Soil Water Regime, Air Temperature, and Precipitation as the Main Drivers of the Future Greenhouse Gas Emissions from West Siberian Peatlands
Abstract
:1. Introduction
2. Materials and Methods
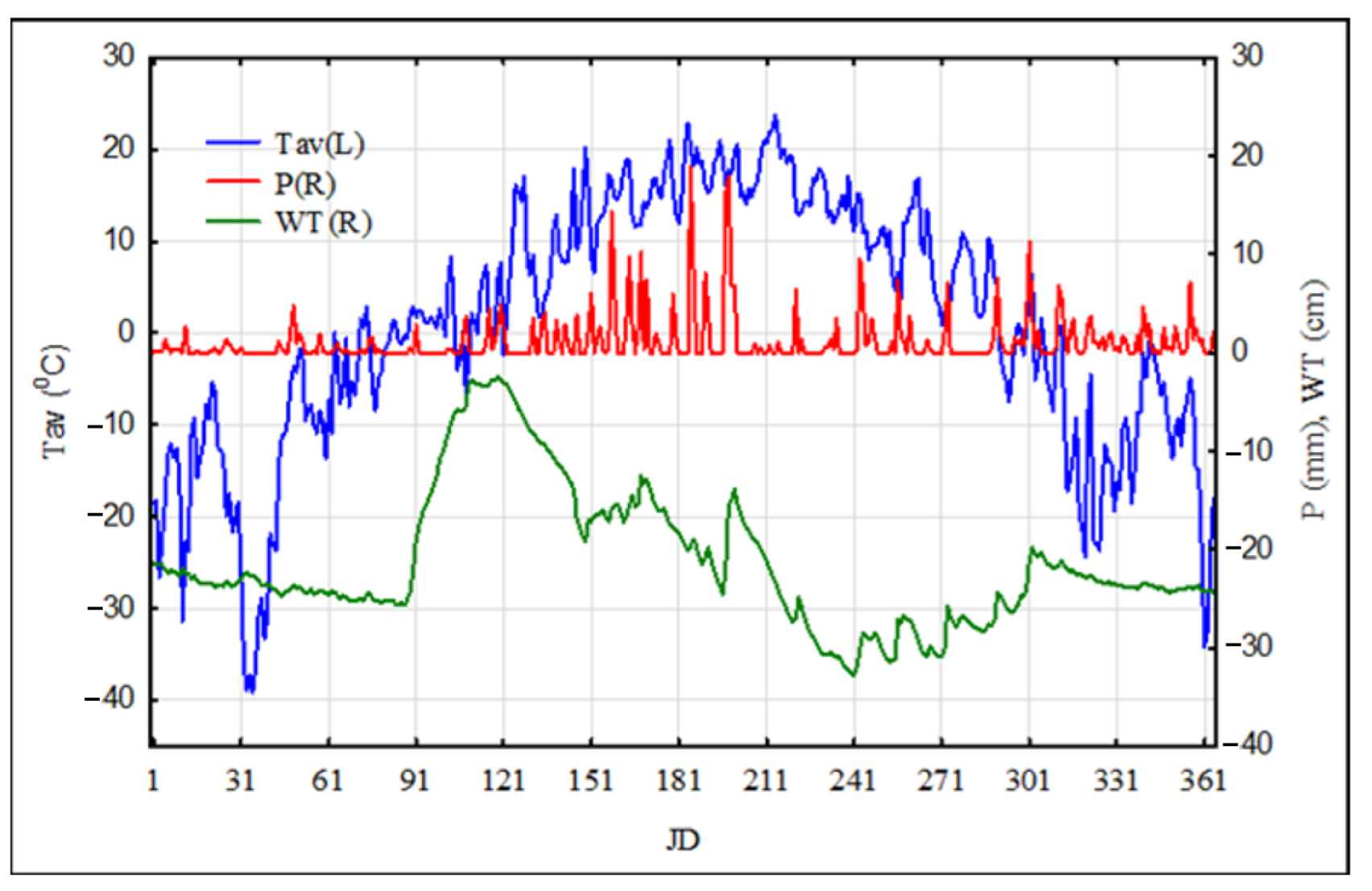
2.1. Materials
2.2. Wetland-DNDC Model Description
2.3. Statistical Analysis
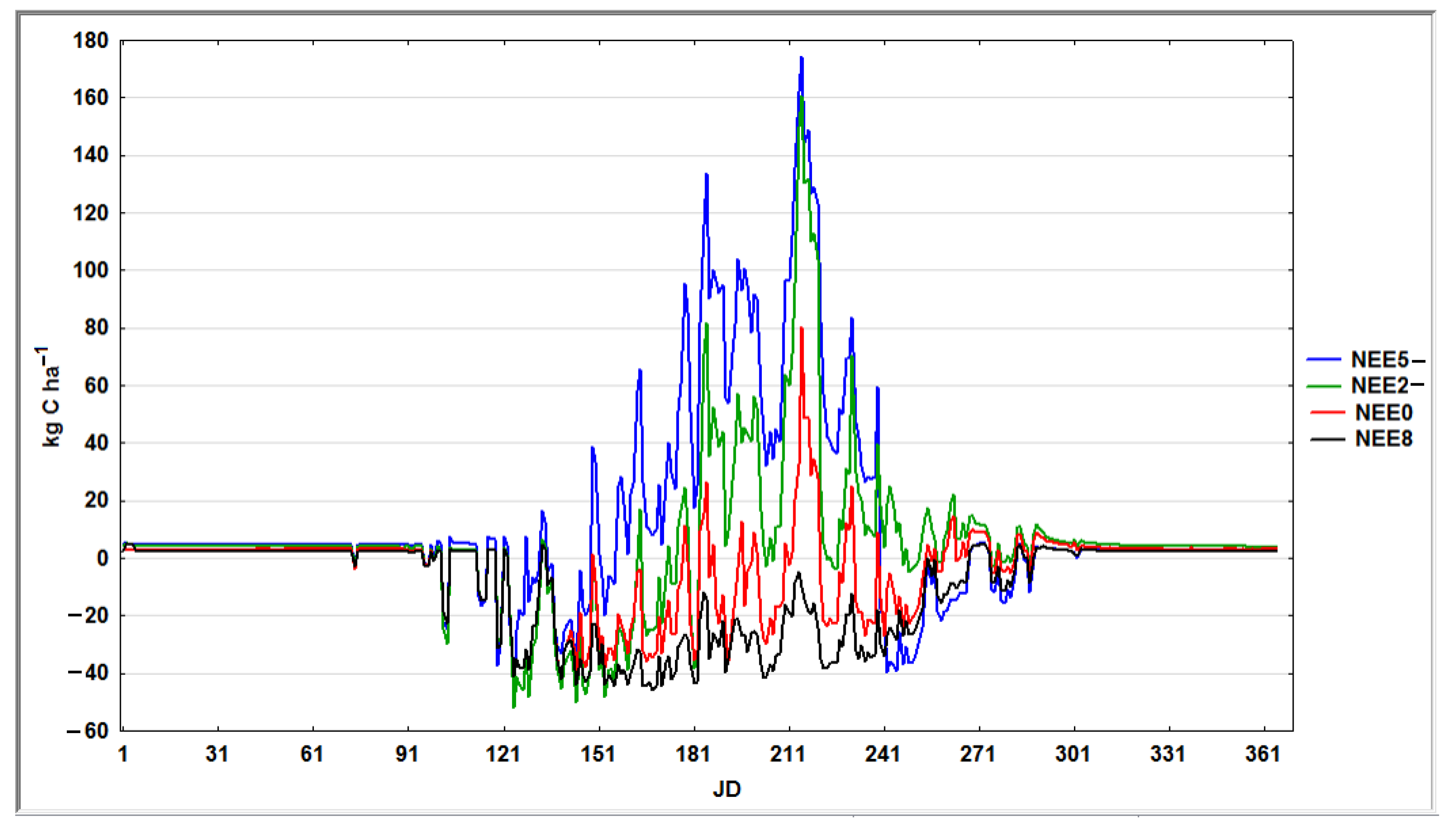
3. Results
3.1. Simulation Modeling of Greenhouse Gas Fluxes with Different Scenarios of Air Temperature
3.2. Simulation Modeling of Precipitation
3.3. Simulation Modeling of Groundwater (Water Table)—WT
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Model Unit | Wetland-DNDC Model Input Parameters | Value |
|---|---|---|
| Climate | [Latitude]: The latitude (decimal unit) of site location; | 56.58 |
| [N in precipitation]: Annually averaged N (dissolved nitrate and ammonium) concentration in rainfall in unit mg N/L or ppm. The ratio between the units of measurement mg/L and ppm is almost equal then, it can be assumed that 1 mg/L = 1 ppm. | 2.79 | |
| [Atmospheric background CO2 concentration (ppm) (350)]: Atmospheric background CO2 concentration (default value set up 350 ppm). | 350 | |
| [Simulated years]: An integer number of total simulated years. | 1 | |
| Daily air maximum and minimum temperatures (°C), Rainfall (mm), and Solar radiation (mj/m2/day) table data file | ||
| Hydrological | Daily observed water table data file (cm) | |
| Forest: Upper story | [Soil fertility]: This is a float number from 1.0 (for fertile soil) to 5.0 (for poor soil). | 1.42857 |
| [Upper-story age]: Age of upper-story trees. | 85 | |
| [Upper-story type]: Dominant type of upper-story trees. | Pine | |
| [Leaf]: Initial leaf biomass, kg C/ha. | 1187 | |
| [Wood]: Initial woody biomass, kg C/ha. | 60,000 | |
| [Root]: Initial root biomass, kg C/ha. | 4349 | |
| [MaxL]: Maximum leaf biomass, kg C/ha. | 1771.5 | |
| [MinL]: Minimum leaf biomass, kg C/ha. | 492 | |
| [PlantN]: Initial plant N storage, kg N/ha. | 8 | |
| [BudC]: Initial available C stored in buds, kg C/ha. | 50 | |
| [WoodC]: Initial available C stored in woody biomass, kg C/ha. | 1380 | |
| [PlantC]: Initial available C stored in forest, kg C/ha. | 9350 | |
| [Initial leaf N content %]: Initial N concentration in foliage, % by weight. | 0.4 | |
| [AmaxA, n mole CO2/g/s] Coefficients for photosynthesis curve. | 2 | |
| [AmaxB]: Coefficients for photosynthesis curve. | 26 | |
| [Optimum Psn temperature]: Optimum temperature for photosynthesis, °C. | 20 | |
| [Minimum Psn temperature]: Minimum temperature for photosynthesis, °C. | 5 | |
| [Amax fraction]: Daily Amax as a fraction of instantaneous Amax. | 0.76 | |
| [Growth respiration fraction]: Growth respiration as a fraction of gross photosynthesis. | 0.2 | |
| [Dark respiration fraction]: | 0.075 | |
| [Wood maintain resp. frac]: Wood maintenance respiration as a fraction of gross photosynthesis. | 0.3 | |
| [Root maintain resp. frac]: Root maintenance respiration as a fraction of gross photosynthesis. | 0.12 | |
| [Light half satur constant]: Half saturation light intensity, µ mole/m2/s. | 200 | |
| [Respiration Q10]: Effect of temperature on respiration. | 2.6 | |
| [Canopy light attenuation k]: Light attenuation constant. | 1.4 | |
| [Water use efficiency]: Water demand for producing a unit of biomass. | 13.9 | |
| [DVPD 1] Coefficients for calculating vapor pressure deficit | 0.05 | |
| [DVPD2]: Coefficients for calculating vapor pressure deficit. | 2 | |
| [Max leaf growth rate]: Maximum foliage growth rate, %/year. | 0.3 | |
| [Max wood growth rate]: Maximum wood growth rate, %/year. | 0.9 | |
| [Leaf start TDD]: Accumulative thermal degree days for starting leaf growth. | 900 | |
| [Wood start TDD]: Accumulative thermal degree days for starting wood growth. | 900 | |
| [Leaf end TDD]: Accumulative thermal degree days for ceasing leaf growth. | 1600 | |
| [Wood end TDD]: Accumulative thermal degree days for ceasing wood growth. | 1600 | |
| [Leaf N retranslocation]: Fraction of leaf N transferred to plant N storage during senescence. | 0.22 | |
| [Senesc start day]: Starting Julian day for senescence. | 246 | |
| [Leaf C/N]: C/N ratio in foliage. | 59 | |
| [Wood C/N]: C/N ratio in woody biomass. | 1364 | |
| [Leaf retention]: Time span of leaf retention, years. | 2 | |
| [C reserve fraction]: Fraction of available C for plant reserve. | 0.75 | |
| [C fraction of dry matter]: C/dry matter ratio. | 0.49 | |
| [Specific leaf weight]: Specific leaf weight, g dry matter/m2 leaf. | 280 | |
| [Min wood/leaf]: Minimum wood/leaf ratio. | 5.5 | |
| [Leaf geometry]: Leaf geometry index. | 2.73 | |
| [Max N storage]: Maximum N content in forest, kg N/ha. | 410 | |
| [SLWdel]: Change in specific leaf weight with foliage biomass, g dry matter/(m2 leaf * g foliage mass). | 0 | |
