.; Zhao, Y.; Shu, L.; Zhang, H. Twenty years of China’s water pollution control: Experiences and challenges. Chemosphere 2022, 295, 133875. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Ronen-Eliraz, G.; Ratner, S.; Aviv, Y.; Wolanov, Y.; Avisar, D. Impact of industrial air pollution on the quality of atmospheric water production. Environ. Pollut. 2023, 325, 121447. [Google Scholar] [CrossRef]
- Aung, P.P.; Mao, Y.; Hu, T.; Qi, S.; Tian, Q.; Chen, Z.; Xing, X. Metal concentrations and pollution assessment in bottom sediments from Inle Lake, Myanmar. J. Geochem. Explor. 2019, 207, 106357. [Google Scholar] [CrossRef]
- Ali, M.M.; Rahman, S.; Islam, M.S.; Rakib, M.R.J.; Hossen, S.; Rahman, M.Z.; Kormoker, T.; Idris, A.M.; Phoungthong, K. Distribution of heavy metals in water and sediment of an urban river in a developing country: A probabilistic risk assessment. Int. J. Sediment Res. 2022, 37, 173–187. [Google Scholar] [CrossRef]
- Raudonytė-Svirbutavičienė, E.; Jokšas, K.; Stakėnienė, R. On the effectiveness of tributyltin ban part II: Temporal and spatial trends of organotin pollution in intense sediment accumulation areas and dumping sites of the Baltic Sea. J. Hazard. Mater. Adv. 2023, 10, 100294. [Google Scholar] [CrossRef]
- Saleem, M.; Jeelani, G.; Pall, I.A.; Ganai, J.; Kumar, S. Water and sediment geochemistry of an urban lake: Implications to weathering and anthropogenic activity. Int. J. Sediment Res. 2022, 37, 809–822. [Google Scholar] [CrossRef]
- Elmorsi, R.R.; Abou-El-Sherbini, K.S.; Abdel-Hafiz Mostafa, G.; Hamed, M.A. Distribution of essential heavy metals in the aquatic ecosystem of Lake Manzala, Egypt. Heliyon 2019, 5, e02276. [Google Scholar] [CrossRef]
- Li, X.; Bing, J.; Zhang, J.; Guo, L.; Deng, Z.; Wang, D.; Liu, L. Ecological risk assessment and sources identification of heavy metals in surface sediments of a river-reservoir system. Sci. Total Environ. 2022, 842, 156683. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, X.; Jin, X.; Jin, P. Characteristics of overflow pollution from combined sewer sediment: Formation, contribution and regulation. Chemosphere 2022, 298, 134254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lin, C.; Zhou, K.; Liu, Y.; Chen, J.; Wang, S.; Pan, Z.; Sun, X.; Wang, W.; Lin, H. Pollution, ecological risk, and source identification of potentially toxic elements in sediments of a landscape urban lagoon, China. Mar. Pollut. Bull. 2022, 174, 113192. [Google Scholar] [CrossRef] [PubMed]
- Adushinov, A.; Borisenko, I.; Zonkhoeva, E.; Rezanov, I.; Parfenov, I.; Shaibonov, B.; Abasheeva, N.; Ayushina, T.; Boikov, T.; Litvinov, A.; et al. Ecology of Lake Gusinoye; Buryat Academic Press: Ulan-Ude, Russia, 1994; p. 199. [Google Scholar]
- Galazy, G. Lake Baikal. Atlas; Roskartografiya: Moscow, Russia, 1993; p. 160. [Google Scholar]
- Radnaeva, L.D.; Bazarzhapov, T.Z.; Shiretorova, V.G.; Zhigzhitzhapova, S.V.; Nikitina, E.P.; Dylenova, E.P.; Shirapova, G.S.; Budaeva, O.D.; Beshentsev, A.N.; Garmaev, E.Z.; et al. Ecological State of Lake Gusinoe—A Cooling Pond of the Gusinoozersk GRES. Water 2021, 14, 4. [Google Scholar] [CrossRef]
- Tsydypov, B.Z.; Andreev, S.G.; Ayurzhanaev, A.A.; Sodnomov, B.V.; Gurzhapov, B.O.; Batotsyrenov, E.A.; Pavlov, I.A.; Shiretorova, V.G.; Ulzetueva, I.D.; Gabeeva, D.A.; et al. Impact of Discharges of Gusinoozerskaya Power Plant on Thermal and Hydrochemical Regime of Lake Gusinoe. ISU Bull. 2017, 22, 135–150. [Google Scholar]
- Naganawa, H. Lake Gusinoe to Baikal via Selenga Delta: Protection-destruction spiral. Lakes Reserv. Ponds 2012, 6, 9–12. [Google Scholar]
- Russian Statistical Yearbook; Federal State Statistics Service: Moscow, Russia, 2022; p. 459.
