Lysimeter Sampling System for Optimal Determination of Trace Elements in Soil Solutions
Abstract
1. Introduction
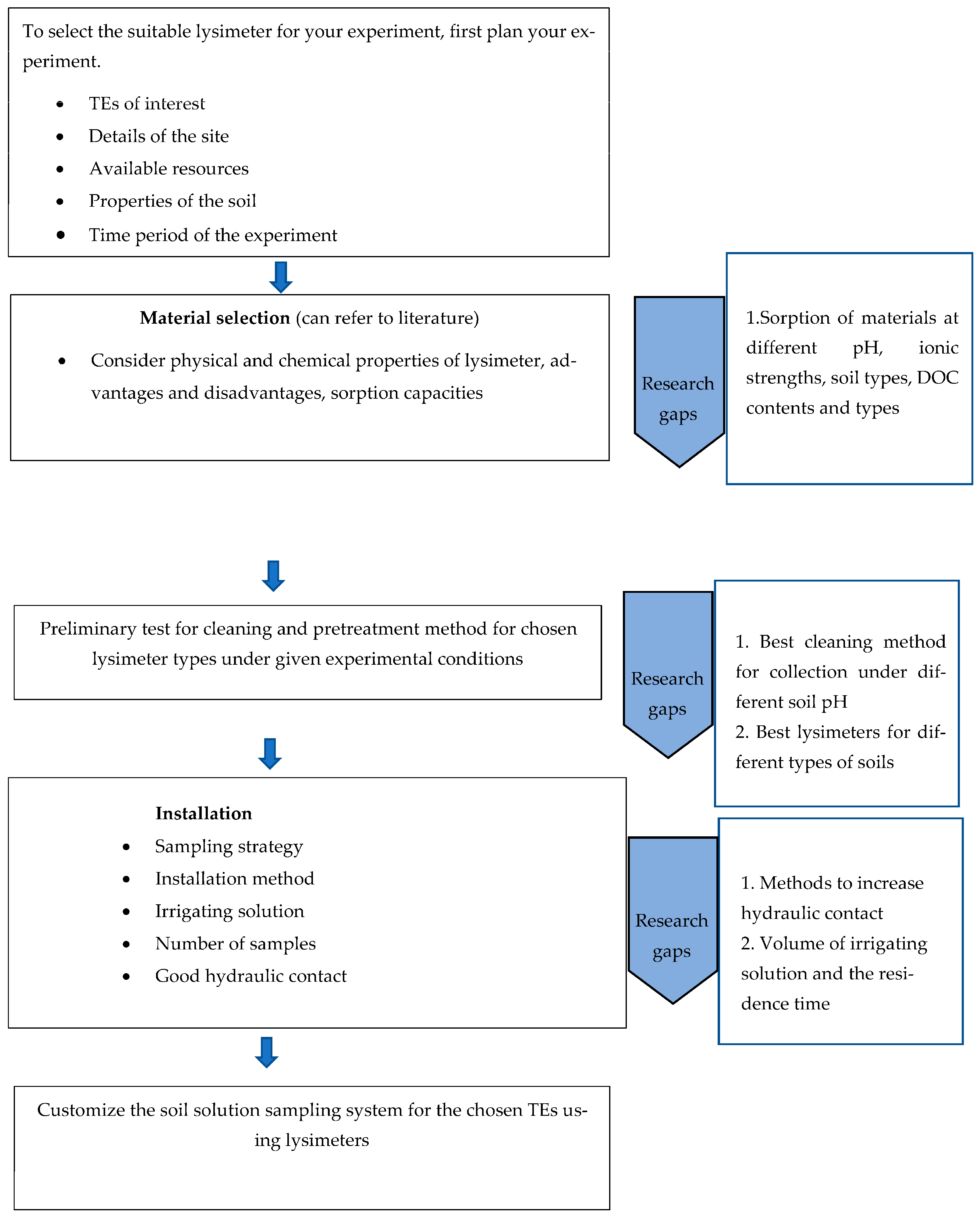
2. Different Types of Lysimeters Used for the Collection of Soil Solution for TE Analysis
2.1. Tension and Zero-Tension Lysimeters
Optimizing Vacuum Pressure in Tension Lysimeters
2.2. Materials of Lysimeter
2.2.1. Critical Properties of Lysimeter Material
Cation Exchange Capacity (CEC) of the Cup Material
Nominal Pore Diameter and Bubbling Pressure
3. Sorption and Release of TEs from Lysimeters
3.1. Effect of Dissolved Organic Carbon (DOC) on Adsorption
3.2. Effect of pH on Adsorption
3.3. Effect of Ionic Strength on Adsorption
4. Methods of Lysimeter Sampling
4.1. Hydraulic Contact
4.2. Cleaning of Lysimeters
4.3. Conditioning the Lysimeter
4.4. Sampling Strategies
5. Other Considerations
5.1. Need for Replicates
5.2. Soil Type
5.3. Colloids and Microorganisms
5.4. Soil Moisture Condition
6. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hooda, P.S. (Ed.) Assessing Bioavailability of Soil Trace Elements. In Trace Elements in Soils; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 227–265. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Soil–plant transfer of trace elements—An environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Beesley, L.; Lepp, N.W.; Dickinson, N.M.; Hartley, W.; Clemente, R. Field sampling of soil pore water to evaluate trace element mobility and associated environmental risk. Environ. Pollut. 2011, 159, 3078–3085. [Google Scholar] [CrossRef]
- Davranche, M.; Bollinger, J.C. A desorption–dissolution model for metal release from polluted soil under reductive conditions. J. Environ. Qual. 2001, 30, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.J.; Brown, P.L.; Byrne, R.H.; Gajda, T.; Hefter, G.; Leuz, A.-K.; Sjöberg, S.; Wanner, H. Chemical Speciation of Environmentally Significant Metals: An IUPAC contribution to reliable and rigorous computer modelling. Chem. Int. 2015, 37, 15–19. [Google Scholar] [CrossRef]
- Di Bonito, M.; Breward, N.; Crout, N.; Smith, B.; Young, S. Overview of selected soil pore water extraction methods for the determination of potentially toxic elements in contaminated soils: Operational and technical aspects. In Environmental Geochemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 213–249. [Google Scholar] [CrossRef]
- Fares, A.; Deb, S.K.; Fares, S. Review of vadose zone soil solution sampling techniques. Environ. Rev. 2009, 17, 215–234. [Google Scholar] [CrossRef]