| Forest: Under story | [Upper-story age]: Age of upper-story trees. | 5 |
| [Upper-story type]: Dominant type of upper-story trees. | Pine | |
| [Leaf]: Initial leaf biomass, kg C/ha. | 1524 | |
| [Wood]: Initial woody biomass, kg C/ha. | 450 | |
| [Root]: Initial root biomass, kg C/ha. | 824 | |
| [MaxL]: Maximum leaf biomass, kg C/ha. | 1517 | |
| [MinL]: Minimum leaf biomass, kg C/ha. | 632 | |
| [PlantN]: Initial plant N storage, kg N/ha. | 0 | |
| [BudC]: Initial available C stored in buds, kg C/ha. | 21 | |
| [WoodC]: Initial available C stored in woody biomass, kg C/ha. | 0 | |
| [PlantC]: Initial available C stored in forest, kg C/ha. | 0 | |
| [Initial leaf N content %]: Initial N concentration in foliage, % by weight. | 0.4 | |
| [AmaxA, n mole CO2/g/s] Coefficients for photosynthesis curve. | 2 | |
| [AmaxB]: Coefficients for photosynthesis curve. | 26 | |
| [Optimum Psn temperature]: Optimum temperature for photosynthesis, °C | 20 | |
| [Minimum Psn temperature]: Minimum temperature for photosynthesis, °C. | 5 | |
| [Amax fraction]: Daily Amax as a fraction of instantaneous Amax. | 0.76 | |
| [Growth respiration fraction]: Growth respiration as a fraction of gross photosynthesis. | 0.2 | |
| [Dark respiration fraction]: | 0.075 | |
| [Wood maintain resp. frac]: Wood maintenance respiration as a fraction of gross photosynthesis. | 0.3 | |
| [Root maintain resp. frac]: Root maintenance respiration as a fraction of gross photosynthesis. | 0.12 | |
| [Light half satur constant]: Half saturation light intensity, µ mole/m2/s. | 200 | |
| [Respiration Q10]: Effect of temperature on respiration. | 2.6 | |
| [Canopy light attenuation k]: Light attenuation constant. | 1.4 | |
| [Water use efficiency]: Water demand for producing a unit of biomass. | 13.9 | |
| [DVPD 1] Coefficients for calculating vapor pressure deficit | 0.05 | |
| [DVPD2]: Coefficients for calculating vapor pressure deficit. | 2 | |
| [Max leaf growth rate]: Maximum foliage growth rate, %/year. | 0.3 | |
| [Max wood growth rate]: Maximum wood growth rate, %/year. | 0.9 | |
| [Leaf start TDD]: Accumulative thermal degree days for starting leaf growth. | 900 | |
| [Wood start TDD]: Accumulative thermal degree days for starting wood growth. | 900 | |
| [Leaf end TDD]: Accumulative thermal degree days for ceasing leaf growth. | 1600 | |
| [Wood end TDD]: Accumulative thermal degree days for ceasing wood growth. | 1600 | |
| [Leaf N retranslocation]: Fraction of leaf N transferred to plant N storage during senescence. | 0.22 | |
| [Senesc start day]: Starting Julian day for senescence. | 246 | |
| [Leaf C/N]: C/N ratio in foliage. | 59 | |
| [Wood C/N]: C/N ratio in woody biomass. | 1364 | |
| [Leaf retention]: Time span of leaf retention, years. | 2 | |
| [C reserve fraction]: Fraction of available C for plant reserve. | 0.75 | |
| [C fraction of dry matter]: C/dry matter ratio. | 0.49 | |
| [Specific leaf weight]: Specific leaf weight, g dry matter/m2 leaf. | 280 | |
| [Min wood/leaf]: Minimum wood/leaf ratio. | 5.5 | |
| [Leaf geometry]: Leaf geometry index. | 2.73 | |
| [Max N storage]: Maximum N content in forest, kg N/ha. | 410 | |
| [SLWdel]: Change in specific leaf weight with foliage biomass, g dry matter/(m2 leaf * g foliage mass). | 0 | |
| Forest: Sedges | Above-ground biomass, kg C/ha | 17.2 |
| [Alpha]: surface inflow relative to precipitation. | 0.05 | |
| [Max Psn, umol CO2/m2/s]: maximumphotosynthesis. | 3.77 | |
| [Min T for Psn]: minimum temperature for photosynthesis, °C. | 5 | |
| [MaxT for Psn]: maximum temperature for photosynthesis, °C. | 30 | |
| [Opt T for Psn]: optimum temperature for photosynthesis, °C. | 20 | |
| [MaxLAI]: maximum leaf area index. | 0.23 | |
| [Rooting depth, m]: Rooting depth. | 0.2 | |
| [Shoot/root ratio]: Shoot/root ratio. | 1.5 | |
| Forest: Mosses | Above-ground biomass, kg C/ha | 1665 |
| [Alpha]: surface inflow relative to precipitation. | 0.01 | |
| [Max Psn, umol CO2/m2/s]: maximum photosynthesis. | 5.5 | |
| [Min T for Psn]: minimum temperature for photosynthesis, °C. | 5 | |
| [MaxT for Psn]: maximum temperature for photosynthesis, °C. | 35 | |
| [Opt T for Psn]: optimum temperature for photosynthesis, °C. | 20 | |
| [MaxLAI]: maximum leaf area index. | 3.18 | |
| [Rooting depth, m]: Rooting depth. | 0.1 | |
| [Shoot/root ratio]: Shoot/root ratio. | 0.1 | |
| Soil | [Forest floor type] is defined based on quality of the organic matter in the forest floor. The categories are rohhumus, moder, and mulls. | rohhumus |
| [Mineral soil type] is defined based on proportions of sand, silt, and clay in a soil. There are 12 soil types, including sand, loamy sand, sandy loam, silt loam, loam, sandy clay loam, silty clay loam, clay loam, sandy clay, silty clay, clay, and organic soil. | organic or peat | |
