Effects of Chemical Activation Conditions on Hierarchical Porous Carbon via Oxytetracycline Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparations of HPCs
2.3. Materials Characterization
2.4. Adsorption Experiments
2.5. Modeling of Adsorption
3. Results and Discussion
3.1. Characterization of HPCs
3.1.1. Microstructure and Pore Structure
3.1.2. FTIR Spectroscopy
3.1.3. XRD
3.1.4. Raman Spectroscopy
3.2. Adsorption of OTC
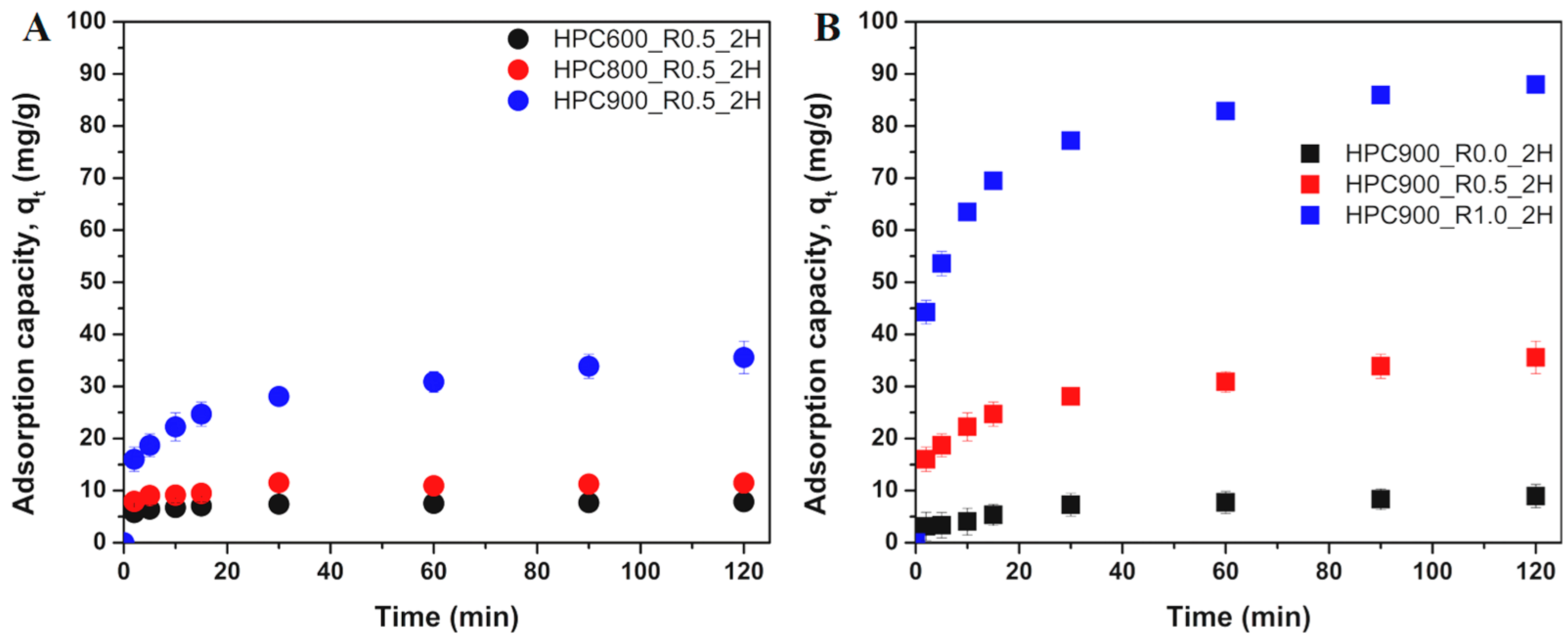
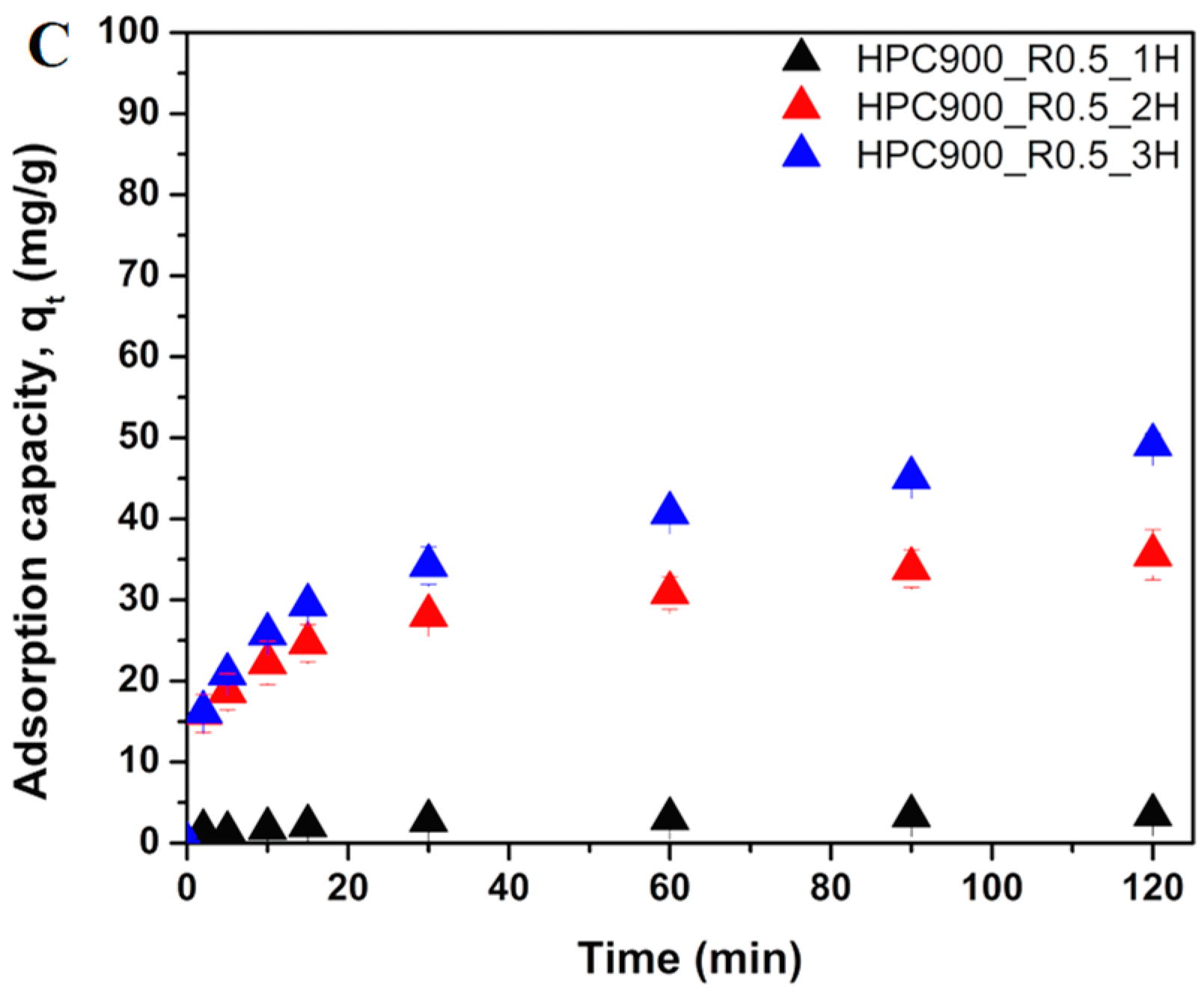
3.2.1. Effect of Activation Conditions of HPCs on OTC Adsorption Kinetics
3.2.2. Effect of pH
3.3. Adsorption Isotherm and Thermodynamic Study
3.4. Discussion about the Stability and Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Cerbo, A.; Palatucci, A.T.; Rubino, V.; Centenaro, S.; Giovazzino, A.; Fraccaroli, E.; Cortese, L.; Ruggiero, G.; Guidetti, G.; Canello, S.; et al. Toxicological Implications and Inflammatory Response in Human Lymphocytes Challenged with Oxytetracycline. J. Biochem. Mol. Toxicol. 2016, 30, 170–177. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Hu, J.; Ren, L.; Zhang, Y.; Li, K. Determination and fate of oxytetracycline and related compounds in oxytetracycline production wastewater and the receiving river. Environ. Toxicol. Chem. 2008, 27, 80–86. [Google Scholar] [CrossRef]
- Cheng, X.; Guan, R.; Chen, Y.; Qian, Y.; Shang, Q.; Sun, Y. Adsorption and photocatalytic degradation process of oxytetracycline using mesoporous Fe-TiO2 based on high-resolution mass spectrometry. Chem. Eng. J. 2023, 460, 141618. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Li, Z.-J.; Qi, W.-N.; Feng, Y.; Liu, Y.-W.; Ebrahim, S.; Long, J. Degradation mechanisms of oxytetracycline in the environment. J. Integr. Agric. 2019, 18, 1953–1960. [Google Scholar] [CrossRef]
- Pelosato, R.; Bolognino, I.; Fontana, F.; Sora, I.N. Applications of Heterogeneous Photocatalysis to the Degradation of Oxytetracycline in Water: A Review. Molecules 2022, 27, 2743. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Si, Q.; Luo, H.; Zhao, Q.; Ren, N. Sludge-derived biochar as efficient persulfate activators: Sulfurization-induced electronic structure modulation and disparate nonradical mechanisms. Appl. Catal. B Environ. 2020, 279, 119361. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, S.; Wang, M.; Zhang, J.; Ding, H.; Song, Y.; Zhu, Y.; Pan, Q.; Zhao, C.; Deng, H. Thiourea-assisted one-step fabrication of a novel nitrogen and sulfur co-doped biochar from nanocellulose as metal-free catalyst for efficient activation of peroxymonosulfate. J. Hazard. Mater. 2021, 416, 125796. [Google Scholar] [CrossRef]
