Meandering Flow Filter for Phosphorus Removal as a Component of Small Wastewater Treatment Plants—A Case Study
Abstract
:1. Introduction
2. Materials and Research Methods
2.1. Characteristics of Rockfos® Material
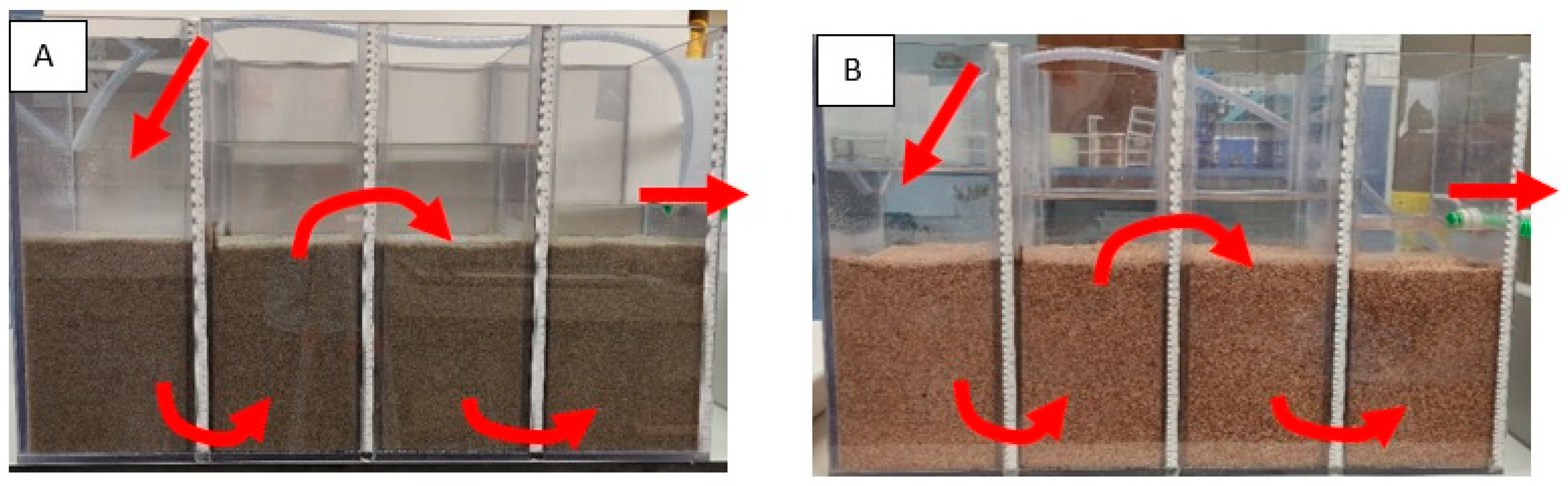
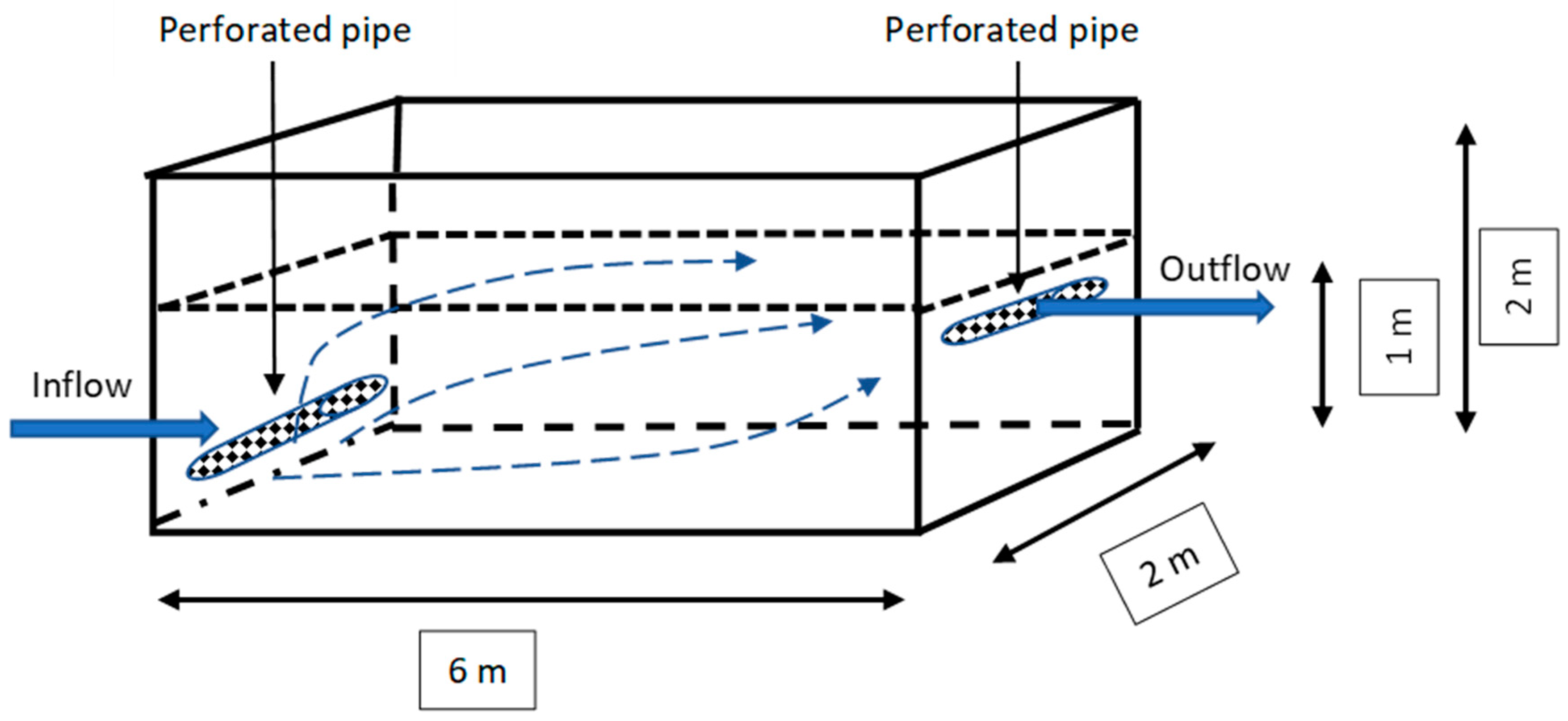
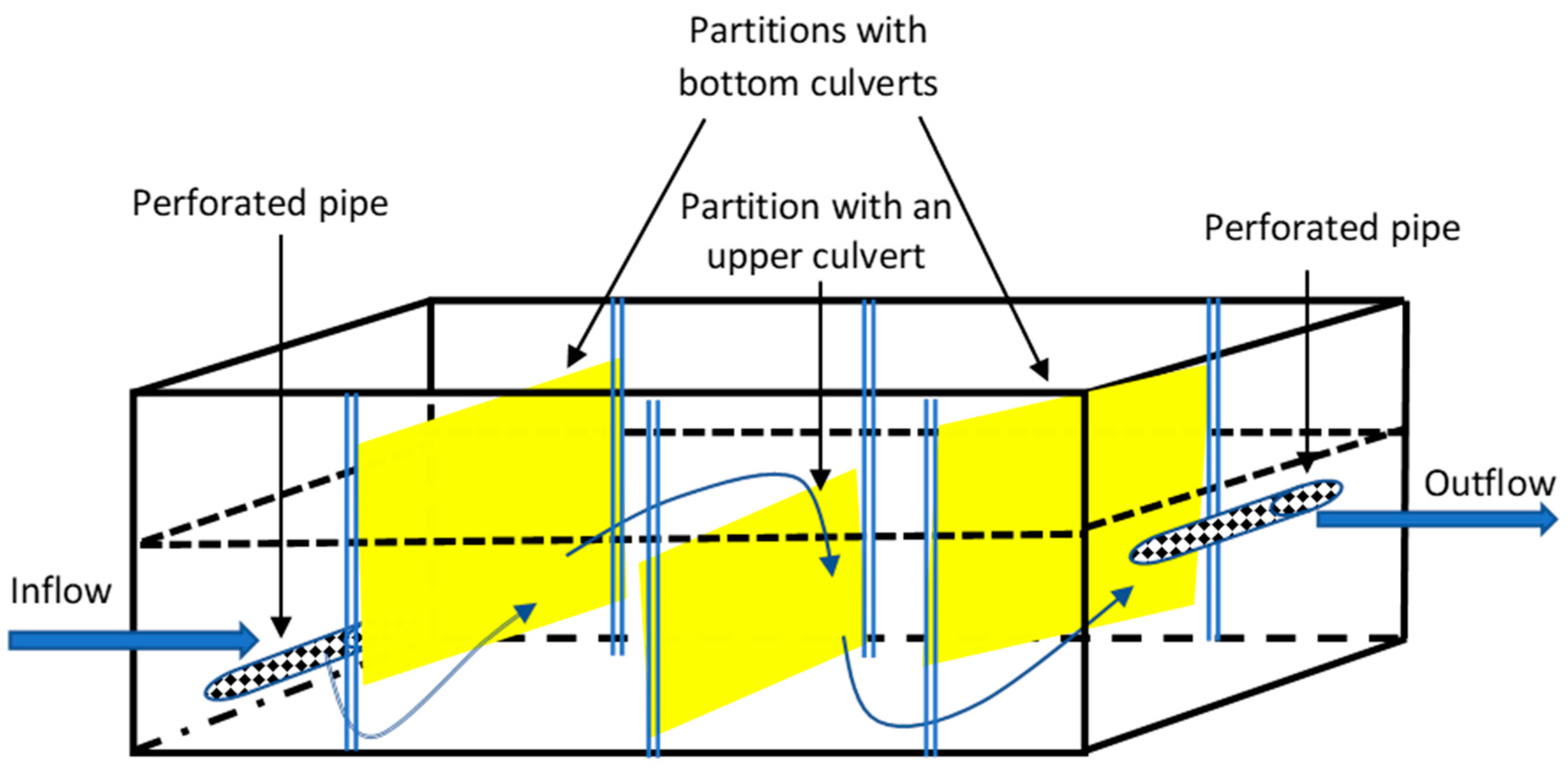
2.2. Construction of the Laboratory Model
2.3. Laboratory Model Testing Methodology
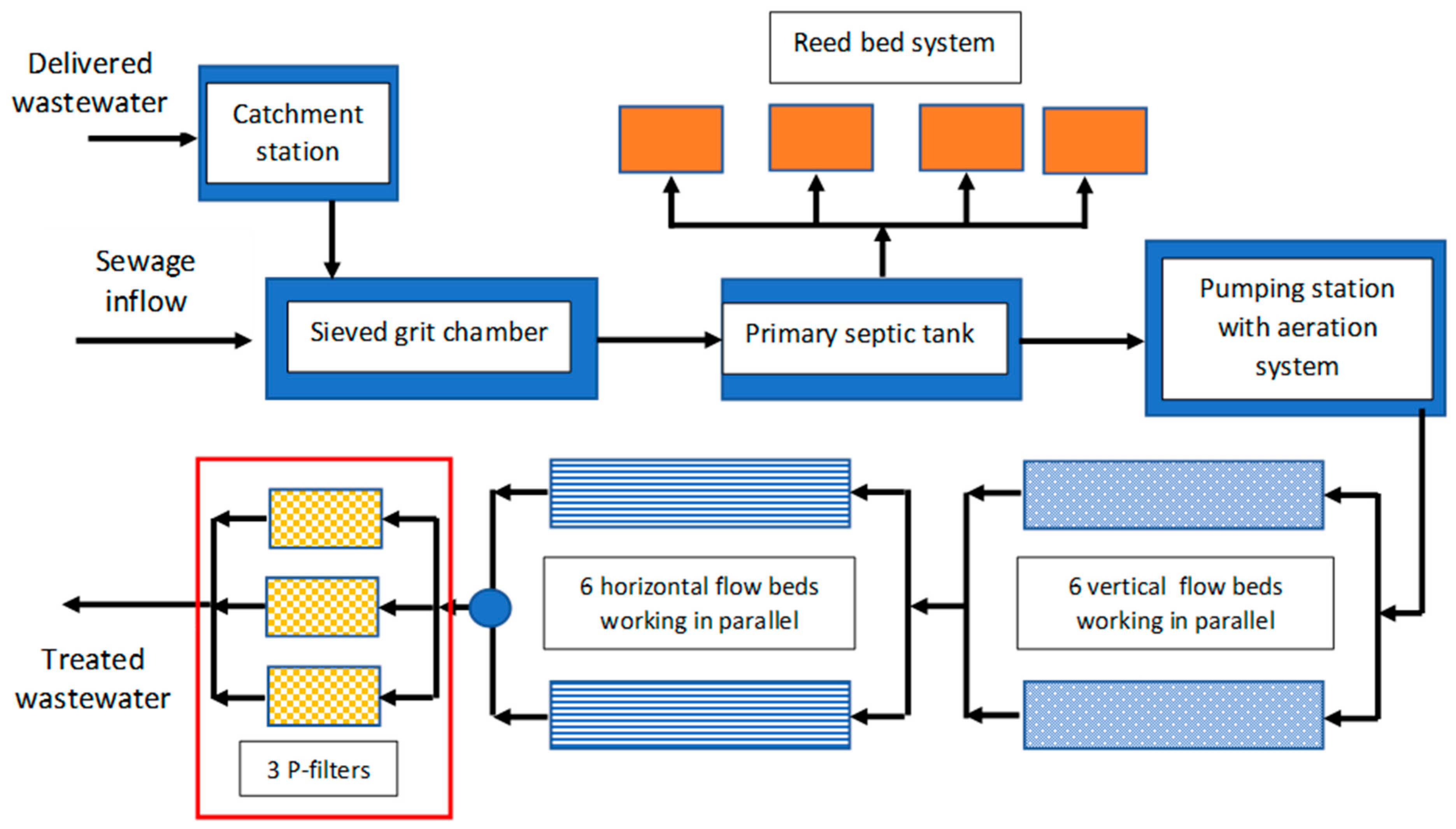
2.4. Construction of a Full-Scale Technical Filter
2.5. Analytical Methods and Statistical Analysis
3. Results and Discussion
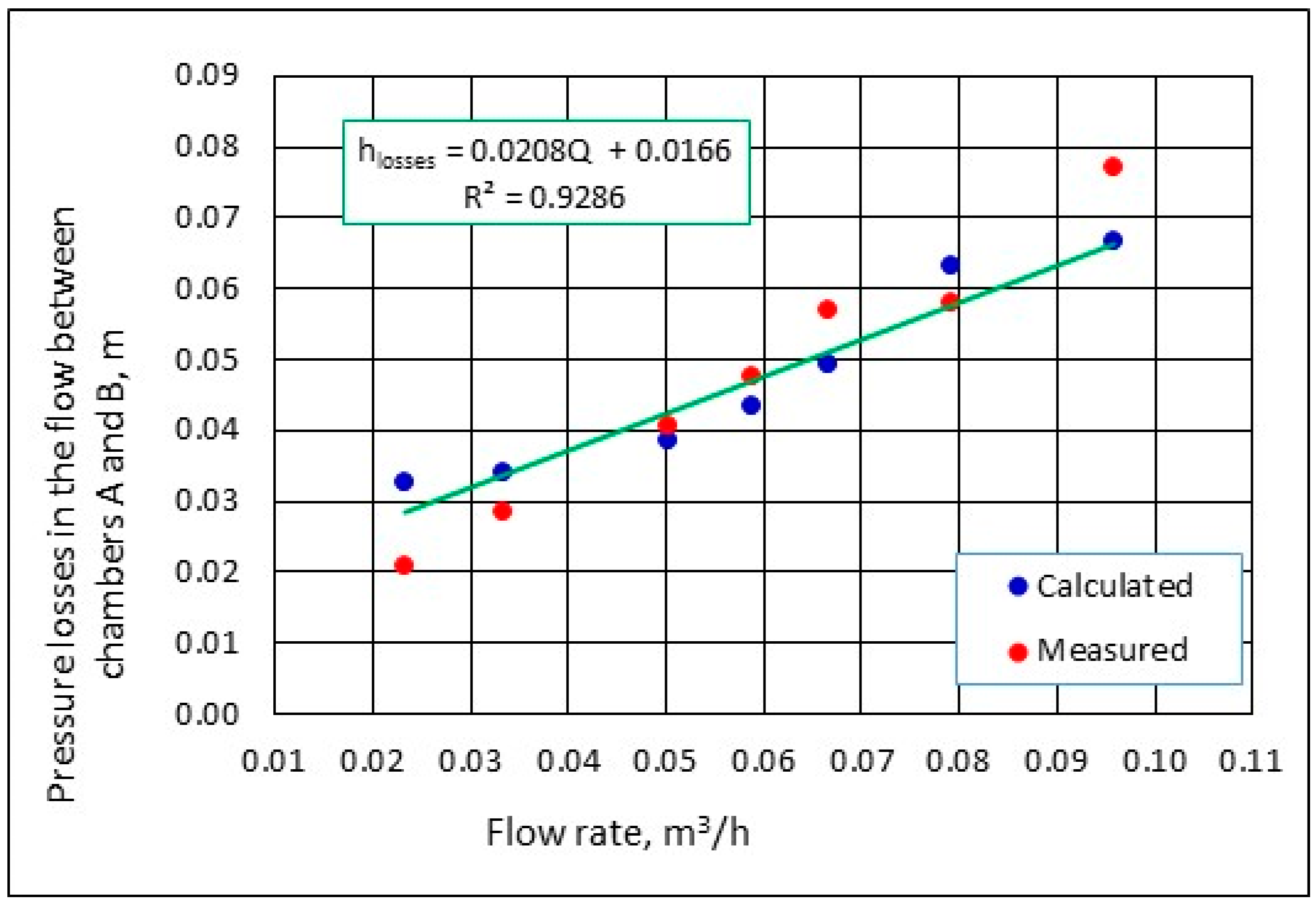
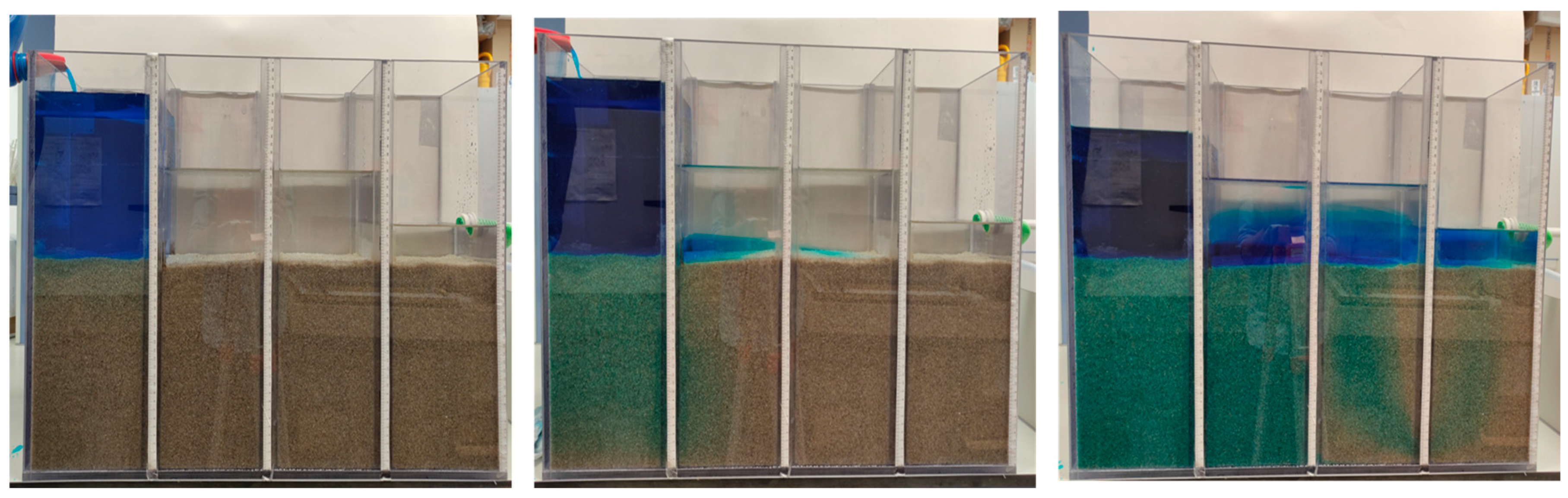
3.1. Hydraulic Flow Conditions through the Bed in a Laboratory Model
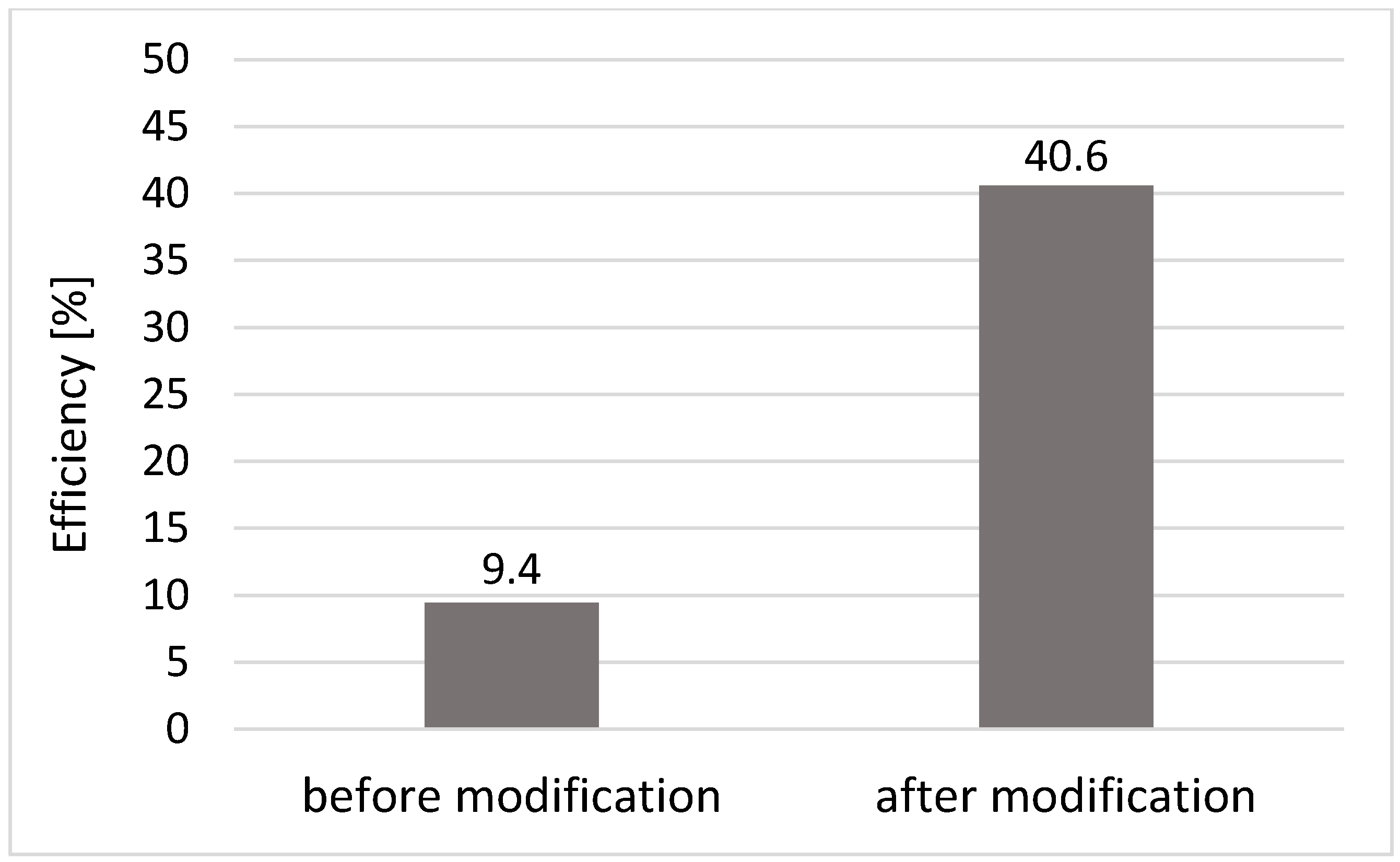
3.2. Efficiency of Phosphorus Removal in a Full-Scale Technical Filter
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and recovery of phosphate from water using sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Zamparas, M.; Gianni, A.; Stathi, P.; Deligiannakis, Y.; Zacharias, I. Removal of phosphate from natural waters using innovative modified bentonites. Appl. Clay Sci. 2012, 62, 101–106. [Google Scholar] [CrossRef]
- Hussain, S.; Aziz, H.A.; Isa, M.H.; Ahmad, A.; Van Leeuwen, J.; Zou, L.; Beecham, S.; Umar, M. Orthophosphate removal from domestic wastewater using limestone and granular activated carbon. Desalination 2011, 271, 265–272. [Google Scholar] [CrossRef]
