Transport and Attenuation of an Artificial Sweetener and Six Pharmaceutical Compounds in a Sequenced Wetland-Steel Slag Wastewater Treatment System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Field Methods
2.2.1. System Configuration
2.2.2. Sampling Procedure
2.3. Analytical Methods
2.4. Quality Control and Quality Assurance
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Wastewater
3.2. Carbonaceous Biochemical Oxygen Demand (cBOD5)
3.3. Pharmaceutical Compounds (PhACs)
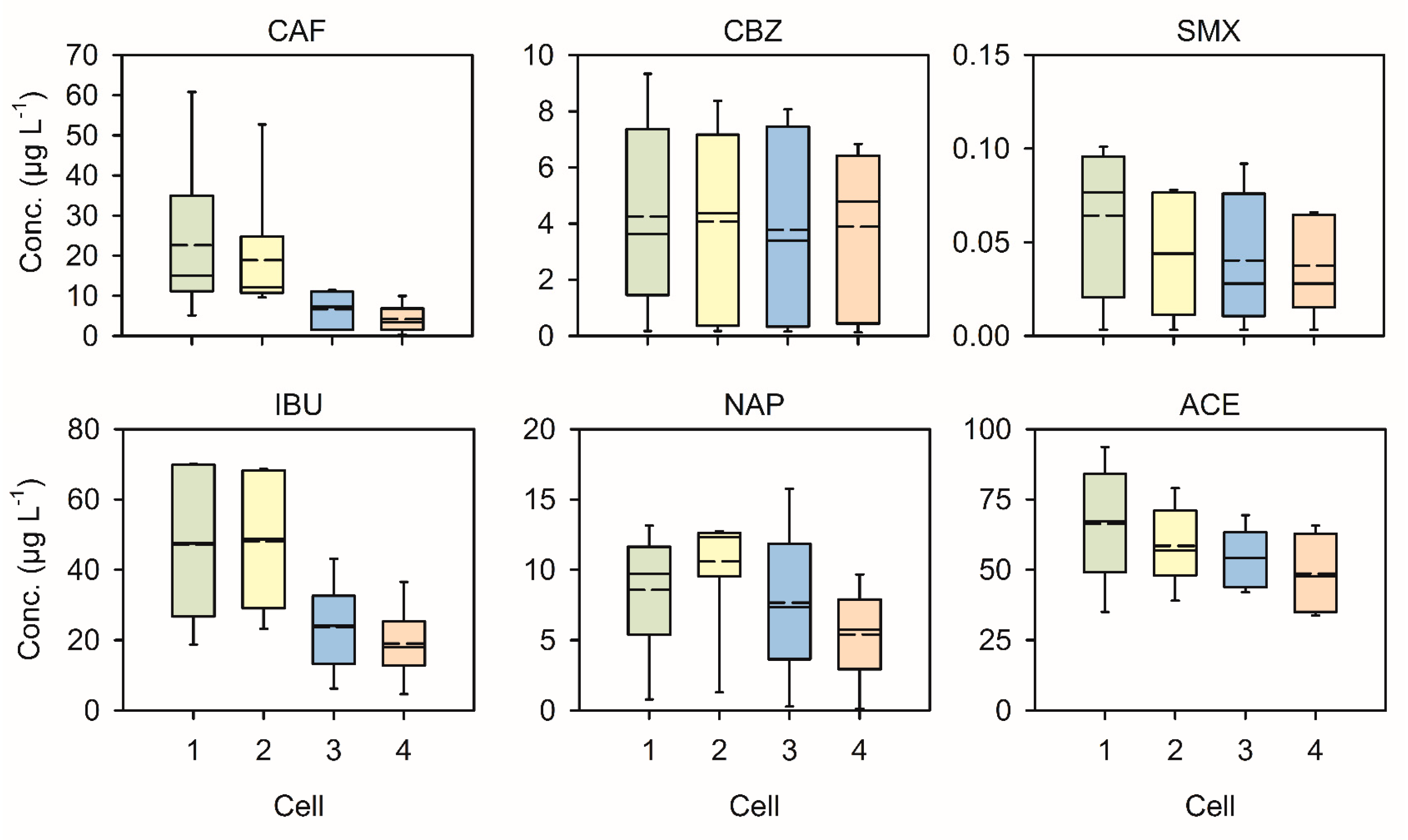
3.3.1. Analgesic Anti-Inflammatory Drugs
3.3.2. Caffeine
3.3.3. Anti-Epileptic Drug
3.3.4. Sulfonamide
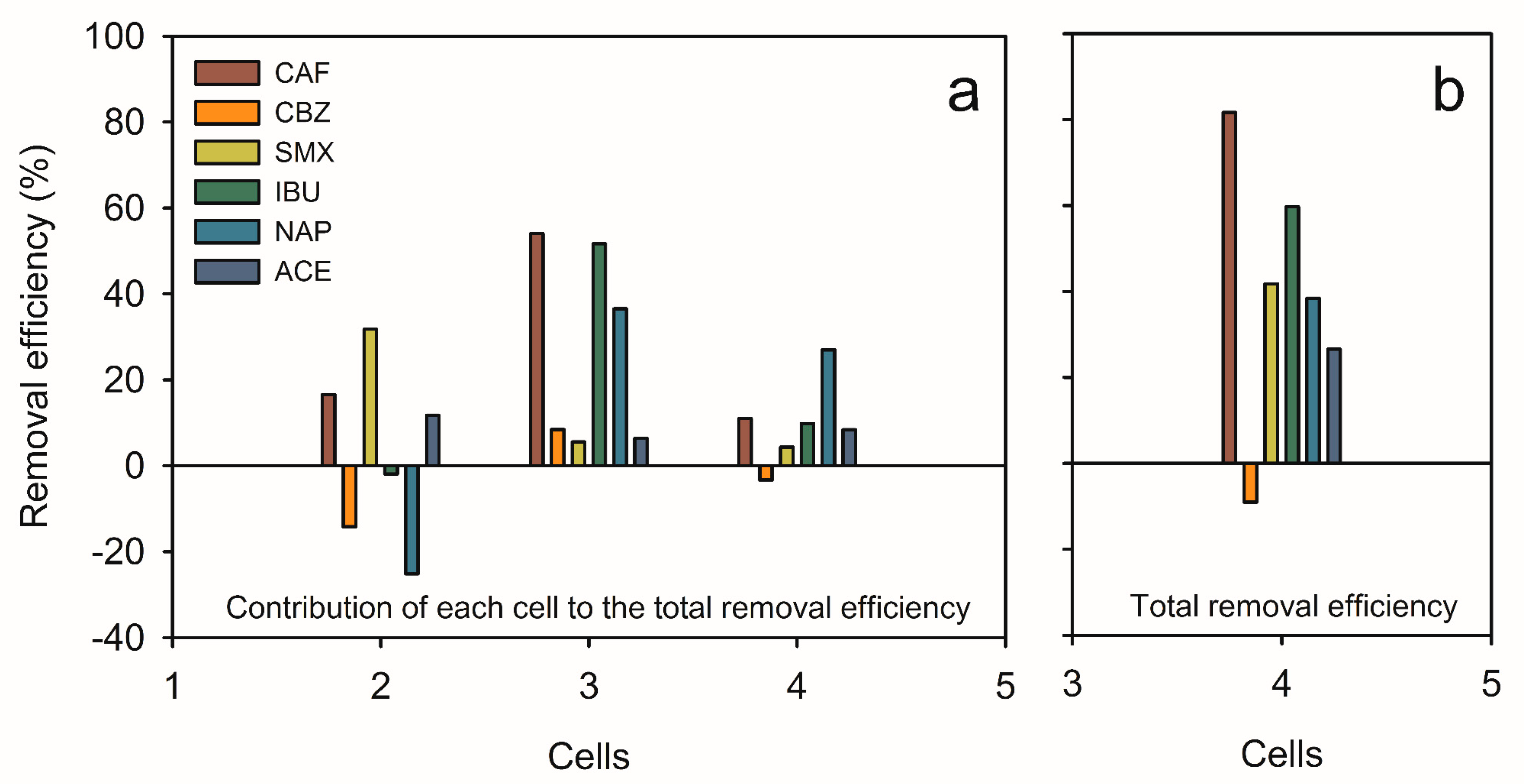
3.3.5. Summary of PhAc Removal Efficiency
3.4. Artificial Sweetener
3.5. Removal Mechanisms
3.5.1. Sorption
3.5.2. Hydrophobic Interactions
3.5.3. Aeration
3.5.4. Biodegradation
3.5.5. Photodegradation
3.5.6. Reduction through ZVI
3.5.7. Plant-Mediated Phytodegradation
3.5.8. Other Potential Removal Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Daughton, C.D. Cradle-to-cradle stewardship of drugs for minimizing their environmental disposition while promoting human health. II. Drug disposal, waste reduction, and future directions. Environ. Health Perspect. 2003, 111, 775–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khetan, S.K.; Collins, T.J. Human pharmaceuticals in the aquatic environment: A challenge to green chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, L.; Beck, A.; Duenk, P.W.; Kleywegt, S.; Lapen, D.R.; Li, H.; Metcalfe, C.D.; Payne, M.; Topp, E. Runoff of pharmaceuticals and personal care products following application of dewatered municipal biosolids to an agricultural field. Sci. Total Environ. 2009, 407, 4596–4604. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacher, F.; Lange, F.T.; Brauch, H.; Blankenhorn, I. Pharmaceuticals in groundwaters: Analytical methods and results of a monitoring program in Baden-Württemberg, Germany. J. Chromatogr. A 2001, 938, 199–210. [Google Scholar] [CrossRef]
- Ternes, T.A.; Bonerz, M.; Herrmann, N.; Teiser, B.; Andersen, H.R. Irrigation of treated wastewater in Braunschweig, Germany: An option to remove pharmaceuticals and musk fragrances. Chemosphere 2007, 66, 894–904. [Google Scholar] [CrossRef]
- Carrara, C.; Ptacek, C.J.; Robertson, W.D.; Blowes, D.W.; Moncur, M.C.; Sverko, E.; Backus, S. Fate of pharmaceutical and trace organic compounds in three septic system plumes, Ontario, Canada. Environ. Sci. Technol. 2008, 42, 2805–2811. [Google Scholar] [CrossRef]
- Fram, M.S.; Belitz, K. Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. Sci. Total Environ. 2011, 409, 3409–3417. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, H. Presence and risk assessment of pharmaceuticals in surface water and drinking water. Water Sci. Technol. 2011, 63, 2143–2148. [Google Scholar] [CrossRef]
- Anumol, T.; Vijayanandan, A.; Park, M.; Philip, L.; Snyder, S.A. Occurrence and fate of emerging trace organic chemicals in wastewater plants in Chennai, India. Environ. Int. 2016, 92, 33–42. [Google Scholar] [CrossRef]
- Zhao, H.; Cao, Z.; Liu, X.; Zhan, Y.; Zhang, J.; Xiao, X.; Yang, Y.; Zhou, J.; Xu, J. Seasonal variation, flux estimation, and source analysis of dissolved emerging organic contaminants in the Yangtze Estuary, China. Mar. Pollut. Bull. 2017, 125, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, W.; Sremski, W.; Piccini, B.; Palluel, O.; Maillot-Maréchal, E.; Betoulle, S.; Jaffal, A.; Aït-Aïssa, S.; Brion, F.; Thybaud, E.; et al. Adverse effects in wild fish living downstream from pharmaceutical manufacture discharges. Environ. Int. 2011, 37, 1342–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, E.; Hornek-Gausterer, R.; Saçan, M.T. Single and mixture toxicity of pharmaceuticals and chlorophenols to freshwater algae Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2016, 2016. 129, 189–198. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Heberer, T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. 2002, 266, 175–189. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Miao, X.; Koenig, B.G.; Struger, J. Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower Great Lakes, Canada. Environ. Toxicol. Chem. 2003, 22, 2881–2889. [Google Scholar] [CrossRef]