- Kim, D.G.; Boldbaatar, S.; Ko, S.O. Enhanced Adsorption of Tetracycline by Thermal Modification of Coconut Shell-Based Activated Carbon. Int. J. Environ. Res. Public Health 2022, 19, 13741. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, L.; Long, C.; Fan, Z. From flour to honeycomb-like carbon foam: Carbon makes room for high energy density supercapacitors. Nano Energy 2015, 13, 527–536. [Google Scholar] [CrossRef]
- Hong, X.; Liu, Y.; Fu, J.; Wang, X.; Zhang, T.; Wang, S.; Hou, F.; Liang, J. A wheat flour derived hierarchical porous carbon/graphitic carbon nitride composite for high-performance lithium–sulfur batteries. Carbon 2020, 170, 119–126. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Song, J.; Lu, L.; Wang, J.; Li, X.; Li, J.; Wang, Q.; Du, H.; Xin, S.; Xu, L.; Yan, Q.; et al. Highly efficient nanocomposite of Y(2)O(3)@biochar for oxytetracycline removal from solution: Adsorption characteristics and mechanisms. Bioresour. Technol. 2023, 385, 129380. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Zhang, L.; Song, X.; Liu, X.; Yang, L.; Pan, F.; Lv, J. Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chem. Eng. J. 2011, 178, 26–33. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Acosta, R.; Fierro, V.; Martinez de Yuso, A.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Wang, M.; Huang, J.; Huang, L. Preparation of activated carbons from Iris tectorum employing ferric nitrate as dopant for removal of tetracycline from aqueous solutions. Ecotoxicol. Environ. Saf. 2013, 98, 273–282. [Google Scholar] [CrossRef]
- Liao, P.; Zhan, Z.; Dai, J.; Wu, X.; Zhang, W.; Wang, K.; Yuan, S. Adsorption of tetracycline and chloramphenicol in aqueous solutions by bamboo charcoal: A batch and fixed-bed column study. Chem. Eng. J. 2013, 228, 496–505. [Google Scholar] [CrossRef]
- Ocampo-Pérez, R.; Rivera-Utrilla, J.; Gómez-Pacheco, C.; Sánchez-Polo, M.; López-Peñalver, J.J. Kinetic study of tetracycline adsorption on sludge-derived adsorbents in aqueous phase. Chem. Eng. J. 2012, 213, 88–96. [Google Scholar] [CrossRef]
- Wohlgemuth, S.-A.; White, R.J.; Willinger, M.-G.; Titirici, M.-M.; Antonietti, M. A one-pot hydrothermal synthesis of sulfur and nitrogen doped carbon aerogels with enhanced electrocatalytic activity in the oxygen reduction reaction. Green Chem. 2012, 14, 1515–1523. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Liu, M.; Zhao, Y.; Ma, X.; Gan, L.; Noonan, O.; Yu, C. Enlargement of uniform micropores in hierarchically ordered micro–mesoporous carbon for high level decontamination of bisphenol A. J. Mater. Chem. A 2014, 2, 8534–8544. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Zhang, H.; Shao, L.-M.; Lü, F.; He, P.-J. Preparation and Application of Hierarchical Porous Carbon Materials from Waste and Biomass: A Review. Waste Biomass Valorization 2020, 12, 1699–1724. [Google Scholar] [CrossRef]
- Lahreche, S.; Moulefera, I.; El Kebir, A.; Sabantina, L.; Kaid, M.H.; Benyoucef, A. Application of Activated Carbon Adsorbents Prepared from Prickly Pear Fruit Seeds and a Conductive Polymer Matrix to Remove Congo Red from Aqueous Solutions. Fibers 2022, 10, 7. [Google Scholar] [CrossRef]