- Kasprzyk, M.; Czerwionka, K.; Gajewska, M. Waste materials assessment for phosphorus adsorption toward sustainable application in circular economy. Resour. Conserv. Recycl. 2021, 168, 105335. [Google Scholar] [CrossRef]
- Xie, J.; Lin, Y.; Li, C.; Wu, D.; Kong, H. Removal and recovery of phosphate from water by activated aluminum oxide and lanthanum oxide. Powder Technol. 2015, 269, 351–357. [Google Scholar] [CrossRef]
- Rayshouni, H.; Wazne, M. Effects of Calcium on the Removal of Ammonium from Aged Landfill Leachate by Struvite Precipitation. Water 2022, 14, 1933. [Google Scholar] [CrossRef]
- Soares, A.; Czajkowska, J.; Colprim, J.; Gali, A.; Johansson, S.; Masic, A.; March, A.; McMeod, A.; Nenov, V.; Ruscalleda, M. Nutrients Recovery from Wastewater Streams; IWA Publishing: London, UK, 2017. [Google Scholar]
- Kasprzyk, M.; Gajewska, M. Phosphorus removal by application of natural and semi-natural materials for possible recovery according to assumptions of circular economy and closed circuit of P. Sci. Total Environ. 2019, 650, 249–256. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhao, J. Phosphate recovery from wastewater using calcium silicate hydrate (CSH): Sonochemical synthesis and properties. Environ. Sci. Water Res. Technol. 2019, 5, 131–139. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Life’s bottleneck: Sustaining the world’s phosphorus for a food secure future. Annu. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Jucherski, A.; Nastawny, M.; Walczowski, A.; Jóźwiakowski, K.; Gajewska, M. Badania przydatności alkalicznych materiałów filtracyjnych do usuwania fosforanów z biologicznie oczyszczonych ścieków bytowych. Ochr. Sr. 2017, 39, 33–38. [Google Scholar]