- Bendz, D.; Paxéus, N.A.; Ginn, T.R.; Loge, F.J. Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Höje River in Sweden. J. Hazard. Mater. 2005, 122, 195–204. [Google Scholar] [CrossRef]
- Dordio, A.; Carvalho, A.J.P.; Teixeira, D.M.; Dias, C.B.; Pinto, A.P. Removal of pharmaceuticals in microcosm constructed wetlands using Typha spp. and LECA. Bioresour. Technol. 2010, 101, 886–892. [Google Scholar] [CrossRef]
- Liu, Y.; Blowes, D.W.; Groza, L.; Sabourin, M.J.; Ptacek, C.J. Acesulfame-K and pharmaceuticals as co-tracers of municipal wastewater in a receiving river. Environ. Sci. Process. Impacts 2014, 16, 2789–2795. [Google Scholar] [CrossRef]
- Tran, N.H.; Gan, J.; Nguyen, V.T.; Chen, H.; You, L.; Zhang, L.; Gin, K.Y.-H. Sorption and biodegradation of artificial sweeteners in activated sludge processes. Bioresour. Technol. 2015, 197, 329–338. [Google Scholar] [CrossRef]
- Buerge, I.J.; Buser, H.; Kahle, M.; Müller, M.D.; Poiger, T. Ubiquitous occurrence of the artificial sweetener acesulfame in the aquatic environment: An ideal chemical marker of domestic wastewater in groundwater. Environ. Sci. Technol. 2009, 43, 4381–4385. [Google Scholar] [CrossRef]
- Van Stempvoort, D.R.; Roy, J.W.; Brown, S.J.; Bickerton, G. Artificial sweeteners as potential tracers in groundwater in urban environments. J. Hydrol. 2011, 401, 126–133. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, K.Y.; Hamm, S.Y.; Kim, M.; Kim, H.K.; Oh, J.E. Occurrence and distribution of pharmaceutical and personal care products, artificial sweeteners, and pesticides in groundwater from an agricultural area in Korea. Sci. Total Environ. 2019, 659, 168–176. [Google Scholar] [CrossRef]
- Scheurer, M.; Brauch, H.; Lange, F.T. Analysis and occurrence of seven artificial sweeteners in German waste water and surface water and in soil aquifer treatment (SAT). Anal. Bioanal. Chem. 2009, 394, 1585–1594. [Google Scholar] [CrossRef]
- Van Stempvoort, D.R.; Robertson, W.D.; Brown, S.J. Artificial sweeteners in a large septic plume. Ground Water Monit. Remediat. 2011, 31, 95–102. [Google Scholar] [CrossRef]
- Toth, J.E.; Rickman, K.A.; Venter, A.R.; Kiddle, J.J.; Mezyk, S.P. Reaction kinetics and efficiencies for the hydroxyl and sulfate radical based oxidation of artificial sweeteners in water. J. Phys. Chem. A 2012, 116, 9819–9824. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; García, J.; Bayona, J.M. Organic micropollutant removal in a full-scale surface flow constructed wetland fed with secondary effluent. Water Res. 2008, 42, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Matamoros, V.; Sidrach-Cardona, R.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Comprehensive assessment of the design configuration of constructed wetlands for the removal of pharmaceuticals and personal care products from urban wastewaters. Water Res. 2010, 44, 3669–3678. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Matamoros, V.; Pedescoll, A.; Martín-Villacorta, J.; Bécares, E.; García, J. Evaluation of primary treatment and loading regimes in the removal of pharmaceuticals and personal care products from urban wastewaters by subsurface-flow constructed wetlands. Int. J. Environ. Anal. Chem. 2011, 91, 632–653. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Matamoros, V.; Sidrach-Cardona, R.; Pedescoll, A.; Martín-Villacorta, J.; García, J. Influence of design, physico-chemical and environmental parameters on pharmaceuticals and fragrances removal by constructed wetlands. Water Sci. Technol. 2011, 63, 2527–2534. [Google Scholar] [CrossRef]