- Vieira, A.L.S.; Ribeiro, R.S.; Lado Ribeiro, A.R.; Ribeiro, A.M.; Silva, A.M.T. Hollow carbon spheres for diclofenac and venlafaxine adsorption. J. Environ. Chem. Eng. 2022, 10, 107348. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, L.; Qi, C.; Zhang, M. Highly Effective Removal of Tetracycline from Water by Hierarchical Porous Carbon: Batch and Column Adsorption. Ind. Eng. Chem. Res. 2019, 58, 20036–20046. [Google Scholar] [CrossRef]
- Ng, S.W.L.; Yilmaz, G.; Ong, W.L.; Ho, G.W. One-step activation towards spontaneous etching of hollow and hierarchical porous carbon nanospheres for enhanced pollutant adsorption and energy storage. Appl. Catal. B Environ. 2018, 220, 533–541. [Google Scholar] [CrossRef]
- González, J.F.; Román, S.; González-García, C.M.; Nabais, J.M.V.; Ortiz, A.L. Porosity Development in Activated Carbons Prepared from Walnut Shells by Carbon Dioxide or Steam Activation. Ind. Eng. Chem. Res. 2009, 48, 7474–7481. [Google Scholar] [CrossRef]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular bamboo-derived activated carbon for high CO2 adsorption: The dominant role of narrow micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Cuong, D.V.; Liu, N.L.; Nguyen, V.A.; Hou, C.H. Meso/micropore-controlled hierarchical porous carbon derived from activated biochar as a high-performance adsorbent for copper removal. Sci. Total Environ. 2019, 692, 844–853. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci. Total Environ. 2019, 646, 1567–1577. [Google Scholar] [CrossRef]
- Lin, Y.; Li, F.; Li, X.; Zhao, H.; Liu, G. Multifunctional Template Prepares N-, O-, and S-Codoped Mesoporous 3D Hollow Nanocage Biochar with a Multilayer Wall Structure for Aqueous High-Performance Supercapacitors. ACS Appl. Energy Mater. 2023, 6, 2265–2275. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Masek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S.; Zhang, Y.; Deng, O.; Wang, L.; Zhang, J.; Wei, L. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Diez, N.; Fuertes, A.B. More Sustainable Chemical Activation Strategies for the Production of Porous Carbons. ChemSusChem 2021, 14, 94–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Wang, J.; Lei, S.; Liang, L. Preparation of porous activated carbon from semi-coke by high temperature activation with KOH for the high-efficiency adsorption of aqueous tetracycline. Appl. Surf. Sci. 2020, 530, 147187. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, T.; Wang, H.; Yang, F.; Yan, J.; Zhu, K.; Ye, K.; Wang, G.; Zhou, L.; Cheng, K.; et al. High-throughput fabrication of porous carbon by chemical foaming strategy for high performance supercapacitor. Chem. Eng. J. 2018, 352, 459–468. [Google Scholar] [CrossRef]