- Khazheeva, Z.I.; Plyusnin, A.M.; Smirnova, O.K.; Peryazeva, E.G.; Sanzhanova, S.S.; Zhambalova, D.I.; Doroshkevich, S.G.; Dabaeva, V.V. Nutrients, Organic Matter, and Trace Elements in Lake Gusinoe (Transbaikalia). Water 2021, 13, 2958. [Google Scholar] [CrossRef]
- Abidueva, E.Y.; Dagurova, O.P.; Garankina, V.P.; Sambueva, G.B. Seasonal changes of the physico-chemical and microbiological parameters of Lake Gusinoe (Western Transbaikalia). BSU Bull. 2014, 3, 74–76. [Google Scholar]
- Bazarova, B.B.; Kuklin, A.P. On the current state and long-term dynamics of the flora and vegetation of Lake Gusinoe (Republic of Buryatia). Ekosistemy 2021, 25, 72–81. [Google Scholar]
- Domysheva, V.M.; Sinyukovich, V.N.; Hodger, T.V. Water regime and hydrochemistry of Lake Gusinoe in the modern period. Geogr. Nat. Resour. 1995, 2, 73–80. [Google Scholar]
- Khazheeva, Z.I.; Plyusnin, A.M. Current state of water resources of Gusinoe Lake (Western Transbaikalia). Water Res. 2018, 45, 104–110. [Google Scholar] [CrossRef]
- Lukyanova, A.; Lukyanova, O.; Yefimova, L.; Yefimov, V. The Anthropogenic and Natural Factors’ Impact on Water Quality in Cooling Pools (the Lake Gusinoye as a Study Case). Water Sect. Russ. Probl. Technol. Manag. 2020, 2, 146–160. [Google Scholar]
- Chebunina, N.S.; Pakhakhinova, Z.Z.; Beshentcev, A.N.; Batoev, V.B. Assessment of impact of warm water dischange of the Gusinoozersk GRES on the ice regime of Gusinoe Lake (in The Western Transbaikalia). Polythematic Netw. Electron. Sci. J. Kuban State Agrar. Univ. 2016, 116, 301–308. [Google Scholar]
- Dampilova, B.V.; Khazheeva, Z.I.; Plyusnin, A.M. Heavy Metal Species in the Bottom Sediments of the Aquatic System of Lake Gusinoe (Buryatia). Geochem. Int. 2022, 60, 279–285. [Google Scholar] [CrossRef]
- Buryat Center for Hydrometeorology and Environmental Monitoring. Available online: http://www.burpogoda.ru (accessed on 30 September 2021).