- Jucherski, A.; Walczowski, A.; Bugajski, P.; Jóźwiakowski, K.; Rodziewicz, J.; Janczukowicz, W.; Wu, S.; Kasprzyk, M.; Gajewska, M.; Mielcarek, A. Long-term operating conditions for different sorption materials to capture phosphate from domestic wastewater. Sustain. Mater. Technol. 2022, 31, e00385. [Google Scholar] [CrossRef]
- Letshwenyo, M.W.; Mokokwe, G. Phosphorus and sulphates removal from wastewater using copper smelter slag washed with acid. SN Appl. Sci. 2021, 3, 854. [Google Scholar] [CrossRef]
- Westholm, L.J. The use of blast furnace slag for removal of phosphorus from wastewater in Sweden—A review. Water 2010, 2, 826–837. [Google Scholar] [CrossRef] [Green Version]
- ÁdÁm, K.; Sovik, A.; Krogstad, T.; Heistad, A. Phosphorous removal by the filter materials light-weight aggregates and shellsand-a review of processes and experimental set-ups for improved design of filter systems for wastewater treatment. Vatten 2007, 63, 245. [Google Scholar]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, Ü. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Gubernat, S.; Masłoń, A.; Czarnota, J.; Koszelnik, P. Reactive materials in the removal of phosphorus compounds from wastewater—A review. Materials 2020, 13, 3377. [Google Scholar] [CrossRef] [PubMed]
- Pytka-Woszczyło, A.; Różańska-Boczula, M.; Gizińska-Górna, M.; Marzec, M.; Listosz, A.; Jóźwiakowski, K. Efficiency of filters filled with rockfos for phosphorus removal from domestic sewage. Adv. Sci. Technol. Res. J. 2022, 16, 176–188. [Google Scholar] [CrossRef]