- Parat, C.; Lévêque, J.; Dousset, S.; Chaussod, R.; Andreux, F. Comparison of three sequential extraction procedures used to study trace metal distribution in an acidic sandy soil. Anal. Bioanal. Chem. 2003, 376, 243–247. [Google Scholar] [CrossRef]
- Rao, C.R.M.; Sahuquillo, A.; Lopez Sanchez, J.F. A Review of the Different Methods Applied in Environmental Geochemistry For Single and Sequential Extraction of Trace Elements in Soils and Related Materials. Water Air Soil Pollut. 2008, 189, 291–333. [Google Scholar] [CrossRef]
- Gleyzes, C.; Tellier, S.; Astruc, M. Fractionation studies of trace elements in contaminated soils and sediments: A review of sequential extraction procedures. TrAC Trends Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jimenez, E.; Clemente, R.; Lepp, N.; Dickinson, N. Mobility of arsenic, cadmium and zinc in a multi-element contaminated soil profile assessed by in-situ soil pore water sampling, column leaching and sequential extraction. Environ. Pollut. 2009, 158, 155–160. [Google Scholar] [CrossRef]
- Raudina, T.V.; Loyko, S.V.; Krickov, I.V.; Lim, A.G. Comparing the composition of soil waters of West Siberian frozen mires sampled by different methods. Vestn. Tomsk. Gos. Univ. Biol. 2016, 35, 26–42. [Google Scholar] [CrossRef][Green Version]
- Zabowski, D.; Ugolini, F.C. Lysimeter and centrifuge soil solutions: Seasonal differences between methods. Soil Sci. Soc. Am. J. 1990, 54, 1130–1135. [Google Scholar] [CrossRef]
- Geibe, C.E.; Danielsson, R.; van Hees, P.A.; Lundström, U.S. Comparison of soil solution chemistry sampled by centrifugation, two types of suction lysimeters and zero-tension lysimeters. J. Appl. Geochem. 2006, 21, 2096–2111. [Google Scholar] [CrossRef]
- Weihermüller, L.; Kasteel, R.; Vanderborght, J.; Pütz, T.; Vereecken, H. Soil water extraction with a suction cup: Results of numerical simulations. Vadose Zone J. 2005, 4, 899–907. [Google Scholar] [CrossRef]
- Wolt, J.D. Soil Solution Chemistry: Applications to Environmental Science and Agriculture; John Wiley & Sons: New York, NY, USA, 1994; Volume 124, p. 475. [Google Scholar]
- Litaor, M.I. Review of soil solution samplers. Water Resour. Res. 1988, 24, 727–733. [Google Scholar] [CrossRef]
- Du, L.; Cuss, C.W.; Dyck, M.F.; Noernberg, T.; Shotyk, W. Size-resolved analysis of trace elements in the dissolved fraction (<0.45 μm) of soil solutions using a novel lysimeter and asymmetrical flow field-flow fractionation coupled to ultraviolet absorbance and inductively coupled plasma mass spectrometry. Can. J. Soil Sci. 2020, 100, 381–392. [Google Scholar] [CrossRef]
