Adsorption Removal of Phosphate from Rural Domestic Sewage by Ca-Modified Biochar Derived from Waste Eggshell and Sawdust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Adsorbents
2.3. Phosphate Adsorption Experiments
2.4. Characterization and Analytical Method
3. Results and Discussion
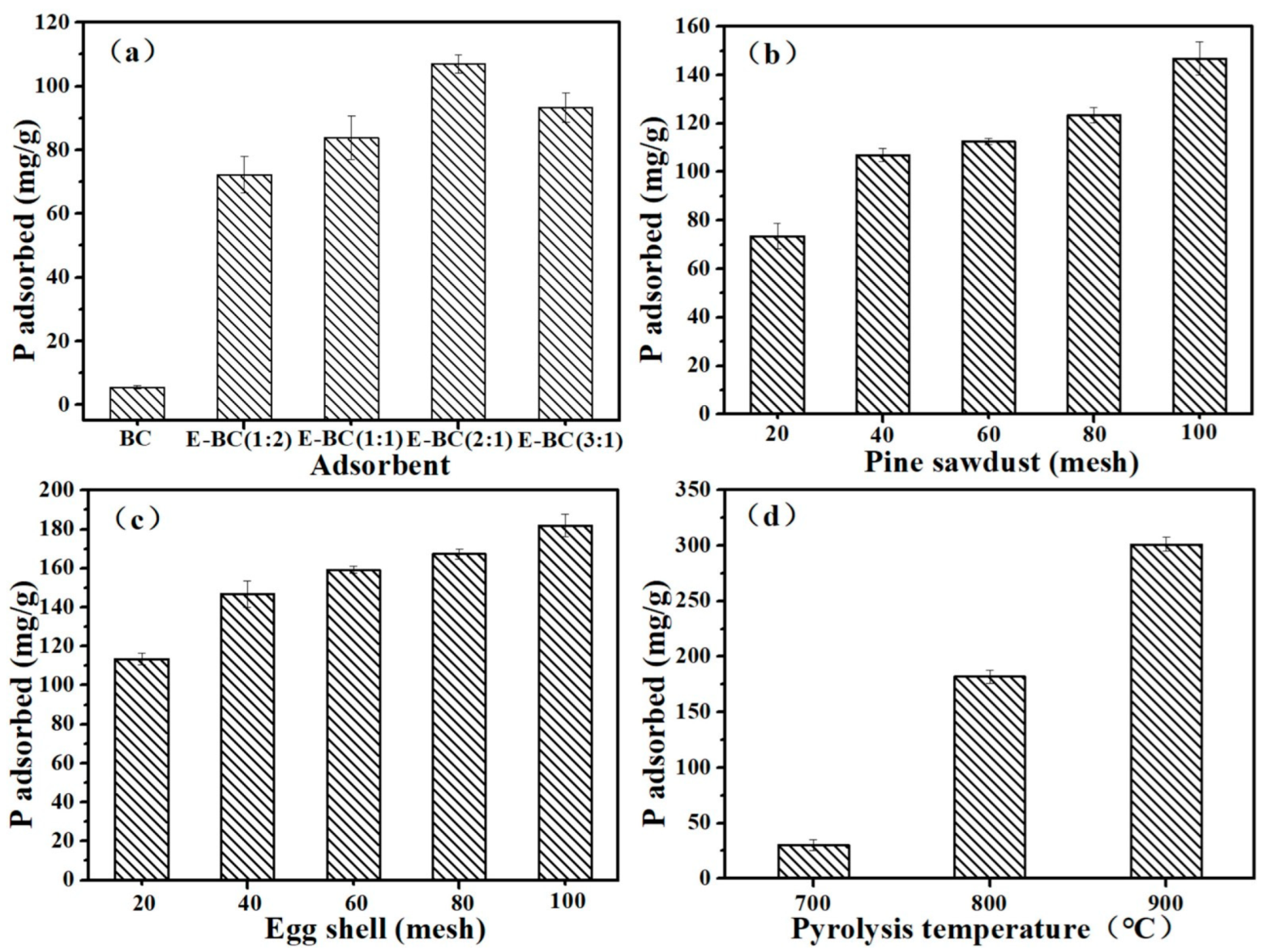
3.1. Sorption of Phosphate on Various E-BC Adsorbents
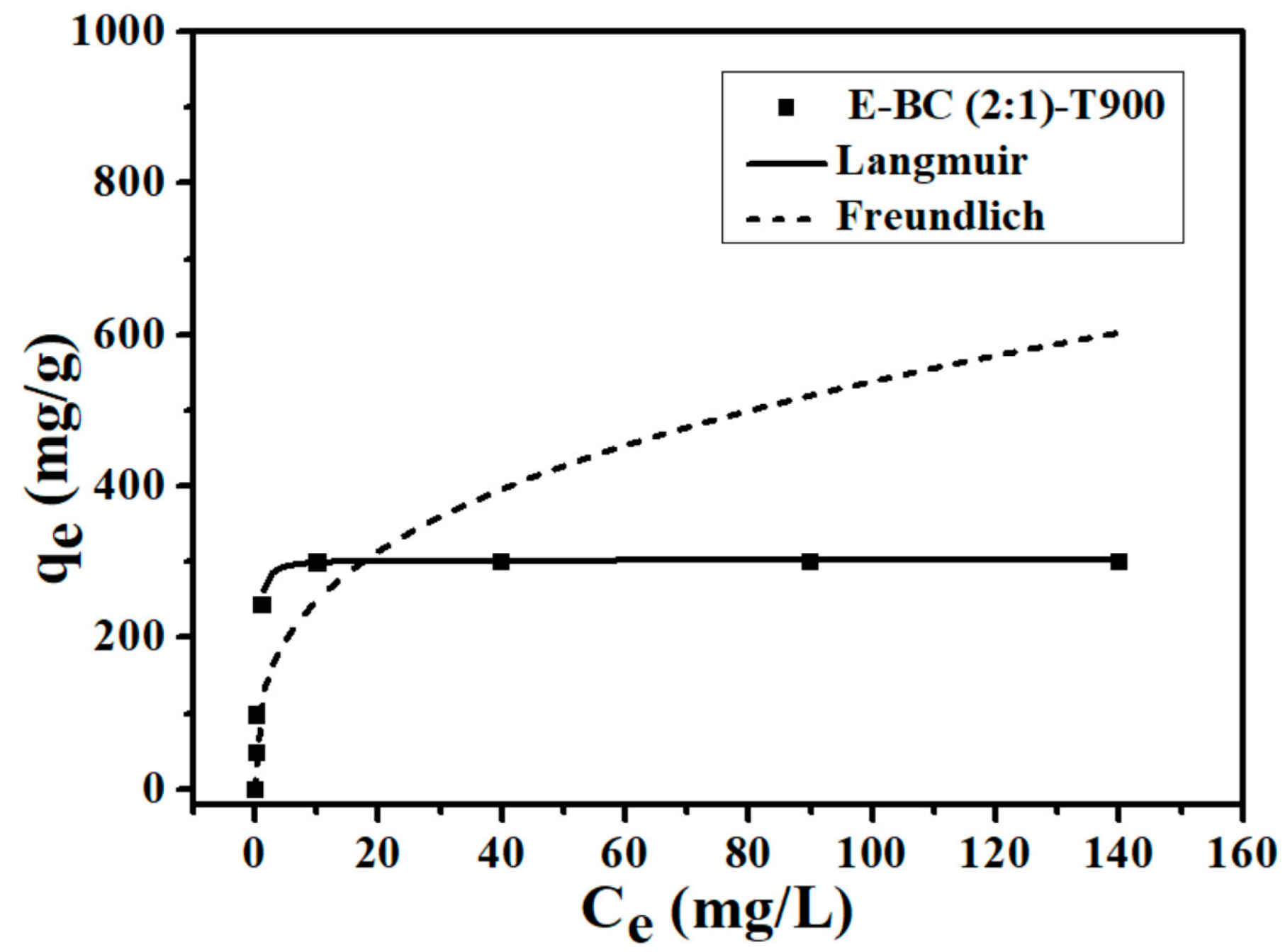
3.2. Adsorption Isotherms
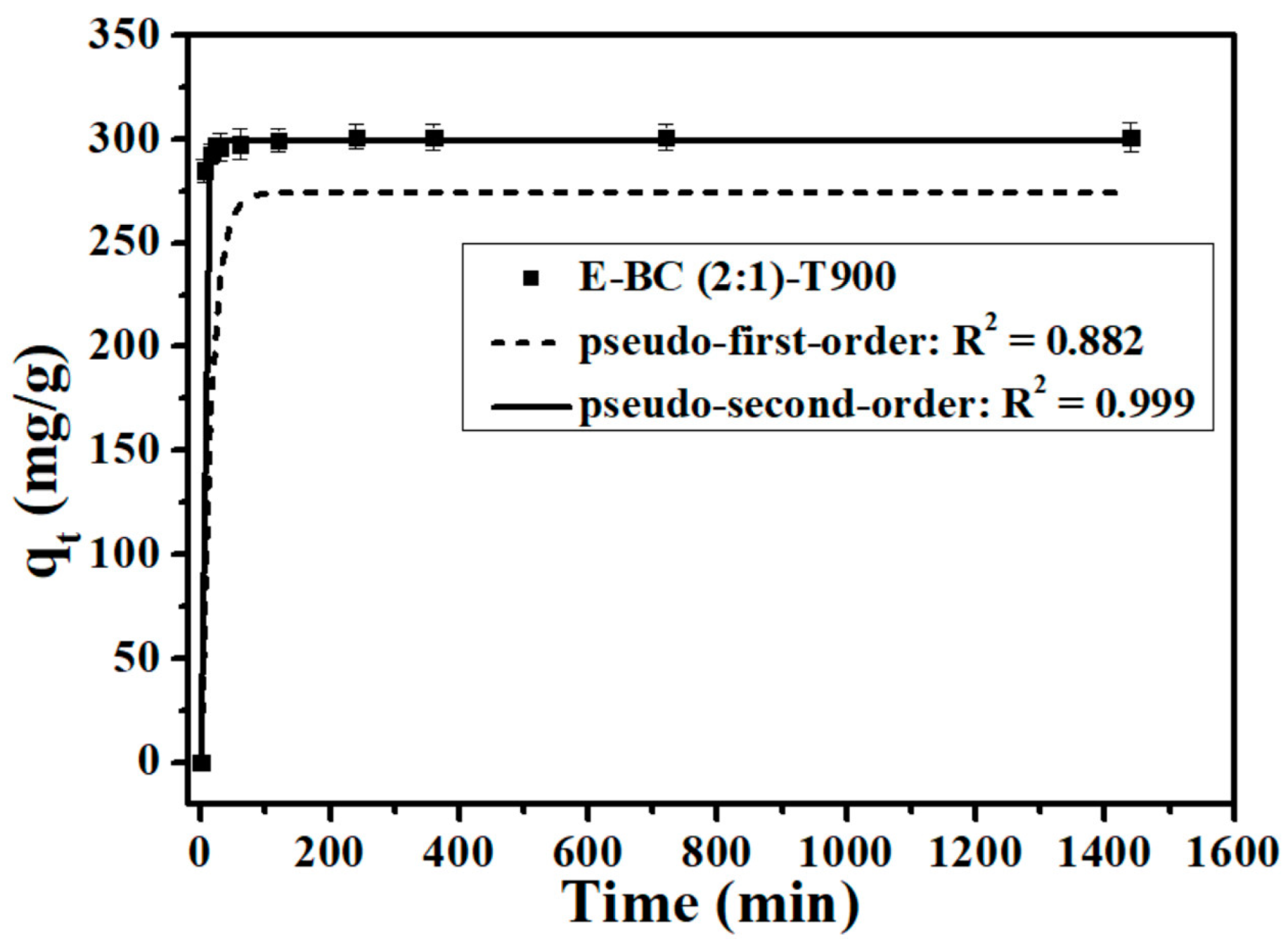
3.3. Adsorption Kinetics
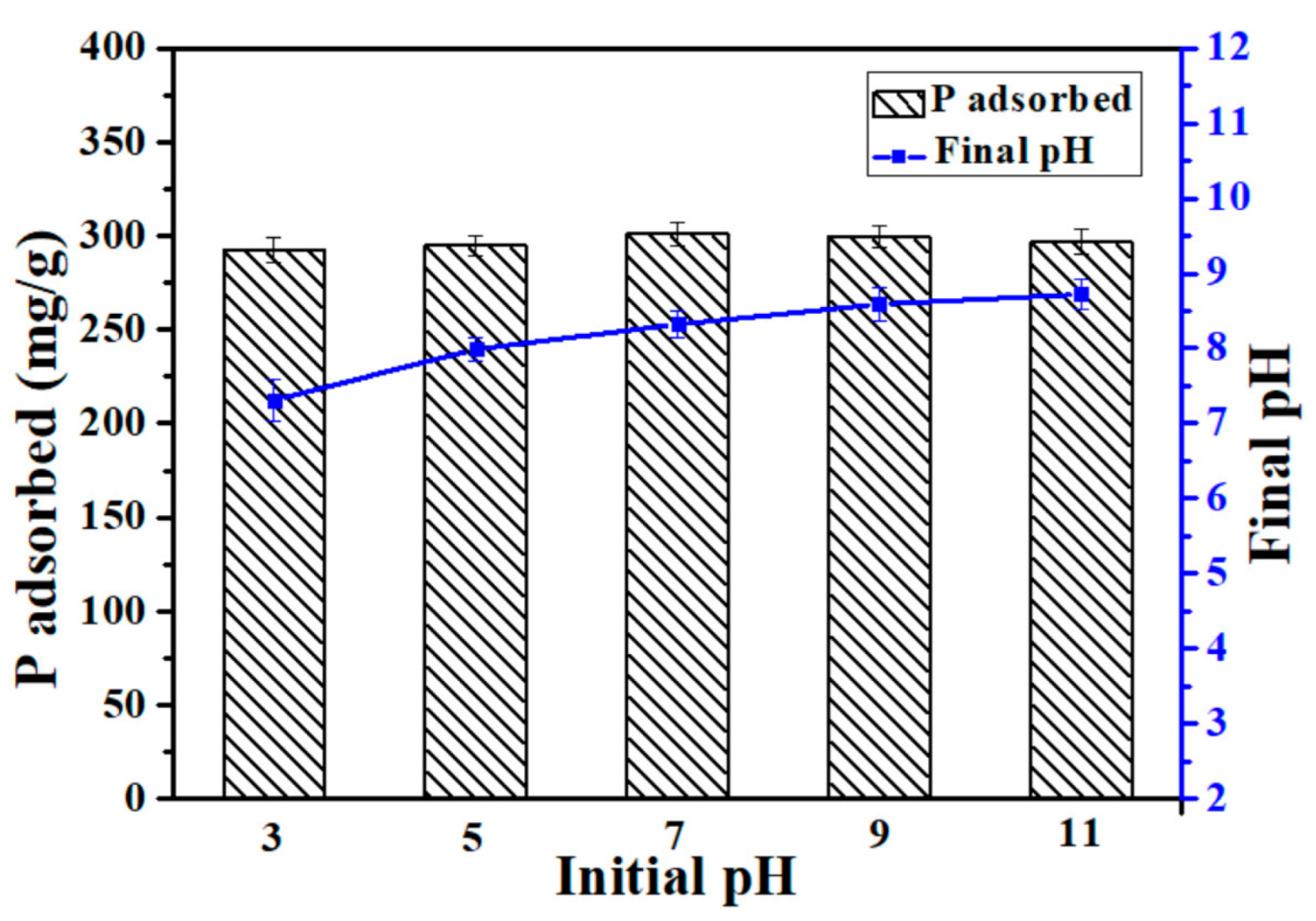
3.4. Effect of pH
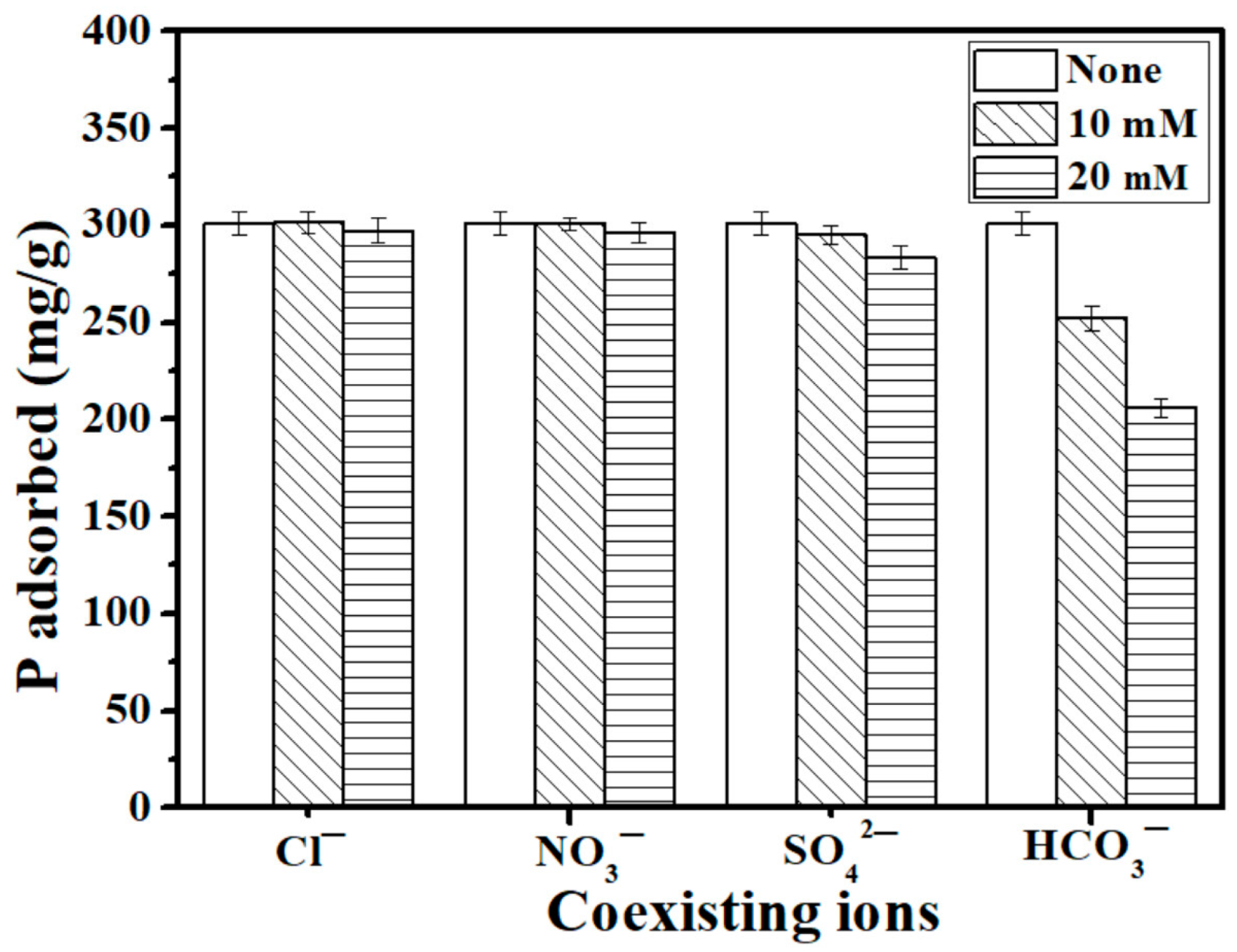
3.5. Influence of coexisting anions
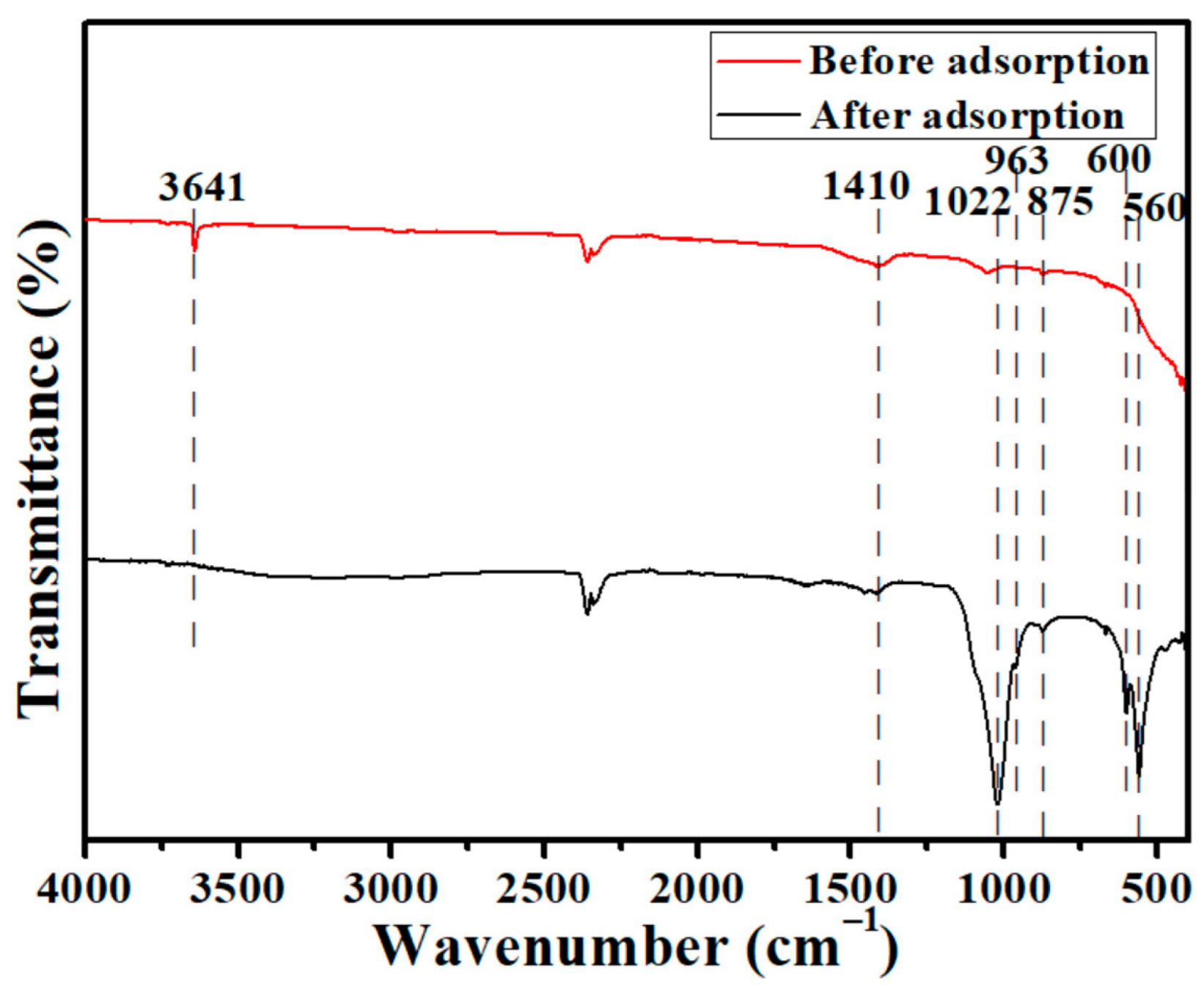
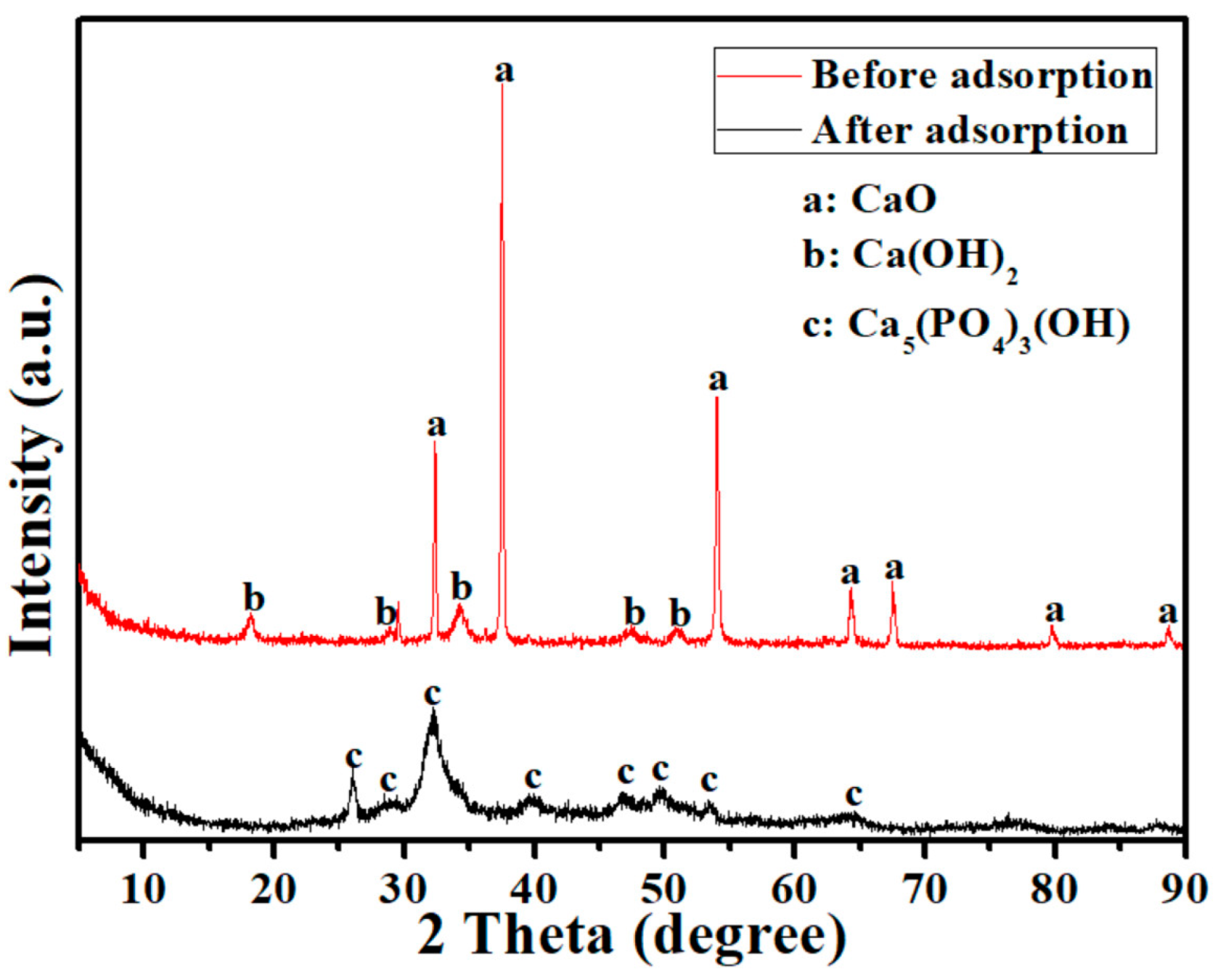
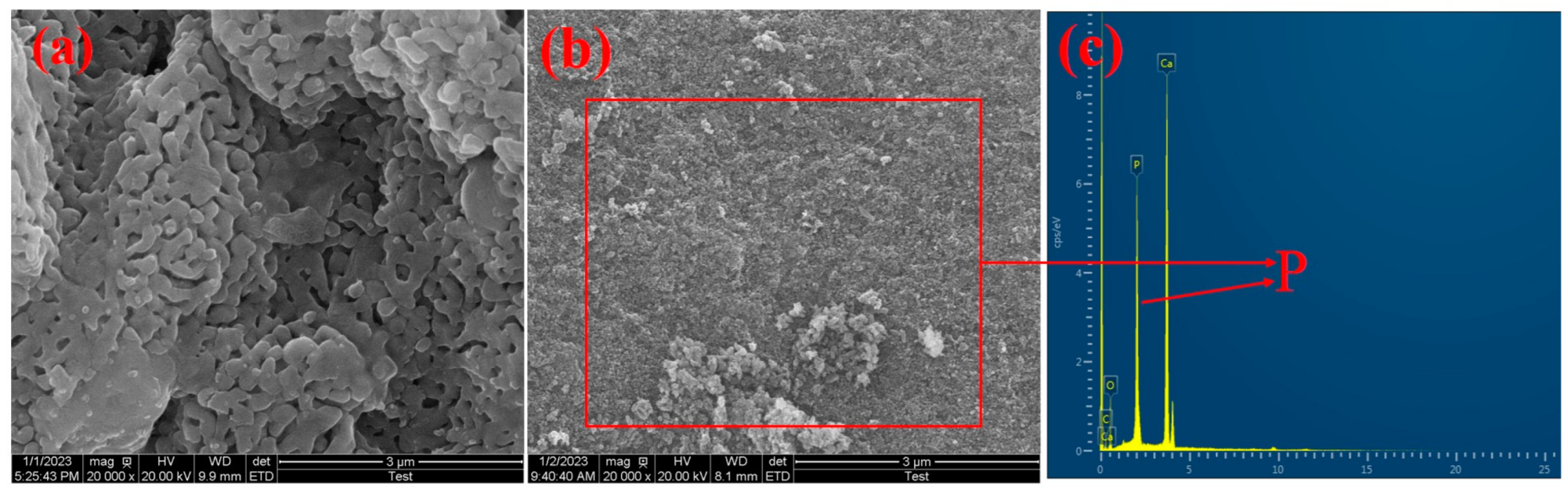
3.6. Adsorption Mechanisms
3.7. Removal of Phosphate by E-BC (2:1)-T900 from Rural Domestic Sewage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gonzalez-Tineo, P.A.; Durán-Hinojosa, U.; Delgadillo-Mirquez, L.R.; Meza-Escalante, E.R.; Gortáres-Moroyoqui, P.; Ulloa-Mercado, R.G.; Serrano-Palacios, D. Performance improvement of an integrated anaerobic-aerobic hybrid reactor for the treatment of swine wastewater. J. Water Process Eng. 2020, 34, 101164. [Google Scholar] [CrossRef]
- Yang, F.L.; Zhang, H.R.; Zhang, X.Z.; Zhang, Y.; Li, J.H.; Jin, F.M.; Zhou, B.X. Performance analysis and evaluation of the 146 rural decentralized wastewater treatment facilities surrounding the Erhai Lake. J. Clean. Prod. 2021, 315, 128159. [Google Scholar] [CrossRef]