- Qu, J.; Tian, X.; Zhang, X.; Yao, J.; Xue, J.; Li, K.; Zhang, B.; Wang, L.; Zhang, Y. Free radicals-triggered reductive and oxidative degradation of highly chlorinated compounds via regulation of heat-activated persulfate by low-molecular-weight organic acids. Appl. Catal. B Environ. 2022, 310, 121359. [Google Scholar] [CrossRef]
- Bech, J.; Duran, P.; Roca, N.; Poma, W.; Sánchez, I.; Roca-Pérez, L.; Boluda, R.; Barceló, J.; Poschenrieder, C. Accumulation of Pb and Zn in Bidens triplinervia and Senecio sp. spontaneous species from mine spoils in Peru and their potential use in phytoremediation. J. Geochem. Explor. 2012, 123, 109–113. [Google Scholar] [CrossRef]
- Chandra, S.; Medha, I.; Tiwari, A.K. The Role of Modified Biochar for the Remediation of Coal Mining-Impacted Contaminated Soil: A Review. Sustainability 2023, 15, 3973. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef]
- Chen, H.; Min, F.; Hu, X.; Ma, D.; Huo, Z. Biochar assists phosphate solubilizing bacteria to resist combined Pb and Cd stress by promoting acid secretion and extracellular electron transfer. J. Hazard. Mater. 2023, 452, 131176. [Google Scholar] [CrossRef]
- Lai, W.; Wu, Y.; Zhang, C.; Dilinuer, Y.; Pasang, L.; Lu, Y.; Wang, Y.; Chen, H.; Li, Z. Combination of Biochar and Phosphorus Solubilizing Bacteria to Improve the Stable Form of Toxic Metal Minerals and Microbial Abundance in Lead/Cadmium-Contaminated Soil. Agronomy 2022, 12, 1003. [Google Scholar] [CrossRef]
- Ferati, F.; Kerolli-Mustafa, M.; Kraja-Ylli, A. Assessment of heavy metal contamination in water and sediments of Trepca and Sitnica rivers, Kosovo, using pollution indicators and multivariate cluster analysis. Environ. Monit. Assess. 2015, 187, 338. [Google Scholar] [CrossRef]
- Liu, P.; Hu, W.; Tian, K.; Huang, B.; Zhao, Y.; Wang, X.; Zhou, Y.; Shi, B.; Kwon, B.O.; Choi, K.; et al. Accumulation and ecological risk of heavy metals in soils along the coastal areas of the Bohai Sea and t Tomlinson he Yellow Sea: A comparative study of China and South Korea. Environ. Int. 2020, 137, 105519. [Google Scholar] [CrossRef] [PubMed]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the Elements in Some Major Units of the Earth’s Crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Solgi, E.; Esmaili-Sari, A.; Riyahi-Bakhtiari, A.; Hadipour, M. Soil contamination of metals in the three industrial estates, Arak, Iran. Bull. Environ. Contam. Toxicol. 2012, 88, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Khazheeva, Z.I.; Pronin, N.M.; Radnaeva, L.D.; Dugarov, Z.N.; Urbazaeva, S.D. The features of heavy metal accumulation in water, bottom sediments and biota of the Cherkalov Sor Bay at Lake Baikal. Chem. Sustain. Dev. 2005, 13, 95–102. [Google Scholar]
- Aradpour, S.; Noori, R.; Tang, Q.; Bhattarai, R.; Hooshyaripor, F.; Hosseinzadeh, M.; Haghighi, A.T.; Klöve, B. Metal contamination assessment in water column and surface sediments of a warm monomictic man-made lake: Sabalan Dam Reservoir, Iran. Hydrol. Res. 2020, 51, 799–814. [Google Scholar] [CrossRef]
- Aradpour, S.; Noori, R.; Vesali Naseh, M.R.; Hosseinzadeh, M.; Safavi, S.; Ghahraman-Rozegar, F.; Maghrebi, M. Alarming carcinogenic and non-carcinogenic risk of heavy metals in Sabalan dam reservoir, Northwest of Iran. Environ. Pollut. Bioavailab. 2021, 33, 278–291. [Google Scholar] [CrossRef]
- Nodefarahani, M.; Aradpour, S.; Noori, R.; Tang, Q.; Partani, S.; Klöve, B. Metal pollution assessment in surface sediments of Namak Lake, Iran. Environ. Sci. Pollut. Res. 2020, 27, 45639–45649. [Google Scholar] [CrossRef]
- Sobczyński, T.; Siepak, J. Speciation of heavy metals in bottom sediments of lakes in the area of Wielkopolski National Park. Pol. J. Environ. Stud. 2001, 10, 463–474. [Google Scholar]
- Sojka, M.; Jaskuła, J.; Siepak, M. Heavy Metals in Bottom Sediments of Reservoirs in the Lowland Area of Western Poland: Concentrations, Distribution, Sources and Ecological Risk. Water 2019, 11, 56. [Google Scholar] [CrossRef]
- Lett, R.; Jackaman, W. Stream Geochemical Survey Guide; British Columbia Ministry of Energy and Mines, Energy and Minerals Division: Vancouver, BC, Canada, 2004.
- Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/3052.pdf (accessed on 30 September 2021).
- Alekin, O.; Semenov, A.; Skopintsev, B. Guidelines for the Chemical Analysis of Land Waters; Hydrometeorological Publishing: Leningrad, Russia, 1973; p. 269. [Google Scholar]
- Sediment from Lake Baikal (Certified Reference Material), BIL-2, GSO 7176-950. A.P. Vinogradov Institute of Geochemistry SB RAS, Irkutsk, Russia. Available online: https://web.archive.org/web/20230926163616/https://nevareaktiv.ru/system/static_attachments/item/pdf/igcir/GRSI-7176-IGC.pdf (accessed on 30 August 2023).
- 24 Element ICP Calibration/Quality Control Standard, STD IV-STOCK-8. Inorganic Ventures, Christiansburg, VA, USA. Available online: https://www.inorganicventures.com/icp-calibration-std-8-125ml (accessed on 30 August 2023).
- Wu, B.; Zhang, Y.; Zhang, X.; Cheng, S. Health risk from exposure of organic pollutants through drinking water consumption in Nanjing, China. Bull. Environ. Contam. Toxicol. 2010, 84, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Islam, M.S.; Proshad, R.; Ahmed, S. Ecological risk of heavy metals in sediment of an urban river in Bangladesh. Hum. Ecol. Risk Assess. Int. J. 2017, 24, 699–720. [Google Scholar] [CrossRef]
- US EPA. Regional Screening Level (RSL) Summary Table. 2017. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 30 August 2023).
- Dagurova, O.P.; Zaitseva, S.V.; Tsydenova, B.V.; Dambaev, V.B.; Matafonov, D.V. Monitoring of physicochemical parameters of Lake Gusinoe. Buryat State Univ. Bull. 2019, 4, 3–8. [Google Scholar]
- Adesakin, T.A.; Oyewale, A.T.; Bayero, U.; Mohammed, A.N.; Aduwo, I.A.; Ahmed, P.Z.; Abubakar, N.D.; Barje, I.B. Assessment of bacteriological quality and physico-chemical parameters of domestic water sources in Samaru community, Zaria, Northwest Nigeria. Heliyon 2020, 6, e04773. [Google Scholar] [CrossRef]
- Jain, N.; Yevatikar, R.; Raxamwar, T.S. Comparative study of physico-chemical parameters and water quality index of river. Mater. Today Proc. 2022, 60, 859–867. [Google Scholar] [CrossRef]
- Li, X.; Huang, T.; Ma, W.; Sun, X.; Zhang, H. Effects of rainfall patterns on water quality in a stratified reservoir subject to eutrophication: Implications for management. Sci. Total Environ. 2015, 521–522, 27–36. [Google Scholar] [CrossRef]
- Tsydenova, B.V.; Dagurova, O.P.; Garankina, V.P.; Dambaev, V.P.; Matafonov, D.V.; Baturina, O.A. Abundance and Taxonomic Composition of Bacterioplankton in Freshwater Lake Gusinoye (Buryatia) in the Warm Water Zone of the Gusinoozerskaya Thermal Power Plant. J. Sib. Fed. Univ. Biol. 2018, 11, 356–366. [Google Scholar]
- Ivanova, E.; Anishenko, O.; Gribovskaya, I.; Zinenko, G.; Nazarenko, N.; Nemchinov, V.; Zuev, I.; Avramov, A. Metal content in higher aquatic plants in a small Siberian water reservoir. Sib. Ecol. J. 2012, 4, 485–495. [Google Scholar] [CrossRef]
- Tsybekmitova, G.T.; Kuklin, A.P.; Tashlykova, N.A.; Afonina, E.Y.; Bazarova, B.B.; Itigilova, M.T.; Gorlacheva, E.P.; Matafonov, P.V.; Afonin, A.V. Ecological state of Lake Kenon as a cooling pond of the Thermal Power Plant-1 (TPP-1) (Zabaykalsky Krai). Novosib. State Pedagog. Univ. Bull. 2017, 7, 194–209. [Google Scholar]