- Lajtha, K.; Jarrell, W.M.; Johnson, D.W.; Sollins, P. Collection of Soil Solution. In Long-Term Ecological Research Network Series; Oxford University Press: New York, NY, USA, 1999; Volume 2, pp. 166–182. [Google Scholar]
- Goldsmith, G.R.; Muñoz-Villers, L.E.; Holwerda, F.; McDonnell, J.J.; Asbjornsen, H.; Dawson, T.E. Stable isotopes reveal linkages among ecohydrological processes in a seasonally dry tropical montane cloud forest. Ecohydrology 2012, 5, 779–790. [Google Scholar] [CrossRef]
- Renée Brooks, J.; Barnard, H.R.; Coulombe, R.; McDonnell, J.J. Ecohydrologic separation of water between trees and streams in a Mediterranean climate. Nat. Geosci. 2010, 3, 100–104. [Google Scholar] [CrossRef]
- Sprenger, M.; Llorens, P.; Cayuela, C.; Gallart, F.; Latron, J. Mechanisms of consistently disjunct soil water pools over (pore) space and time. Hydrol. Earth Syst. Sci. 2019, 23, 2751–2762. [Google Scholar] [CrossRef]
- Landon, M.K.; Delin, G.N.; Komor, S.C.; Regan, C.P. Comparison of the stable-isotopic composition of soil water collected from suction lysimeters, wick samplers, and cores in a sandy unsaturated zone. J. Hydrol. 1999, 224, 45–54. [Google Scholar] [CrossRef]
- Andersen, M.K.; Raulund-Rasmussen, K.; Strobel, B.W.; Hansen, H.C.B. Adsorption of Cadmium, Copper, Nickel, and Zinc to a Poly(tetrafluorethene) Porous Soil Solution Sampler. J. Environ. Qual. 2002, 31, 168–175. [Google Scholar] [CrossRef]
- Rais, D.; Nowack, B.; Schulin, R.; Luster, J. Sorption of trace metals by standard and micro suction cups in the absence and presence of dissolved organic carbon. J. Environ. Qual. 2006, 35, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, J.; Udluft, P. The extraction of soil water by the suction-cup method: A review. J. Soil Sci. 1991, 42, 83–93. [Google Scholar] [CrossRef]
- Creasey, C.L.; Dreiss, S.J. Porous cup samplers; Cleaning procedures and potential sample bias from trace element contamination. Soil Sci. 1988, 145, 93–101. [Google Scholar] [CrossRef]
- McGuire, P.E.; Lowery, B.; Helmke, P.A. Potential Sampling Error: Trace Metal Adsorption on Vacuum Porous Cup Samplers. Soil Sci. Soc. Am. J. 1992, 56, 74–82. [Google Scholar] [CrossRef]
- Kohnke, H.; Dreibelbis, F.R.; Davidson, J.M. A Survey and Discussion of Lysimeters and A Bibliography on Their Construction and Performance; U.S. Department of Agriculture: Washington, DC, USA, 1940; pp. 2–27. [Google Scholar]
- Haines, B.L.; Waide, J.B.; Todd, R.L. Soil solution nutrient concentrations sampled with tension and zero-tension lysimeters: Report of discrepancies. Soil Sci. Soc. Am. J. 1982, 46, 658–661. [Google Scholar] [CrossRef]