- Zong, Y.T.; Chen, H.; Malik, Z.; Xiao, Q.; Lu, S.G. Comparative study on the potential risk of contaminated-rice straw, its derived biochar and phosphorus modified biochar as an amendment and their implication for environment. Environ. Pollut. 2022, 293, 118515. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wu, Y.H.; Huang, Y.P.; Song, L.X.; Chen, H.F.; Zhu, S.J.; Tang, C.L. Enhanced adsorption of phosphate on orange peel-based biochar activated by Ca/Zn composite: Adsorption efficiency and mechanisms. Collids Surf. A 2022, 651, 129728. [Google Scholar] [CrossRef]
- Zhang, M.D.; He, M.Z.; Chen, Q.P.; Huang, Y.L.; Zhang, C.Y.; Yue, C.; Yang, L.Y.; Mu, J.L. Feasible synthesis of a novel and low-cost seawater-modified biochar and its potential application in phosphate removal/recovery from wastewater. Sci. Total Environ. 2022, 824, 153833. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wu, X.; Syed-Hassan, S.S.; Zhang, S.; Mood, S.H.; Milan, Y.J.; Garcia-Perez, M. Characteristics and mechanisms of phosphorous adsorption by rape straw-derived biochar functionalized with calcium from eggshell. Bioresour. Technol. 2020, 318, 124063. [Google Scholar] [CrossRef]
- Caneghem, J.V.; Brems, A.; Lievens, P.; Block, C.; Billen, P.; Vermeulen, I.; Dewil, R.; Baeyens, J.; Vandecasteele, C. Potential of pistachio shell biochar and dicalcium phosphate combination to reduce Pb speciation in spinach, improved soil enzymatic activities, plant nutritional quality, and antioxidant defense system. Chemosphere 2020, 245, 125611. [Google Scholar]