- Grace, M.A.; Clifford, E.; Healy, M.G. The potential for the use of waste products from a variety of sectors in water treatment processes. J. Clean. Prod. 2016, 137, 788–802. [Google Scholar] [CrossRef] [Green Version]
- Penn, C.; Chagas, I.; Klimeski, A.; Lyngsie, G. A Review of Phosphorus Removal Structures: How to Assess and Compare Their Performance. Water 2017, 9, 583. [Google Scholar] [CrossRef]
- Ezzati, G.; Healy, M.G.; Christianson, L.; Feyereisen, G.; Thornton, S.; Daly, K.; Fenton, O. Developing and validating a decision support tool for media selection to mitigate drainage waters. Ecol. Eng. 2019, 142, 100010. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.M.; Santos, S.C.; Boaventura, R.A.; Botelho, C.M. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Bus, A.; Karczmarczyk, A. Charakterystyka skały wapienno-krzemionkowej opoki w aspekcie jej wykorzystania jako materiału reaktywnego do usuwania fosforu z wód i ścieków. Infrastrukt. I Ekol. Teren. Wiej. 2014, II/1, 227–238. [Google Scholar]
- Brogowski, Z.; Renman, G. Characterization of opoka as a basis for its use in wastewater treatment. Pol. J. Environ. Stud. 2004, 13, 15–20. [Google Scholar]
- Jóźwiakowski, K.; Gajewska, M.; Pytka, A.; Marzec, M.; Gizińska-Górna, M.; Jucherski, A.; Walczowski, A.; Nastawny, M.; Kamińska, A.; Baran, S. Influence of the particle size of carbonate-siliceous rock on the efficiency of phosphorous removal from domestic wastewater. Ecol. Eng. 2017, 98, 290–296. [Google Scholar] [CrossRef]