- Pütz, T.; Fank, J.; Flury, M. Lysimeters in vadose zone research. Vadose Zone J. 2018, 17, 1–4. [Google Scholar] [CrossRef]
- Hendershot, W.H.; Courchesne, F. Comparison of soil solution chemistry in zero tension and ceramic-cup tension lysimeters. J. Soil Sci. 1991, 42, 577–583. [Google Scholar] [CrossRef]
- Giesler, R.; Lundström, U.S.; Grip, H. Comparison of soil solution chemistry assessment using zero-tension lysimeters or centrifugation. Eur. J. Soil Sci. 1996, 47, 395–405. [Google Scholar] [CrossRef]
- Mohamed, R.M.S.R. Zero-Tension Lysimeter for use in greywater irrigation monitoring. Int. J. Integr. Eng. 2012, 4, 15–21. Available online: https://penerbit.uthm.edu.my/ojs/index.php/ijie/article/view/189 (accessed on 18 November 2022).
- Soil Water Extraction Methods. Available online: https://library.metergroup.com/Application%20Notes/UMS_Soil-Water-Extraction-Methods.pdf (accessed on 6 November 2022).
- Titus, B.D.; Kingston, D.G.O.; Pitt, C.M.; Mahendrappa, M.K. A lysimeter system for monitoring soil solution chemistry. Can. J. Soil Sci. 2000, 80, 219–226. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Sletten, R.S.; Brandstetter, A.; Wieshammer, G.; Stingeder, G. Adsorption of trace metals by tension lysimeters: Nylon membrane vs. porous ceramic cup. J. Environ. Qual. 1997, 26, 1430–1434. [Google Scholar] [CrossRef]
- Hansen, E.A.; Harris, A.R. Validity of soil-water samples collected with porous ceramic cups. Soil Sci. Soc. Am. J. 1975, 39, 528–536. [Google Scholar] [CrossRef]
- Morell, I.; Sánchez-Pérez, J.M. Evaluation of porous cup soil-water samplers under laboratory conditions: Comparison of ceramic and PTFE cups. Toxicol. Environ. Chem. 2000, 74, 231–244. [Google Scholar] [CrossRef]
- Salaita, G.N. Determination of the spatial distribution of trace elements in stainless steel by imaging microprobe secondary ion mass spectrometry. Appl. Surf. Sci. 1995, 90, 465–479. [Google Scholar] [CrossRef]
- Vandenbruwane, J.; De Neve, S.; De Schrijver, A.; Geudens, G.; Verheyen, K.; Hofman, G. Comparison of ceramic and polytetrafluoroethene/quartz suction cups for sampling inorganic ions in soil solution. Commun. Soil Sci. Plant Anal. 2008, 39, 1105–1121. [Google Scholar] [CrossRef]
- Grossmann, J.; Bredemeier, M.; Udluft, P. Sorption of trace metals by suction cups of aluminium oxide, ceramic and plastics. J. Plant Nutr. Soil Sci. 1990, 153, 359–364. [Google Scholar] [CrossRef]
- User Manual SiC 20. Available online: https://publications.metergroup.com/Manuals/UMS/SIC20_Manual (accessed on 11 September 2023).