- Yang, J.; Zhang, M.L.; Wang, H.X.; Xue, J.B.; Lv, Q.; Pang, G.B. Efficient Recovery of Phosphate from Aqueous Solution Using Biochar Derived from Co-pyrolysis of Sewage Sludge with Eggshell. J. Environ. Chem. Eng. 2021, 9, 105354. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar-compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef]
- Novais, S.V.; Zenero, M.D.O.; Conz, R.F.; Cerri, C.E.P.; Tronto, J. Poultry manure and sugarcane straw biochars modified with MgCl2 for phosphorus adsorption. J. Environ. Manag. 2018, 214, 36–44. [Google Scholar] [CrossRef]
- Cheng, F.L.; Wang, Y.N.; Fan, Y.T.; Huang, D.; Pan, J.; Li, W. Optimized Ca-Al-La modified biochar with rapid and efficient phosphate removal performance and excellent pH stability. Arab. J. Chem. 2023, 16, 104880. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Luo, Y.N.; He, Q.P.; Zhao, D.; Zhang, K.Q.; Shen, S.Z.; Wang, F. Performance and mechanism of a biochar-based Ca-La composite for the adsorption of phosphate from water. J. Environ. Chem. Eng. 2021, 9, 105267. [Google Scholar] [CrossRef]
- Wang, C.W.; Qiu, C.; Song, Z.G.; Gao, M.L. A novel Ca/Mn-modified biochar recycles P from solution: Mechanisms and phosphate efficiency. Environ. Sci. Process. Impacts 2022, 24, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.H.; Li, M.M.; Zhang, L.S.; Luo, Y.; Zhao, D.; Yuan, M.Y.; Zhang, K.Q.; Wang, F. Oyster Shell Modified Tobacco Straw Biochar: Efficient Phosphate Adsorption at Wide Range of pH Values. Int. J. Environ. Res. Public Health 2022, 19, 7227. [Google Scholar] [CrossRef]