- Reyes-Contreras, C.; Hijosa-Valsero, M.; Sidrach-Cardona, R.; Bayona, J.M.; Bécares, E. Temporal evolution in PPCP removal from urban wastewater by constructed wetlands of different configuration: A medium-term study. Chemosphere 2012, 88, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Nivala, J.; Kahl, S.; Boog, J.; van Afferden, M.; Reemtsma, T.; Muller, R.A. Dynamics of emerging organic contaminant removal in conventional and intensified subsurface flow treatment wetlands. Sci. Total Environ. 2019, 649, 1144–1156. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Matamoros, V.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Assessment of full-scale natural systems for the removal of PPCPs from wastewater in small communities. Water Res. 2010, 44, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Conkle, J.L.; White, J.R.; Metcalfe, C.D. Reduction of pharmaceutically active compounds by a lagoon wetland wastewater treatment system in Southeast Louisiana. Chemosphere 2008, 73, 1741–1748. [Google Scholar] [CrossRef]
- Reyes-Contreras, C.; Matamoros, V.; Ruiz, I.; Soto, M.; Bayona, J.M. Evaluation of PPCPs removal in a combined anaerobic digester-constructed wetland pilot plant treating urban wastewater. Chemosphere 2011, 84, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Tan, S.K.; Gersberg, R.M.; Sadreddini, S.; Zhu, J.; Tuan, N.A. Removal of pharmaceutical compounds in tropical constructed wetlands. Ecol. Eng. 2011, 37, 460–464. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Gersberg, R.M.; Hua, T.; Zhu, J.; Tuan, N.A.; Tan, S.K. Pharmaceutical removal in tropical subsurface flow constructed wetlands at varying hydraulic loading rates. Chemosphere 2012, 87, 273–277. [Google Scholar] [CrossRef]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D.; Al Aukidy, M.; Zambello, E. Removal of selected pharmaceuticals from domestic wastewater in an activated sludge system followed by a horizontal subsurface flow bed-analysis of their respective contributions. Sci. Total Environ. 2013, 454, 411–425. [Google Scholar] [CrossRef]
- Hussain, S.A.; Prasher, S.O. Understanding the sorption of ionophoric pharmaceuticals in a treatment wetland. Wetlands 2011, 31, 563–571. [Google Scholar] [CrossRef]
- Hussain, S.A.; Prasher, S.O.; Patel, R.M. Removal of ionophoric antibiotics in free water surface constructed wetlands. Ecol. Eng. 2012, 41, 13–21. [Google Scholar] [CrossRef]
- Ranieri, E.; Verlicchi, P.; Young, T.M. Paracetamol removal in subsurface flow constructed wetlands. J. Hydrol. 2011, 404, 130–135. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Araújo, J.L.; Mucha, A.P.; Basto, M.C.P.; Almeida, C.M.R. Potential of constructed wetlands microcosms for the removal of veterinary pharmaceuticals from livestock wastewater. Bioresour. Technol. 2013, 134, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, C.; Zheng, J.; Huang, X.; Wang, Z.; Liu, Y. Elimination of veterinary antibiotics and antibiotic resistance genes from swine wastewater in the vertical flow constructed wetlands. Chemosphere 2013, 91, 1088–1093. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A review on removing pharmaceutical contaminants from wastewater by constructed wetlands: Design, performance and mechanism. Sci. Total Environ. 2014, 468, 908–932. [Google Scholar] [CrossRef]
- Dordio, A.V.; Carvalho, A.J.P. Organic xenobiotics removal in constructed wetlands, with emphasis on the importance of the support matrix. J. Hazard Mater. 2013, 252, 272–292. [Google Scholar] [CrossRef] [Green Version]
