Integrative Taxonomic Reappraisal and Evolutionary Biogeography of the Most Diverse Freshwater Mussel Clade from Southeast Asia (Pseudodontini)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection and Morphological Studies
2.2. Molecular Analyses
2.3. Phylogenetic Analyses and Divergence Time Estimation
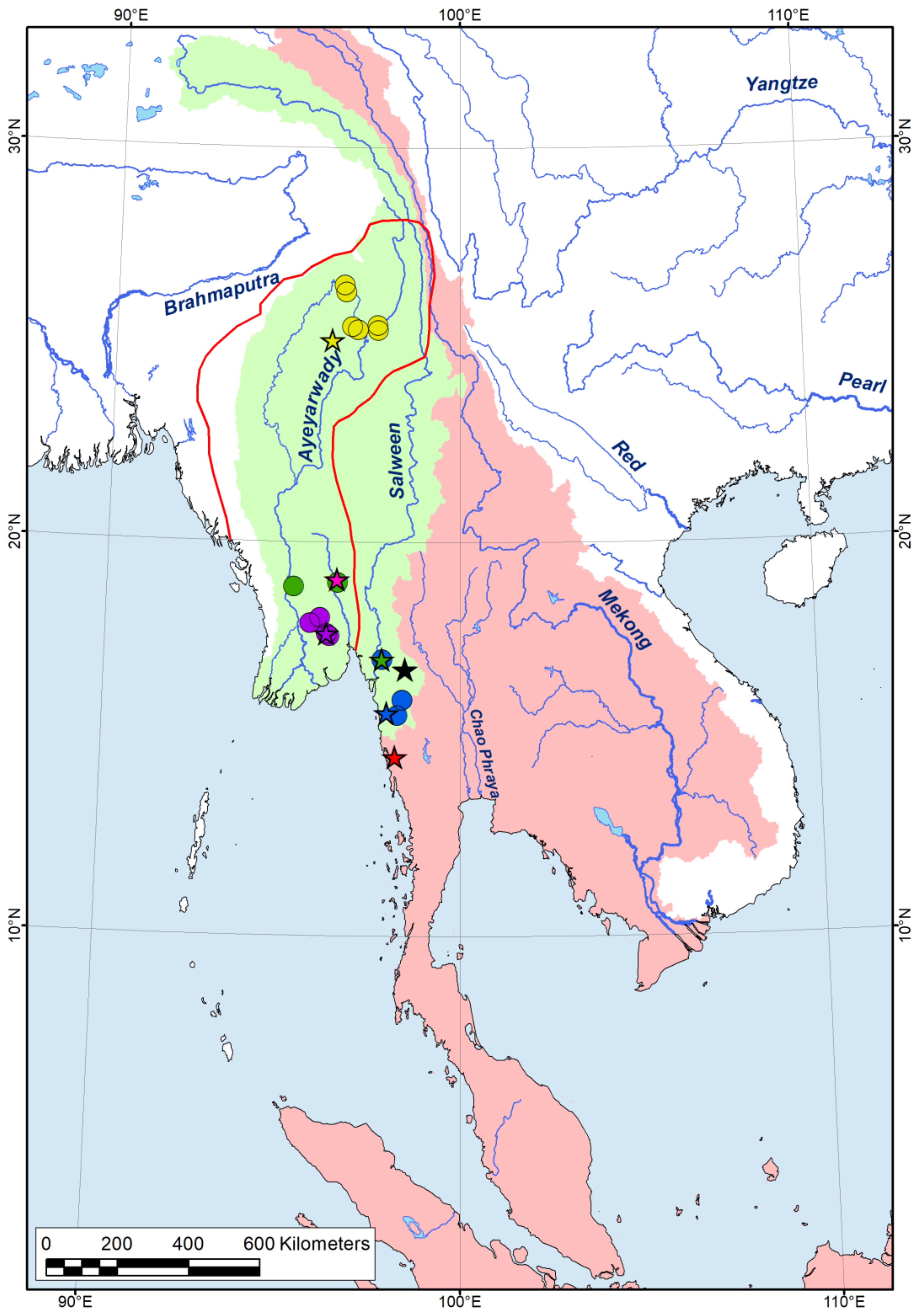
2.4. Biogeographic Modeling
3. Results
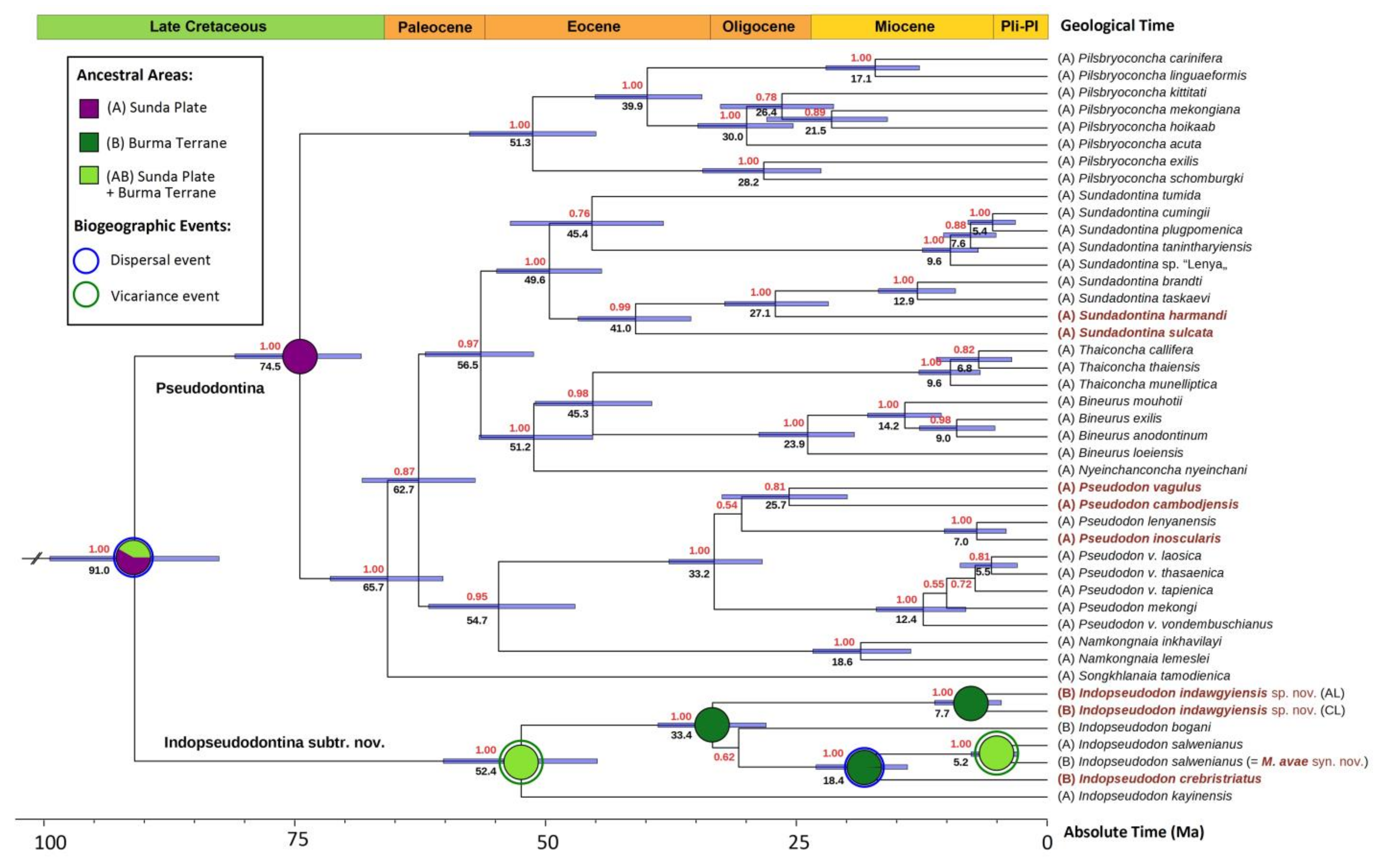
3.1. Macroevolution and Biogeography of the Tribe Pseudodontini

3.2. Integrative Taxonomic Revision of the Genus Indopseudodon
3.3. Newly Sequenced Samples of Little-Known Pseudodontini Species
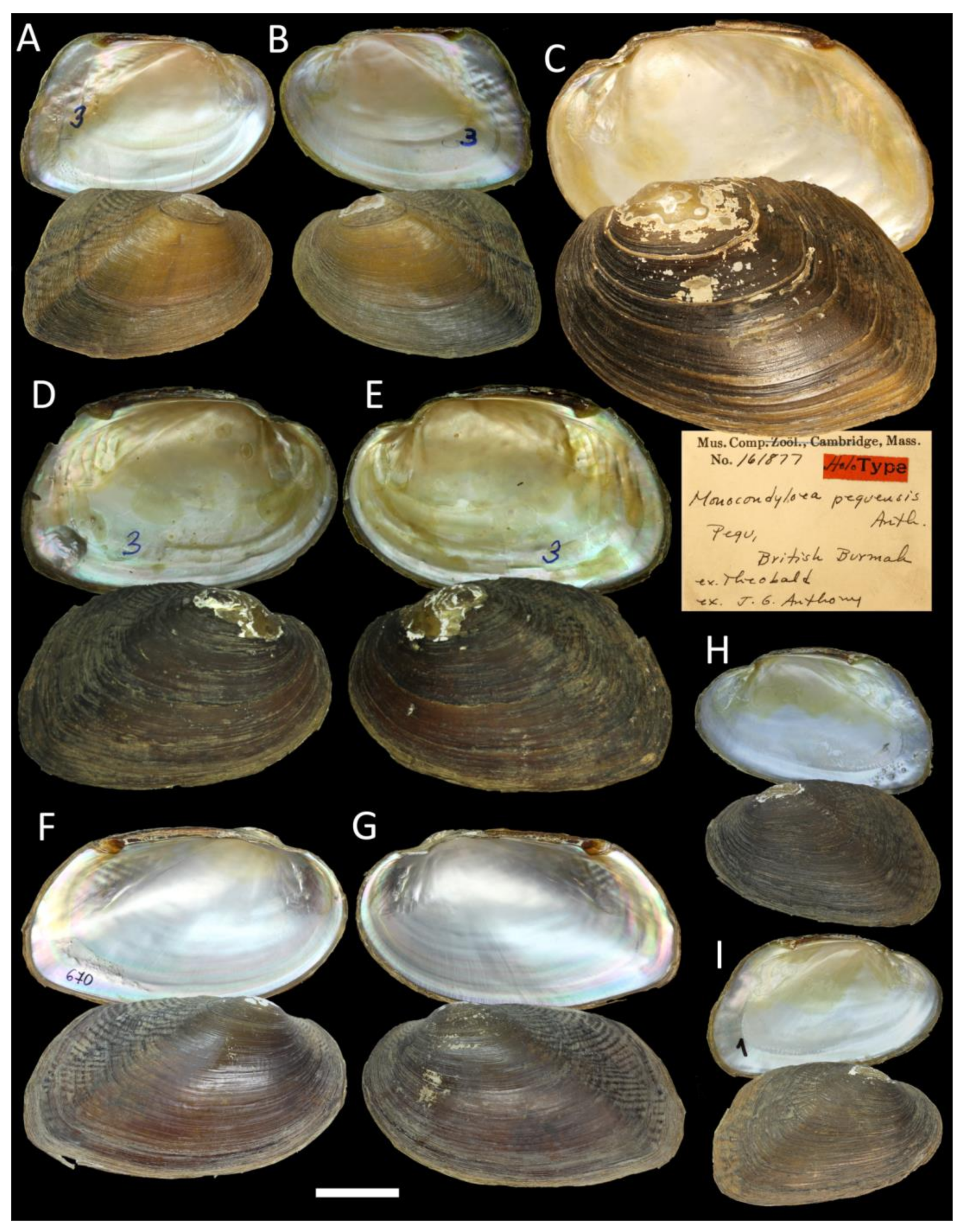
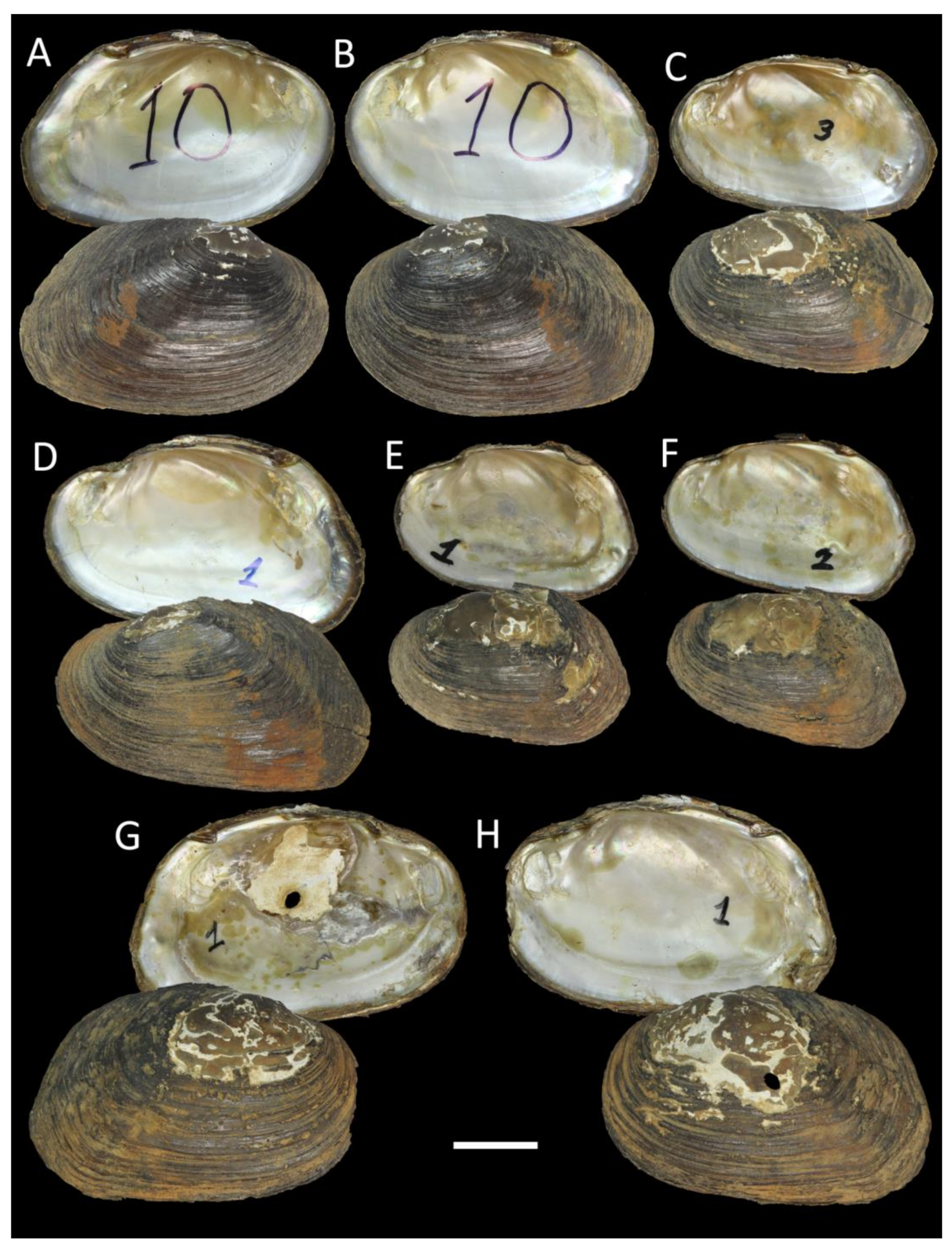
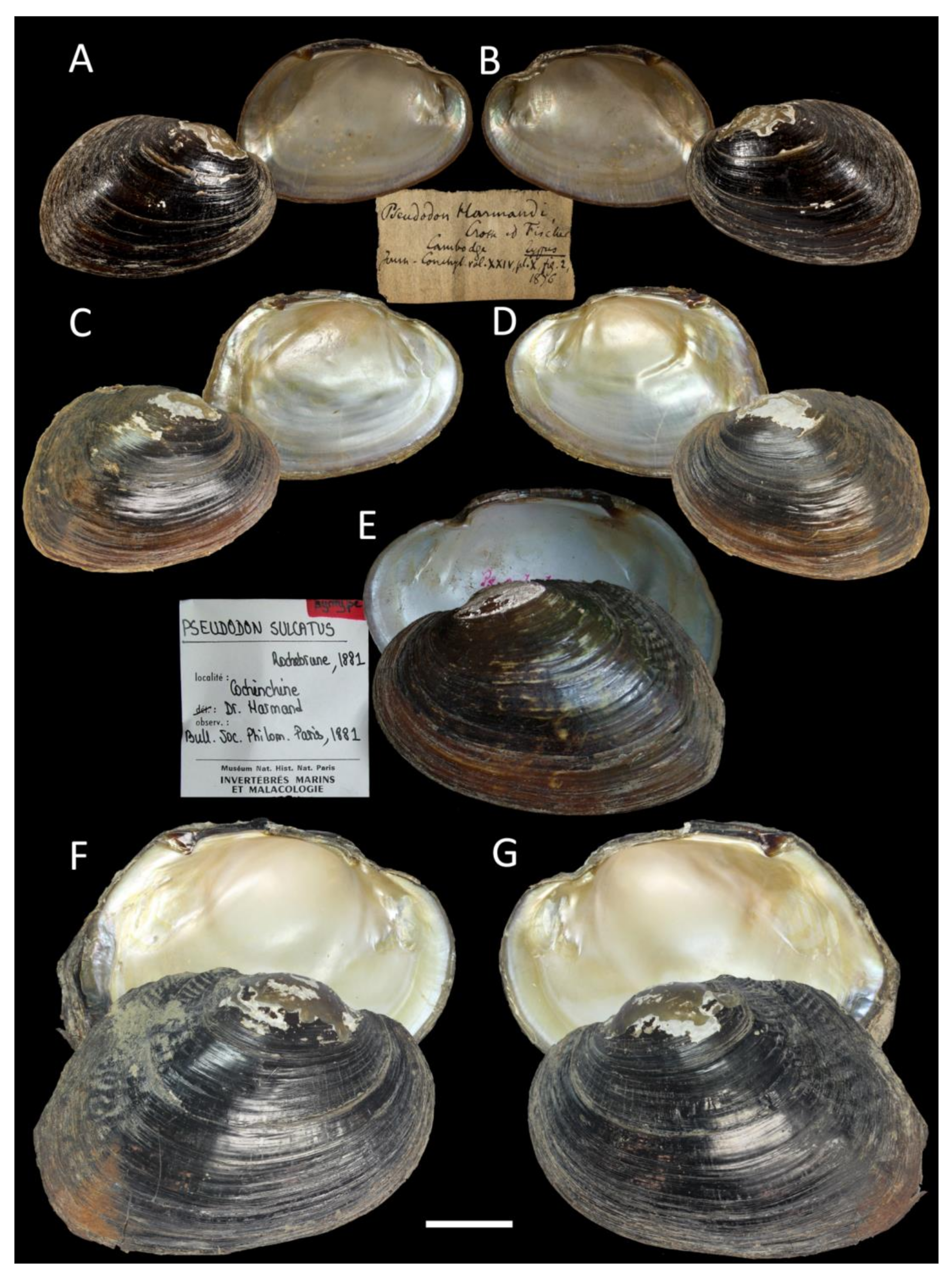
3.4. Taxonomic Account of the Tribe Pseudodontini with a New Species Description
- Family Unionidae Rafinesque, 1820
- Subfamily Gonideinae Ortmann, 1916
- Tribe Pseudodontini Frierson, 1927
- Indopseudodon crebristriatus (Anthony, 1865)
- Indopseudodon indawgyiensis Bolotov, Konopleva, Kondakov & Vikhrev sp. nov.
- Subtribe Pseudodontina Frierson, 1927 (type genus: Pseudodon Gould, 1844; by original designation).
- Genus Pseudodon Gould, 1844 stat. rev. (type species: Anodon inoscularis Gould, 1844; by original designation).
- Pseudodon cambodjensis (Petit de la Saussaye, 1865) comb. rev.
- Pseudodon inoscularis (Gould, 1844)
- Genus Bineurus Simpson, 1900 (type species: Monocondyloea mouhotii Lea, 1863; by original designation).
- Genus Namkongnaia Jeratthitikul et al., 2021 (type species: Namkongnaia inkhavilayi Jeratthitikul et al., 2021; by original designation).