- Linden, D.R. Design, Installation, and Use of Porous Ceramic Samplers for Monitoring Soil-Water Quality; Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1977. [Google Scholar] [CrossRef]
- Abdulkareem, J.H.; Abdulkadir, A.; Abdu, N. A review of different types of lysimeters used in solute transport studies. Int. J. Plant Soil Sci. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Wiese, G.R.; James, R.O.; Healy, T.W. Discreteness of charge and solvation effects in cation adsorption at the oxide/water interface. Discuss. Faraday Soc. 1971, 52, 302–311. [Google Scholar] [CrossRef]
- Raudina, T.V.; Loiko, S.V.; Lim, A.G.; Krickov, I.V.; Shirokova, L.S.; Istigechev, G.I.; Kuzmina, D.M.; Kulizhsky, S.P.; Vorobyev, S.N.; Pokrovsky, O.S. Dissolved organic carbon and major and trace elements in peat porewater of sporadic, discontinuous, and continuous permafrost zones of western Siberia. Biogeosciences 2017, 14, 3561–3584. [Google Scholar] [CrossRef]
- Raudina, T.V.; Loiko, S.V.; Kuzmina, D.M.; Shirokova, L.S.; Kulizhskiy, S.P.; Golovatskaya, E.A.; Pokrovsky, O.S. Colloidal organic carbon and trace elements in peat porewaters across a permafrost gradient in Western Siberia. Geoderma 2021, 390, 114971. [Google Scholar] [CrossRef]
- Hart, G.L.; Lowery, B. Axial-radial influence of porous cup soil solution samplers in a sandy soil. Soil Sci. Soc. Am. J. 1997, 61, 1765–1773. [Google Scholar] [CrossRef]
- Shira, J.M.; Williams, B.C.; Flury, M.; Czigány, S.; Tuller, M. Sampling silica and ferrihydrite colloids with fiberglass wicks under unsaturated conditions. J. Environ. Qual. 2006, 35, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Czigány, S.; Flury, M.; Harsh, J.B.; Williams, B.C.; Shira, J.M. Suitability of fiberglass wicks to sample colloids from vadose zone pore water. Vadose Zone J 2005, 4, 175–183. [Google Scholar] [CrossRef]
- Swistock, B.R.; Yamona, J.J.; Dewalle, D.R.; Sharpe, W.E. Comparison of soil water chemistry and sample size requirements for pan vs tension lysimeters. Water Air Soil Pollut. 1990, 50, 387–396. [Google Scholar] [CrossRef]
- Shipitalo, M.J.; Edwards, W.M. Effects of initial water content on macropore/matrix flow and transport of surface-applied chemicals. J. Environ. Qual. 1996, 25, 662–670. [Google Scholar] [CrossRef]
- MacDonald, J.D.; Bélanger, N.; Hendershot, W.H. Column leaching using dry soil to estimate solid-solution partitioning observed in zero-tension lysimeters. 1. Method development. Soil Sediment Contam. 2004, 13, 361–374. [Google Scholar] [CrossRef]
- Reemtsma, T.; Bredow, A.; Gehring, M. The nature and kinetics of organic matter release from soil by salt solutions. Eur. J. Soil Sci. 1999, 50, 53–64. [Google Scholar] [CrossRef]