- Kurwadkar, S.T.; Adams, C.D.; Meyer, M.T.; Kolpin, D.W. Effects of sorbate speciation on sorption of selected sulfonamides in three loamy soils. J. Agric. Food Chem. 2007, 55, 1370–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lertpaitoonpan, W.; Ong, S.K.; Moorman, T.B. Effect of organic carbon and pH on soil sorption of sulfamethazine. Chemosphere 2009, 76, 558–564. [Google Scholar] [CrossRef]
- Sassman, S.A.; Lee, L.S. Sorption and degradation in soils of veterinary ionophore antibiotics: Monensin and lasalocid. Environ. Chem. 2009, 26, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, D.; Bruland, G.L.; Torrance, B.S.; Upchurch, V.G.; MacKay, A.A. pH-dependent ciprofloxacin sorption to soils: Interaction mechanisms and soil factors influencing sorption. Geoderma. 2009, 151, 68–76. [Google Scholar] [CrossRef]
- Baker, M.J.; Blowes, D.W.; Ptacek, C.J. Laboratory development of permeable reactive mixtures for the removal of phosphorus from onsite wastewater disposal systems. Environ. Sci. Technol. 1998, 32, 2308–2316. [Google Scholar] [CrossRef]
- Smyth, D.J.A.; Blowes, D.W.; Ptacek, C.J.; Baker, M.J.; Ford, G.; Foss, S.; Bernstene, E. Removal of phosphate and waterborne pathogens from wastewater effluent using permeable reactive materials. In Proceedings of the 55th Canadian Geotechnical and 3rd Joint IAH-CNC and CGS Groundwater Specialty Conference, Niagara, CA, USA, 20–23 October 2002; pp. 1123–1127. [Google Scholar]
- Kim, E.-H.; Hwang, H.-K.; Yim, S.-B. Phosphorus removal characteristics in hydroxyapatite crystallization using converter slag. J. Environ. Sci. Health A 2006, 41, 2531–2545. [Google Scholar] [CrossRef]
- Bowden, L.I.; Jarvis, A.P.; Younger, P.L.; Johnson, K.L. Phosphorus removal from waste waters using basic oxygen steel slag. Environ. Sci. Technol. 2009, 43, 2476–2481. [Google Scholar] [CrossRef]
- Hussain, S.I.; Blowes, D.W.; Ptacek, C.J.; Olding, D. Phosphorus removal from lake water using basic oxygen furnace slag: System performance and characterization of reaction products. Environ. Eng. Sci. 2014, 31, 631–642. [Google Scholar] [CrossRef]
- Han, C.; Wang, Z.; Yang, H.; Xue, X. Removal kinetics of phosphorus from synthetic wastewater using basic oxygen furnace slag. J. Environ. Sci. 2015, 30, 21–29. [Google Scholar] [CrossRef]
- Hussain, S.I.; Blowes, D.W.; Ptacek, C.J.; Jamieson-Hanes, J.H.; Wootton, B.; Balch, G.; Higgins, J. Mechanism of phosphorus removal in a pilot-scale constructed wetland/BOF slag wastewater treatment system. Environ. Eng. Sci. 2015, 32, 340–352. [Google Scholar]
- Ludwig, R.D.; McGregor, R.G.; Blowes, D.W.; Benner, S.G.; Mountjoy, K. A permeable reactive barrier for treatment of heavy metals. Ground Water 2002, 40, 59–66. [Google Scholar] [CrossRef]
- Puls, R.W.; Blowes, D.W.; Gillham, R.W. Long-term performance monitoring for a permeable reactive barrier at the U.S. Coast Guard Support Center, Elizabeth City, North Carolina. J. Hazard. Mater. 1999, 68, 109–124. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Ptacek, C.J.; Blowes, D.W. Treatment of dissolved perchlorate, nitrate, and sulfate using zero-valent iron and organic carbon. J. Environ. Qual. 2014, 43, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, J.; Ma, L.; Wang, Y.; Liang, M.; Zhang, Z.; Li, L. Iron foam combined ozonation for enhanced treatment of pharmaceutical wastewater. Environ. Res. 2020, 183, 109205. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Blowes, D.W.; Ptacek, C.J.; Groza, L.G. Removal of pharmaceutical compounds, artificial sweeteners, and perfluoroalkyl substances from water using a passive treatment system containing zero-valent iron and biochar. Sci. Total Environ. 2019, 691, 165–177. [Google Scholar] [CrossRef]