- Genus Nyeinchanconcha Bolotov et al., 2020 (type species: Nyeinchanconcha nyeinchani Bolotov et al., 2020; by original designation).
- Genus Pilsbryoconcha Simpson, 1900 (type species: Anodonta exilis Lea, 1838; by original designation).
- Genus Sundadontina Bolotov et al., 2020 (type species: Anodonta cumingii Lea, 1851; by original designation).
- Sundadontina harmandi (Crosse & Fischer, 1876)
- Sundadontina sulcata (Rochebrune, 1882)
- Genus Thaiconcha Bolotov et al., 2020 (type species: Anodonta callifera Martens, 1860; by original designation).
3.5. A Brief Overview of Taxa That Should Be Excluded from the Tribe Pseudodontini
- Family Unionidae Rafinesque, 1820
- Subfamily Gonideinae Ortmann, 1916
- Tribe Gonideini Ortmann, 1916
- Genus Ptychorhynchus Simpson, 1900 (type species: Unio pfisteri Heude, 1874; by original designation).
- Genus Simpsonasus Bolotov & Konopleva nom. nov. (replacement name for Nasus Simpson, 1900).
- Subfamily Unioninae Rafinesque, 1820
- Tribe Unionini Rafinesque, 1820
- Genus Pseudobaphia Simpson, 1900 (type species: Unio biesianus Heude, 1877; by original designation [90]).
4. Discussion
4.1. Taxonomic Novelties and Their Consequences for the Modern Concept of the Tribe Pseudodontini
4.2. Ancient Radiations of the Pseudodontini in Mainland Southeast Asia
4.3. Ecology and Larval Hosts of the Pseudodontini
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graf, D.L.; Cummings, K.S. A “big data” approach to global freshwater mussel diversity (Bivalvia: Unionoida), with an updated checklist of genera and species. J. Molluscan Stud. 2021, 87, eyaa034. [Google Scholar] [CrossRef]
- Haas, F. Superfamilia Unionacea. Das Tierreich 1969, 88, 1–663. [Google Scholar]
- Brandt, R.A.M. The non-marine aquatic mollusca of Thailand. Arch. Molluskenkd. 1974, 105, 1–423. [Google Scholar]
- Bolotov, I.N.; Kondakov, A.V.; Vikhrev, I.V.; Aksenova, O.V.; Bespalaya, Y.V.; Gofarov, M.Y.; Kolosova, Y.S.; Konopleva, E.S.; Spitsyn, V.M.; Tanmuangpak, K.; et al. Ancient river inference explains exceptional Oriental freshwater mussel radiations. Sci. Rep. 2017, 7, 2135. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Konopleva, E.S.; Vikhrev, I.V.; Gofarov, M.Y.; Lopes-Lima, M.; Bogan, A.E.; Lunn, Z.; Chan, N.; Win, T.; Aksenova, O.V.; et al. New freshwater mussel taxa discoveries clarify biogeographic division of Southeast Asia. Sci. Rep. 2020, 10, 6616. [Google Scholar] [CrossRef]
- Zieritz, A.; Bogan, A.E.; Froufe, E.; Klishko, O.; Kondo, T.; Kovitvadhi, U.; Kovitvadhi, S.; Lee, J.H.; Lopes-Lima, M.; Pfeiffer, J.M.; et al. Diversity, biogeography and conservation of freshwater mussels (Bivalvia: Unionida) in East and Southeast Asia. Hydrobiologia 2018, 810, 29–44. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Vikhrev, I.V.; Kondakov, A.V.; Konopleva, E.S.; Gofarov, M.Y.; Aksenova, O.V.; Tumpeesuwan, S. New taxa of freshwater mussels (Unionidae) from a species-rich but overlooked evolutionary hotspot in Southeast Asia. Sci. Rep. 2017, 7, 11573. [Google Scholar] [CrossRef]
- Konopleva, E.S.; Bolotov, I.N.; Pfeiffer, J.M.; Vikhrev, I.V.; Kondakov, A.V.; Gofarov, M.Y.; Tomilova, A.A.; Tanmuangpak, K.; Tumpeesuwan, S. New freshwater mussels from two Southeast Asian genera Bineurus and Thaiconcha (Pseudodontini, Gonideinae, Unionidae). Sci. Rep. 2021, 11, 8244. [Google Scholar]
- Konopleva, E.S.; Lheknim, V.; Sriwoon, R.; Kondakov, A.V.; Tomilova, A.A.; Gofarov, M.Y.; Vikhrev, I.V.; Bolotov, I.N. Diversity and Phylogenetics of Freshwater Mussels (Unionidae) from Southern Thailand with the Description of One New Genus and Five New Species-Group Taxa. Diversity 2023, 15, 10. [Google Scholar] [CrossRef]
- Zieritz, A.; Lopes-Lima, M. Handbook and National Red-List of the Freshwater Mussels of Malaysia; Zieritz & Lopes-Lima: Kuala Lumpur, Malaysia, 2018. [Google Scholar]
- Jeratthitikul, E.; Sutcharit, C. Multi-locus Phylogeny Reveals a New Freshwater Mussel in the Genus Bineurus Simpson, 1900 (Unionidae: Pseudodontini) from Thailand. Trop. Nat. Hist. 2023, 7, 173–180. [Google Scholar]
- Lopes-Lima, M.; Burlakova, L.E.; Karatayev, A.Y.; Mehler, K.; Seddon, M.; Sousa, R. Conservation of freshwater bivalves at the global scale: Diversity, threats and research needs. Hydrobiologia 2018, 810, 1–14. [Google Scholar] [CrossRef]
- Zieritz, A.; Bogan, A.E.; Rahim, K.A.A.; Sousa, R.; Jainih, L.; Harun, S.; Razak, N.F.A.; Gallardo, B.; McGowan, S.; Hassan, R.; et al. Changes and drivers of freshwater mussel diversity and distribution in northern Borneo. Biol. Conserv. 2018, 219, 126–137. [Google Scholar] [CrossRef]
- Zieritz, A.; Lopes-Lima, M.; Bogan, A.E.; Sousa, R.; Walton, S.; Rahim, K.A.A.; Wilson, J.J.; Ng, P.Y.; Froufe, E.; McGowan, S. Factors driving changes in freshwater mussel (Bivalvia, Unionida) diversity and distribution in Peninsular Malaysia. Sci. Total Environ. 2016, 571, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.H.; Tan, S.K.; Wong, W.H.; Meier, R.; Chan, S.-Y.; Tan, H.H.; Yeo, D.C.J. Molluscs for sale: Assessment of freshwater gastropods and bivalves in the ornamental pet trade. PLoS ONE 2016, 11, e0161130. [Google Scholar]
- Yang, S.L. Record of a freshwater bivalve, Pseudodon vondembuschianus (Mollusca: Unionidae) in Singapore. Raffles Bull. Zool. 1990, 38, 83–84. [Google Scholar]
- Ng, P.K.; Chou, L.M.; Lam, T.J. The status and impact of introduced freshwater animals in Singapore. Biol. Conserv. 1993, 64, 19–24. [Google Scholar] [CrossRef]
- Clements, R.; Koh, L.P.; Lee, T.M.; Meier, R.; Li, D. Importance of reservoirs for the conservation of freshwater molluscs in a tropical urban landscape. Biol. Conserv. 2006, 128, 136–146. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Bespalaya, Y.V.; Gofarov, M.Y.; Kondakov, A.V.; Konopleva, E.S.; Vikhrev, I.V. Spreading of the Chinese pond mussel, Sinanodonta woodiana, across Wallacea: One or more lineages invade tropical islands and Europe. Biochem. Syst. Ecol. 2016, 67, 58–64. [Google Scholar] [CrossRef]
- Sahidin, A.; Muhammad, G.; Hasan, Z.; Arief, M.C.W.; Marwoto, R.M.; Komaru, A. Indonesian freshwater bivalves: A meta-analysis of endemicity, ecoregion distributions, and conservation status. Aquac. Aquar. Conserv. Legis. 2021, 14, 3750–3775. [Google Scholar]
- Affandi, M.; Candra, L.A.; Priatama, A.B.; Irawan, B.; Soegianto, A. Diversity of the Unionid Freshwater Mussels (Bivalvia: Unionidae) in Brantas River, East Java, Indonesia. J. Biol. Res. 2013, 18, 111–115. [Google Scholar] [CrossRef]
- Do, V.T.; Tuan, L.Q.; Bogan, A.E. Freshwater mussels (Bivalvia: Unionida) of Vietnam: Diversity, distribution, and conservation status. Freshw. Mollusk Biol. Conserv. 2018, 21, 1–18. [Google Scholar] [CrossRef]
- Razak, N.F.A.; Supramaniam, C.V.; Zieritz, A. A dichotomous PCR–RFLP identification key for the freshwater mussels (Bivalvia: Unionida) of Peninsular Malaysia. Conserv. Genet. Resour. 2019, 11, 457–464. [Google Scholar] [CrossRef]
- Subba Rao, N.V. Handbook. Freshwater Molluscs of India; Zoological Survey of India: Calcutta, India, 1989. [Google Scholar]
- Graf, D.L.; Cummings, K.S. Review of the systematics and global diversity of freshwater mussel species (Bivalvia: Unionoida). J. Molluscan Stud. 2007, 73, 291–314. [Google Scholar] [CrossRef]
- Jeratthitikul, E.; Sutcharit, C.; Ngor, P.B.; Prasankok, P. Molecular phylogeny reveals a new genus of freshwater mussels from the Mekong River Basin (Bivalvia: Unionidae). Eur. J. Taxon. 2021, 775, 119–142. [Google Scholar] [CrossRef]
- Gould, A.A.D. Gould read descriptions of two Anodon, from the river Salwen, in British Burmah, sent him by Rev. F. Mason. Proc. Boston Soc. Nat. Hist. 1844, 1, 160–161. [Google Scholar]
- Bolotov, I.N.; Konopleva, E.S.; Vikhrev, I.V.; Win, T.; Lunn, Z.; Chan, N.; Gofarov, M.Y.; Kondakov, A.V.; Tomilova, A.A.; Pasupuleti, R.; et al. Follow the Footsteps of Leonardo Fea: An Example of an Integrative Revision of Freshwater Mussel Taxa Described from the Former British Burma (Myanmar). J. Zool. Syst. Evol. Res. 2022, 2022, 6600359. [Google Scholar] [CrossRef]