- Cheng, F.; Li, X. Preparation and Application of Biochar-Based Catalysts for Biofuel Production. Catalysts 2018, 8, 346. [Google Scholar] [CrossRef]
- Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. C 2021, 7, 39. [Google Scholar] [CrossRef]
- Song, G.; Deng, R.; Yao, Z.; Chen, H.; Romero, C.; Lowe, T.; Driscoll, G.; Kreglow, B.; Schobert, H.; Baltrusaitis, J. Anthracite coal-based activated carbon for elemental Hg adsorption in simulated flue gas: Preparation and evaluation. Fuel 2020, 275, 117921. [Google Scholar] [CrossRef]
- Schott, J.A.; Do-Thanh, C.-L.; Shan, W.; Puskar, N.G.; Dai, S.; Mahurin, S.M. FTIR investigation of the interfacial properties and mechanisms of CO2 sorption in porous ionic liquids. Green Chem. Eng. 2021, 2, 392–401. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Chang, C. Novel Method for Preparing Activated Carbons with High Specific Surface Area from Rice Husk. Ind. Eng. Chem. Res. 2012, 51, 15075–15081. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Ding, L.; Yu, J.; Zhou, Q.; Kong, Y.; Ma, J. Novel sodium bicarbonate activation of cassava ethanol sludge derived biochar for removing tetracycline from aqueous solution: Performance assessment and mechanism insight. Bioresour. Technol. 2021, 330, 124949. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Wu, X.; Chen, Q.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Effect of acetylation on the properties of corn starch. Food Chem. 2008, 106, 923–928. [Google Scholar] [CrossRef]
- Leblanc, N.; Saiah, R.; Beucher, E.; Gattin, R.; Castandet, M.; Saiter, J.M. Structural investigation and thermal stability of new extruded wheat flour based polymeric materials. Carbohydr. Polym. 2008, 73, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.T.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Q.; Cao, F.; Qin, Q.; Jiao, M. Silkworm cocoon derived N, O-codoped hierarchical porous carbon with ultrahigh specific surface area for efficient capture of methylene blue with exceptionally high uptake: Kinetics, isotherm, and thermodynamics. RSC Adv. 2019, 9, 33872–33882. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A.; Skrzypczyńska, K.; Błażewicz, S.; Hryniewicz, J. The effects of the thermal treatment of activated carbon on the phenols adsorption. Korean J. Chem. Eng. 2017, 34, 1081–1090. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, Y.; Lai, B.; Wang, L.; Yang, S.; Li, K.; Wang, D.; Wu, Y.; Chen, G.-H.; Qian, J. Nitrogen and phosphorus co-doped porous carbons (NPCs) for peroxydisulfate (PDS) activation towards tetracycline degradation: Defects enhanced adsorption and non-radical mechanism dominated by electron transfer. Chem. Eng. J. 2023, 455, 140615. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Ryu, Z.; Zheng, J.; Zhang, Y. Influence of heat treatment of rayon-based activated carbon fibers on the adsorption of formaldehyde. J. Colloid Interface Sci. 2003, 261, 207–212. [Google Scholar] [CrossRef]