| [Thickness of forest floor] is the total thickness of the organic layer. The default thickness is 1.5 and 0.2 m for wetland and upland forests, respectively. | 0.5 | |
| [Thickness of mineral soil] is the total thickness of the mineral layers of the soil profile. The default thickness is 0.02 and 0.3 m for wetland and upland forests, respectively. | 2.5 | |
| [pH] is soil acidity. | 3.7 | |
| [SOC, kg C/kg 5 cm] is soil organic carbon concentration at the top soil (0–5 cm). The unit is kg C/kg soil: | ||
| forest floor | 0.5 | |
| mineral soil | 0.05 | |
| [SOC, kg C/ha] is soil organic carbon content in the entire organic or mineral profile. The unit is kg C/ha: | ||
| forest floor | 770 | |
| mineral soil | 20,343.8 | |
| [Bypass flow] is water flow through the macro pore. 0 is no bypass flow; 1 indicates there is bypass flow. | 0 | |
| [Stone fraction] is fraction of stone content in the soil. | 0 | |
| [Soil profile thickness (m)] is the total thickness of the entire soil profile, including the forest floor and the mineral layers. | 3 | |
| [Total layers] is the number of total organic and mineral layers. | 34 | |
| [Bulk Density (g/cm3)] is soil bulk density. The unit is g soil per cubic cm. | ||
| Organic | 0.1 | |
| Mineral | 0.1 | |
| [Clay % (0–1)] is clay fraction by weight. | ||
| Organic | 0.01 | |
| Mineral | 0.001 | |
| [Hydrologic conductivity] is soil-saturated hydrological conductivity. The unit is cm per minute. | ||
| Organic | 1.33 | |
| Mineral | 0.001 | |
| [Porosity] is pore volumetric fraction of the soil. | ||
| Organic | 0.92 | |
| Mineral | 0.001 | |
| [Field Capacity] is the maximum water-filled fraction of total porosity in a freely drained soil. | ||
| Organic | 0.75 | |
| Mineral | 0.001 | |
| [Wilting Point] is the maximum water-filled fraction of total porosity at which the plant starts wilting permanently. | ||
| Organic | 0.7 | |
| Mineral | 0.001 | |
| [Litter fraction] is decomposing plant or animal residue C percent of total SOC. | ||
| Organic | 0.099 | |
| Mineral | 0.001 | |
| [Humads fraction] is living microbial biomass C and active humus C percent of total SOC. | ||
| Organic | 0.99 | |
| Mineral | 0.2312 | |
| [Humus fraction] is resistant humus C percent of total SOC. | ||
| Organic | 0.001 | |
| Mineral | 0.7678 | |
| Manage | Missing | 0 |
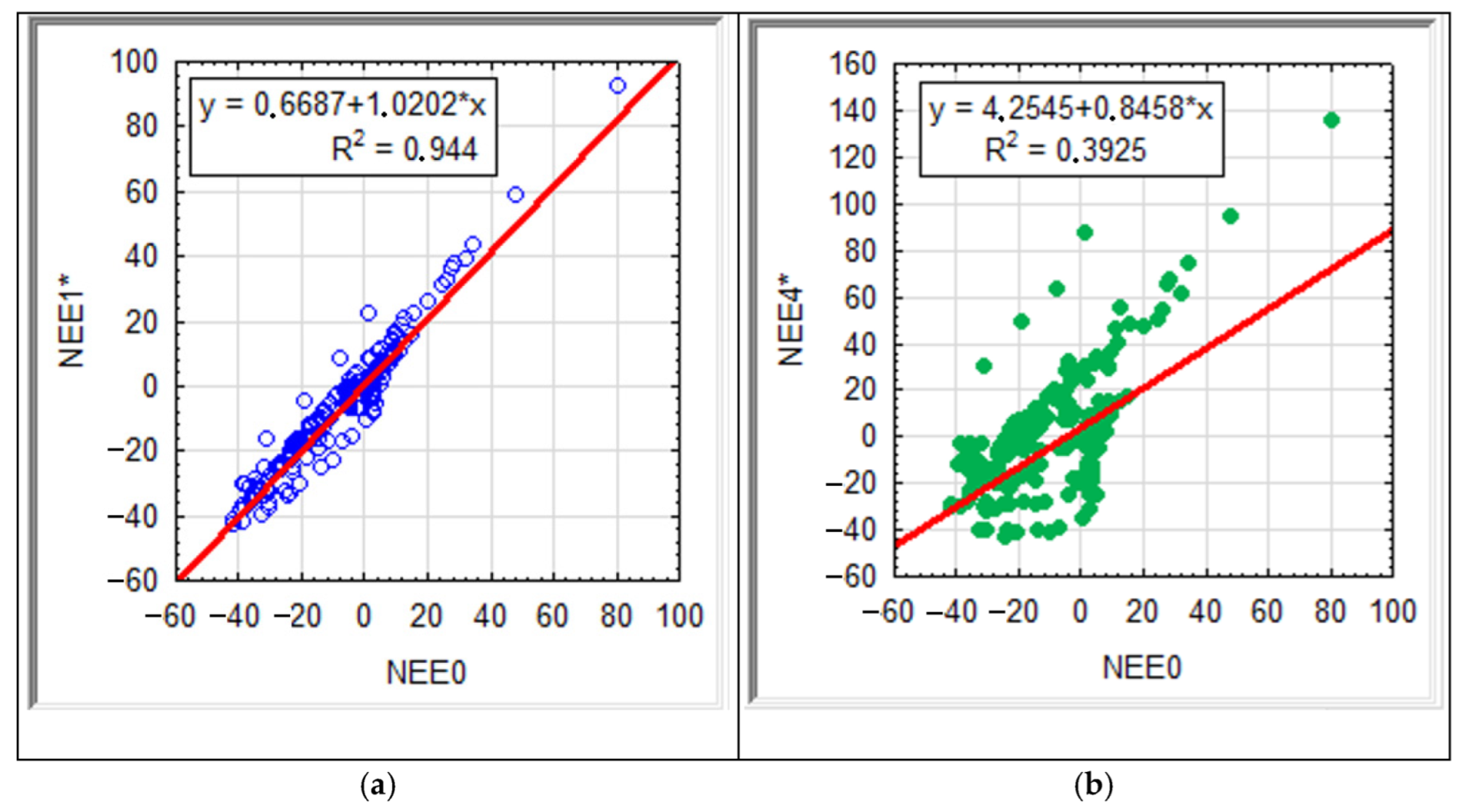
| NEE0 | NEE1 | NEE2 | NEE3 | NEE4 | NEE1* | NEE2* | NEE3* | NEE4* | ||
|---|---|---|---|---|---|---|---|---|---|---|
| NEE0 | 1.000 | 0.889 | 0.757 | 0.521 | 0.383 | 0.915 | 0.800 | 0.687 | 0.573 | Rs |
| NEE1 | 0.958 | 1.000 | 0.886 | 0.677 | 0.545 | 0.935 | 0.898 | 0.794 | 0.687 | |
| NEE2 | 0.846 | 0.963 | 1.000 | 0.861 | 0.749 | 0.849 | 0.909 | 0.880 | 0.818 | |
| NEE3 | 0.706 | 0.877 | 0.974 | 1.000 | 0.933 | 0.635 | 0.773 | 0.793 | 0.807 | |
| NEE4 | 0.574 | 0.781 | 0.920 | 0.984 | 1.000 | 0.503 | 0.685 | 0.757 | 0.811 | |
| NEE1* | 0.972 | 0.984 | 0.920 | 0.815 | 0.704 | 1.000 | 0.900 | 0.799 | 0.684 | |
| NEE2* | 0.893 | 0.967 | 0.965 | 0.907 | 0.830 | 0.968 | 1.000 | 0.944 | 0.858 | |
| NEE3* | 0.746 | 0.870 | 0.922 | 0.913 | 0.873 | 0.870 | 0.960 | 1.000 | 0.945 | |
| NEE4* | 0.626 | 0.787 | 0.882 | 0.911 | 0.901 | 0.771 | 0.900 | 0.980 | 1.000 | |
| r | ||||||||||

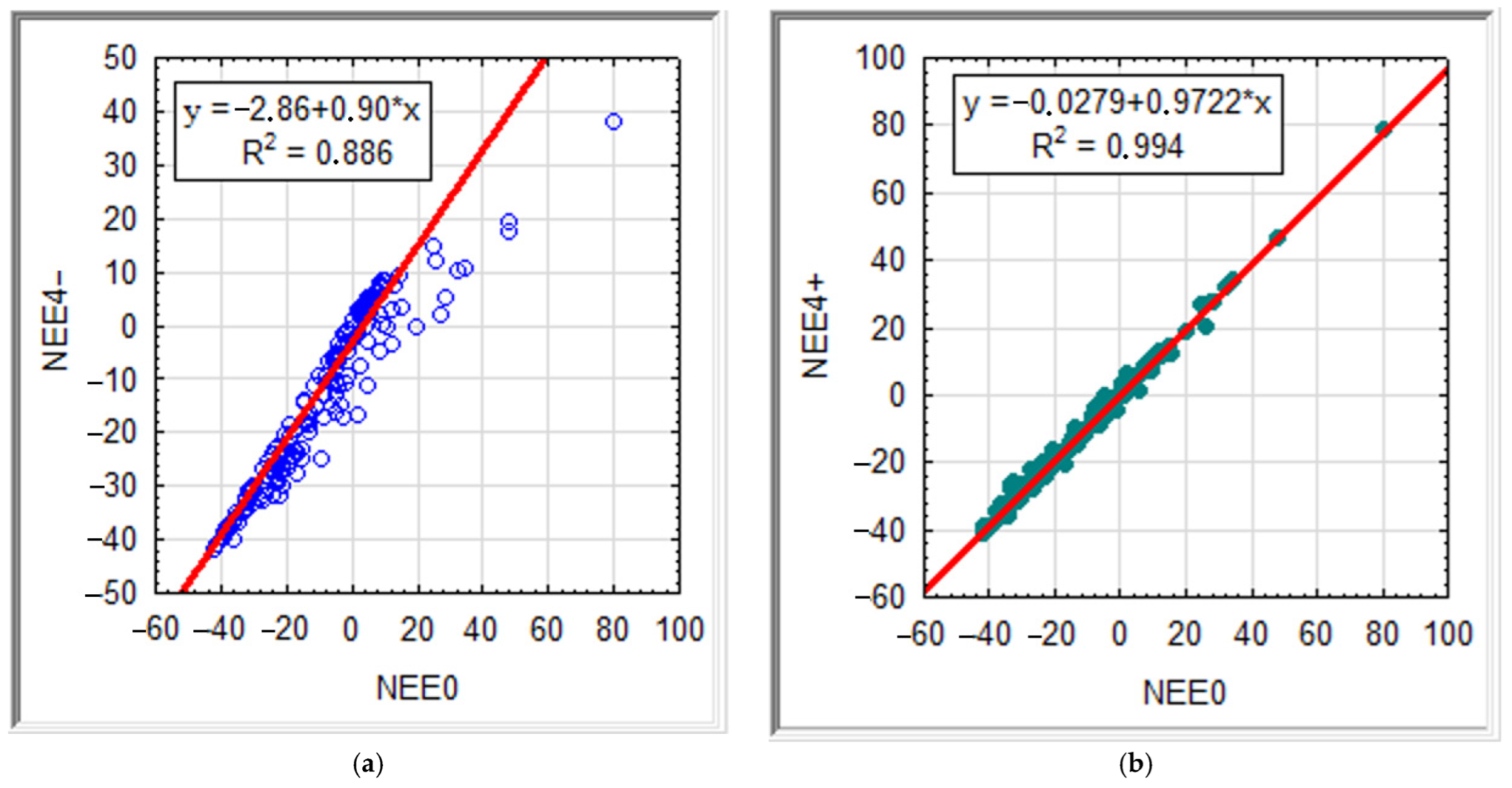
| NEE4− | NEE3− | NEE2− | NEE1− | NEE0 | NEE1+ | NEE2+ | NEE3+ | NEE4+ | ||
|---|---|---|---|---|---|---|---|---|---|---|
| NEE4− | 1.000 | 0.961 | 0.937 | 0.906 | 0.910 | 0.900 | 0.902 | 0.891 | 0.890 | Rs |
| NEE3− | 0.994 | 1.000 | 0.987 | 0.956 | 0.955 | 0.948 | 0.952 | 0.942 | 0.936 | |
| NEE2− | 0.986 | 0.998 | 1.000 | 0.975 | 0.974 | 0.968 | 0.972 | 0.961 | 0.953 | |
| NEE1− | 0.955 | 0.978 | 0.988 | 1.000 | 0.992 | 0.995 | 0.988 | 0.983 | 0.975 | |
| NEE0 | 0.941 | 0.967 | 0.980 | 0.998 | 1.000 | 0.994 | 0.981 | 0.976 | 0.969 | |
| NEE1+ | 0.940 | 0.967 | 0.980 | 0.998 | 0.999 | 1.000 | 0.987 | 0.983 | 0.974 | |
| NEE2+ | 0.940 | 0.967 | 0.980 | 0.996 | 0.997 | 0.999 | 1.000 | 0.989 | 0.981 | |
| NEE3+ | 0.944 | 0.970 | 0.982 | 0.997 | 0.997 | 0.998 | 0.999 | 1.000 | 0.993 | |
| NEE4+ | 0.944 | 0.969 | 0.981 | 0.996 | 0.997 | 0.998 | 0.999 | 1.000 | 1.000 | |
| r | ||||||||||
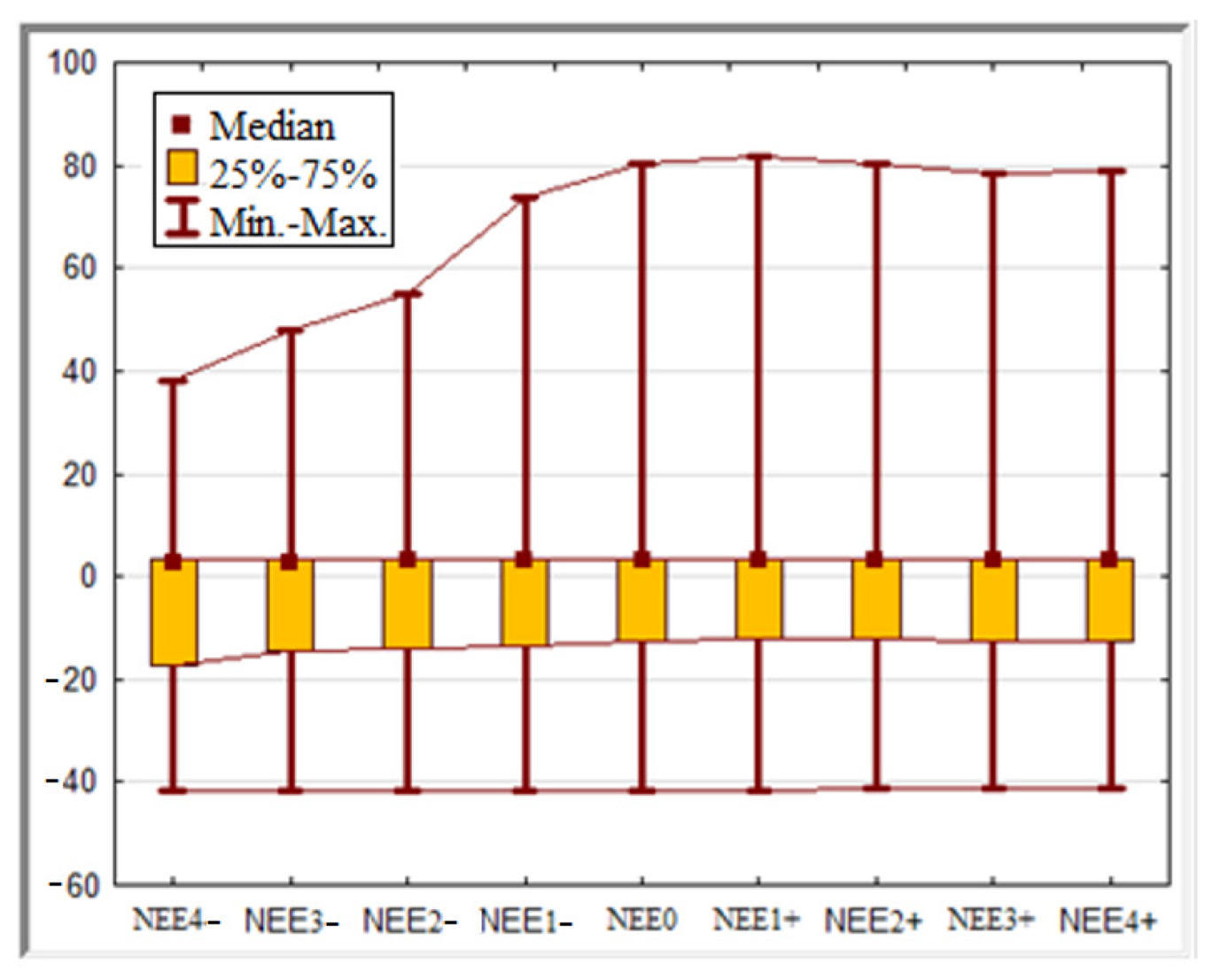
| NEEx | No. of Observ. | Mean | Median | Minimum | Maximum | Low Quartile | Upper Quartile | Mean Deviation |
|---|---|---|---|---|---|---|---|---|
| NEE4− | 365 | −6.02 | 2.86 | −41.71 | 38.02 | −17.07 | 3.27 | 14.46 |
| NEE3− | 365 | −5.12 | 2.90 | −41.86 | 47.80 | −14.45 | 3.40 | 14.39 |
| NEE2− | 365 | −4.59 | 2.91 | −41.85 | 54.78 | −13.90 | 3.41 | 14.39 |
| NEE1− | 365 | −3.78 | 2.91 | −41.86 | 73.79 | −13.51 | 3.42 | 14.83 |
| NEE0 | 365 | −3.51 | 2.93 | −41.85 | 80.53 | −12.54 | 3.44 | 15.13 |