- Pinińska, J. Właściwości geomechaniczne opok. Górnictwo I Geoinżynieria 2008, 32, 293–301. [Google Scholar]
- Cucarella, V.; Zaleski, T.; Mazurek, R. Phosphorus sorption capacity of different types of opoka. Ann. Wars. Univ. Life Sci.-SGGW Land Reclam. 2007, 38, 11–18. [Google Scholar] [CrossRef]
- Nilsson, C.; Renman, G.; Westholm, L.J.; Renman, A.; Drizo, A. Effect of organic load on phosphorus and bacteria removal from wastewater using alkaline filter materials. Water Res. 2013, 47, 6289–6297. [Google Scholar] [CrossRef] [Green Version]
- Jóźwiakowski, K.; Marzec, M.; Gizińska-Górna, M.; Pytka, A.; Skwarzyńska, A.; Słowik, T.; Kowalczyk-Juśko, A.; Gajewska, M.; Steszuk, A.; Grabowski, T. The Concept of Construction of Hybrid Constructed Wetland for Wastewater Treatment in Roztocze National Park. Barom. Reg. Anal. I Prognozy 2014, 12, 91–102. [Google Scholar] [CrossRef]
- Jóźwiakowski, K.; Gajewska, M.; Marzec, M.; Gizińska-Górna, M.; Pytka, A.; Kowalczyk-Juśko, A.; Sosnowska, B.; Baran, S.; Malik, A.; Kufel, R. Hybrid constructed wetlands for the national parks in Poland–The case study, requirements, dimensioning and preliminary results. In Natural and Constructed Wetlands: Nutrients, Heavy Metals and Energy Cycling, and Flow; Springer: Berlin/Heidelberg, Germany, 2016; pp. 247–265. [Google Scholar]
- Jóźwiakowski, K.; Marzec, M.; Kowalczyk-Juśko, A.; Gizińska-Górna, M.; Pytka-Woszczyło, A.; Malik, A.; Listosz, A.; Gajewska, M. 25 years of research and experiences about the application of constructed wetlands in southeastern Poland. Ecol. Eng. 2019, 127, 440–453. [Google Scholar] [CrossRef]