- Yu, F.; Pan, J.; Li, Y.; Yang, Y.; Zhang, Z.; Nie, J.; Ma, J. Batch and continuous fixed-bed column adsorption of tetracycline by biochar/MOFs derivative covered with κ-carrageenan/calcium alginate hydrogels. J. Environ. Chem. Eng. 2022, 10, 107996. [Google Scholar] [CrossRef]
- Jang, H.M.; Kan, E. Engineered biochar from agricultural waste for removal of tetracycline in water. Bioresour. Technol. 2019, 284, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xing, R.; Zhou, W. Adsorption of ciprofloxacin and Cu2+ onto biochars in the presence of dissolved organic matter derived from animal manure. Environ. Sci. Pollut. Res. Int. 2019, 26, 14382–14392. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Hu, F.; Huang, J.; Wang, Y.; Dai, H.; Liu, Z. Preparation of a graphitic ordered mesoporous carbon and its application in sorption of ciprofloxacin: Kinetics, isotherm, adsorption mechanisms studies. Microporous Mesoporous Mater. 2016, 228, 196–206. [Google Scholar] [CrossRef]
- Mozaffari Majd, M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpaa, M. Adsorption isotherm models: A comprehensive and systematic review (2010–2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Graphene-based materials for effective adsorption of organic and inorganic pollutants: A critical and comprehensive review. Sci. Total Environ. 2023, 863, 160871. [Google Scholar] [CrossRef]
- El Hassani, A.A.; Tanji, K.; El Mrabet, I.; Fahoul, Y.; El Gaidoumi, A.; Benjelloun, A.T.; Sfaira, M.; Zaitan, H.; Kherbeche, A. A combined molecular dynamics simulation, DFT calculations, and experimental study of the adsorption of Rhodamine B dye on kaolinite and hydroxyapatite in aqueous solutions. Surf. Interfaces 2023, 36, 102647. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Kadhim, M.M.; Taban, T.Z.; Baigenzhenov, O.; Ivanets, A.; Lal, B.; Kumar, N.; Hosseini-Bandegharaei, A. Adsorption performance of Enterobacter cloacae towards U(VI) ion and application of Enterobacter cloacae/carbon nanotubes to preconcentration and determination of low-levels of U(VI) in water samples. Chemosphere 2023, 311, 136804. [Google Scholar] [CrossRef]
- Oh, W.D.; Zaeni, J.R.J.; Lisak, G.; Lin, K.A.; Leong, K.H.; Choong, Z.Y. Accelerated organics degradation by peroxymonosulfate activated with biochar co-doped with nitrogen and sulfur. Chemosphere 2021, 277, 130313. [Google Scholar] [CrossRef]