- Missau, J.S.; Rodriguesb, M.A.; Bertuola, D.A.; Tanabea, E.H. Phosphate adsorption improvement using a novel adsorbent by CaFe/LDH supported onto CO2 activated biochar. Water Sci. Technol. 2022, 86, 2396–2414. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.D.; Kong, L.J.; Long, J.Y.; Su, M.H.; Diao, Z.H.; Chang, X.Y.; Chen, D.Y.; Song, G.; Shih, K. Adsorption of phosphorus by calcium-flour biochar: Isotherm, kinetic and transformation studies. Chemosphere 2018, 195, 666–672. [Google Scholar] [CrossRef]
- Deng, W.D.; Zhang, D.Q.; Zheng, X.X.; Ye, X.Y.; Niu, X.J.; Lin, Z.; Fu, M.L.; Zhou, S.Q. Adsorption recovery of phosphate from waste streams by Ca/Mg biochar synthesis from marble waste, calcium-rich sepiolite and bagasse. J. Clean. Prod. 2021, 288, 125638. [Google Scholar] [CrossRef]
- Liu, D.D.; Hao, Z.K.; Chen, D.Q.; Jiang, L.P.; Li, T.Q.; Tian, B.; Yan, C.Q.; Luo, Y.; Chen, G.; Ai, H.F. Use of eggshell-catalyzed biochar adsorbents for Pb removal from aqueous solution. ACS Omega 2022, 7, 21808–21819. [Google Scholar] [CrossRef]
- Waheed, M.; Yousaf, M.; Shehzad, A.; Inam-Ur-Raheem, M.; Kashif Iqbal Khan, M.; Rafiq Khan, M.; Ahmad, N.; Abdullah; Muhammad Aadil, R. Channelling eggshell waste to valuable and utilizable products: A comprehensive review. Trends Food Sci. Technol. 2020, 106, 78–90. [Google Scholar] [CrossRef]