- Franco-Uria, A.; Lopez-Mateo, C.; Roca, E.; Fernandez-Marcos, M.L. Source identification of heavy metals in pastureland by multivariate analysis in NW Spain. J. Hazard. Mater. 2009, 165, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Tulokhonov, A.K.; Garmaev, E.A.; Andreev, S.G. Experience of Socio-Ecological Rehabilitation of the Consequences of Ammunition Explosions (on the Example of the Disaster at the Goose Lake Station, Republic of Buryatia). Problems of Protection of the Population and Territories from Emergency Situations; Federal Center of Science and High Technologies: Moscow, Russia, 2012. [Google Scholar]
- Zhigzhitzhapova, S.V.; Pavlov, I.A.; Shiretorova, V.G.; Dylenova, E.P.; Radnaeva, L.D.; Tulokhonov, A.K. Metal content in aquatic plants of Lake Gusinoye. Water Chem. Ecol. 2019, 1, 34–40. [Google Scholar]
- Mehantiev, I. Health Risks for the Population of the Voronezh Region Related to Drinking Water Quality. Public Health Life Environ. 2020, 4, 37–42. [Google Scholar] [CrossRef]
- Kiku, P.F.; Kislitsyna, L.V.; Bogdanova, V.D.; Sabirova, K.M. Hygienic evaluation of the quality of drinking water and risks for the health of the population of the Primorye territory. Hyg. Sanit. 2019, 1, 94–101. [Google Scholar] [CrossRef]
- Elumalai, V.; Brindha, K.; Lakshmanan, E. Human Exposure Risk Assessment Due to Heavy Metals in Groundwater by Pollution Index and Multivariate Statistical Methods: A Case Study from South Africa. Water 2017, 9, 234. [Google Scholar] [CrossRef]
- Cymes, I.; Glińska-Lewczuk, K.; Szymczyk, S.; Sidoruk, M.; Potasznik, A. Distribution and potential risk assessment of heavy metals and arsenic in sediments of a dam reservoir: A case study of the Łoje retention reservoir, NE Poland. J. Elementol. 2017, 22, 843–856. [Google Scholar] [CrossRef]
- Skwierawski, A. Carbon Sequestration Potential in the Restoration of Highly Eutrophic Shallow Lakes. Int. J. Environ. Res. Public Health 2022, 19, 6308. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Gracia, I. Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere 2004, 55, 431–442. [Google Scholar] [CrossRef]
- Lee, P.K.; Kang, M.J.; Yu, S.; Ko, K.S.; Ha, K.; Shin, S.C.; Park, J.H. Enrichment and geochemical mobility of heavy metals in bottom sediment of the Hoedong reservoir, Korea and their source apportionment. Chemosphere 2017, 184, 74–85. [Google Scholar] [CrossRef]
- Leblebici, Z.; Aksoy, A.; Duman, F. Influence of salinity on the growth and heavy metal accumulation capacity of Spirodela polyrhiza (Lemnaceae). Turk. J. Biol. 2011, 35, 215–220. [Google Scholar] [CrossRef]
- Liu, L.; Luo, D.; Lu, Y.; Huang, X.; Liu, Y.; Wei, L.; Xiao, T.; Wu, Q.; Liu, G. Risk assessment of groundwater pollution during GLDA-assisted phytoremediation of Cd- and Pb-contaminated soil. Ecol. Indic. 2022, 139, 108913. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking Water Quality Recommendations; 1 (2004) 515; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- MAC. Nature Protection. Hydrosphere. Indices of State and Requlation for Valuation Survey of Tisnery Waters. Available online: https://gost.ruscable.ru/Index/33/33538.htm (accessed on 7 November 2011).