- Liu, Y.; Ptacek, C.J.; Baldwin, R.J.; Cooper, J.M.; Blowes, D.W. Application of zero-valent iron coupled with biochar for removal of perfluoroalkyl carboxylic and sulfonic acids from water under ambient environmental conditions. Sci. Total Environ. 2020, 719, 137372. [Google Scholar] [CrossRef]
- Parenky, A.C.; Gevaerd De Souza, N.; Asgari, P.; Jeon, J.; Nadagouda, M.N.; Choi, H. Removal of perfluorooctanesulfonic acid in water by combining zerovalent iron particles with common oxidants. Environ. Eng. Sci. 2020, 37, 472–481. [Google Scholar] [CrossRef]
- Gross, B.; Montgomery-Brown, J.; Naumann, A.; Reinhard, M. Occurrence and fate of pharmaceuticals and alkylphenol ethoxylate metabolites in an effluent-dominated river and wetland. Environ. Toxicol. Chem. 2004, 23, 2074–2083. [Google Scholar] [CrossRef]
- Vanderford, B.J.; Snyder, S.A. Analysis of pharmaceuticals in water by isotope dilution liquid chromatography/tandem mass spectrometry. Environ. Sci. Technol. 2006, 40, 7312–7320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafiej, A.; Pyrzynska, K.; Regan, F. Determination of anti-inflammatory drugs and estrogens in water by HPLC with UV detection. J. Sep. Sci. 2007, 30, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Agrawal, A. Reduction of nitrate and nitrite by iron metal: Implications for ground water remediation. In Proceedings of the 213th ACS National Meeting, San Francisco, CA, USA, 13–17 April 1997; Volume 37, pp. 157–159. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters, 2nd ed.; John Wiley & Sons Ltd.: New York, NY, USA, 1981. [Google Scholar]
- Kadlec, R.H.; Wallace, S. Treatment Wetland, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Vymazal, J. Constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2010, 36, 891–908. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.J.; Schlautman, M.A.; Bilinski, H. Rates of abiotic MnII oxidation by O2: Influence of various multidentate ligands at high pH. Environ. Sci. Technol. 2021, 55, 14426–14435. [Google Scholar] [CrossRef] [PubMed]
- Brix, H.; Arias, C.A. The use of vertical flow constructed wetlands for on-site treatment of domestic wastewater: New Danish guidelines. Ecol. Eng. 2005, 25, 491–500. [Google Scholar] [CrossRef]
- Matamoros, V.; Arias, C.; Brix, H.; Bayona, J.M. Removal of pharmaceuticals and personal care products (PPCPs) from urban wastewater in a pilot vertical flow constructed wetland and a sand filter. Environ. Sci. Technol. 2007, 41, 8171–8177. [Google Scholar] [CrossRef]
- Lancheros, J.C.; Madera-Parra, C.A.; Caselles-Osorio, A.; Torres-López, W.A.; Vargas-Ramírez, X.M. Ibuprofen and naproxen removal from domestic wastewater using a horizontal subsurface flow constructed wetland coupled to ozonation. Ecol. Eng. 2019, 135, 89–97. [Google Scholar] [CrossRef]
- de Oliveira, M.; Atalla, A.A.; Frihling, B.E.F.; Cavalheri, P.S.; Migliolo, L.; Filho, F.J.C.M. Ibuprofen and caffeine removal in vertical flow and free-floating macrophyte constructed wetlands with Heliconia rostrata and Eichornia crassipes. Chem. Eng. J. 2019, 373, 458–467. [Google Scholar] [CrossRef]
- Matamoros, V.; Uggetti, E.; García, J.; Bayona, J.M. Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater: A laboratory scale study. J. Hazard. Mater. 2016, 301, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Matamoros, V.; Bayona, J.M. Elimination of pharmaceuticals and personal care products in subsurface flow constructed wetlands. Environ. Sci. Technol. 2006, 40, 5811–5816. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Caselles-Osorio, A.; García, J.; Bayona, J.M. Behaviour of pharmaceutical products and biodegradation intermediates in horizontal subsurface flow constructed wetland. A microcosm experiment. Sci. Total Environ. 2008, 394, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Brownawell, B.J. Microbial degradation of pharmaceuticals in estuarine and coastal seawater. Environ. Pollut. 2009, 157, 994–1002. [Google Scholar] [CrossRef]