- Du, L.; Cuss, C.W.; Dyck, M.; Noernberg, T.; Shotyk, W. Size Fractionation of Dissolved (<0.45 Μm) Trace Elements from Extracted Soil with Water and CaCl2 Using Af4-Uv-Icpms to Predict Their Bioavailability. Available online: https://ssrn.com/abstract=4352031 (accessed on 16 August 2023).
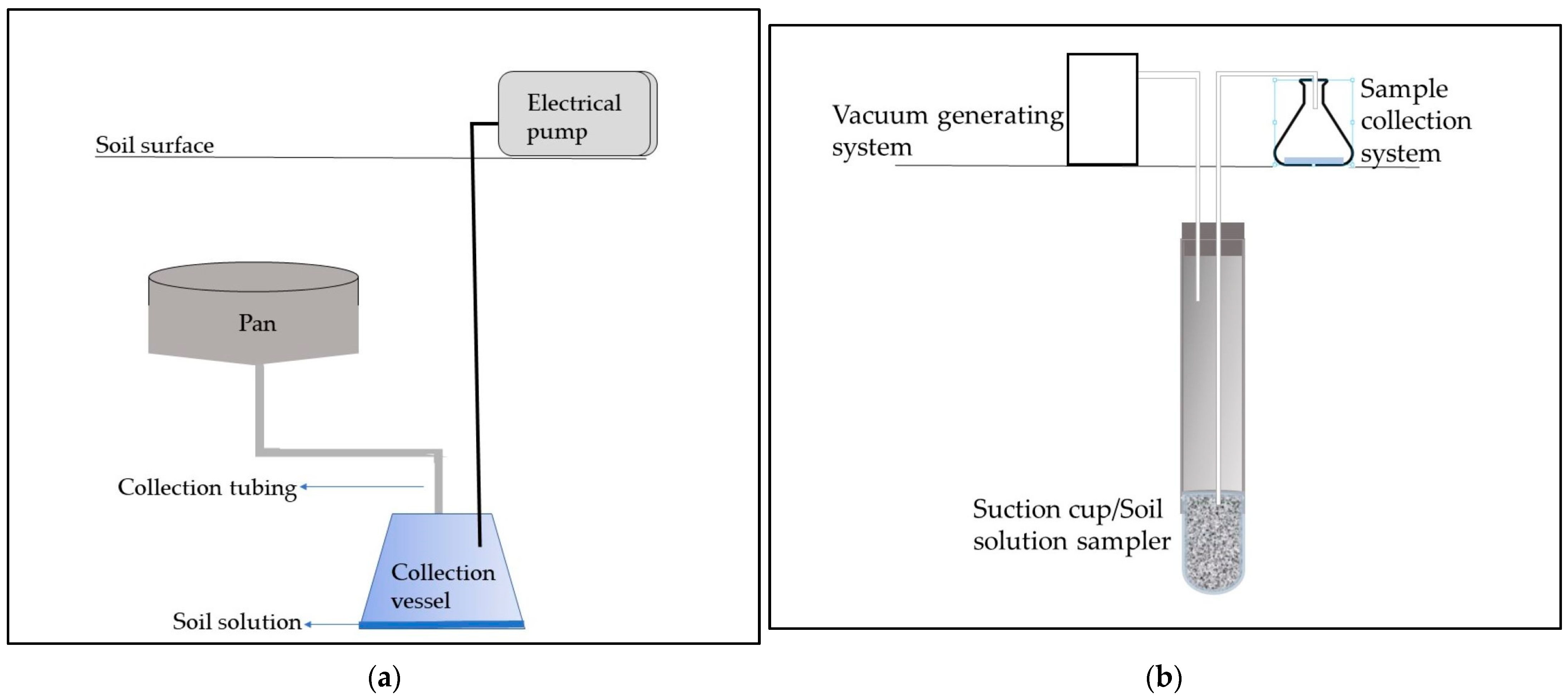

| Lysimeter Type | Features | Advantages | Disadvantages | Literature |
|---|---|---|---|---|
| Ceramic | Ceramic cups are composed mainly of alumina and small amounts of SiO2, Fe2O3, and TiO2. | Simple and versatile | Adsorption of considerable amounts of relatively soluble TEs such as Cd, Co, Mn, Ni, and Zn to the cup at pH 4 and 5; removal of these TEs from the soil solution was significantly greater at pH 6; Cu, Cr, As, and Pb are almost completely re-moved from solution at pH 4, 5, and 6. Quite brittle; special care is required to avoid cracking. | [37,38,39] |
| Surgical (316L) stainless steel (SS) lysimeter | This is composed of powdered 316 L stainless steel (SS) that is resistant to corrosion and relatively inert material. This lysimeter consists of five major parts: (1) a bottom disc to close the collection chamber, (2) a 316 L SS collection chamber, (3) a filter section made from 316 L SS-type, (4) an upper disk, and (5) 316 sst tubing that connects the lysimeter to the vacuum hose. A tungsten inert gas weld is used to connect these discs and tubings. | High mechanical strength and corrosion resistance due to the formation of a Cr2O3 surface layer | Presence of Cr and Fe were observed in localized areas of the SS tube [40]. High heat which is required to weld the steel can destroy the protective chromium oxide layer. Then, the material will begin to oxidize. This can happen if lysimeters are scratched while inserting them to the soil as well. Thus, not suitable for analyzing Fe, Cr, or Ni compared to concentrations in soils and soil solutions. | [17,36] |
| PTFE | This is produced from PTFE polymer. Some PTFE cups are manufactured by mixing PTFE with quartz flour to create the porous cup, thus combining the characteristics of PTFE with the good hydraulic conductivity of silica flour (Prenart Super Quartz; Prenart Equipment, Frederiksberg, Denmark). | Less reactive A flexible material | At low ionic strength of the soil solution, the adsorption of Cd, Cu, Ni, and Zn to the PTFE cup is high. Pressure operating range is extremely narrow (with the use of silica flour it can be expanded up to about 7 centibars of suction). Sample intake rate is considerably higher compared to ceramic cups. Thus, prolonged exposure of the bulk solution to the open-air increases evaporation and ultimately the ion concentration. | [24,41] |