- Luo, J.Y.; Huang, W.X.; Guo, W.; Ge, R.; Zhang, Q.; Fang, F.; Feng, Q.; Cao, J.S.; Wu, Y. Novel strategy to stimulate the food wastes anaerobic fermentation performance by eggshell wastes conditioning and the underlying mechanisms. Chem. Eng. J. 2020, 398, 125560. [Google Scholar] [CrossRef]
- Matej, B.; Elena, V.B.; Dmitry, R.; Stefan, P.; Daily, R.P.; Tihana, M.; Rafael, L. State-of-the-art of eggshell waste in materials science: Recent advances in catalysis, pharmaceutical applications, and mechanochemistry. Trends Food Sci. Technol. 2021, 8, 612567. [Google Scholar]
- Li, C.; Zhou, S.X.; Li, Q.Y.; Gao, G.M.; Zhang, L.J.; Zhang, S.; Huang, Y.; Ding, K.; Hu, X. Activation of sawdust with eggshells. J. Anal. Appl. Pyrol. 2023, 171, 105968. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Q.; Cissoko, N.; Wo, J.; Xu, X. Removal of phosphates using eggshells and calcined eggshells in high phosphate solutions. Appl. Biol. Chem. 2022, 65, 75. [Google Scholar] [CrossRef]
- Sarker, P.; Liu, X.; Hata, N.; Takeshita, H.; Miyamura, H.; Maruo, M. Thermally modified bamboo-eggshell adsorbent for phosphate recovery and its sustainable application as fertilizer. Environ. Res. 2023, 231, 115992. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Korpinen, R.; Mikkonen, K.S.; Willför, S.; Xu, C.L. Nanofibrillated cellulose originated from birch sawdust after sequential extractions: A promising polymeric material from waste to films. Cellulose 2014, 21, 2587–2598. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Emam, H.E. Sono-chemical synthesis of cellulose nanocrystals from wood sawdust using acid hydrolysis. Int. J. Biol. Marcomol. 2018, 107, 1599–1606. [Google Scholar] [CrossRef]