| Element | Determining Threshold | BIL-2 | ||
|---|---|---|---|---|
| Certified | Measured | Recovery | ||
| Fe | 4 | 37,000 | 33,800 | 92 |
| Mn | 0.1 | 929 | 892 | 96 |
| Zn | 0.1 | 64 | 66 | 103 |
| Cu | 0.1 | 18 | 17 | 94 |
| Cr | 0.2 | 158 | 160 | 101 |
| Ni | 0.5 | 31 | 24.3 | 97 |
| Pb | 0.2 | 14 | 13 | 93 |
| Index | Category | Description | |
|---|---|---|---|
| Hazard index (HI) | HI < 0.1 | Low risk | [50] |
| 0.1 ≤ HI < 1 | Low to moderate risk | ||
| 1 ≤ HI < 5 | Moderate risk | ||
| 5 ≤ HI < 10 | High risk | ||
| HI > 10 | Very high risk | ||
| Contamination factor (Cf) | Cf < 1 | Low contamination | |
| 1 ≤ Cf < 2 | Low to moderate contamination | [34,35] | |
| 2 ≤ Cf < 3 | Moderate contamination | ||
| 3 ≤ Cf < 4 | Moderate to high contamination | ||
| 4 ≤ Cf < 5 | High contamination | ||
| 5 ≤ Cf < 6 | High to very high contamination | ||
| Cf ≥ 6 | Extreme contamination | ||
| Degree of contamination (Cd) | Cd < 6 | Low degree of contamination | [51] |
| 6 ≤ Cd < 12 | Moderate degree of contamination | ||
| 12 ≤ Cd < 24 | Significant degree of contamination | ||
| Cd > 24 | High degree of contamination | ||
| Pollution load index (PLI) | PLI < 1 | Unpolluted | [38] |
| 1 ≤ PLI < 2 | Moderately polluted | ||
| 2 ≤ PLI < 3 | Significant degree of pollution | ||
| PLI > 3 | Highly polluted | ||
| Geoaccumulation index (Igeo) | Igeo ≤ 0 | Unpolluted | [36,37] |
| 0 ≤ Igeo < 1 | Unpolluted to moderately polluted | ||
| 1 ≤ Igeo < 2 | Moderately polluted | ||
| 2 ≤ Igeo < 3 | Moderately to highly polluted | ||
| 3 ≤ Igeo < 4 | Highly polluted | ||
| 4 ≤ Igeo < 5 | Highly to very highly polluted | ||
| Igeo > 5 | Extremely polluted | ||
| EF < 2 | No enrichment | [52] | |
| Enrichment factor (EF) | 2 ≤ EF < 5 | Moderate enrichment | |
| 5 ≤ EF < 10 | Moderate to high enrichment | ||
| 10 ≤ EF < 25 | High enrichment | ||
| 25 ≤ EF < 50 | Very high enrichment | ||
| EF > 50 | Extreme enrichment | ||
| Ecological risk factor (Er) | Er < 40 | Low risk | [51] |
| 40 ≤ Er < 80 | Moderate risk | ||
| 80 ≤ Er < 160 | Considerable risk | ||
| 160 ≤ Er < 320 | High risk | ||
| Er > 80 | Very high risk | ||
| Potential Ecological Risk Index (RI) | RI < 95 | Low risk | [36,51] |
| 95 ≤ RI < 190 | Moderate risk | ||
| 190 ≤ RI < 380 | Considerable risk | ||
| RI > 380 | High risk |
| Site | Fe | Mn | Zn | Cu | Cr | Ni | Cd | Pb |