- Available online: http://www.ceramika-kufel.pl/rockfos/ (accessed on 20 May 2023).
- Siwiec, T. The sphericity of grains of filtration beds applied for water treatment on examples of selected minerals. Electron. J. Pol. Agric. Univ. Civ. Eng. 2007, 10, #30. [Google Scholar]
- Radoičić, T.K.; Đuriš, M.; Garić-Grulović, R.; Arsenijević, Z.; Grbavčić, Ž. Particle characterization of polydisperse quartz filtration sand. Powder Technol. 2014, 254, 63–71. [Google Scholar] [CrossRef]
- Gibson, I.A.; Slim, C.J.; Zheng, Y.; Scott, S.A.; Davidson, J.F.; Hayhurst, A. An examination of Wen and Yu’s formula for predicting the onset of fluidisation. Chem. Eng. Res. Des. 2018, 135, 103–111. [Google Scholar] [CrossRef]
- Pang, M.; Zhang, T.; Meng, Y.; Ling, Z. Experimental study on the permeability of crushed coal medium based on the Ergun equation. Sci. Rep. 2021, 11, 23030. [Google Scholar] [CrossRef] [PubMed]
- Graciano-Uribe, J.; Pujol, T.; Puig-Bargués, J.; Duran-Ros, M.; Arbat, G.; Cartagena, F. Assessment of Different Pressure Drop-Flow Rate Equations in a Pressurized Porous Media Filter for Irrigation Systems. Water 2021, 13, 2179. [Google Scholar] [CrossRef]
- Cleasby, J.L.; Fan, K.-S. Predicting fluidization and expansion of filter media. J. Environ. Eng. Div. 1981, 107, 455–471. [Google Scholar] [CrossRef]
- Abbas, M.N. Modeling of porosity equation for water flow through packed bed of monosize spherical packing. J. Eng. Sustain. Dev. 2011, 15, 205–226. [Google Scholar]