- Konopleva, E.S.; Pfeiffer, J.M.; Vikhrev, I.V.; Kondakov, A.V.; Gofarov, M.Y.; Aksenova, O.V.; Lunn, Z.; Chan, N.; Bolotov, I.N. A new genus and two new species of freshwater mussels (Unionidae) from western Indochina. Sci. Rep. 2019, 9, 4106. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Makhrov, A.A.; Gofarov, M.Y.; Aksenova, O.V.; Aspholm, P.E.; Bespalaya, Y.V.; Kabakov, M.B.; Kolosova, Y.S.; Kondakov, A.V.; Ofenböck, T.; et al. Climate Warming as a Possible Trigger of Keystone Mussel Population Decline in Oligotrophic Rivers at the Continental Scale. Sci. Rep. 2018, 8, 35. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Konopleva, E.S.; Vikhrev, I.V.; Lopes-Lima, M.; Bogan, A.E.; Lunn, Z.; Chan, N.; Win, T.; Aksenova, O.V.; Gofarov, M.Y.; et al. Eight new freshwater mussels (Unionidae) from tropical Asia. Sci. Rep. 2019, 9, 12053. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Pasupuleti, R.; Subba Rao, N.V.; Unnikrishnan, S.K.; Chan, N.; Lunn, Z.; Win, T.; Gofarov, M.Y.; Kondakov, A.V.; Konopleva, E.S.; et al. Oriental freshwater mussels arose in East Gondwana and arrived to Asia on the Indian Plate and Burma Terrane. Sci. Rep. 2022, 12, 1518. [Google Scholar]
- Pfeiffer, J.M.; Graf, D.L. Evolution of bilaterally asymmetrical larvae in freshwater mussels (Bivalvia: Unionoida: Unionidae). Zool. J. Linn. Soc. 2015, 175, 307–318. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Villesen, P. FaBox: An online toolbox for fasta sequences. Mol. Ecol. Notes 2007, 7, 965–968. [Google Scholar] [CrossRef]
- Chernomor, O.; von Haeseler, A.; Minh, B.Q. Terrace aware data structure for phylogenomic inference from supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Zieritz, A.; Froufe, E.; Bolotov, I.; Gonçalves, D.V.; Aldridge, D.C.; Bogan, A.E.; Gan, H.M.; Gomes-Dos-Santos, A.; Sousa, R.; Teixeira, A.; et al. Mitogenomic phylogeny and fossil-calibrated mutation rates for all F-and M-type mtDNA genes of the largest freshwater mussel family, the Unionidae (Bivalvia). Zool. J. Linn. Soc. 2021, 193, 1088–1107. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Matzke, N.J. Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Syst. Biol. 2014, 63, 951–970. [Google Scholar] [CrossRef]
- Matzke, N.J. Probabilistic historical biogeography: New models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front. Biogeogr. 2013, 5, 242–248. [Google Scholar] [CrossRef]
- Yu, Y.; Blair, C.; He, X.J. RASP 4: Ancestral State Reconstruction Tool for Multiple Genes and Characters. Mol. Biol. Evol. 2020, 37, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Preston, H.B. A catalogue of the Asiatic naiades in the collection of the Indian Museum, Calcutta, with descriptions of new species. Rec. Indian Mus. 1912, 7, 279–308. [Google Scholar] [CrossRef]
- Anthony, J.G. Descriptions of two new species of Monocondylaea. Am. J. Conch. 1865, 1, 205–206. [Google Scholar]
- Gregory, J.W. The Geological Relations of the Oil Shales of Southern Burma. Geol. Mag. 1923, 60, 152–159. [Google Scholar] [CrossRef]
- Annandale, N. Fossil molluscs from the oil-measures of the Dawna Hills, Tenasserim. Rec. Geol. Surv. India 1924, 55, 97–104. [Google Scholar]
- Swinton, W.E. XLIX.—Daunophis langi, gen. et sp. n. (Pliocene, Burma). Ann. Mag. Nat. Hist. 1926, 17, 342–348. [Google Scholar] [CrossRef]
- Brown, G.F.; Buravas, S.; Charaljavanaphet, J.; Jalichandra, N.; Johnston, W.D.; Sresthaputra, V.; Taylor, G.C. Geologic Reconnaissance of the Mineral Deposits of Thailand; US Government Printing Office: Washington, DC, USA, 1951. [Google Scholar]
- Gurung, D.; Takayasu, K.; Matsuoka, K. Middle Miocene-Pliocene freshwater gastropods of the Churia Group, west-central Nepal. Paleontol. Res. 1997, 1, 166–179. [Google Scholar]
- Theobald, W. Descriptions of new species of Unionidæ. J. Asiat. Soc. Bengal 1873, 42, 207–209. [Google Scholar]
- He, J.; Zhuang, Z. The Freshwater Bivalves of China; Conch Books: Harxheim, Germany, 2013. [Google Scholar]
- Bolotov, I.N.; Konopleva, E.S.; Vikhrev, I.V.; Gofarov, M.Y.; Lopes-Lima, M.; Bogan, A.E.; Lunn, Z.; Chan, N.; Win, T.; Aksenova, O.V.; et al. Nominal taxa of freshwater Mollusca from Southeast Asia described by Dr. Nguyen, N. Thach: A brief overview with new synonyms and fixation of a publication date. Ecol. Montenegrina 2021, 41, 73–83. [Google Scholar] [CrossRef]
- Jeratthitikul, E.; Paphatmethin, S.; Sutcharit, C.; Ngor, P.B.; Inkhavilay, K.; Prasankok, P. Phylogeny and biogeography of Indochinese freshwater mussels in the genus Pilsbryoconcha Simpson, 1900 (Bivalvia: Unionidae) with descriptions of four new species. Sci. Rep. 2022, 12, 20458. [Google Scholar] [CrossRef] [PubMed]
- Panha, S. The site survey and the study on reproductive cycles of freshwater pearl mussels in the central part of Thailand. Venus 1990, 49, 240–257. [Google Scholar]
- Martens, E.v. Neue Unioniden aus Tonkin und Anam. Nachrichtsblatt Dtsch. Malakozool. Ges. 1902, 34, 130–135. [Google Scholar]
- Martens, E.v. On the Mollusca of Siam. Proc. Zool. Soc. Lond. 1860, 1860, 6–18. [Google Scholar]
- Drouët, H.; Chaper, M. Voyage de M. Chaper a Bornéo. Unionidae. Mém. Soc. Zool. Fr. 1892, 5, 145–154. [Google Scholar]
- Morelet, A. Diagnoses de coquilles nouvelles de l’Indo-Chine. Rev. Mag. Zool. 1866, 18, 165–168. [Google Scholar]
- Petit de la Saussaye, S. Note sur le genre Monocondylea de d’Orbigny, et description d’une espèce nouvelle. J. Conchyliol. 1865, 13, 15–19. [Google Scholar]
- Sowerby, G.B. Genus Unio. Conchol. Iconica 1867, 16, 55–60. [Google Scholar]
- Fischer, P. Catalogue et distribution géographique des mollusques terrestres, fluviatiles & marins d’une partie de l’Indo-chine (Siam, Laos, Cambodge, Cochinchine, Annam, Tonkin). Bull. Soc. Hist. Nat. Autun 1891, 4, 87–276. [Google Scholar]
- Lea, I. Descriptions of new fresh water and land shells. Proc. Am. Philos. Soc. 1840, 1, 284–289. [Google Scholar]
- Van Benthem Jutting, T. Non marine Mollusca from fossil horizons in Java with special reference to the Trinil fauna. Zool. Meded. 1937, 20, 83–180. [Google Scholar]
- Joordens, J.; d’Errico, F.; Wesselingh, F.P.; Munro, S.; de Vos, J.; Wallinga, J.; Ankjærgaard, C.; Reimann, T.; Wijbrans, J.R.; Kuiper, K.F.; et al. Homo erectus at Trinil on Java used shells for tool production and engraving. Nature 2015, 518, 228–231. [Google Scholar] [CrossRef]
- Gibran, A.K.; Ananda, D.R.; Setijadi, R.; Nabil, M.I.; Purwasatriya, E.B. Paleocurrents and paleogeography of the Kalibiuk, Kaliglagah, Mengger, and Gintung Formation, Bumiayu-Tonjong, Central Java. AIP Conf. Proc. 2023, 2482, 080002. [Google Scholar]
- Tolstikova, N.V. New Paleogene Freshwater Mollusks of the Zaisan Depression. In Development and Change of Organic World at the Boundary of Mesozoic and Cenozoic. New Data on the Development of Fauna; Menner, V.V., Ed.; Nauka: Moscow, Russia, 1975; pp. 51–68, 154–155. (In Russian) [Google Scholar]
- Tolstikova, N.V. Mollusks of the Ancient Lakes of the Zaysan Depression (South-Eastern Kazakhstan, Cretaceous, Paleogene, Miocene). In Fossil Freshwater Mollusks and Their Significance for Paleolimnology; Martinson, G.G., Ed.; Nauka: Leningrad, Russia, 1976; pp. 51–256. (In Russian) [Google Scholar]
- Modell, H. Paläontologische und Geologische Untersuchungen im Tertiär von Pakistan. 4. Die tertiären Najaden des Punjab und Vorderindiens. Abh. Bayer. Akad. Wiss. Math.-Naturwissenschaftliche Kl. Neue Folge 1969, 135, 1–49. [Google Scholar]
- Heude, M.P. Conchyliologie Fluviatile de la Province de Nanking et de la Chine Centrale, Fascicule VIII; Librairie, F., Ed.; Savy: Paris, France, 1883; pp. 57–65. [Google Scholar]
- Haas, F. Neue ostasiatische Najaden. Nachrichtsblatt Dtsch. Malakozool. Ges. 1911, 43, 43–46. [Google Scholar]
- Heude, M.P. Conchyliologie Fluviatile de la Province de Nanking et de la Chine Centrale, Fascicule III; Librairie, F., Ed.; Savy: Paris, France, 1877; pp. 17–24. [Google Scholar]
- Heude, M.P. Diagnoses molluscoum in fluminibus provinciae Nankingensis collectorum. J. Conchyliol. 1874, 22, 112–118. [Google Scholar]
- Tolstikova, N.V. Paleogene Freshwater Mollusks of the Zaysan Depression and Their Paleolimnological Importance. In Problems of Studies of Ancient Lakes of Eurasia; Martinson, G.G., Kyansep-Romashkina, N.P., Eds.; Nauka: Leningrad, Russia, 1974; pp. 70–95. (In Russian) [Google Scholar]
- MolluscaBase. Pseudodon politus (Tolstikova, 1974). Available online: https://www.molluscabase.org/aphia.php?p=taxdetails&id=1604505 (accessed on 21 July 2023).