- Pham, V.L.; Kim, D.G.; Ko, S.O. Cu@Fe3O4 core-shell nanoparticle-catalyzed oxidative degradation of the antibiotic oxytetracycline in pre-treated landfill leachate. Chemosphere 2018, 191, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.L.; Kim, D.G.; Ko, S.O. Advanced oxidative degradation of acetaminophen by carbon catalysts: Radical vs non-radical pathways. Environ. Res. 2020, 188, 109767. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhu, K.; Huang, J.; Shen, Y.; Lei, L.; He, H.; Chen, W. N, S co-doped magnetic mesoporous carbon nanosheets for activating peroxymonosulfate to rapidly degrade tetracycline: Synergistic effect and mechanism. J. Hazard. Mater. 2022, 424, 127569. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Hou, C.; Gao, Z.; Wang, L.; Yang, C.; Li, Y.; Liu, K.; Sun, Y. Preparation of Biochar with Developed Mesoporous Structure from Poplar Leaf Activated by KHCO(3) and Its Efficient Adsorption of Oxytetracycline Hydrochloride. Molecules 2023, 28, 3188. [Google Scholar] [CrossRef]
- Yang, J.-G.; Bai, X.-J.; Wang, Y.-L. The preparation of synthetic graphite materials with hierarchical pores from lignite by one-step impregnation and their characterization as dye absorbents. RSC Adv. 2019, 9, 12737–12746. [Google Scholar] [CrossRef]
- Xu, K.; Yang, X.; Ruan, L.; Qi, S.; Liu, J.; Liu, K.; Pan, S.; Feng, G.; Dai, Z.; Yang, X.; et al. Superior Adsorption and Photocatalytic Degradation Capability of Mesoporous LaFeO3/g-C3N4 for Removal of Oxytetracycline. Catalysts 2020, 10, 301. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, S.; Wang, Y. Mechanism of Oxytetracycline Removal by Coconut Shell Biochar Loaded with Nano-Zero-Valent Iron. Int. J. Environ. Res. Public Health 2021, 18, 13107. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, P.; Shen, J.; Ren, S. Development of Vitamin B6-Mediated Biochar with Nano Zero-Valent Iron Coating for Oxytetracycline Removal through Adsorption and Degradation under Harsh Acidic Conditions. Water 2022, 14, 2734. [Google Scholar] [CrossRef]
- Kumar, R.; Oves, M.; Ansari, M.O.; Taleb, M.A.; Baraka, M.A.E.; Alghamdi, M.A.; Makishah, N.H.A. Biopolymeric Ni(3)S(4)/Ag(2)S/TiO(2)/Calcium Alginate Aerogel for the Decontamination of Pharmaceutical Drug and Microbial Pollutants from Wastewater. Nanomaterials 2022, 12, 3642. [Google Scholar] [CrossRef]
- Kumar, R.; Ansari, M.O.; Taleb, M.A.; Oves, M.; Barakat, M.A.; Alghamdi, M.A.; Al Makishah, N.H. Integrated Adsorption-Photocatalytic Decontamination of Oxytetracycline from Wastewater Using S-Doped TiO2/WS2/Calcium Alginate Beads. Catalysts 2022, 12, 1676. [Google Scholar] [CrossRef]








| Specific Surface Area (SSA) | Total Pore Volume (Vtotal) | Micropore Volume (Vmicro) | Mesopore Volume (Vmeso) | Vmeso/ Vtotal | Average Pore Diameter (da) | |
|---|---|---|---|---|---|---|
| (m2/g) | (cm3/g) | (cm3/g) | (cm3/g) | (nm) | ||
| HPC600_R0.5_2H | 460.54 | 0.2281 | 0.1779 | 0.0366 | 0.1605 | 1.9811 |
| HPC800_R0.5_2H | 622.29 | 0.2879 | 0.2513 | 0.0369 | 0.1282 | 1.8505 |
| HPC900_R0.5_2H | 648.13 | 0.2908 | 0.2539 | 0.0502 | 0.1726 | 1.7947 |
| Specific Surface Area (SSA) | Total Pore Volume (Vtotal) | Micropore Volume (Vmicro) | Mesopore Volume (Vmeso) | Vmeso/ Vtotal | Average Pore Diameter (da) | |