- Suarez, S.; Lema, J.M.; Omil, F. Removal of pharmaceutical and personal care products (PPCPs) under nitrifying and denitrifying conditions. Water Res. 2010, 44, 3214–3224. [Google Scholar] [CrossRef]
- Alexy, R.; Kümpel, T.; Kümmerer, K. Assessment of degradation of 18 antibiotics in the Closed Bottle Test. Chemosphere 2004, 57, 505–512. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Daschner, F.D.; Kummerer, K. Biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G, and sulfamethoxazole and inhibition of waste water bacteria. Arch. Environ. Contam. Toxicol. 1999, 37, 158–163. [Google Scholar] [CrossRef]
- Batt, A.L.; Kim, S.; Aga, D.S. Comparison of the occurrence of antibiotics in full-scale wastewater treatment plants with varying designs and operations. Chemosphere 2007, 68, 428–435. [Google Scholar] [CrossRef]
- Hanawa, T.; Kon, M.; Doi, H.; Ukai, H.; Murakami, K.; Hamanaka, H.; Asaoka, K. Amount of hydroxyl radical on calcium-ion-implanted titanium and point of zero charge of constituent oxide of the surface-modified layer. J. Mater. Sci. Mater. Med. 1998, 9, 89–92. [Google Scholar] [CrossRef]
- Parks, G.A. The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Davis, J.A.; Kent, D.B. Surface complexation modeling in aqueous geochemistry. Rev. Mineral 1990, 23, 177–260. [Google Scholar]
- Wang, Y.; Cai, Z.; Sheng, S.; Pan, F.; Chen, F.; Fu, J. Comprehensive evaluation of substrate materials for contaminants removal in constructed wetlands. Sci. Total Environ. 2020, 701, 134736. [Google Scholar] [CrossRef] [PubMed]
- Ney, R.E. Where did that Chemical Go: A Practical Guide to Chemical Fate and Transport in the Environment. Nostrand Reinhold 1990, 192, 192. [Google Scholar]
- Verliefde, A.R.D.; Heijman, S.G.J.; Cornelissen, E.R.; Amy, G.L.; Van Der Bruggen, B.; Van Dijk, J.C. Rejection of trace organic pollutants with high pressure membranes (NF/RO). Environ. Prog. 2008, 27, 180–188. [Google Scholar] [CrossRef]
- Amiard-Triquet, C.; Rainbow, P.S.; Roméo, M. Tolerance to environmental contaminants. In Environmental and Ecological Risk Assessment; Newman, M.C., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 299–332. [Google Scholar]
- Nghiem, L.D.; Hawkes, S. Effects of membrane fouling on the nanofiltration of trace organic contaminants. Desalination 2009, 236, 273–281. [Google Scholar] [CrossRef]
- Stuer-Lauridsen, F.; Birkved, M.; Hansen, L.P.; Holten Lützhøft, H.; Halling-Sørensen, B. Environmental risk assessment of human pharmaceuticals in Denmark after normal therapeutic use. Chemosphere 2000, 40, 783–793. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M. Calculation methods to perform mass balances of micropollutants in sewage treatment plants. Application to pharmaceutical and personal care products (PPCPs). Environ. Sci. Technol. 2007, 41, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Al-Baldawi, I.A.; Mohammed, A.A.; Mutar, Z.H.; Abdullah, S.R.S.; Jasim, S.S.; Almansoory, A.F.; Ismail, N.I. Application of phytotechnology in alleviating pharmaceuticals and personal care products (PPCPs) in wastewater: Source, impacts, treatment, mechanisms, fate, and SWOT analysis. J. Clean. Prod. 2021, 319, 128584. [Google Scholar] [CrossRef]
- Kahl, S.; Nivala, J.; van Afferden, M.; Müller, R.A.; Reemtsma, T. Effect of design and operational conditions on the performance of subsurface flow treatment wetlands: Emerging organic contaminants as indicators. Water Res. 2017, 125, 490–500. [Google Scholar] [CrossRef]