- Nigmatova, S.A.; Bayshashov, B.U.; Pirogova, T.E.; Billia, E.M.E.; Zhamangara, A.K. Geology, stratigraphy and palaeontology of the Eocene Shynzhyly Locality (Eastern Kazakhstan) and comparison with the continental Eocene of Italy. Gortania Geol. Paleontol. Paletnol. 2020, 42, 37–53. [Google Scholar]
- Heude, M.P. Conchyliologie Fluviatile de la Province de Nanking et de la Chine Centrale, Fascicule IX; Librairie, F., Ed.; Savy: Paris, France, 1885; pp. 65–72. [Google Scholar]
- Otuka, Y. Some new Unionidae from North China and southern Mongolia. Proc. Imp. Acad. 1942, 18, 479–483. [Google Scholar] [CrossRef]
- Takayasu, K.; Gurung, D.D.; Matsuoka, K. Some new species of freshwater bivalves from the Mio-Pliocene Churia Group, west-central Nepal. Trans. Proc. Palaeontol. Soc. Jpn. New Ser. 1995, 179, 157–168. [Google Scholar]
- Nesemann, H.; Sharma, S.; Sharma, G.; Sinha, K.R. Illustrated checklist of large freschwater Bivalves of the Ganga River System (Molluska: Bivalvia: Solecurtidae, Unionidae, Amblemidae). Nachrichtenblatt Ersten Vorarlb. Malakol. Ges. 2005, 13, 1–51. [Google Scholar]
- Thiele, J. Handbuch der Systematischen Weichtierkunde. Bd. 1, Teil 3 (Scaphopoda, Bivalvia, Cephalopoda); Gustav Fischer: Jena, Germany, 1934. [Google Scholar]
- Kongim, B.; Sutcharit, C.; Jeratthitikul, E. Discovery of a New Endangered Freshwater Mussel Species in the Genus Chamberlainia Simpson, 1900 (Bivalvia: Unionidae) from Mekong Basin. Trop. Nat. Hist. 2023, 7, 242–250. [Google Scholar]
- Mason, F. Tenasserim: Or Notes on the Fauna, Flora, Minerals, and Nations of British Burmah and Pegu: With Systematic Catalogues of the Known Minerals, Plants, Mammals, Fishes, Mollusks, Sea-Nettles, Corals, Sea-Urchins, Worms, Insects, Crabs, Reptiles, and Birds; with Vernacular Names; American Mission Press, T.S. Ranney: Maulmain, Burma, 1851. [Google Scholar]
- Anonymous. Tavoy District. Imp. Gazet. India 1908, 23, 258. [Google Scholar]
- Mason, F. Quarterly Paper. V. Descriptive notices of Tavoy Province. Baptist Mission. Mag. 1838, 18, 28–32. [Google Scholar]
- Anonymous. American Baptist Board of Foreign Missions. Recent Intelligence—Burmah. Baptist Mission. Mag. 1844, 24, 330–332. [Google Scholar]
- Wyeth, W.N. The Wades: Jonathan Wade, Deborah BL Wade; A Memorial; Wyeth: Philadelphia, PA, USA, 1891. [Google Scholar]
- Conrad, T.A. Monography of the Family Unionidae, or Naiades of Lamarck, (Fresh Water Bivalve Shells) of North America, Illustrated by Figures Drawn on Stone from Nature; J. Dobson: Philadelphia, PA, USA, 1837; Issue 8. [Google Scholar]
- Simpson, C.T. Synopsis of the naiades, or pearly fresh-water mussels. Proc. U.S. Natl. Mus. 1900, 22, 501–1044. [Google Scholar] [CrossRef]
- Watters, G.T.; Hoggarth, M.A.; Stansberry, D.H. The Freshwater Mussels of Ohio; The Ohio State University Press: Columbus, OH, USA, 2009. [Google Scholar]
- Crosse, H.; Fischer, P. Mollusques fluviatiles, recueillis au Cambodge, par la mission scientifique française de 1873. J. Conchyliol. 1876, 24, 313–334. [Google Scholar]
- Rochebrune, A.-T. Documents sur la faune malacologique de la Cochinchine et du Cambodge. Bull. Soc. Philomath. Paris 1882, 6, 35–74. [Google Scholar]
- Haas, F. Die Unioniden; Küster, H.C., Ed.; Systematisches Conchylien-Cabinet Martini Chemnitz; Bauer&Raspe: Nuremberg, Germany, 1920; Volume 9, pp. 305–344. [Google Scholar]
- Dai, Y.; Huang, X.; Wu, C.; Chen, Z.; Guo, L.; Shu, F.; Ouyang, S.; Wu, X. Multilocus and mitogenomic phylogenetic analyses reveal a new genus and species of freshwater mussel (Bivalvia: Unionidae) from Guangxi, China. Invertebr. Syst. 2023, 37, 152–166. [Google Scholar] [CrossRef]
- Bogan, A.E.; Do, V.T. An overlooked new species of freshwater bivalve from northern Vietnam (Mollusca: Bivalvia: Unionidae). Raffles Bull. Zool. 2018, 66, 78–86. [Google Scholar]
- Bolotov, I.N.; Pfeiffer, J.; Konopleva, E.S.; Vikhrev, I.V.; Kondakov, A.V.; Aksenova, O.V.; Gofarov, M.Y.; Tumpeesuwan, S.; Win, T. A new genus and tribe of freshwater mussel (Unionidae) from Southeast Asia. Sci. Rep. 2018, 8, 10030. [Google Scholar] [CrossRef]
- Schneider, S.; Böhme, M.; Prieto, J. Unionidae (Bivalvia; Palaeoheterodonta) from the Palaeogene of northern Vietnam: Exploring the origins of the modern East Asian freshwater bivalve fauna. J. Syst. Palaeontol. 2013, 11, 337–357. [Google Scholar] [CrossRef]
- Huang, H.; Morley, R.J.; Licht, A.; Dupont-Nivet, G.; Pérez-Pinedo, D.; Westerweel, J.; Win, Z.; Aung, D.W.; Lelono, E.B.; Aleksandrova, G.N.; et al. A proto-monsoonal climate in the late Eocene of Southeast Asia: Evidence from a sedimentary record in central Myanmar. Geosci. Front. 2023, 14, 101457. [Google Scholar] [CrossRef]
- Huang, H.; Pérez-Pinedo, D.; Morley, R.J.; Dupont-Nivet, G.; Philip, A.; Win, Z.; Aung, D.W.; Licht, A.; Jardine, P.E.; Hoorn, C. At a crossroads: The late Eocene flora of central Myanmar owes its composition to plate collision and tropical climate. Rev. Palaeobot. Palynol. 2021, 291, 104441. [Google Scholar] [CrossRef]
- Yuan, J.; Deng, C.; Yang, Z.; Krijgsman, W.; Thubtantsering; Qin, H.; Shen, Z.; Hou, Y.; Zhang, S.; Yu, Z.; et al. Triple-stage India-Asia collision involving arc-continent collision and subsequent two-stage continent-continent collision. Glob. Planet. Chang. 2022, 212, 103821. [Google Scholar] [CrossRef]
- Klaus, S.; Morley, R.J.; Plath, M.; Zhang, Y.-P.; Li, J.-T. Biotic interchange between the Indian subcontinent and mainland Asia through time. Nat. Commun. 2016, 7, 12132. [Google Scholar] [CrossRef]
- Panha, S. Infection experiment of the glochidium of a freshwater pearl mussel, Hyriopsis (Limnoscapha) myersiana (Lea, 1856). Venus 1992, 51, 303–314. [Google Scholar]
- Panha, S. Glochidiosis and juvenile production in a freshwater pearl mussel, Chamberlainia hainesiana. Invertebr. Reprod. Dev. 1993, 24, 157–160. [Google Scholar] [CrossRef]
- Goncalves, A.; Zieritz, A.; Lopes-Lima, M.; Deein, G.; Pfeiffer, J. Taxonomic revision and conservation assessment of the Southeast Asian freshwater mussel genus Chamberlainia Simpson, 1900. J. Molluscan Stud. 2022, 88, eyac008. [Google Scholar] [CrossRef]
- Bolotov, I.N.; Konopleva, E.S.; Chan, N.; Lunn, Z.; Win, T.; Gofarov, M.Y.; Kondakov, A.V.; Tomilova, A.A.; Vikhrev, I.V. A riverine biodiversity hotspot in northern Myanmar supports three new and narrowly endemic freshwater mussel species. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1490–1508. [Google Scholar] [CrossRef]
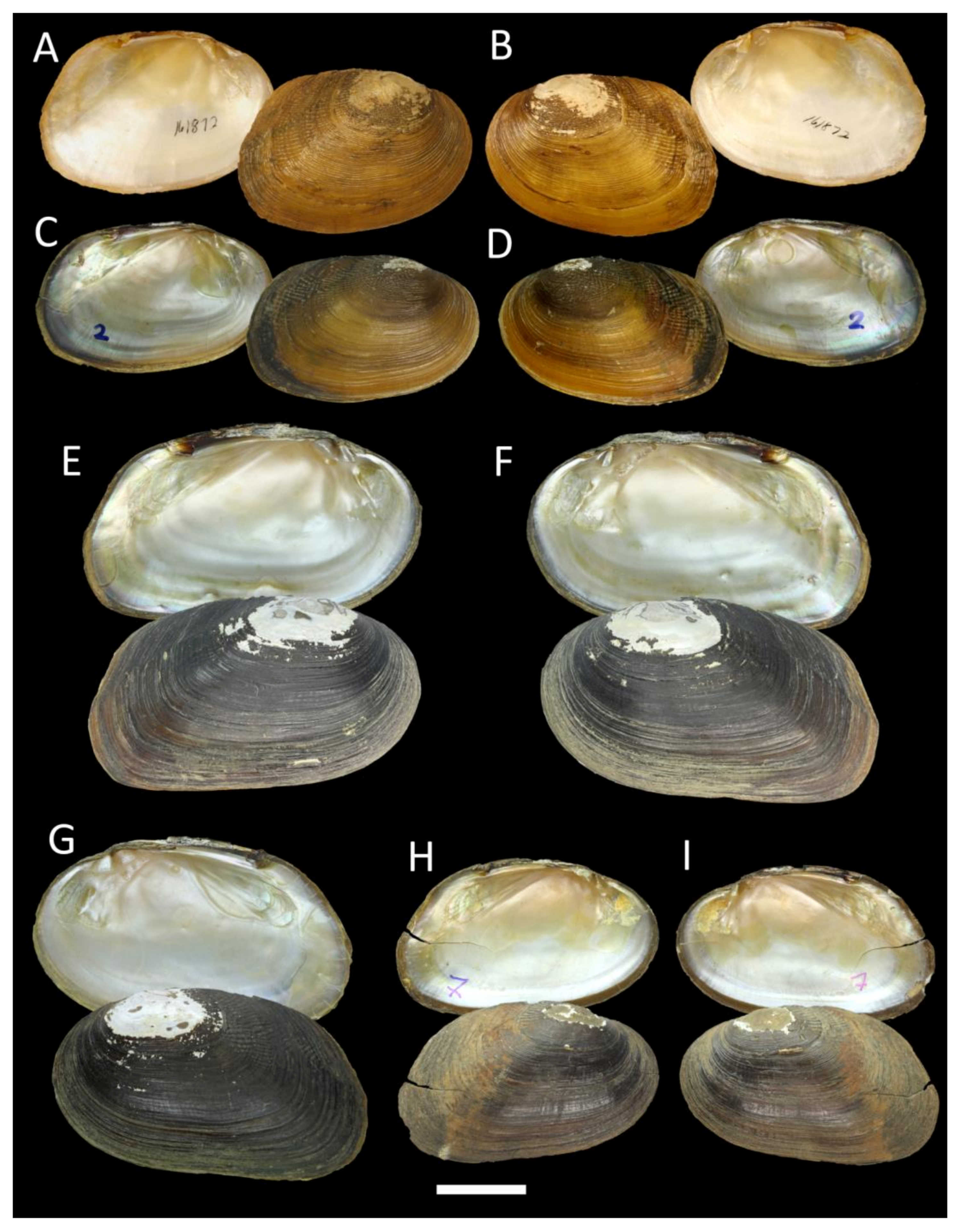

| Taxa | Type Locality | Distribution | Habitat Requirements and Larval Hosts |
|---|---|---|---|
| Subfamily GONIDEINAE Ortmann, 1916 | |||
| Tribe Pseudodontini Frierson, 1927 | |||
| Subtribe Indopseudodontina Bolotov, Konopleva, Kondakov & Vikhrev subtr. nov. | |||
| Genus Indopseudodon Prashad, 1922 stat. rev. | |||
| I. bogani (Bolotov, Kondakov & Konopleva, 2017) comb. nov. =Pseudodon bogani Bolotov, Kondakov & Konopleva, 2017 | Kanni River, 19.0545° N, 96.5131° E, Sittaung Basin, Myanmar [7] | Kanni River, Sittaung Basin, Myanmar | River specialist; host fish unknown |
| I. crebristriatus (Anthony, 1865) comb. nov. =Monocondyloea crebristriata Anthony, 1865; =Pseudodon (Trigonodon) crebristriatus var. curvata Preston, 1912; TL: Pegu [45] | Pegu, British Burmah (Bago River, Myanmar) [46] | Bago River basin, Myanmar | River/stream specialist; host fish unknown |