| NEE1+ | 365 | −3.51 | 2.92 | −41.75 | 81.59 | −12.21 | 3.43 | 15.12 |
| NEE2+ | 365 | −3.33 | 2.98 | −41.45 | 80.22 | −11.95 | 3.44 | 14.82 |
| NEE3+ | 365 | −3.39 | 2.98 | −41.08 | 78.22 | −12.55 | 3.45 | 14.74 |
| NEE4+ | 365 | −3.44 | 2.98 | −41.11 | 78.70 | −12.66 | 3.45 | 14.75 |
| NEEx | 5− | 3− | 2− | 1− | 0 | 1 | 2 | 3 | 6 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 5− | 1.00 | 0.529 | 0.400 | 0.291 | 0.108 | 0.213 | 0.159 | 0.047 | 0.004 | Rs |
| 3− | 0.867 | 1.000 | 0.885 | 0.677 | 0.424 | 0.382 | 0.407 | 0.229 | 0.153 | |
| 2− | 0.781 | 0.959 | 1.00 | 0.792 | 0.531 | 0.488 | 0.515 | 0.352 | 0.282 | |
| 1− | 0.645 | 0.865 | 0.932 | 1.000 | 0.722 | 0.685 | 0.678 | 0.551 | 0.487 | |
| 0 | 0.332 | 0.590 | 0.714 | 0.822 | 1.00 | 0.820 | 0.811 | 0.717 | 0.677 | |
| 1 | 0.255 | 0.466 | 0.562 | 0.716 | 0.877 | 1.000 | 0.909 | 0.862 | 0.797 | |
| 2 | 0.179 | 0.475 | 0.592 | 0.740 | 0.885 | 0.913 | 1.00 | 0.892 | 0.826 | |
| 3 | −0.092 | 0.164 | 0.310 | 0.483 | 0.784 | 0.863 | 0.883 | 1.000 | 0.905 | |
| 6 | −0.230 | 0.034 | 0.194 | 0.407 | 0.750 | 0.828 | 0.840 | 0.943 | 1.00 | |
| r | ||||||||||
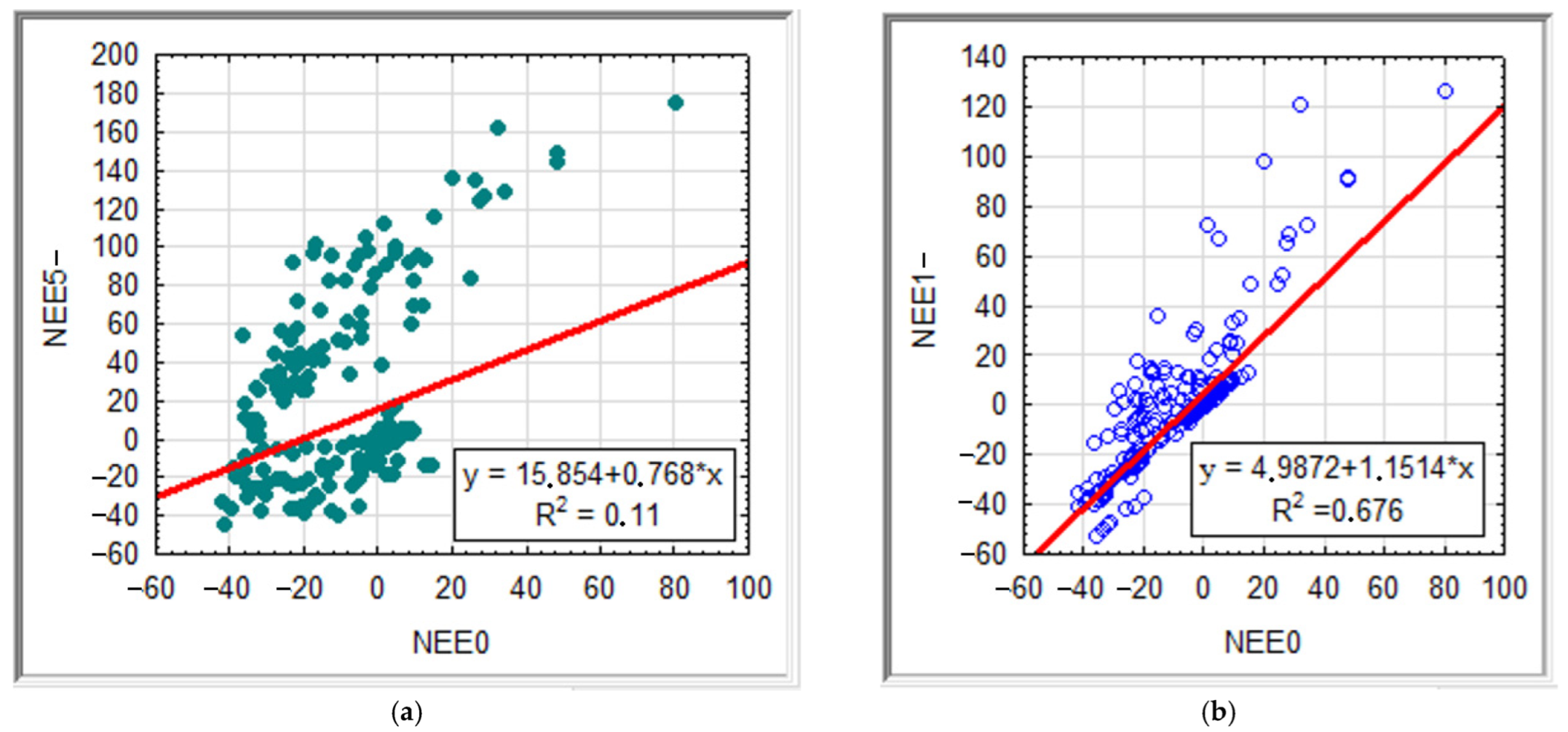
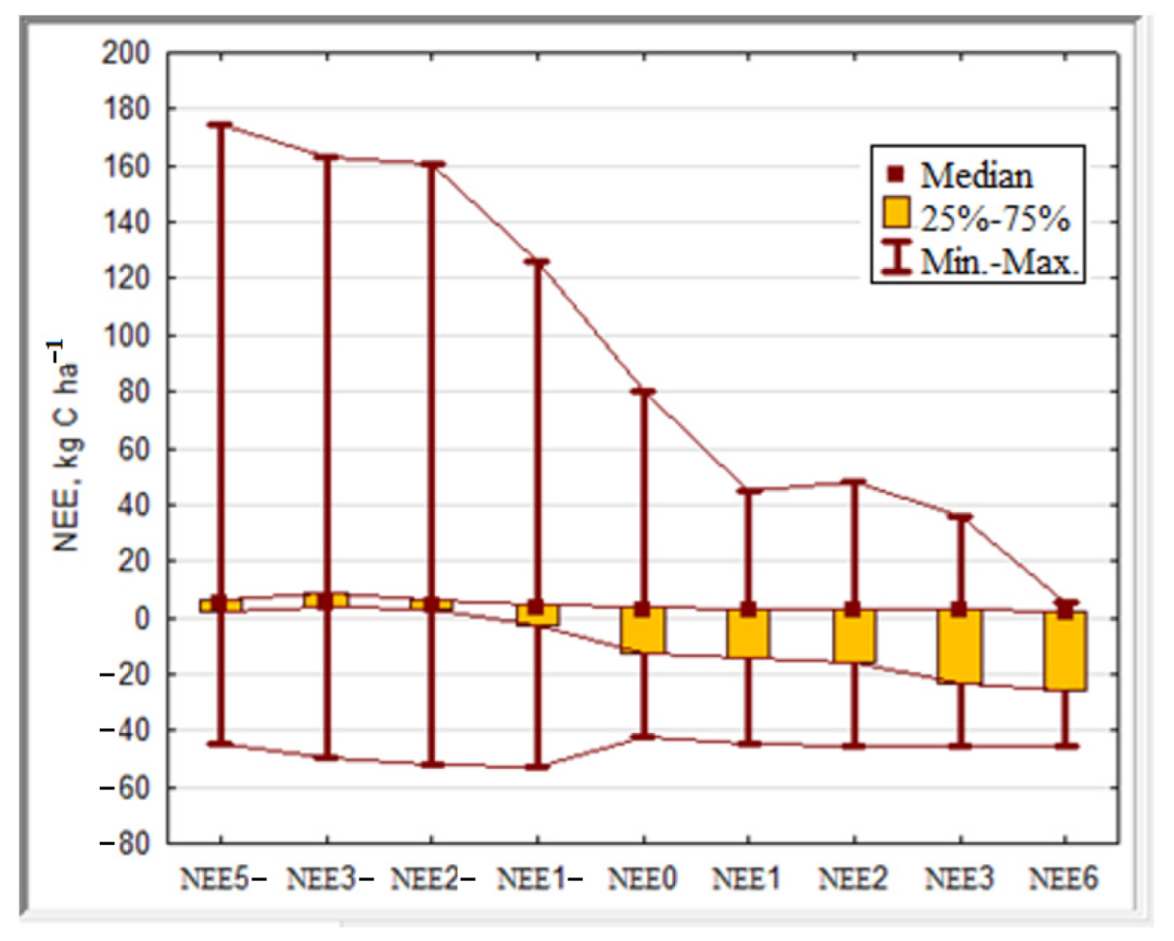
| NEEx | No. of Observ. | Mean | Median | Minimum | Maximum | Low Quartile | Upper Quartile | Mean Deviation |
|---|---|---|---|---|---|---|---|---|
| NEE5− | 365 | 13.15 | 4.75 | −44.63 | 174.40 | 2.34 | 5.97 | 35.05 |
| NEE3− | 365 | 9.61 | 4.79 | −49.49 | 163.37 | 4.12 | 9.10 | 28.61 |
| NEE2− | 365 | 5.43 | 4.15 | −52.06 | 160.44 | 2.86 | 6.27 | 26.29 |
| NEE1− | 365 | 0.94 | 3.48 | −52.57 | 125.86 | −2.86 | 4.21 | 21.18 |
| NEE0 | 365 | −3.51 | 2.93 | −41.85 | 80.53 | −12.54 | 3.44 | 15.13 |
| NEE1 | 365 | −5.87 | 2.82 | −44.47 | 44.65 | −14.62 | 3.06 | 15.89 |
| NEE2 | 365 | −5.88 | 2.77 | −45.09 | 48.32 | −16.09 | 2.96 | 16.31 |
| NEE3 | 365 | −8.99 | 2.67 | −45.57 | 35.58 | −23.45 | 2.91 | 16.89 |
| NEE6 | 365 | −10.26 | 2.17 | −45.59 | 5.00 | −25.93 | 2.35 | 16.42 |
References
- Eswaran, H.; Van den Berg, E.; Reich, P.; Kimble, J. Global soil carbon resources. In Soils and Global Change; Lal, R., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 27–43. [Google Scholar]
- Bubier, J.L.; Crill, P.M.; Moore, T.R.; Savage, K.; Varner, R.K. Seasonal patterns of controls on net ecosystem CO2 exchange in a boreal peatland complex. Global Biogeochem. Cycles 1998, 12, 703–714. [Google Scholar] [CrossRef]
- Silvola, J.J.; Alm, U.A.; Ahlholm, U.; Nykanen, H.; Martikainen, P.J. CO2 fluxes from peat in boreal mires under varying temperature and moisture conditions. J. Ecol. 1996, 84, 219–228. [Google Scholar] [CrossRef]
- Shurpali, N.J.; Verma, S.B.; Kim, J.; Arkebauer, T.J. Carbon dioxide exchange in a peatland ecosystem. J. Geophys. Res. 1995, 100, 14319–14326. [Google Scholar] [CrossRef]
- Li, C. Quantifying greenhouse gas emissions from soils: Scientific basis and modeling approach. Soil Sci. Plant Nutr. 2007, 53, 344–352. [Google Scholar] [CrossRef]
- Giltrap, D.L.; Li, C.; Saggar, S. DNDC: A process-based model of greenhouse gas fluxes from agricultural soils. Agric. Ecosyst. Environ. 2010, 136, 292–300. [Google Scholar] [CrossRef]
- Gilhespy, S.L.; Anthony, S.; Cardenas, L.; Chadwick, D.; del Prado, A.; Li, C.; Misselbrook, T.; Rees, R.M.; Salas, W.; Sanz-Cobena, A.; et al. First 20 years of DNDC (DeNitrificationDeComposition): Model evolution. Ecol. Model. 2014, 292, 51–62. [Google Scholar] [CrossRef]
- Janse, J.H.; Van Dam, A.A.; Hes, E.M.; de Klein, J.J.; Finlayson, C.M.; Janssen, A.B.; van Wijk, D.; Mooij, W.M.; Verhoeven, J.T. Towards a global model for wetlands ecosystem services. Curr. Opin. Environ. Sustain. 2019, 36, 11–19. [Google Scholar] [CrossRef]
- Song, C.; Luo, F.; Zhang, L.; Yi, L.; Wang, C.; Yang, Y.; Li, J.; Chen, K.; Wang, W.; Li, Y.; et al. Nongrowing Season CO2 Emissions Determine the Distinct Carbon Budgets of Two Alpine Wetlands on the Northeastern Qinghai—Tibet. Atmosphere 2021, 12, 1695. [Google Scholar] [CrossRef]
- Sukhoveeva, O.E. Modeling of greenhouse gas fluxes and nitrogen and carbon cycles in soils (review). J. Nat. Sci. Res. 2017, 2, 61–76. (In Russian) [Google Scholar]
- Mitsch, W.J.; Sraskraba, M.; Jorgensen, S.E. (Eds.) Development in Environmental Modeling. In Wetland Modeling; Elsevier Science: New York, NY, USA, 1988; Volume 12, p. 227. [Google Scholar]
- Cui, J.B.; Li, C.S.; Trettin, C. Analyzing the ecosystem carbon and hydrologic characteristics of forested wetland using a biogeochemical process model. Glob. Change Biol. 2005, 11, 278–289. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Trettin, C.C.; Li, H.; Sun, G. An integrated model of soil, hydrology, and vegetation for carbon dynamics in wetland ecosystems. Glob. Biogeochem. Cycles 2002, 16, 1061. [Google Scholar] [CrossRef]