- PN-ISO 5667-10:2021-11; Water Quality—Sampling—Part 10: Guidance on Waste Water Sampling. ISO: London, UK, 2021.
- PN-EN ISO 6878:2006; Water Quality—Determination of Phosphorus—Spectrometric Method with Ammonium Molybdate. ISO: London, UK, 2006.
- PN-EN ISO 10523:2012; Water Quality—Determination of pH. ISO: London, UK, 2012.
- Yang, T.; Hu, L.; Yu, D.; Xiong, X.; Chvojka, J.; Venkataraman, M.; Petrů, M.; Tomková, B.; Morikawa, H.; Militký, J. Simple determination of key structural parameters for fibrous materials enabled by Ergun-Type and Kozeny-type equations. Polym. Test. 2022, 108, 107514. [Google Scholar] [CrossRef]
- Mucha, J. Metody Geostatystyczne w Dokumentowaniu Złóż; Wydaw. AGH: Kraków, Poland, 1994. [Google Scholar]
- Kasprzyk, M.; Węgler, J.; Gajewska, M. Analysis of efficiency of phosphates sorption by different granulation of selected reactive material. E3S Web Conf. 2018, 26, 00002. [Google Scholar] [CrossRef] [Green Version]
- Albright, M.; Waterfield, H. Evaluating Phosphorus-Removal Media for Use in Onsite Wastewater Treatment Systems (Interim Report). 42nd Ann. Rept. (2009). SUNY Oneonta Biol. Fld. Sta., SUNY Oneonta 2010. Available online: https://oneonta365.sharepoint.com/sites/BFS/Shared%20Documents/Forms/AllItems.aspx?id=%2Fsites%2FBFS%2FShared%20Documents%2FWeb%20Documents%2FAnnual%2DReports%2F2009%2F19%20P%20removal%20report%2Epdf&parent=%2Fsites%2FBFS%2FShared%20Documents%2FWeb%20Documents%2FAnnual%2DReports%2F2009&p=true&ga=1 (accessed on 20 May 2023).