| I. indawgyiensis sp. nov. =Pseudodon cf. avae Bolotov et al., 2017 (identification error) [7]; =Pseudodon cf. inoscularis Bolotov et al., 2020 (identification error) [5] | Nanuinhka Chaung River near Lonton village, 25.1209° N, 96.2812° E, Indawgyi Lake basin, Ayeyarwady River drainage, Myanmar | Upper Chindwin and Upper Ayeyarwady basins, including tributaries and outflow of Lake Indawgyi, Myanmar | River/stream specialist; host fish unknown |
| I. kayinensis (Bolotov et al., 2020) comb. nov. =Pseudodon kayinensis Bolotov et al., 2020 | Winyaw River, 15.6685° N, 97.9496° E, Ataran River basin, Myanmar [5] | Salween, Ataran, and Haungthayaw basins, Myanmar | River/stream specialist; host fish unknown |
| †I. rostratus Annandale, 1924 | A limestone rock in a stream bed about 2.5 miles east of Tichara village, Dawna Hills, Tenasserim (a limestone rock in a stream bed about 2.5 miles east of Hteechara village, 16.7783° N, 98.4581° E, Dawna Range, southern Myanmar) [47,48] | A fossil lacustrine or fluviatile limestone deposit of pre-Pliocene [47] or Pliocene [49,50,51] age on the eastern slope of the Dawna Range, the Cenozoic Mae Sot Series (within the recent Moei River drainage, a tributary of Salween), southern Myanmar | Fossil species |
| I. salwenianus (Gould, 1844) comb. rev. =Anodon salweniana Gould, 1844; =Monocondyloea peguensis Anthony, 1865 syn. nov.; TL: Pegu, British Burmah [46]; =Monocondylaea avae Theobald, 1873 syn. nov.; TL: Mandalay, Burmah [52]; =Pseudodon manueli Konopleva, Kondakov & Vikrev, 2017 syn. nov.; TL: Pyowne Stream, 18.9694° N, 96.5309° E, Sittaung Basin, Myanmar [7] | Salwen River, British Burmah [27] | Salween, Ataran, Haungthayaw, Sittaung, Bago, and Ayeyarwady basins, Myanmar | River/stream specialist; host fish unknown |
| Subtribe Pseudodontina Frierson, 1927 =Pilsbryoconchina Bolotov, Vikhrev & Tumpeesuwan, 2017 syn. nov. | |||
| Genus Bineurus Simpson, 1900 | |||
| B. anodontinum (Rochebrune, 1882) =Pseudodon anodontinum Rochebrune, 1882 | Sombor-Sombor, Mekong, Cochinchine (Mekong River at Sambour, approx. 12.7726° N, 105.9629° E, Cambodia) [8] | Lower Mekong in Cambodia [8] and Laos | River/stream specialist; host fish unknown |
| B. exilis (Morelet, 1866) =Monocondylaea exilis Morelet, 1866 | In torrentibus montanis Cambodia; the lectotype label reads as follows: ‘lac Tonli-Sap, Cambodia’ (Tonlé Sap Lake, Cambodia); it cannot be found in the lake but was probably collected from a stream or river belonging to the Tonlé Sap Drainage [8] | Mekong Basin in Thailand, Cambodia, southern Vietnam [8], and Laos | River/stream specialist; host fish unknown |
| B. loeiensis Konopleva et al., 2021 | Loei River, 17.0982° N, 101.4814° E, Mekong Basin, Thailand [8] | Loei River, Mekong Basin, northeastern Thailand [8] | River specialist; host fish unknown |
| B. mouhotii (Lea, 1863) =Monocondyloea mouhotii Lea, 1863 | Laos Mts., Cambodia, Siam (most likely Laos Mountains; see [8] for explanation) | Mekong Basin in western and northern Laos, including Nam Ou River; northern Thailand [8]; and southern China: Puwen River, Yunnan [53] | River/stream specialist; host fish unknown |
| B. panhai Jeratthitikul & Sutcharit, 2023 | Phra Sathueng Stream, 13.3346° N, 102.1329° E, Bang Pakong Basin, Wang Thong Subdistrict, Wang Sombun District, Sa Kaeo Province, Thailand [11] | Bang Pakong Basin, eastern Thailand and headwater tributaries of Tonle Sap Basin in Thailand and Cambodia [11] | Stream specialist; host fish unknown [11] |
| Genus Namkongnaia Jeratthitikul et al., 2021 | |||
| N. inkhavilayi Jeratthitikul et al., 2021 | Bunghona Market, 7 km N of Xe Bangfai River, 17.13674° N, 104.98591° E, Kammoune Province, Laos [26] | Lower Mekong Basin in Laos and northeastern Thailand [26] | Probably generalist; host fish unknown [26] |
| N. lemeslei (Morelet, 1875) =Anodonta lemeslei Morelet, 1875 | Cambodge, Marecages de Battambang (Battambang, approx. 13.0929° N, 103.2001° E, Mekong Basin, Cambodia) [26] | Tonle Sap Lake and its tributaries in Cambodia [26] | Generalist; host fish unknown [26] |
| Genus Nyeinchanconcha Bolotov et al., 2020 | |||
| N. nyeinchani Bolotov et al., 2020 = Pseudodon artbogani Thach, 2020 syn. nov.; TL: Las Freci River, near Thakhek, approx. 17.4352° N, 104.8359° E, Mekong River basin, Laos [54] | Small stream arising at a cave near Ban Kouanphavang, 17.4578° N, 104.9263° E, Nam Done River drainage, Mekong Basin, Laos [5] | Mountain tributaries of the Mekong River in Laos | River/stream specialist; host fish unknown |
| Genus Pilsbryoconcha Simpson, 1900 | |||
| P. acuta Jeratthitikul & Prasankok, 2022 | Dom Yai River at Pho Sai, 15.2311° N, 105.1571° E, Phibun Mangsahan District, Ubon Ratchathani Province, Thailand [55] | Thailand, Laos, and Vietnam [55] | Generalist; host fish unknown [55] |
| P. carinifera (Conrad, 1837) =Anodonta carinifera Conrad, 1837; =Anodonta sempervivens Deshayes & Jullien, 1876; TL: Arroyo de Peam-Chelang, Cambodge; =Anodonta laminata Rochebrune, 1882; TL: Rapides du Mekong, Cochinchine; =Pilsbryoconcha exilis sensu Pfeiffer & Graf, 2015 (identification error) [55] | Huai Yang Stream at Nong Muang, 13.8695° N, 102.5899° E, Khok Sung District, Sa Kaeo Province, Thailand (based on the neotype) [55] | Headwater of Tonle Sap Basin in Thailand and Cambodia, and probably the Mekong River in Cambodia [55] | Generalist; host fish unknown [55] |
| P. exilis (Lea, 1838) =Anodonta exilis Lea, 1838; =Anodonta polita Mousson, 1849; TL: Java; =Spatha compressa Martens, 1860; TL: Khao-kho, north-east of Pakpriau, Siam; =Monocondyloea compressa Lea, 1863; unavailable: homonym of Spatha compressa Martens, 1860; TL: Siam; =Anodonta javona Sowerby, 1867; TL: Java (original label), Japan (protologue, erroneous); =Anodon kelletii Sowerby, 1867; TL: unknown [55] | Java? (from the label of a syntype; in the protologue, Lea noted that its locality is unknown) [55] | Indochina, the Malay Peninsula, Singapore, Sumatra, Java, and Borneo [2,3,55] | Generalist [55]; an unspecified cyprinid fish [56] |
| *P. expressa (Martens, 1900) =Anodonta expressa Martens, 1900 | Lake Danau-Baru, Indragiri, Sumatra [57] | Sumatra, Indonesia [55] | No data |
| P. hoikaab Jeratthitikul & Prasankok, 2022 | Kam River at Na Khu, 16.9581° N, 104.5045° E, Na Kae District, Nakhon Phanom Province, Thailand [55] | Songkhram River and tributaries of the Middle Mekong in Thailand and Laos [55] | Probably river specialist; host fish unknown [55] |
| P. kittitati Jeratthitikul & Prasankok, 2022 | Unnamed pond near Nong Ya Sai, 16.9798° N, 103.3371° E, Wang Sam Mo District, Udon Thani Province, Thailand [55] | Unknown beyond the type locality, Chi River drainage, Mekong Basin, Thailand [55] | No data (probably generalist as it was described from a pond) [55] |
| P. linguaeformis (Morelet, 1875) =Anodonta linguaeformis Morelet, 1875 | Cambodia [55] | Mekong Basin in Cambodia, Thailand, and southern Vietnam [55] | Generalist; host fish unknown [55] |
| P. mekongiana Jeratthitikul & Prasankok, 2022 | Tributary of Mekong River at Khok Kong, 18.3382° N, 103.7624° E, Mueang District, Bueng Kan Province, Thailand [55] | Tributaries of Mekong River in Sakon Nakhon Basin, Thailand [55]; and Nam Kadan River in Laos | Generalist; host fish unknown [55] |