- Dong, X.F.; Gan, W.T.; Shang, Y.; Tang, J.F.; Wang, Y.X.; Cao, Z.F.; Xie, Y.J.; Liu, J.Q.; Bai, L.; Li, J.; et al. Low-value wood for sustainable high-performance structural materials. Nat. Sustain. 2022, 5, 628–635. [Google Scholar] [CrossRef]
- Xie, F.Z.; Wu, F.C.; Liu, G.J.; Mu, Y.S.; Feng, C.L.; Wang, H.H.; Giesy, J.P. Removal of phosphate from eutrophic lakes through adsorption by in situ formation of magnesium hydroxide from diatomite. Environ. Sci. Technol. 2014, 48, 582–590. [Google Scholar] [CrossRef]
- Pan, W.L.; Xie, H.M.; Zhou, Y.; Wu, Q.Y.; Zhou, J.Q.; Guo, X. Simultaneous adsorption removal of organic and inorganic phosphorus from discharged circulating cooling water on biochar derived from agricultural waste. J. Clean. Prod. 2023, 383, 135496. [Google Scholar] [CrossRef]
- Luo, D.; Wang, L.Y.; Nan, H.Y.; Cao, Y.J.; Wang, H.; Kumar, T.V.; Wang, C.Q. Phosphorus adsorption by functionalized biochar: A review. Environ. Chem. Lett. 2023, 21, 497–524. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.M.; Santos, S.C.; Boaventura, R.A.; Botelho, C.M. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Liu, M.C.; Wang, C.Z.; Guo, J.B.; Zhang, L.H. Removal of phosphate from wastewater by lanthanum modified bio-ceramisite. J. Environ. Chem. Eng. 2021, 9, 106123. [Google Scholar] [CrossRef]