- User’s Guide for Wetland-DNDC, USA. Available online: https://www.dndc.sr.unh.edu/model/ForestUserGuide.pdf (accessed on 1 April 2023).
- Kurbatova, J.; Li, C.; Tatarinov, F.; Varlagin, A.; Shalukhina, N.; Olchev, A. Modeling of the carbon dioxide fluxes in European Russia peat bogs. Environ. Res. Lett. 2009, 4, 045022. [Google Scholar] [CrossRef]
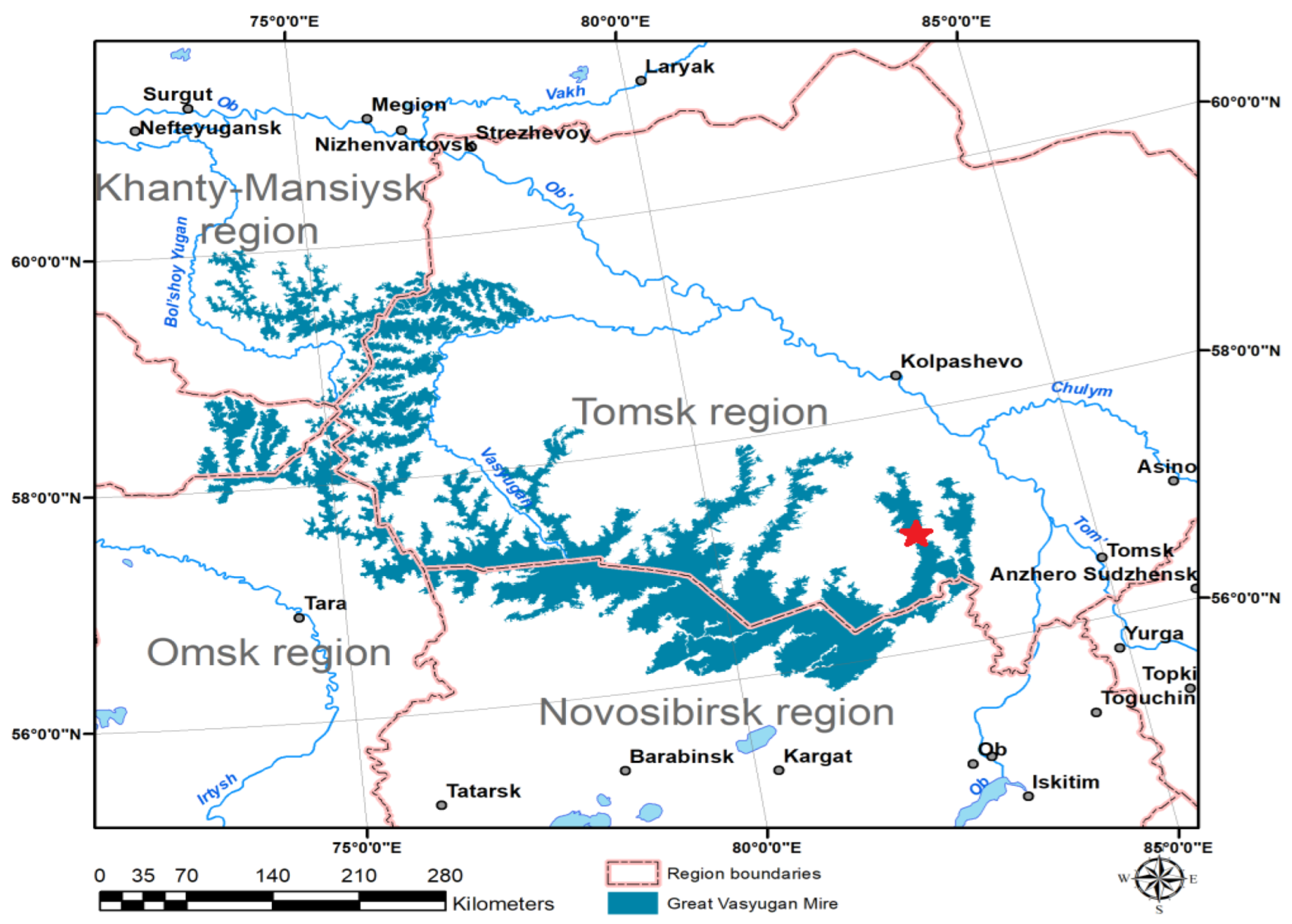
- Mikhalchuk, A.; Borilo, L.; Burnashova, E.; Kharanzhevskaya, Y.; Akerman, E.; Chistyakova, N.; Kirpotin, S.N.; Pokrovsky, O.S.; Vorobyev, S. Assessment of Greenhouse Gas Emissions into the Atmosphere from the Northern Peatlands Using the Wetland-DNDC Simulation Model: A Case Study of the Great Vasyugan Mire, Western Siberia. Atmosphere 2022, 13, 2053. [Google Scholar] [CrossRef]
- Līcīte, I.; Popluga, D.; Rivža, P.; Lazdiņš, A.; Meļņiks, R. Nutrient-Rich Organic Soil Management Patterns in Light of Climate Change Policy. Civ. Eng. J. 2022, 8, 2290–2304. [Google Scholar] [CrossRef]
- Kurbatova, J.; Li, C.; Varlagin, A.; Xiao, X.; Vygodskaya, N. Modeling carbon dynamics in two adjacent spruce forests with different soil conditions in Russia. Biogeosciences 2008, 5, 969–980. [Google Scholar] [CrossRef]
- Cui, J.; Li, C.; Sun, G.; Trettin, C. Linkage of MIKE SHE to Wetland-DNDC for carbon budgeting and anaerobic biogeochemistry simulation. Biogeochemistry 2005, 72, 147–167. [Google Scholar] [CrossRef]
- Webster, K.L.; Mclaughlin, J.W.; Packalen, M.S.; Kim, Y. Modelling carbon dynamics and response to environmental change along a boreal fen nutrient gradient. Ecol. Model. 2013, 248, 148–164. [Google Scholar] [CrossRef]
- Berezin, A.E.; Bazanov, V.A.; Skugarev, A.A.; Rybina, T.A.; Parshina, N.V. Great Vasyugan Mire: Landscape structure and peat deposit structure features. Int. J. Environ. Stud. 2014, 71, 618–623. [Google Scholar] [CrossRef]
- Peregon, A.; Uchida, M.; Yamagata, Y. Lateral extension in Sphagnum mires along the southern margin of the boreal region, Western Siberia. Environ. Res. Lett. 2009, 4, 045028. [Google Scholar] [CrossRef]
- Makarov, B.N. A simplified method for determining soil respiration (biochemical activity). Soil Sci. 1957, 9, 119–122. [Google Scholar]
- Golovatskaya, E.A. Carbon Fluxes in Mire Ecosystems of the Southern Taiga of Western Siberia. Ph.D. Thesis, Sukachev Institute of Forest SB RAS, Federal Research Center “Krasnoyarsk Science Center SB RAS”, Krasnoyarsk, Russia, 2013; 33p. [Google Scholar]
- Bazarov, A.V.; Badmaev, N.B.; Kurakov, S.A.; Gonchikov, B.-M.N. Erratum to: A Mobile Measurement System for the Coupled Monitoring of Atmospheric and Soil Parameters. Russ. Meteorol. Hydrol. 2018, 43, 795–796. [Google Scholar] [CrossRef]
- Frolking, S.; Goulden, M.; Wofsy, S.; Fan, S.-M.; Sutton, D.; Munger, J.; Bazzaz, A.M.; Daube, B.; Crill, P.M.; Aber, J.D.; et al. Modelling temporal variability in the carbon balance of a spruce/moss boreal forest. Glob. Change Biol. 1996, 2, 343–366. [Google Scholar] [CrossRef]
- Walter, B.P.; Heimann, M. A process-based, climate-sensitive model to derive methane emissions from natural wetlands: Application to five wetland sites, sensitivity to model parameters, and climate. Glob. Biogeochem. Cycles 2000, 14, 745–765. [Google Scholar] [CrossRef]
- Cao, M.; Marshall, S.; Gregson, K. Global carbon exchange and methane emissions from natural wetlands: Application of a process-based model. J. Geophys. Res. 1996, 101, 399–414. [Google Scholar] [CrossRef]
- Potter, C.S. An ecosystem simulation model for methane production and emission from wetlands. Glob. Biogeochem. Cycles 1997, 11, 495–506. [Google Scholar] [CrossRef]
- Fiedler, S.; Sommer, M. Methane emissions, ground water levels and redox potentials of common wetland soils in a temperate-humid climate. Glob. Biogeochem. Cycles 2000, 14, 1081–1093. [Google Scholar] [CrossRef]
- Dyukarev, E.A. Partitioning of net ecosystem exchange using chamber measurements data from bare soil and vegetated sites. Agric. For. Meteorol. 2017, 239, 236–248. [Google Scholar] [CrossRef]
- Abdalla, M.; Kumar, S.; Jones, M.; Burke, J.; Williams, M. Testing DNDC model for simulating soil respiration and assessing the effects of climate change on the CO2 gas flux from Irish agriculture. Glob. Planet. 2011, 78, 106–115. [Google Scholar] [CrossRef]
- Smith, P.; Smith, J.U.; Powlson, D.S.; McGill, W.B.; Arah, J.R.M.; Chertov, O.G.; Coleman, K.; Franko, U.; Frolking, S.; Jenkinson, D.S.; et al. A comparison of the performance of nine soil organic models using datasets from seven long-term experiments. Geoderma 1997, 81, 153–225. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Data Science Textbook, 2020. Available online: https://docs.tibco.com/data-science/textbook (accessed on 21 August 2023).