|---|---|---|---|---|---|---|---|---|
| Clarke (mg/kg), [38] | 47,200 | 850 | 95 | 45 | 90 | 68 | 0.3 | 20 |
| Tsagan-gol | 12,704.67 | 267 | 32.33 | 5.0 | 15.1 | 5.47 | 0.09 | 0.09 |
| Tsagan-gol mouth | 10,424.0 | 208.0 | 20.0 | 2.2 | 9.3 | 3.82 | 0.33 | 1.48 |
| Zagustai | 21,684.33 | 846.33 | 34.67 | 5.17 | 9.15 | 4.09 | 0.15 | 3.52 |
| Tel | 18,806.33 | 443.33 | 30.67 | 6.03 | 12.00 | 2.49 | 0.66 | 3.26 |
| Bayan-gol | 16,931.00 | 481.00 | 36.67 | 5.93 | 15.20 | 3.19 | 0.11 | 0.98 |
| GRES discharge | 14,038.67 | 224.67 | 46.00 | 23.60 | 14.05 | 8.06 | 0.23 | 4.26 |
| Mean ± SD | 15,765 ± 4160 | 412 ± 242 | 33 ± 8 | 8 ± 8 | 12 ± 3 | 5 ± 2 | 0.26 ± 0.21 | 2 ± 2 |
| 8 | 33,251.00 | 375.00 | 87.00 | 12.57 | 26.27 | 7.08 | 0.15 | 3.79 |
| 9 | 13,432.00 | 209.00 | 44.67 | 20.43 | 16.74 | 9.35 | 0.08 | 4.16 |
| 10 | 15,101.67 | 483.00 | 55.00 | 13.10 | 14.15 | 4.17 | 0.13 | 2.83 |
| 11 | 11,646.00 | 344.33 | 65.67 | 13.13 | 7.03 | 12.09 | 0.07 | 2.04 |
| 12 | 5942.33 | 147.33 | 16.33 | 2.13 | 3.16 | 7.02 | 0.42 | 5.59 |
| 13 | 7396.33 | 277.67 | 15.33 | 6.70 | 4.36 | 1.52 | 0.20 | 3.11 |
| 14 | 6646.33 | 166.33 | 16.67 | 2.27 | 5.24 | 2.11 | 0.04 | 4.48 |
| 15 | 40,516.67 | 587.67 | 83.67 | 2.33 | 4.07 | 6.13 | 0.03 | 3.60 |
| 16 | 53,588.00 | 807.33 | 125.33 | 33.67 | 41.60 | 18.25 | 0.24 | 14.79 |
| 17 | 42,249.00 | 874.67 | 112.67 | 45.80 | 34.59 | 16.80 | 0.30 | 13.52 |
| 18 | 52,433.00 | 1015.67 | 147.00 | 43.17 | 51.64 | 24.55 | 0.54 | 19.45 |
| Mean ± SD | 25,654.76 ± 18,951.43 | 480.73 ± 302.38 | 69.94 ± 45.70 | 17.75 ± 16.14 | 18.99 ± 17.09 | 9.92 ± 7.29 | 0.20 ± 0.16 | 7.03 ± 5.95 |
| Element | Fe | Mn | Zn | Cu | Cr | Ni | Cd | Pb | HI |
|---|---|---|---|---|---|---|---|---|---|
| RfD | 7 × 10−1 | 1.4 × 10−1 | 3 × 10−1 | 4 × 10−2 | 3 × 10−3 | 2 × 10−2 | 1 × 10−3 | 2 × 10−3 | 0.0157 |
| HQ | 0.0004 | 0.0004 | 0.0004 | 0.0004 | 0.0086 | 0.0004 | 0.0030 | 0.0021 |
| Index | Point | Index Value | Category | Description |
|---|---|---|---|---|
| Hazard Index | All samples | 0.0157 | HI < 0.1 | Low risk |
| Contamination factor (Cf) | Avr | 0.12–0.74 | Cf < 1 | Low contamination |
| Tel | Cd-2.2 | 1 ≤ Cf < 2 | Low to moderate contamination | |
| 16 | Fe-1.14, Zn-1.32 | |||
| 18 | Fe-1.11, Mn-1.19, Zn-1.55 | |||
| 17 | Zn-1.19 | |||
| Degree of contamination | Avr | 3.23 | Cd < 6 | Low degree of contamination |