- Lee, J.I.; Kim, J.M.; Yoo, S.C.; Jho, E.H.; Lee, C.G.; Park, S.J. Restoring phosphorus from water to soil: Using calcined eggshells for P adsorption and subsequent application of the adsorbent as a P fertilizer. Chemosphere 2022, 287, 132267. [Google Scholar] [CrossRef]
- Khemthong, P.; Luadthong, C.; Nualpaeng, W. Industrial eggshell wastes as the heterogeneous catalysts for microwave-assisted biodiesel production. Catal. Today 2012, 190, 112–116. [Google Scholar] [CrossRef]
- Liu, X.N.; Shen, F.; Qi, X.H. Adsorption recovery of phosphate from aqueous solution by CaO-biochar composites prepared from eggshell and rice straw. Sci. Total Environ. 2019, 666, 694–702. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Psychoyou, M.; Baziotis, I.; Inglezakis, V.J.; Koukouzas, N.; Tsoukalas, N.; Palles, D.; Kamitsos, E.; Oikonomou, G.; Markou, G. Removal of phosphate from aqueous solutions by adsorption onto Ca(OH)2 treated natural clinoptilolite. Chem. Eng. J. 2017, 320, 510–522. [Google Scholar] [CrossRef]
- Liu, X.N.; Shen, F.; Smith, R.L., Jr.; Qi, X.H. Black liquor-derived calcium-activated biochar for recovery of phosphate from aqueous solutions. Bioresour. Technol. 2019, 294, 122198. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.N.; Dai, T.C.; Ren, H.Y.; Liu, B.F. Simultaneous adsorption of phosphate and tetracycline by calcium modified corn stover biochar: Performance and mechanism. Bioresour. Technol. 2022, 359, 127477. [Google Scholar] [CrossRef]
- DB33/973-2021; Discharge Standard of Water Pollutants for Centralized Rural Sewage Treatment Facilities. The People’s Government of Zhejiang Province: Hangzhou, China, 2021. (In Chinese)