|---|---|---|---|---|---|---|
| (m2/g) | (cm3/g) | (cm3/g) | (cm3/g) | (nm) | ||
| HPC900_R0.0_2H | 662.75 | 0.2750 | 0.2611 | 0.0139 | 0.0505 | 1.6595 |
| HPC900_R0.5_2H | 648.13 | 0.2908 | 0.2539 | 0.0369 | 0.1269 | 1.7947 |
| HPC900_R1.0_2H | 633.30 | 0.4382 | 0.3070 | 0.1312 | 0.2994 | 2.7678 |
| Specific Surface Area (SSA) | Total Pore Volume (Vtotal) | Micropore Volume (Vmicro) | Mesopore Volume (Vmeso) | Vmeso/ Vtotal | Average Pore Diameter (da) | |
|---|---|---|---|---|---|---|
| (m2/g) | (cm3/g) | (cm3/g) | (cm3/g) | (nm) | ||
| HPC900_R0.5_1H | 259.01 | 0.1169 | 0.0975 | 0.0194 | 0.1659 | 1.8054 |
| HPC900_R0.5_2H | 648.13 | 0.2908 | 0.2539 | 0.0369 | 0.1269 | 1.7947 |
| HPC900_R0.5_3H | 720.81 | 0.3720 | 0.3044 | 0.0676 | 0.1817 | 2.0643 |
| D4 | D | D3 | G | D2 | D+G | 2D | ID/IG | I2D/IG | ID/ID2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HPC600_ | Center (cm−1) | 1210 | 1350 | 1540 | 1580 | 1610 | 2900 | 2660 | 4.528 | 1.579 | 15.700 |
| R0.5_2H | Fraction (%) | 0.126 | 0.584 | 0.124 | 0.129 | 0.037 | 0.599 | 0.401 | |||
| HPC900_ | Center (cm−1) | 1210 | 1350 | 1541 | 1580 | 1610 | 2906 | 2670 | 7.887 | 1.766 | 11.883 |
| R0.5_2H | Fraction (%) | 0.097 | 0.637 | 0.131 | 0.081 | 0.054 | 0.567 | 0.433 |
| D4 | D | D3 | G | D2 | D+G | 2D | ID/IG | I2D/IG | ID/ID2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HPC900_ | Center (cm−1) | 1210 | 1350 | 1540 | 1580 | 1610 | 2915 | 2670 | 4.821 | 1.638 | 12.607 |
| R0.0_2H | Fraction (%) | 0.114 | 0.592 | 0.124 | 0.123 | 0.047 | 0.410 | 0.590 | |||
| HPC900_ | Center (cm−1) | 1210 | 1350 | 1541 | 1580 | 1610 | 2906 | 2670 | 7.887 | 1.766 | 11.883 |
| R0.5_2H | Fraction (%) | 0.097 | 0.637 | 0.131 | 0.081 | 0.054 | 0.567 | 0.433 | |||
| HPC900_ | Center (cm−1) | 1210 | 1351 | 1540 | 1580 | 1610 | 2911 | 2670 | 7.959 | 2.818 | 10.765 |
| R1.0_2H | Fraction (%) | 0.105 | 0.625 | 0.133 | 0.079 | 0.058 | 0.448 | 0.552 |
| D4 | D | D3 | G | D2 | D+G | 2D | ID/IG | I2D/IG | ID/ID2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HPC900_ | Center (cm−1) | 1210 | 1351 | 1541 | 1580 | 1610 | 2911 | 2670 | 5.729 | 1.603 | 19.451 |
| R0.5_1H | Fraction (%) | 0.107 | 0.621 | 0.132 | 0.108 | 0.032 | 0.493 | 0.507 | |||
| HPC900_ | Center (cm−1) | 1210 | 1350 | 1541 | 1580 | 1610 | 2906 | 2670 | 7.887 | 1.766 | 11.883 |
| R0.5_2H | Fraction (%) | 0.097 | 0.637 | 0.131 | 0.081 | 0.054 | 0.567 | 0.433 | |||
| HPC900_ | Center (cm−1) | 1210 | 1350 | 1540 | 1580 | 1610 | 2900 | 2660 | 7.888 | 1.953 | 9.764 |
| R0.5_3H | Fraction (%) | 0.088 | 0.647 | 0.117 | 0.082 | 0.066 | 0.542 | 0.458 |
| Model | Temperature Effect | Activator Ratio Effect | Activation Time Effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HPC600_ R0.5_2H | HPC800_ R0.5_2H | HPC900_ R0.5_2H | HPC900_ R0.0_2H | HPC900_ R0.5_2H | HPC900_ R1.0_2H | HPC900_ R0.5_1H | HPC900_ R0.5_2H | HPC900_ R0.5_3H | ||