- Petrie, B.; Rood, S.; Smith, B.D.; Proctor, K.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Biotic phase micropollutant distribution in horizontal sub-surface flow constructed wetlands. Sci. Total Environ. 2018, 630, 648–657. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, T.; Zhang, Y.; Stein, O.R.; Arias, C.A.; Brix, H.; Carvalho, P.N. Effects of constructed wetland design on ibuprofen removal–A mesocosm scale study. Sci. Total Environ. 2017, 609, 38–45. [Google Scholar] [CrossRef]
- Chen, J.; Tong, T.; Jiang, X.; Xie, S. Biodegradation of sulfonamides in both oxic and anoxic zones of vertical flow constructed wetland and the potential degraders. Environ. Pollut. 2020, 265, 115040. [Google Scholar] [CrossRef] [PubMed]
- Hijosa-Valsero, M.; Fink, G.; Schlüsener, M.P.; Sidrach-Cardona, R.; Martín-Villacorta, J.; Ternes, T. Removal of antibiotics from urban wastewater by constructed wetland optimization. Chemosphere 2011, 83, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Hua, T.; Gersberg, R.M.; Zhu, J.; Ng, W.J.; Tan, S.K. Carbamazepine and naproxen: Fate in wetland mesocosms planted with Scirpus validus. Chemosphere 2013, 91, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.W.; Young, C.J.; Brain, R.J.; Johnson, D.J.; Hanson, M.A.; Wilson, C.J.; Richards, S.M.; Solomon, K.R.; Mabury, S.A. Aquatic persistence of eight pharmaceuticals in a microcosm study. Environ. Toxicol. Chem. 2004, 23, 1431–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tixier, C.; Singer, H.P.; Canonica, S.; Müller, S.R. Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ. Sci. Technol. 2002, 36, 3482–3489. [Google Scholar] [CrossRef]
- Li, A.J.; Schmitz, O.J.; Stephan, S.; Lenzen, C.; Yue, P.Y.-K.; Li, K.; Li, H.; Leun, K.S.-Y. Photocatalytic transformation of acesulfame: Transformation products identification and embryotoxicity study. Water Res. 2016, 89, 68–75. [Google Scholar] [CrossRef]
- Blowes, D.W.; Ptacek, C.J.; Benner, S.G.; McRae, C.W.T.; Bennett, T.A.; Puls, R.W. Treatment of inorganic contaminants using permeable reactive barriers. J. Contam. Hydrol. 2000, 45, 123–137. [Google Scholar] [CrossRef]
- Morrison, S.J.; Metzler, D.R.; Dwyer, B.P. Removal of As, Mn, Mo, Se, U, V and Zn from groundwater by zero-valent iron in a passive treatment cell: Reaction progress modeling. J. Contam. Hydrol. 2002, 56, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A.; Tuqan, A.; Assi, H.A. Antibiotic removal from water: Elimination of amoxicillin and ampicillin by microscale and nanoscale iron particles. Environ. Pollut. 2009, 157, 1626–1635. [Google Scholar] [CrossRef]
- Bautitz, I.R.; Velosa, A.C.; Nogueira, R.F.P. Zero valent iron mediated degradation of the pharmaceutical diazepam. Chemosphere 2012, 88, 688–692. [Google Scholar] [CrossRef] [Green Version]
- Reardon, E.J. Zerovalent irons: Styles of corrosion and inorganic control on hydrogen pressure buildup. Environ. Sci. Technol. 2005, 39, 7311–7317. [Google Scholar] [CrossRef]
- Zhang, W.-X. Nanoscale iron particles for environmental remediation: An overview. J. Nanopart. Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Wei, Y.-T.; Wu, S.-C.; Chou, C.-M.; Che, C.-H.; Tsai, S.-M.; Lien, H.-L. Influence of nanoscale zero-valent iron on geochemical properties of groundwater and vinyl chloride degradation: A field case study. Water Res. 2010, 44, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.R.S.; Al-Baldawi, I.A.; Almansoory, A.F.; Purwanti, I.F.; Al-Sbani, N.H.; Sharuddin, S.S.N. Plant-assisted remediation of hydrocarbons in water and soil: Application, mechanisms, challenges and opportunities. Chemosphere 2020, 247, 125932. [Google Scholar] [CrossRef]