- Manasypov, R.M.; Lim, A.G.; Krickov, I.V.; Shirokova, L.S.; Vorobyev, S.N.; Kirpotin, S.N.; Pokrovsky, O.S. Spatial and Seasonal Variations of C, Nutrient, and Metal Concentration in Thermokarst Lakes of Western Siberia Across a Permafrost Gradient. Water 2020, 12, 1830. [Google Scholar] [CrossRef]
- Nash, J.E.; Sutcliffe, J.V. River flow forecasting through conceptual models part I—A discussion of principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Kim, Y.; Roulet, N.T.; Peng, C.; Li, C.; Frolking, S.; Strachan, I.B.; Tremblay, A. Multi-year carbon dioxide flux simulations for mature canadian black spruce forests and ombrotrophic bogs using FOREST-DNDC. Boreal Environ. Res. 2014, 19, 417–440. [Google Scholar]
- Li, T.; Huang, Y.; Zhang, W.; Song, C. CH4MODwetland: A biogeophysical model for simulating methane emissions from natural wetlands. Ecol. Model. 2010, 221, 666–680. [Google Scholar] [CrossRef]
- Kang, X.; Li, Y.; Wang, J.; Yan, L.; Zhang, X.; Wu, H.; Yan, Z.; Zhang, K.; Hao, Y. Precipitation and temperature regulate the carbon allocation process in alpine wetlands: Quantitative simulation. J. Soils Sediments 2020, 20, 3300–3315. [Google Scholar] [CrossRef]
- Dai, Z.; Trettin, C.C.; Li, C.; Li, H.; Sun, G.; Amatya, D.M. Effect of assessment scale on spatial and temporal variations in CH4, CO2, and N2O fluxes in a forested Wetland. Water Air Soil Pollut. 2012, 223, 253–265. [Google Scholar] [CrossRef]
- Park, S.-B.; Knohl, A.; Migliavacca, M.; Thum, T.; Vesala, T.; Peltola, O.; Mammarella, I.; Prokushkin, A.; Kolle, O.; Lavrič, J.; et al. Temperature Control of Spring CO2 Fluxes at a Coniferous Forest and a Peat Bog in Central Siberia. Atmosphere 2021, 12, 984. [Google Scholar] [CrossRef]
- Lund, M.; Lafleur, P.M.; Roulet, N.T.; Lindroth, A.; Christensen, T.R.; Aurela, M.; Chojnicki, B.H.; Flanagan, L.B.; Humphreys, E.R.; Laurila, T.; et al. Variability in exchange of CO2 across 12 northern peatland and tundra sites. Glob. Change Biol. 2010, 16, 2436–2448. [Google Scholar] [CrossRef]
- Dyukarev, E.A.; Martynova Yu, V.; Golovatskaya, E.A. Assessment of the carbon balance of treed bogs under climate change with observation and modelling data. In Proceedings of the IOP Conference Series: Earth and Environmental Science, International Young Scientists School and Conference on Computational Information Technologies for Environmental Sciences, Moscow, Russia, 27 May–6 June 2019; Volume 386, p. 012028. [Google Scholar] [CrossRef]
- Guidelines for the Drainage of Forest Lands: Part 2; Design-Soyuzgipro-Leskhoz: Moscow, Russia, 1986; 100p. (In Russian)
- McLaughlin, J.W.; Packalen, M.S. Packalen Peat Carbon Vulnerability to Projected Climate Warming in the Hudson Bay Lowlands, Canada: A Decision Support Tool for Land Use Planning in Peatland Dominated Landscapes. Front. Earth Sci. 2021, 9, 650662. [Google Scholar] [CrossRef]
- Gong, J.; Kellomäki, S.; Wang, K.; Zhang, C.; Shurpali, N.; Martikainen, P.J. Martikainen Modeling CO2 and CH4 flux changes in pristine peatlands of Finland under changing climate conditions. Ecol. Model. 2013, 263, 64–80. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, X.; Wu, H.; Kang, E.; Li, Y.; Wang, J.; Yan, Z.; Zhang, K.; Kang, X. Disproportionate Changes in the CH4 Emissions of Six Water Table Levels in an Alpine Peatland. Atmosphere 2020, 11, 1165. [Google Scholar] [CrossRef]
- Naumov, A.V. Carbon status of Russia and dynamic equilibrium of the biosphere. Soils Environ. 2022, 5, e166. (In Russian) [Google Scholar] [CrossRef]
- Golovatskaya, E.A.; Dyukarev, E.A.; Ippolitov, I.I.; Kabanov, M.V. Influence of landscape and hydrometeorological conditions on CO2 emission in peatland ecosystems. Dokl. Earth Sci. 2008, 418, 187–190. [Google Scholar] [CrossRef]
- Golovatskaya, E.A.; Dyukarev, E.A. Influence of environmental factors on CO2 emission from the surface of oligotrophic peat soils in Western Siberia. Eurasian Soil Sci. 2012, 45, 658–667. [Google Scholar] [CrossRef]