| P. schomburgki (Martens, 1860) =Anodonta (Lamproscapha) schomburgki Martens, 1860 | Siam [58] | Chi River drainage, Mae Klong River basin, the headwaters of Mun River and Tonle Sap basin, Khlong Phraphut stream in Thailand; the Tha Taphao and Tapi River basins in southern Thailand [9,55] | Generalist; host fish unknown [55] |
| Genus Pseudodon Gould, 1844 stat. rev. =Monodontina Conrad, 1853 | |||
| *P. aeneolus Drouet & Chaper, 1892 comb. rev. =Pseudodon aeneolus Drouet & Chaper, 1892 | Sebruang River (approx. 0.4937° N, 111.8931° E), Kapuas Basin, western Borneo [59] | Kapuas Basin, western Borneo | No data |
| P. cambodjensis (Petit de la Saussaye, 1865) comb. rev. =Monocondylea cambodjensis Petit de la Saussaye, 1865; =Monocondylus orbicularis Morelet, 1866; TL: Battambang, Siam [60] | Battambang (approx. 13.0929° N, 103.2001° E), Mekong Basin, Cambodia [61] | Mekong Basin in Thailand and Cambodia | River specialist; host fish unknown |
| P. inoscularis (Gould, 1844) =Anodon inoscularis Gould, 1844 | River Salwen, Tavoy, Brit. Burmah [27] (here, we recommend to restrict its type locality to Tavoy (now Dawei) River, Myanmar: see Taxonomic Account for explanation) | Dawei River, Myanmar | River/stream specialist; host fish unknown |
| P. lenyanensis (Bolotov et al., 2020) comb. nov. =Monodontina lenyanensis Bolotov et al., 2020 | 14 Mile Stream, 11.3508° N, 99.1093° E, Lenya River basin, Myanmar [5] | Lenya Basin, Myanmar [5] | Stream specialist; host fish unknown |
| P. mekongi (Bolotov et al., 2020) comb. nov. =Monodontina mekongi Bolotov et al., 2020 | Headwater of the Phong River, 16.8616° N, 101.9105° E, Mekong Basin, Thailand [5] | Phong River, Mekong Basin, Thailand [5] | River specialist; host fish unknown |
| *P. nicobaricus (Mörch, 1872) comb. nov. =Alasmodonta nicobarica Mörch, 1872 | Nicobar Islands [32] | Nicobar Islands, India | No data |
| *P. thomsoni Morlet, 1884 | Cambodge [8] | Mekong Basin in Cambodia [8] | No data |
| P. vagulus (Fischer, 1891) comb. rev. =Unio subtrigonus Sowerby, 1867; unavailable name: homonym of †U. subtrigonus Noulet, 1864 [62]; =Unio vagulus Fischer, 1891 (new name for U. subtrigonus Sowerby, 1867) [63]; =Pseudodon cambodjensis tenerrimus Brandt, 1974; TL: Songkram River at Sri Songkram, Thailand [3]; =Pseudodon cambodjensis sensu Pfeiffer & Graf, 2015 (identification error) [33]; =Pseudodon cambodjensis sensu Zieritz et al., 2016 (identification error) [14] | Siam [62] | Mekong Basin in Thailand, Laos, and Cambodia; Malaysia [3,14,33] | River specialist; host fish unknown [3] |
| P. vondembuschianus vondembuschianus (Lea, 1840) comb. rev. =Margaritana vondembuschiana Lea, 1840; =Alasmodonta crispata Mousson, 1849; TL: Java; =Alasmodonta zollingeri Mousson, 1849; TL: Java; =Monodontina buschiana Conrad, 1853 (new name for Margaritana vondembuschiana); =Monocondyloea planulata Lea, 1859; TL: Java; =Monocondyloea hageni Strubell, 1897; TL: S. Sumatra [5] | Java [64] | Malaysia, Sumatra, and Java [5,9] | River/stream specialist; host fish unknown [3] |
| P. vondembuschianus laosica (Bolotov et al., 2020) comb. nov. =Monodontina laosica Bolotov et al., 2020 | Ca. 300 m upstream of the mouth of Houai Pin Stream, 14.7944° N, 106.4842° E, a tributary of the Vang Ngao River, Mekong Basin, Laos [5] | Mekong River basin in Laos [5,9] | River/stream specialist; host fish unknown |
| P. vondembuschianus tapienica (Konopleva et al., 2023) comb. nov. =Monodontina vondembuschiana tapienica Konopleva et al., 2023 | Main Klong Min, Tapi River basin, Tambon Kaew San, Nabon District, Nakhon Si Thammarat Province, 8.2997° N, 99.5580° E, southern Thailand [9] | Southern Thailand [9] | River/stream specialist; host fish unknown |
| P. vondembuschianus thasaenica (Konopleva et al., 2023) comb. nov. =Monodontina vondembuschiana thasaenica Konopleva et al., 2023 | Main Klong Thasae, Tha Taphao River Basin, nearby Wat Na Srang, Thasae District, Chumphon Province, 10.6753° N, 99.1737° E, southern Thailand [9] | Southern Thailand [9] | River/stream specialist; host fish unknown |
| †P. vondembuschianus trinilensis (Dubois, 1908) =†Unio trinilensis Dubois, 1908 | ‘Hauptknochenschicht’ deposits at Trinil site, 7.3667° S, 111.3500° E, Solo River valley, Ngawi Regency, east Java, Indonesia [65,66] | Middle Pleistocene (40Ar/39Ar maximum and minimum age of 0.54 and 0.43 Ma, respectively), Ngawi Regency, east Java, Indonesia [66] | Fossil insular subspecies |
| †P. vondembuschianus vandervlerki Oostingh, 1935 =†Pseudodon (Trigonodon) vandervlerki Oostingh, 1935 | Kali Glagah beds at Boemiajoe, Central Java (Bumiayu District, approx. 7.2620° S, 108.9864° E, Brebes Regency, Central Java, Indonesia) [65] | Early Pleistocene (age ca. 1.8 Ma), Kaliglagah Formation, Bumiayu District, Brebes Regency, central Java, Indonesia [67] | Fossil insular subspecies |
| *P. walpolei (Hanley, 1871) comb. rev. =Monocondylaea walpolei Hanley, 1871; =Pseudodon crassus Drouet & Chaper, 1892; TL: le Sarawak [59] | Sarawak, Borneo (by the lectotype designation) [5] | Northern Borneo [5] | No data |
| Genus Songkhlanaia Konopleva et al., 2023 | |||
| S. tamodienica Konopleva et al., 2023 | Klong Plug Pom, 7.3324° N, 100.0917° E, middle reach of Klong Tamod, SLB, Ban Kok Sai, Tambon Mae Kree, Tamod District, Phatthalung Province, southern Thailand [9] | Songkhla Lake, southern Thailand [9] | Probably stream specialist; host fish unknown |
| Genus Sundadontina Bolotov et al., 2020 | |||
| S. brandti Bolotov et al., 2020 | Headwater of the Mun River, 14.4138° N, 102.0821° E, Mekong Basin, Thailand [5] | Mun River, Mekong Basin, Thailand [5] | River specialist; host fish unknown |
| S. cumingii (Lea, 1850) =Anodonta cumingii Lea, 1850; =Pseudodus chaperi Morgan, 1885; TL: tous affluents de la rivière Kinta, de Pèrak, Malacca [5] | Malacca [5] | Malaysia [5] | River/stream specialist; host fish unknown [3] |
| S. harmandi (Crosse & Fischer, 1876) =Pseudodon harmandi Crosse & Fischer, 1876 | Cambodia [5] | Lower Mekong Basin in Laos and Cambodia [5] | River/stream specialist; host fish unknown [3] |
| *S. mabilli (Rochebrune, 1881) =Pseudodon mabilli Rochebrune, 1881 | Mekong, Shigloni Breithon, Cochinchina [5] | Lower Mekong Basin in southern Vietnam [5] | No data |
| *S. moreleti (Crosse & Fischer, 1876) =Pseudodon moreleti Crosse & Fischer, 1876 | Mekong, Kompang Cham Province, Cambodia [5] | Lower Mekong Basin in Cambodia [5] | No data |
| *S. ovalis (Morlet, 1889) comb. nov. =Pseudodon ovalis Morlet, 1889 | Srakeo River, Siam (Bang Pakong River, Thailand) [8] | Bang Pakong Basin, Thailand [8] | No data |
| S. plugpomenica Konopleva et al., 2023 | Klong Pa-Payom, 7.8456° N, 99.9933° E, SLB, Ban Teng, Tambon Laem Tanod, Khuan Kanun District, Phatthalung Province, southern Thailand [9] | Songkhla Lake basin, southern Thailand [9] | Stream specialist; host fish unknown |
| *S. ponderosa (Preston, 1909) =Pseudodon ponderosa Preston, 1909 | Nan-ko, Siam (Nan River, Chao Phraya Basin, Thailand) [5] | Chao Phraya Basin, Thailand [5] | No data |
| S. sulcata (Rochebrune, 1881) =Pseudodon sulcatum Rochebrune, 1881 | Mouth of the Mekong River, Cochinchina [5] | Mekong Basin in southern Vietnam [5] and Laos | Probably river specialist; host fish unknown |
| S. tanintharyiensis Bolotov et al., 2020 | Chaung Nauk Pyan Stream, 11.7620° N, 99.1124° E, Lenya River basin, Myanmar [5] | Lenya Basin, Myanmar [5] | Stream specialist; host fish unknown |