- Zhang, X.; Jing, R.; Feng, X.; Dai, Y.; Tao, R.; Vymazal, J.; Cai, N.; Yang, Y. Removal of acidic pharmaceuticals by small-scale constructed wetlands using different design configurations. Sci. Total Environ. 2018, 639, 640–647. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.C.; Zheng, Y.; Dzakpasu, M. Removal of pharmaceutical active compounds in wastewater by constructed wetlands: Performance and mechanisms. J. Environ. Manag. 2023, 325, 116478. [Google Scholar] [CrossRef]
- He, Y.; Langenhoff, A.A.M.; Sutton, N.B.; Rijnaarts, H.H.M.; Blokland, M.H.; Chen, F.; Huber, C.; Schroder, P. Metabolism of ibuprofen by Phragmites australis: Uptake and phytodegradation. Environ. Sci. Technol. 2017, 51, 4576–4584. [Google Scholar] [CrossRef]
- Gonz’alez, M.; Fern’andez-l´opez, C.; Pedrero-salcedo, F.; Jose, J. Absorption of carbamazepine and diclofenac in hydroponically cultivated lettuces and human health risk assessment. Agric. Water Manag. 2018, 206, 42–47. [Google Scholar] [CrossRef]
- Dordio, A.V.; Duarte, C.; Barreiros, M.; Carvalho, A.J.P.; Pinto, A.P.; da Costa, C.T. Toxicity and removal efficiency of pharmaceutical metabolite clofibric acid by Typha spp.–Potential use for phytoremediation? Bioresour. Technol. 2009, 100, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Lyman, W.J.; Reehl, W.; Rosenblatt, D. Handbook of Chemical Property Estimation Methods. Am. Chem. Soc. 1990, 4, 15–29. [Google Scholar]


| Cell ID | Cell Type | Cell Description | Cell Volume (m3) | Media Volume (m3) | Porosity (θ) | Hydraulic Retention Time (HRT) (Day) |
|---|---|---|---|---|---|---|
| Cell one | Pre-treatment septic tank effluent chamber | The influent of the treatment system, wastewater, was received from this pre-treatment septic tank and periodically pumped to cell two | 1 | - | - | - |
| Cell two | Subsurface flow constructed wetland (HSSF CW Cell) | Filled with granitic gravel and vegetated with cattails (Typha spp.) | 24 | 19.4 | 0.35 | 7.64 |
| Cell three | Subsurface flow aerated constructed wetland (Aerated VSSF CW Cell) | Filled with granitic gravel and vegetated with cattails (Typha spp.) | 24.2 | 19.3 | 0.35 | 7.61 |
| Cell four | Downward vertical flow cell (BOFS Cell) | Filled with BOFS and ZVI to remove phosphate. BOFS media were covered by a granitic gravel layer and then a plastic trap and a sand layer to avoid atmospheric oxygen ingress. A sacrificial BOFS chamber was placed before cell four to prevent the formation of a CaCO3 scale around the cell four inlet | 27.4 | 13.3 | 0.4 | 5.97 |
| Cell five | pH-adjustment unit | Equipped with a CO2 sparger and an adjusted pH between 6.5 and 8.5 before releasing into the City of Kawartha Lakes sewer system | 2 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.I.; Ptacek, C.J.; Blowes, D.W.; Liu, Y.; Wootton, B.C.; Balch, G.; Higgins, J. Transport and Attenuation of an Artificial Sweetener and Six Pharmaceutical Compounds in a Sequenced Wetland-Steel Slag Wastewater Treatment System. Water 2023, 15, 2835. https://doi.org/10.3390/w15152835
Hussain SI, Ptacek CJ, Blowes DW, Liu Y, Wootton BC, Balch G, Higgins J. Transport and Attenuation of an Artificial Sweetener and Six Pharmaceutical Compounds in a Sequenced Wetland-Steel Slag Wastewater Treatment System. Water. 2023; 15(15):2835. https://doi.org/10.3390/w15152835
Chicago/Turabian StyleHussain, Syed I., Carol J. Ptacek, David W. Blowes, YingYing Liu, Brent C. Wootton, Gordon Balch, and James Higgins. 2023. "Transport and Attenuation of an Artificial Sweetener and Six Pharmaceutical Compounds in a Sequenced Wetland-Steel Slag Wastewater Treatment System" Water 15, no. 15: 2835. https://doi.org/10.3390/w15152835
APA StyleHussain, S. I., Ptacek, C. J., Blowes, D. W., Liu, Y., Wootton, B. C., Balch, G., & Higgins, J. (2023). Transport and Attenuation of an Artificial Sweetener and Six Pharmaceutical Compounds in a Sequenced Wetland-Steel Slag Wastewater Treatment System. Water, 15(15), 2835. https://doi.org/10.3390/w15152835