| 17 | 6.41 | 6 ≤ Cd < 12 | Moderate degree of contamination | |
| 16 | 6.42 | |||
| 18 | 8.52 | |||
| Pollution load index (PLI) | All samples | 0.10–0.96 | PLI < 1 | Unpolluted |
| Geoaccumulation index (Igeo) | Avr | −4.08-(−1.73) | Igeo ≤ 0 | Unpolluted |
| Tel | Cd-0.55 | 0 ≤ Igeo < 1 | Unpolluted to moderately polluted | |
| 18 | Cd-0.26 | |||
| Enrichment factor (EF) | Avr | 0.44–2.23 | EF < 2 | No enrichment |
| GRES discharge | Cd-2.89 | 2 ≤ EF < 5 | Moderate enrichment | |
| 17 | Avg—2.93, Zn-4.41 | |||
| 16 | Avg—2.8, Zn-4.9 | |||
| 18 | Avg-3.93 | |||
| 15 | Mn-2.57, Zn-3.27 | |||
| Tel | Cd-8.17 | 5 ≤ EF < 10 | Moderate to high enrichment | |
| 18 | Cd-6.69 | |||
| 12 | Cd-5.24 | |||
| Ecological risk factor (Er) | Avr | Er < 40 | Low risk | |
| Tel | Cd-66 | 40 ≤ Er < 80 | Moderate risk | |
| 18 | Cd-54 | |||
| Potential ecological risk index (RI) | All samples | 6.49–70.46 | RI < 95 | Low risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazarzhapov, T.Z.; Shiretorova, V.G.; Radnaeva, L.D.; Nikitina, E.P.; Bazarsadueva, S.V.; Shirapova, G.S.; Dong, S.; Li, Z.; Liu, S.; Wang, P. Distribution of Heavy Metals in Water and Bottom Sediments in the Basin of Lake Gusinoe (Russia): Ecological Risk Assessment. Water 2023, 15, 3385. https://doi.org/10.3390/w15193385
Bazarzhapov TZ, Shiretorova VG, Radnaeva LD, Nikitina EP, Bazarsadueva SV, Shirapova GS, Dong S, Li Z, Liu S, Wang P. Distribution of Heavy Metals in Water and Bottom Sediments in the Basin of Lake Gusinoe (Russia): Ecological Risk Assessment. Water. 2023; 15(19):3385. https://doi.org/10.3390/w15193385
Chicago/Turabian StyleBazarzhapov, Tcogto Zh., Valentina G. Shiretorova, Larisa D. Radnaeva, Elena P. Nikitina, Selmeg V. Bazarsadueva, Galina S. Shirapova, Suocheng Dong, Zehong Li, Shiqi Liu, and Ping Wang. 2023. "Distribution of Heavy Metals in Water and Bottom Sediments in the Basin of Lake Gusinoe (Russia): Ecological Risk Assessment" Water 15, no. 19: 3385. https://doi.org/10.3390/w15193385
APA StyleBazarzhapov, T. Z., Shiretorova, V. G., Radnaeva, L. D., Nikitina, E. P., Bazarsadueva, S. V., Shirapova, G. S., Dong, S., Li, Z., Liu, S., & Wang, P. (2023). Distribution of Heavy Metals in Water and Bottom Sediments in the Basin of Lake Gusinoe (Russia): Ecological Risk Assessment. Water, 15(19), 3385. https://doi.org/10.3390/w15193385