| Adsorbent | Physical Properties | Major Elements | ||||
|---|---|---|---|---|---|---|
| Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | C (%) | O (%) | Ca (%) | |
| E-BC (2:1)-T700 | 28.960 | 0.019 | 2.683 | 72.16 | 19.64 | 5.03 |
| E-BC (2:1)-T800 | 58.931 | 0.059 | 4.031 | 9.61 | 59.35 | 30.95 |
| E-BC (2:1)-T900 | 76.565 | 0.081 | 4.251 | 7.14 | 43.9 | 48.94 |
| BC-T800 | 18.25 | 0.021 | 3.052 | 79.21 | 14.85 | 1.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Liu, R.; Tang, Q.; Hou, Y.; Chen, L.; Wang, Q. Adsorption Removal of Phosphate from Rural Domestic Sewage by Ca-Modified Biochar Derived from Waste Eggshell and Sawdust. Water 2023, 15, 3087. https://doi.org/10.3390/w15173087
Xu C, Liu R, Tang Q, Hou Y, Chen L, Wang Q. Adsorption Removal of Phosphate from Rural Domestic Sewage by Ca-Modified Biochar Derived from Waste Eggshell and Sawdust. Water. 2023; 15(17):3087. https://doi.org/10.3390/w15173087
Chicago/Turabian StyleXu, Cancan, Rui Liu, Qi Tang, Yifan Hou, Lvjun Chen, and Quanxi Wang. 2023. "Adsorption Removal of Phosphate from Rural Domestic Sewage by Ca-Modified Biochar Derived from Waste Eggshell and Sawdust" Water 15, no. 17: 3087. https://doi.org/10.3390/w15173087
APA StyleXu, C., Liu, R., Tang, Q., Hou, Y., Chen, L., & Wang, Q. (2023). Adsorption Removal of Phosphate from Rural Domestic Sewage by Ca-Modified Biochar Derived from Waste Eggshell and Sawdust. Water, 15(17), 3087. https://doi.org/10.3390/w15173087