- Karczmarczyk, A.; Woja, K.; Bliska, P.; Baryła, A.; Bus, A. The efficiency of filtration materials (Polonite® and Leca®) supporting phosphorus removal in on site treatment systems with wastewater infiltration. Infrastrukt. Ekol. Teren. Wiej. 2017, IV/1, 1401–1413. [Google Scholar]
- Renman, A.; Renman, G. Long-term phosphate removal by the calcium-silicate material Polonite in wastewater filtration systems. Chemosphere 2010, 79, 659–664. [Google Scholar] [CrossRef]
- Sobieski, W.; Zhang, Q. Sensitivity analysis of Kozeny-Carman and Ergun equations. Technol. Sci./Univ. Warm. Mazury Olszt. 2014, 17, 235–248. [Google Scholar]








| Component | Percentage [% by Weight] |
|---|---|
| CaO | 43.336 |
| SiO2 | 36.047 |
| Al2O3 | 5.932 |
| Na2O | 2.856 |
| Fe | 1.340 |
| MgO | 0.938 |
| TiO2 | 0.960 |
| S | 0.654 |
| K2O | 0.489 |
| P | 0.480 |
| Cl | 0.237 |
| MnO | 0.117 |
| Parameter | Unit | Sand Bed Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Flow rate | m3/h | 0.091 | 0.081 | 0.067 | 0.060 | 0.053 | 0.047 | 0.045 |
| Height “a” | cm | 47.2 | 43.5 | 40.5 | 39.7 | 39.2 | 37.7 | 37.4 |
| Height “b” | cm | 36.3 | 35.1 | 34.0 | 33.5 | 33.2 | 32.5 | 32.4 |
| Height “c” | cm | 29.7 | 29.6 | 29.5 | 29.4 | 29.4 | 29.4 | 29.4 |
| Parameter | Unit | Rockfos® Bed Model | ||||||
| Flow rate | m3/h | 0.096 | 0.079 | 0.067 | 0.059 | 0.050 | 0.033 | 0.023 |
| Height “a” | cm | 47.5 | 43.5 | 42.0 | 40.3 | 38.45 | 35.4 | 33.6 |
| Height “b” | cm | 39.8 | 37.7 | 36.3 | 35.5 | 34.4 | 32.55 | 31.5 |
| Height “c” | cm | 29.85 | 29.8 | 29.6 | 29.6 | 29.45 | 29.3 | 29.2 |
| Statistics Indicators | Before Modification (n = 16) | After Modification (n = 8) | ||||||
|---|---|---|---|---|---|---|---|---|
| TP [mg/L] | pH | TP [mg/L] | pH | |||||
| In | Out | In | Out | In | Out | In | Out | |
| Average | 0.90 | 0.82 | - | - | 1.25 | 0.74 | - | - |
| Median | 0.48 | 0.47 | 7.245 | 7.73 | 0.89 | 0.52 | 7.115 | 9.275 |
| Min | 0.18 | 0.14 | 6.81 | 6.86 | 0.29 | 0.11 | 6.76 | 8.28 |
| Max | 4.62 | 3.80 | 7.91 | 8.55 | 3.36 | 2.01 | 7.49 | 10.43 |
| SD | 1.04 | 0.89 | - | - | 0.99 | 0.58 | - | - |
| Cv [%] | 115.1 | 109.7 | - | - | 79.1 | 78.0 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzka, B.; Siwiec, T.; Marzec, M.; Jóźwiakowski, K.; Listosz, A. Meandering Flow Filter for Phosphorus Removal as a Component of Small Wastewater Treatment Plants—A Case Study. Water 2023, 15, 2703. https://doi.org/10.3390/w15152703
Zawadzka B, Siwiec T, Marzec M, Jóźwiakowski K, Listosz A. Meandering Flow Filter for Phosphorus Removal as a Component of Small Wastewater Treatment Plants—A Case Study. Water. 2023; 15(15):2703. https://doi.org/10.3390/w15152703
Chicago/Turabian StyleZawadzka, Beata, Tadeusz Siwiec, Michał Marzec, Krzysztof Jóźwiakowski, and Agnieszka Listosz. 2023. "Meandering Flow Filter for Phosphorus Removal as a Component of Small Wastewater Treatment Plants—A Case Study" Water 15, no. 15: 2703. https://doi.org/10.3390/w15152703
APA StyleZawadzka, B., Siwiec, T., Marzec, M., Jóźwiakowski, K., & Listosz, A. (2023). Meandering Flow Filter for Phosphorus Removal as a Component of Small Wastewater Treatment Plants—A Case Study. Water, 15(15), 2703. https://doi.org/10.3390/w15152703