- Parazoo, N.C.; Koven, C.D.; Lawrence, D.M.; Romanovsky, V.; Miller, C.E. Detecting the permafrost carbon feedback: Talik formation and increased cold-season respiration as precursors to sink-to-source transitions. Cryosphere 2018, 12, 123–144. [Google Scholar] [CrossRef]
- Dyukarev, E.; Zarov, E.; Alekseychik, P.; Nijp, J.; Filippova, N.; Mammarella, I.; Filippov, I.; Bleuten, W.; Khoroshavin, V.; Ganasevich, G.; et al. The Multiscale Monitoring of Peatland Ecosystem Carbon Cycling in the Middle Taiga Zone of Western Siberia: The Mukhrino Bog Case Study. Land 2021, 10, 824. [Google Scholar] [CrossRef]
- Golovatskaya, E.A.; Dyukarev, E.A. Carbon budget of oligotrophic mire sites in the Southern Taiga of Western. Plant Soil. 2009, 315, 19–34. [Google Scholar] [CrossRef]
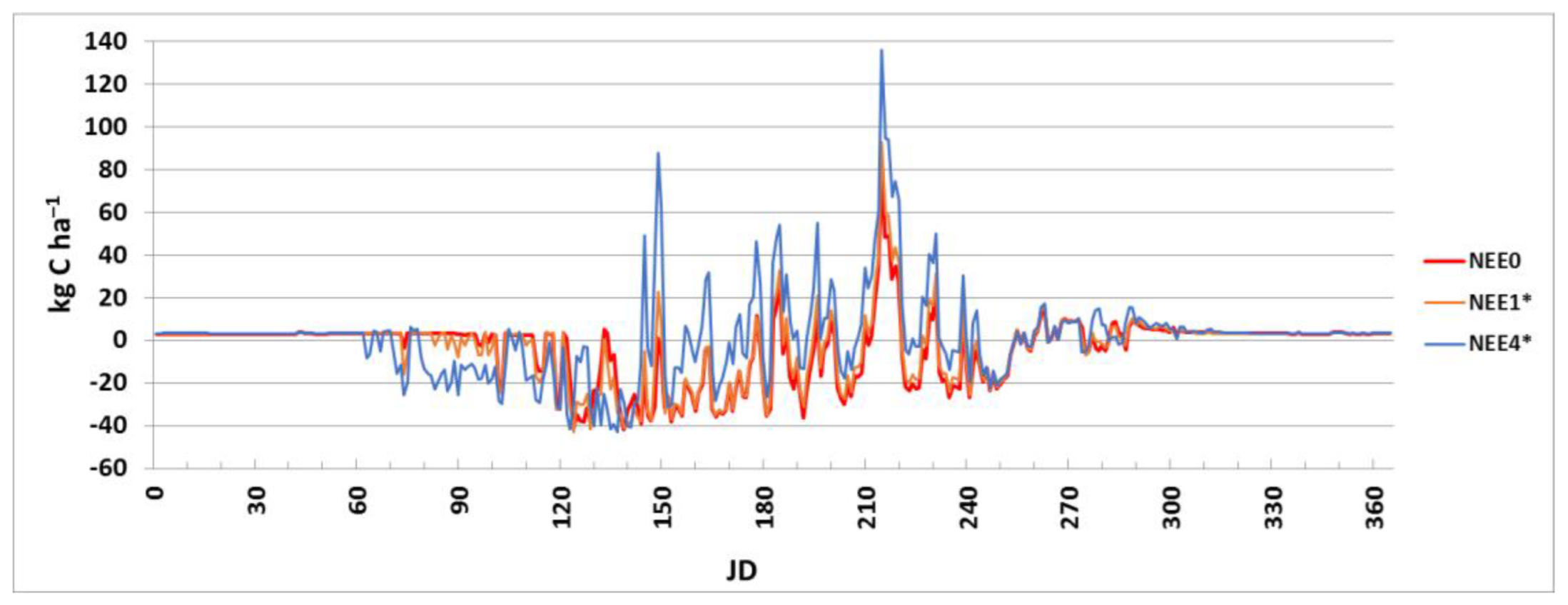
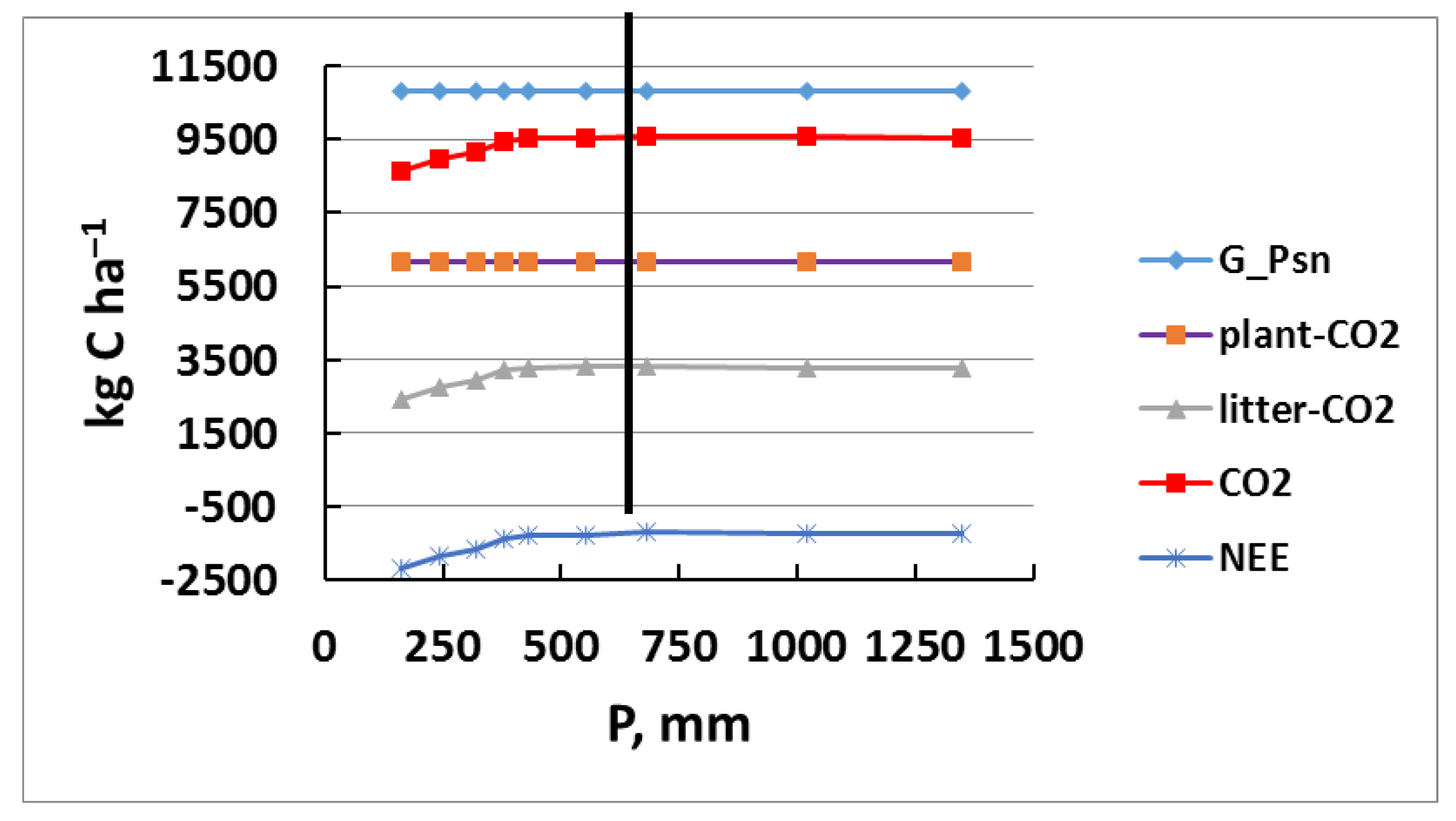
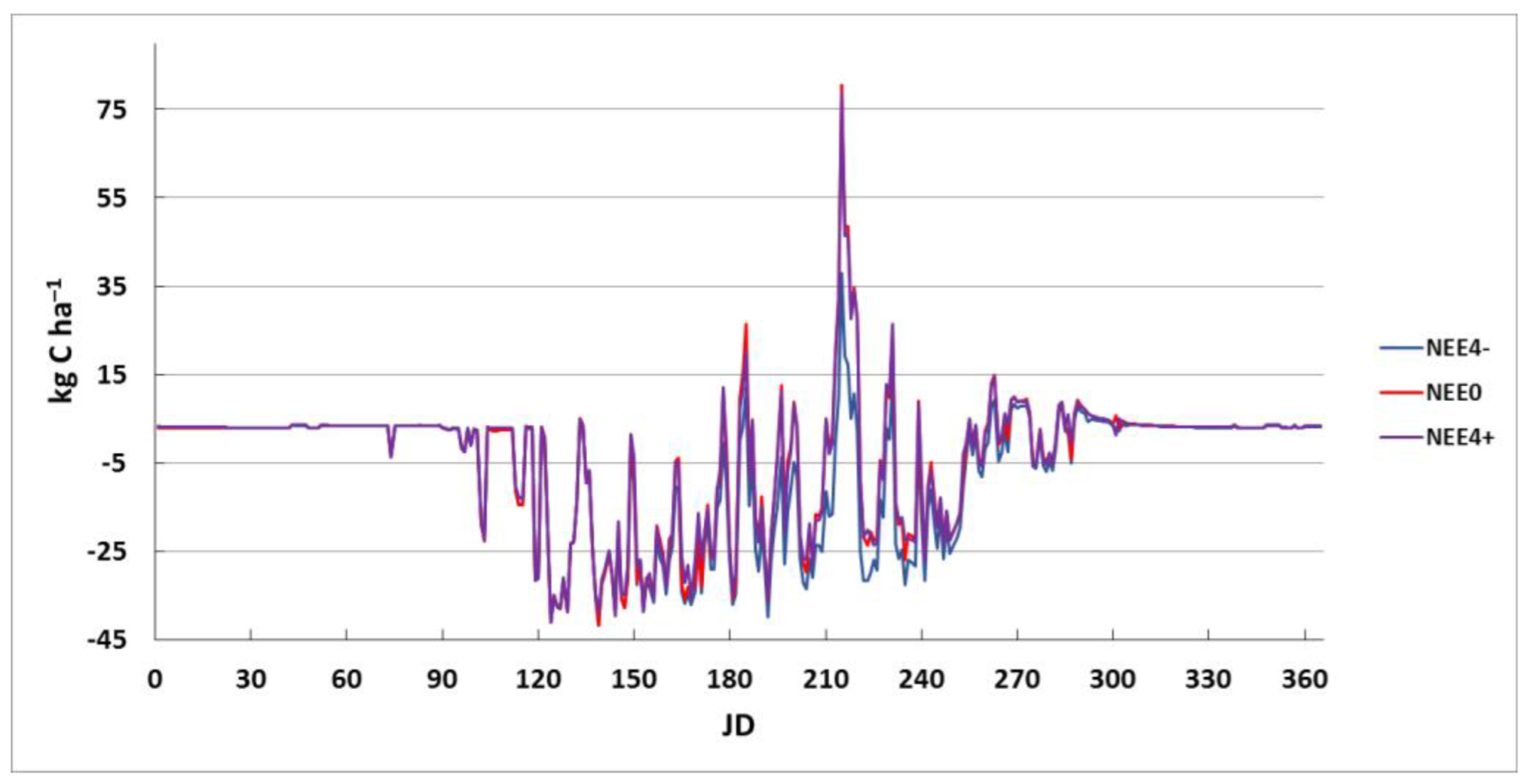
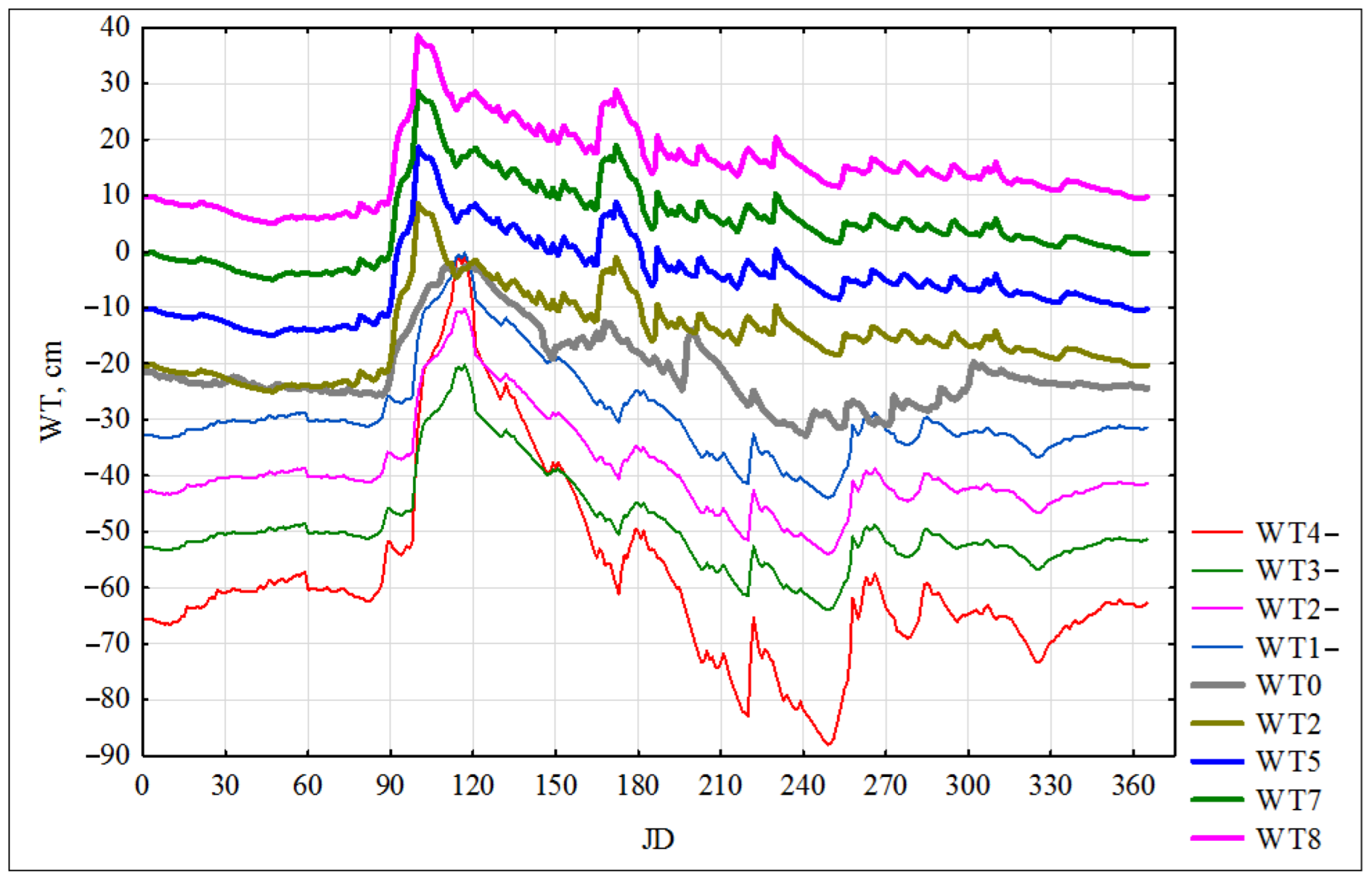

| Month | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Year |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1970–1987/ 1988–2019 | 0 | 2.5 | 2.2 * | 1.22 | 2.04 | 0 | 0.6 | 0.5 | 0.1 | 1.7 * | 0.4 | 0.5 | 0.99 |
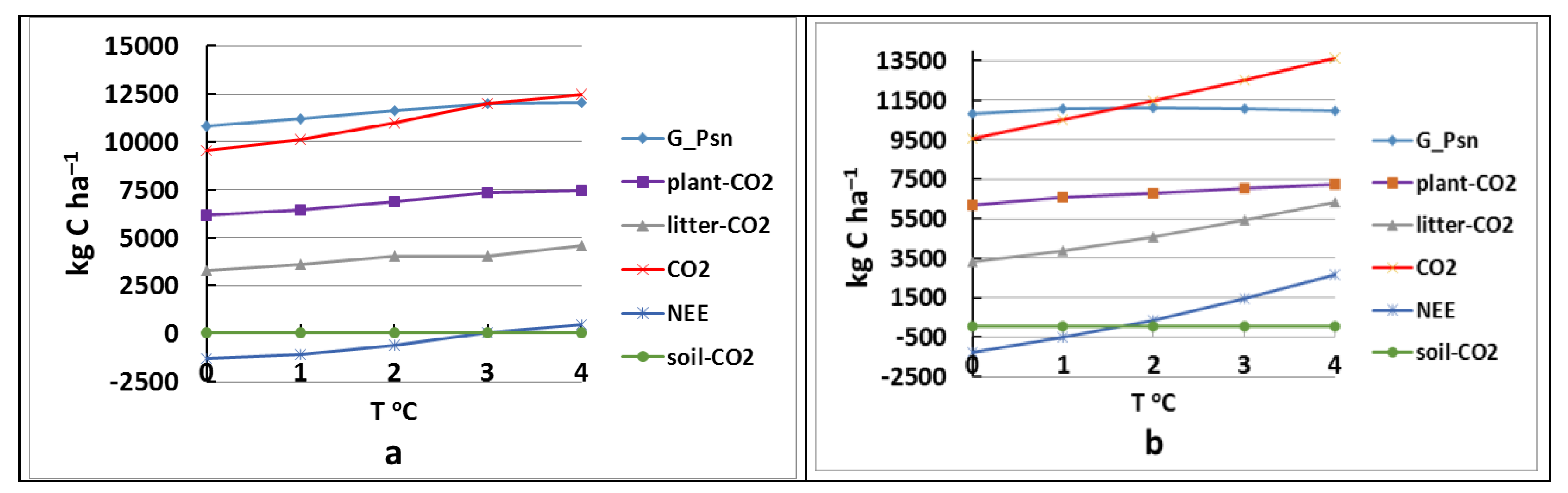
| Tk °C | G_Psn | Plant-CO2 | Litter-CO2 | Soil-CO2 | CO2 | NEE | CH4 | NO | N2O |
|---|---|---|---|---|---|---|---|---|---|
| base model | 10,818 | 6189 | 3291 | 56 | 9535 | −1283 | 118 | 8229 | 3614 |
| +1 | 11,057 | 6576 | 3893 | 57 | 10,526 | −531 | 145 | 9394 | 3769 |
| +2 | 11,096 | 6803 | 4598 | 60 | 11,461 | 365 | 177 | 10,567 | 3897 |
| +3 | 11,069 | 7031 | 5412 | 63 | 12,506 | 1437 | 214 | 11,725 | 4006 |
| +4 | 10,991 | 7237 | 6352 | 67 | 13,656 | 2665 | 254 | 12,947 | 4302 |
| +1 * | 11,178 | 6457 | 3600 | 57 | 10,113 | −1065 | 144 | 8620 | 3718 |
| +2 * | 11,604 | 6880 | 4064 | 59 | 11,003 | −601 | 177 | 9356 | 3806 |
| +3 * | 11,980 | 7369 | 4566 | 62 | 11,997 | 17 | 227 | 9817 | 3881 |
| +4 * | 12,034 | 7439 | 4998 | 65 | 12,502 | 468 | 264 | 9787 | 3980 |
| Tk °C | G_Psn | Plant-CO2 | Litter-CO2 | Soil-CO2 | CO2 | NEE | CH4 | NO | N2O |
|---|---|---|---|---|---|---|---|---|---|
| +1 | 2.2 | 6.3 | 18.3 | 1.8 | 10.4 | 58.6 | 22.9 | 14.2 | 4.3 |
| +2 | 2.6 | 9.9 | 39.7 | 7.1 | 20.2 | 128.4 | 50.0 | 28.4 | 7.8 |
| +3 | 2.3 | 13.6 | 64.4 | 12.5 | 31.2 | 212.0 | 81.4 | 42.5 | 10.8 |
| +4 | 1.6 | 16.9 | 93.0 | 19.6 | 43.2 | 307.7 | 115.3 | 57.3 | 19.0 |
| +1 * | 3.3 | 4.3 | 9.4 | 1.8 | 6.1 | 17.0 | 22.0 | 4.8 | 2.9 |
| +2 * | 7.3 | 11.2 | 23.5 | 5.4 | 15.4 | 53.2 | 50.0 | 13.7 | 5.3 |
| +3 * | 10.7 | 19.1 | 38.7 | 10.7 | 25.8 | 101.3 | 92.4 | 19.3 | 7.4 |
| +4 * | 11.2 | 20.2 | 51.9 | 16.1 | 31.1 | 136.5 | 123.7 | 18.9 | 10.1 |
| P, mm | G_Psn | Plant-CO2 | Litter-CO2 | Soil-CO2 | CO2 | NEE | CH4 | NO | N2O |
|---|---|---|---|---|---|---|---|---|---|
| 160 | 10,818 | 6173 | 2398 | 48 | 8620 | −2198 | 113 | 1164 | 1688 |
| 240 | 10,818 | 6167 | 2730 | 50 | 8948 | −1870 | 113 | 2164 | 2269 |
| 320 | 10,818 | 6170 | 2919 | 53 | 9143 | −1675 | 115 | 3463 | 2791 |
| 380 | 10,818 | 6178 | 3206 | 53 | 9437 | −1381 | 116 | 5546 | 2731 |
| 430 * | 10,818 | 6189 | 3291 | 56 | 9535 | −1283 | 118 | 8229 | 3614 |
| 550 | 10,818 | 6183 | 3298 | 57 | 9538 | −1280 | 117 | 9447 | 3727 |
| 680 | 10,818 | 6185 | 3298 | 119 | 9602 | −1216 | 117 | 9896 | 4128 |
| 1020 | 10,818 | 6173 | 3278 | 130 | 9580 | −1238 | 114 | 10,675 | 5002 |
| 1350 | 10,818 | 6166 | 3257 | 139 | 9561 | −1257 | 112 | 10,897 | 5542 |
| NEEk | P, mm | G_Psn | Plant-CO2 | Litter-CO2 | Soil-CO2 | CO2 | NEE | CH4 | NO | N2O |
|---|---|---|---|---|---|---|---|---|---|---|
| NEE4− | −62.79 | 0 | −0.26 | −27.13 | −14.29 | −9.60 | −71.32 | −4.24 | −85.85 | −53.29 |
| NEE3− | −44.19 | 0 | −0.36 | −17.05 | −10.71 | −6.16 | −45.75 | −4.24 | −73.70 | −37.22 |
| NEE2− | −25.58 | 0 | −0.31 | −11.30 | −5.36 | −4.11 | −30.55 | −2.54 | −57.92 | −22.77 |