| Nylon | Nylon membrane is attached to the acrylic ends and sealed along the seam where the nylon membrane is wrapped around the polyethylene support using the acrylic adhesive. | Low adsorption of TEs at pH 4 and 5; trace metal concentrations in the collected solution are representative | Slight sorption of As, Cr, Cu, and Pb in solutions was observed at pH 6. | [37] |
| pH | Lysimeter Material | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nylon | PTFE | Al2O3 | Ceramic | Borosilicate | ||||||
| <4 | Cd, Cu | Cu | Cd, Cu | Cu | ||||||
| 4–5 | Cd, Cu, Mn, Ni, Pb, Zn | Cu, Ni, Pb, Zn | Cd, Cu, Mn, Ni, Pb, Zn | Cd, Co, * Cr, * Cu, * Pb, Zn | Mn, Ni | |||||
| 5–6 | Cu, Ni, Zn | Cr | ||||||||
| >6 | Cd, Co, Cr, Cu, Mn, Ni, Zn | Co, Cr, Mn, Ni | Cd, Cu, Pb, Zn | Mn, Ni | Cd, Cu, Pb, Zn | Mn, Ni | Co, Cr, Cu, Mn, Ni, Pb, Zn | |||
| Lysimeter Type | Diagram | CEC (mmolc/cup) | Nominal Pore Diameter (μm) | Reference |
|---|---|---|---|---|
| Surgical (316L) stainless steel (SS) lysimeter |  | Unknown | 5 | [18] |
| Ceramic cup lysimeter |  | 0.40–0.65 | 1 | [43] |
| PTFE |  | Unknown | <2 | [24] |
| Nylon membrane | 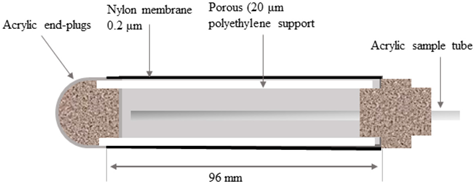 | 0.06 | 0.2 | [37] |
| Method of Cleaning | Material | Primary Elements of Interest |
|---|---|---|
| Ceramic cup | As, Cd, Cr, Cu, Co, Mn, Ni, Pb, Zn | |
| Surgical (316L) stainless steel | Mn, Fe, Ni, Cu, Zn, Mo, V, Cr, Co, Li, Al, Ba, As, Ag, Cd, Tl, Pb, Th, U |
| Fritted glass | Cd, Cr, Co, Zn |
| PTFE | Al, Ca, Cd, Cr, Co, Fe, Hf, Mn, Ni, Sr, Th, Ti, V, Zn, Zr |
| Nylon | As, Cd, Cr, Cu, Co, Mn, Ni, Pb, Zn |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernando, S.U.; Galagedara, L.; Krishnapillai, M.; Cuss, C.W. Lysimeter Sampling System for Optimal Determination of Trace Elements in Soil Solutions. Water 2023, 15, 3277. https://doi.org/10.3390/w15183277
Fernando SU, Galagedara L, Krishnapillai M, Cuss CW. Lysimeter Sampling System for Optimal Determination of Trace Elements in Soil Solutions. Water. 2023; 15(18):3277. https://doi.org/10.3390/w15183277
Chicago/Turabian StyleFernando, Salani U., Lakshman Galagedara, Mano Krishnapillai, and Chad W. Cuss. 2023. "Lysimeter Sampling System for Optimal Determination of Trace Elements in Soil Solutions" Water 15, no. 18: 3277. https://doi.org/10.3390/w15183277
APA StyleFernando, S. U., Galagedara, L., Krishnapillai, M., & Cuss, C. W. (2023). Lysimeter Sampling System for Optimal Determination of Trace Elements in Soil Solutions. Water, 15(18), 3277. https://doi.org/10.3390/w15183277