| S. taskaevi Bolotov et al., 2020 | Headwater of the Mun River, 14.4138° N, 102.0821° E, Mekong Basin, Thailand [5] | Mun River, Mekong Basin, Thailand [5] | River specialist; host fish unknown |
| S. tumida (Morelet, 1866) =Monocondylus tumidus Morelet, 1866 | Cambodia [5] | Lower Mekong Basin in Cambodia and southern Vietnam [5] | Probably river specialist; host fish unknown |
| Genus Thaiconcha Bolotov et al., 2020 | |||
| T. callifera (Martens, 1860) =Anodonta callifera Martens, 1860; =Pseudodon ellipticum Conrad, 1865; TL: Cambodia [8] | Siam (Thailand) [8] | Mekong Basin in Cambodia and Thailand [8] | River specialist; host fish unknown |
| T. munelliptica Konopleva et al., 2021 | Pool site with clay bottom, Mun River upstream of Tha Tum village, 15.3575° N, 103.6637° E, Mekong Basin, Surin Province, Thailand [8] | Mun and Chi rivers, Mekong Basin, northeastern Thailand [8] | River specialist; host fish unknown |
| T. thaiensis Konopleva et al., 2021 | Kham Nong Bua River, trib. Of Mekong River, at Rt. 1016 bridge 0.3 mi west of Rt. 1290, 20.2681° N, 100.0721° E, Chiang Rai, Thailand [8] | Mekong and Upper Chao Phraya basins, Thailand [8] | River specialist; host fish unknown |
| Tribe Gonideini Ortmann, 1916 | |||
| Genus Parvasolenaia Huang & Wu, 2019 | |||
| †Parvasolenaia sublinguaeformis (Tolstikova, 1975) comb. nov. =†Pilsbryoconcha sublinguaeformis Tolstikova, 1975 | Dyusyumbay Strata, Chakelmes Mountain, Zaysan Depression, Kazakhstan [68] | Paleocene, Zaysan Depression, Kazakhstan [68] | Fossil species |
| †Parvasolenaia praeexilis (Tolstikova, 1976) comb. nov. =†Pilsbryoconcha praeexilis Tolstikova, 1976 | Kiin-Kerish Mountain, Zaysan Depression, Kazakhstan [69] | Late Eocene, Zaysan Depression, Kazakhstan [69] | Fossil species |
| Genus Pseudodontopsis Kobelt, 1913 | |||
| †Pseudodontopsis oettingenae (Modell, 1969) comb. nov. =†Pseudodon oettingenae Modell, 1969; =†Monodontina mogul Modell, 1969 syn. nov.; TL: Charigambir, 6.5 km WSW of Chinji Village, Punjab, Pakistan [70]; our first reviser’s action on the precedence of simultaneous synonyms | Pirawalaban, 9 km ENE of Chinji Village, Punjab, Pakistan [70] | Upper Miocene deposits near Chinji, Punjab, Pakistan [70] | Fossil species |
| Genus Ptychorhynchus Simpson, 1900 =Cosmopseudodon Haas, 1920 syn. nov.; =Heudeana Frierson, 1922 | |||
| *Ptychorhynchus liuovatus (He & Zhuang, 2013) comb. nov. =Unio ovatus Liu, Duan & Wang, 1994; unavailable name: homonym of Unio ovatus Say, 1817; =Lamellidens liuovatus He & Zhuang, 2013 (new name for Unio ovatus); =Pseudobaphia liuovata (He & Zhuang, 2013) [1,53] | Jiangkou, Guizhou Province, China [53] | Pearl Basin, China [53] | No data |
| *P. murinum (Heude, 1883) =Unio murinus Heude, 1883; =Unio pinchonianus Heude, 1883 syn. nov.; TL: Les canaux de la plaine élevée de Tch’eng-tou fou, province du Setchouan (canals of the Chengdu High Plain, Yangtze Basin, Sichuan, China) [71]; =Pseudodon solidus Haas, 1911 syn. nov.; TL: Hunan, Mittelchina [72]; our first reviser action on the precedence of simultaneous synonyms: Unio murinus over U. pinchonianus | Le grand torrent du Kien-té sud, vers ses sources (the main channel of Yangtze River towards its sources, Anhui Province, China) [71] | Yangtze Basin, China | No data |
| *P. resupinatus (Martens, 1902) comb. nov. =Pseudodon resupinatus Martens, 1902 | Than Moi, Tonkin, French Indo China (approx. 21.6313° N, 106.5532° E, Thuong Basin, flowing into Thái Bình River, northern Vietnam) [57] | Thuong Basin, northern Vietnam [57] | No data |
| Genus Simpsonasus Bolotov & Konopleva nom. nov. (replacement name for Nasus Simpson, 1900) | |||
| *Simpsonasus nankingensis (Heude, 1874) comb. nov. =Monocondylea nankingensis Heude, 1874; =Pseudodon secundus Heude, 1877 syn. nov.; TL: Ngan-houe, China [73] | Rivière de Nanking [74] | Lower Yangtze Basin, China | No data |
| †Simpsonasus politus (Tolstikova, 1974) comb. nov. =†Nasus politus Tolstikova, 1974; =†Pseudodon politus (Tolstikova, 1974) [75,76] | Obaylin Formation, Zaysan Depression, Ulkun-Ulasty River, Kazakhstan [75] | Middle Eocene (ca. 40–50 Ma), Zaysan Depression, Ulkun-Ulasty River, Kazakhstan [77] | Fossil species |
| Subfamily UNIONINAE Rafinesque, 1820 | |||
| Tribe Unionini Rafinesque, 1820 | |||
| Genus Pseudobaphia Simpson, 1900 =Chrysopseudodon Haas, 1920 syn. nov. | |||
| *Pseudobaphia aurea (Heude, 1885) comb. nov. =Psudodon (sic!) aureus Heude, 1885 | Un torrent du district de Kien-té, Nanking (a watercourse within a former district in Anhui, China) [78] | Lower Yangtze Basin, China | No data |
| †Pseudobaphia pingi (Otuka, 1942) comb. nov. =†Pseudodon pingi Otuka, 1942 | Loc. Kwodo No 30, grey mud of the Lower part of Nihowan beds on a valley floor, SW of Liuchiashaopu, San-Kien-Ho basin, Tsanan, Menchiang, Inner Mongolia, north China [79] | Middle Neogene or pre-Upper Pliocene, Nihowan beds, San-Kien-Ho basin, Inner Mongolia, China [79] | Fossil species |
| Subfamily PARREYSIINAE Henderson, 1935 | |||
| Tribe Lamellidentini Modell, 1942 | |||
| Genus Balwantia Prashad, 1919 | |||
| †Balwantia longiformis (Takayasu, Gurung & Matsuoka, 1995) comb. nov. =†Lamellidens longiformis Takayasu, Gurung & Matsuoka, 1995; =†Pilsbryoconcha longiformis (Takayasu, Gurung & Matsuoka, 1995) [80,81] | The right bank of the Narayani River, 500 m south of the confluence with the Binai Khola; middle member of the Binai Khola Formation, west-central Nepal [80] | Mio-Pliocene Churia Group, west-central Nepal [80] | Fossil species |
| Genus Lamellidens Simpson, 1900 | |||
| †Lamellidens indicus (Modell, 1969) comb. nov. =†Cosmopseudodon indicus Modell, 1969 | Parlewali, 4–5 km WSW of Dhok Pathan Village, Punjab, Pakistan [70] | Pliocene deposits near Dhok Pathan, Punjab, Pakistan [70] | Fossil species |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolotov, I.N.; Konopleva, E.S.; Vikhrev, I.V.; Gofarov, M.Y.; Kondakov, A.V.; Lyubas, A.A.; Soboleva, A.A.; Chan, N.; Lunn, Z.; Win, T.; et al. Integrative Taxonomic Reappraisal and Evolutionary Biogeography of the Most Diverse Freshwater Mussel Clade from Southeast Asia (Pseudodontini). Water 2023, 15, 3117. https://doi.org/10.3390/w15173117
Bolotov IN, Konopleva ES, Vikhrev IV, Gofarov MY, Kondakov AV, Lyubas AA, Soboleva AA, Chan N, Lunn Z, Win T, et al. Integrative Taxonomic Reappraisal and Evolutionary Biogeography of the Most Diverse Freshwater Mussel Clade from Southeast Asia (Pseudodontini). Water. 2023; 15(17):3117. https://doi.org/10.3390/w15173117
Chicago/Turabian StyleBolotov, Ivan N., Ekaterina S. Konopleva, Ilya V. Vikhrev, Mikhail Y. Gofarov, Alexander V. Kondakov, Artem A. Lyubas, Alena A. Soboleva, Nyein Chan, Zau Lunn, Than Win, and et al. 2023. "Integrative Taxonomic Reappraisal and Evolutionary Biogeography of the Most Diverse Freshwater Mussel Clade from Southeast Asia (Pseudodontini)" Water 15, no. 17: 3117. https://doi.org/10.3390/w15173117
APA StyleBolotov, I. N., Konopleva, E. S., Vikhrev, I. V., Gofarov, M. Y., Kondakov, A. V., Lyubas, A. A., Soboleva, A. A., Chan, N., Lunn, Z., Win, T., & Inkhavilay, K. (2023). Integrative Taxonomic Reappraisal and Evolutionary Biogeography of the Most Diverse Freshwater Mussel Clade from Southeast Asia (Pseudodontini). Water, 15(17), 3117. https://doi.org/10.3390/w15173117