| NEE1− | −11.63 | 0 | −0.18 | −2.58 | −5.36 | −1.03 | −7.64 | −1.69 | −32.60 | −24.43 |
| NEE0 | 0.00 * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NEE1+ | 27.91 | 0 | −0.10 | 0.21 | 1.79 | 0.03 | 0.23 | −0.85 | 14.80 | 3.13 |
| NEE2+ | 58.14 | 0 | −0.06 | 0.21 | 112.50 | 0.70 | 5.22 | −0.85 | 20.26 | 14.22 |
| NEE3+ | 137.21 | 0 | −0.26 | −0.40 | 132.14 | 0.47 | 3.51 | −3.39 | 29.72 | 38.41 |
| NEE4+ | 213.95 | 0 | −0.37 | −1.03 | 148.21 | 0.27 | 2.03 | −5.08 | 32.42 | 53.35 |
| WT, cm | G_Psn | Plant-CO2 | Litter-CO2 | Soil-CO2 | CO2 | NEE | CH4 | NO | N2O | |
|---|---|---|---|---|---|---|---|---|---|---|
| WT5- | −102 | 10,293 | 6244 | 4233 | 4617 | 15,094 | 4801 | 0 | 18,135 | 9436 |
| WT3- | −49 | 12,062 | 6243 | 4768 | 4560 | 15,571 | 3509 | 1 | 17,141 | 9463 |
| WT2- | −39 | 12,011 | 6241 | 4686 | 3065 | 13,992 | 1981 | 11 | 16,054 | 7397 |
| WT1- | −29 | 11,618 | 6229 | 4494 | 1239 | 11,961 | 343 | 45 | 13,567 | 5544 |
| WT0 | −21 * | 10,818 | 6189 | 3291 | 56 | 9535 | −1283 | 118 | 8229 | 3614 |
| WT1 | −18 | 10,485 | 6182 | 2110 | 50 | 8342 | −2143 | 165 | 5663 | 2291 |
| WT2 | −15 | 9927 | 6132 | 1600 | 48 | 7779 | −2148 | 256 | 4459 | 1794 |
| WT3 | −12 | 9927 | 6123 | 478 | 45 | 6646 | −3281 | 729 | 1380 | 702 |
| WT6 | −1 | 9925 | 6114 | 20 | 46 | 6179 | −3746 | 2893 | 12 | 49 |
| WT8 | 15 | 9925 | 6114 | 20 | 46 | 6179 | −3746 | 2893 | 12 | 49 |
| WT, cm | G_Psn | Plant-CO2 | Litter-CO2 | Soil-CO2 | CO2 | NEE | CH4 | NO | N2O |
|---|---|---|---|---|---|---|---|---|---|
| −385.71 | −4.85 | 0.89 | 28.62 | 8144.64 | 58.30 | 474.20 | −100.00 | 120.38 | 161.10 |
| −133.33 | 11.50 | 0.87 | 44.88 | 8042.86 | 63.30 | 373.50 | −99.15 | 108.30 | 161.84 |
| −85.71 | 11.03 | 0.84 | 42.39 | 5373.21 | 46.74 | 254.40 | −90.68 | 95.09 | 104.68 |
| −38.10 | 7.40 | 0.65 | 36.55 | 2112.50 | 25.44 | 126.73 | −61.86 | 64.87 | 53.40 |
| 0 * | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14.29 | −3.08 | −0.11 | −35.89 | −10.71 | −12.51 | −67.03 | 39.83 | −31.18 | −36.61 |
| 28.57 | −8.24 | −0.92 | −51.38 | −14.29 | −18.42 | −67.42 | 116.95 | −45.81 | −50.36 |
| 42.86 | −8.24 | −1.07 | −85.48 | −19.64 | −30.30 | −155.73 | 517.80 | −83.23 | −80.58 |
| 95.24 | −8.25 | −1.21 | −99.39 | −17.86 | −35.20 | −191.97 | 2351.69 | −99.85 | −98.64 |
| 171.43 | −8.25 | −1.21 | −99.39 | −17.86 | −35.20 | −191.97 | 2351.69 | −99.85 | −98.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhalchuk, A.; Kharanzhevskaya, Y.; Burnashova, E.; Nekhoda, E.; Gammerschmidt, I.; Akerman, E.; Kirpotin, S.; Nikitkin, V.; Khovalyg, A.; Vorobyev, S. Soil Water Regime, Air Temperature, and Precipitation as the Main Drivers of the Future Greenhouse Gas Emissions from West Siberian Peatlands. Water 2023, 15, 3056. https://doi.org/10.3390/w15173056
Mikhalchuk A, Kharanzhevskaya Y, Burnashova E, Nekhoda E, Gammerschmidt I, Akerman E, Kirpotin S, Nikitkin V, Khovalyg A, Vorobyev S. Soil Water Regime, Air Temperature, and Precipitation as the Main Drivers of the Future Greenhouse Gas Emissions from West Siberian Peatlands. Water. 2023; 15(17):3056. https://doi.org/10.3390/w15173056
Chicago/Turabian StyleMikhalchuk, Alexander, Yulia Kharanzhevskaya, Elena Burnashova, Evgeniya Nekhoda, Irina Gammerschmidt, Elena Akerman, Sergey Kirpotin, Viktor Nikitkin, Aldynai Khovalyg, and Sergey Vorobyev. 2023. "Soil Water Regime, Air Temperature, and Precipitation as the Main Drivers of the Future Greenhouse Gas Emissions from West Siberian Peatlands" Water 15, no. 17: 3056. https://doi.org/10.3390/w15173056
APA StyleMikhalchuk, A., Kharanzhevskaya, Y., Burnashova, E., Nekhoda, E., Gammerschmidt, I., Akerman, E., Kirpotin, S., Nikitkin, V., Khovalyg, A., & Vorobyev, S. (2023). Soil Water Regime, Air Temperature, and Precipitation as the Main Drivers of the Future Greenhouse Gas Emissions from West Siberian Peatlands. Water, 15(17), 3056. https://doi.org/10.3390/w15173056