| ka1 (min−1) | 0.7164 | 0.0603 | 0.1746 | 0.0789 | 0.1746 | 0.2472 | 0.0938 | 0.1746 | 0.0981 | |
| Pseudo-first-order | qe1 (mg/g) | 7.3002 | 10.5649 | 31.0715 | 7.9271 | 31.0715 | 79.9331 | 3.1866 | 31.0715 | 42.8964 |
| r2 | 0.9715 | 0.9356 | 0.8836 | 0.9172 | 0.8836 | 0.9252 | 0.8746 | 0.8836 | 0.8985 | |
| SSE | 1.36 | 6.68 | 112.04 | 5.21 | 112.04 | 463.43 | 1.27 | 112.04 | 194.00 | |
| ka2 (g/mg·min) | 0.1752 | 0.0898 | 0.0079 | 0.0120 | 0.0079 | 0.0044 | 0.0399 | 0.0079 | 0.0029 | |
| Pseudo-second-order | qe2 (mg/g) | 7.6021 | 11.1119 | 33.5996 | 9.1863 | 33.5996 | 85.8779 | 3.4861 | 33.5996 | 47.4636 |
| r2 | 0.9930 | 0.9716 | 0.9545 | 0.9550 | 0.9545 | 0.9812 | 0.9234 | 0.9545 | 0.9551 | |
| SSE | 0.33 | 2.95 | 43.79 | 1.05 | 43.79 | 116.59 | 0.78 | 43.79 | 85.86 | |
| Elovich | αEl (mg/g) | 16,253.980 | 1600.437 | 32.7913 | 2.5162 | 32.7913 | 202.7550 | 1.4056 | 32.7913 | 35.1593 |
| Equation | βEl (g/mg) | 1.9062 | 1.0417 | 0.1878 | 0.5795 | 0.1879 | 0.0843 | 1.6265 | 0.1879 | 0.1135 |
| r2 | 0.9575 | 0.8793 | 0.9964 | 0.9595 | 0.9964 | 0.9805 | 0.9433 | 0.9964 | 0.9929 | |
| SSE | 1.36 | 6.68 | 111.98 | 5.21 | 111.98 | 463.53 | 1.27 | 111.98 | 194.00 | |
| Isotherm | 298 °K | 308 °K | 318 °K | ||
|---|---|---|---|---|---|
| Langmuir | qmax | (mg/g) | 87.184 | 128.370 | 162.707 |
| KL | (L/g) | 0.052 | 0.025 | 0.030 | |
| r2 | 0.999 | 0.995 | 0.996 | ||
| SSE | 67.38 | 110.44 | 303.40 | ||
| Freundlich | KF | ((mg/g)∙(L/g)1/n) | 13.596 | 14.912 | 17.194 |
| n | 3.082 | 2.763 | 2.571 | ||
| r2 | 0.936 | 0.973 | 0.953 | ||
| SSE | 901.64 | 683.14 | 1609.76 | ||
| Temkin | bT | (J/mol) | 0.006 | 0.008 | 0.010 |
| AT | (L/mg) | 1.274 | 0.883 | 0.877 | |
| r2 | 0.936 | 0.939 | 0.951 | ||
| SSE | 380.57 | 69.22 | 231.37 |
| 298 K | 308 K | 318 K | ||
|---|---|---|---|---|
| ΔG⁰ | (kJ/mol) | −3.093 | −4.109 | −6.028 |
| ΔH⁰ | (kJ/mol) | 40.788 | ||
| ΔS⁰ | (kJ/mol·°K) | 0.147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayrera, F.O.; Kim, D.-G.; Ko, S.-O. Effects of Chemical Activation Conditions on Hierarchical Porous Carbon via Oxytetracycline Adsorption. Water 2023, 15, 3146. https://doi.org/10.3390/w15173146
Hayrera FO, Kim D-G, Ko S-O. Effects of Chemical Activation Conditions on Hierarchical Porous Carbon via Oxytetracycline Adsorption. Water. 2023; 15(17):3146. https://doi.org/10.3390/w15173146
Chicago/Turabian StyleHayrera, Fernando Oscar, Do-Gun Kim, and Seok-Oh Ko. 2023. "Effects of Chemical Activation Conditions on Hierarchical Porous Carbon via Oxytetracycline Adsorption" Water 15, no. 17: 3146. https://doi.org/10.3390/w15173146
APA StyleHayrera, F. O., Kim, D.-G., & Ko, S.-O. (2023). Effects of Chemical Activation Conditions on Hierarchical Porous Carbon via Oxytetracycline Adsorption. Water, 15(17), 3146. https://doi.org/10.3390/w15173146






