Mg and Sr Isotopes in Cap Dolostone: Implications for Oceanic Mixing after a Neoproterozoic Snowball Earth Event
Abstract
:1. Introduction
1.1. Implications of Sr and Mg Isotopes
1.2. Previous Leaching Studies in Literature
1.3. Aims of This Study
2. Materials and Analytical Methods
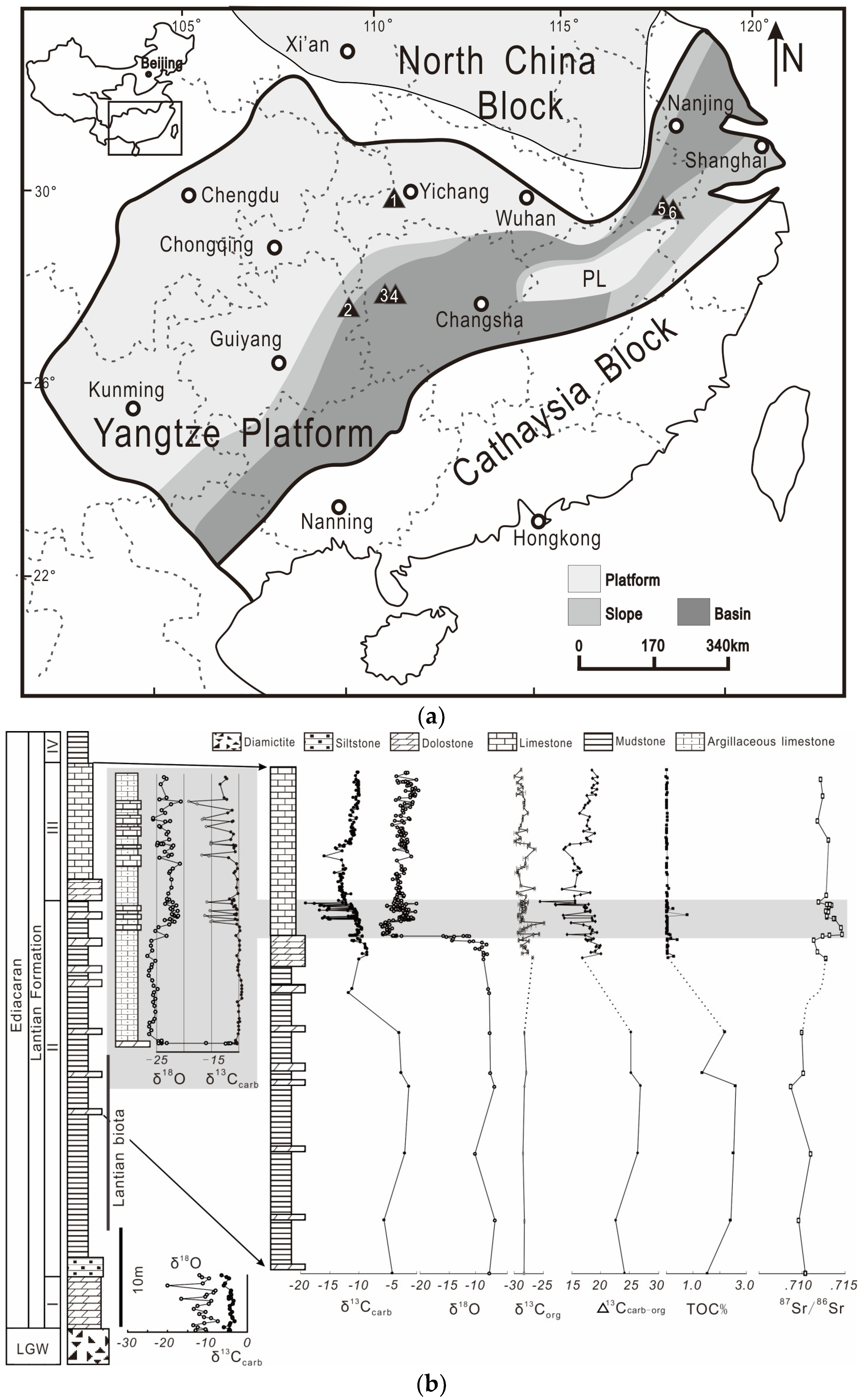
2.1. Geological Background
To Develop New Sequential-Extraction Procedures in Cap Dolostone
2.2. Analytical Methods
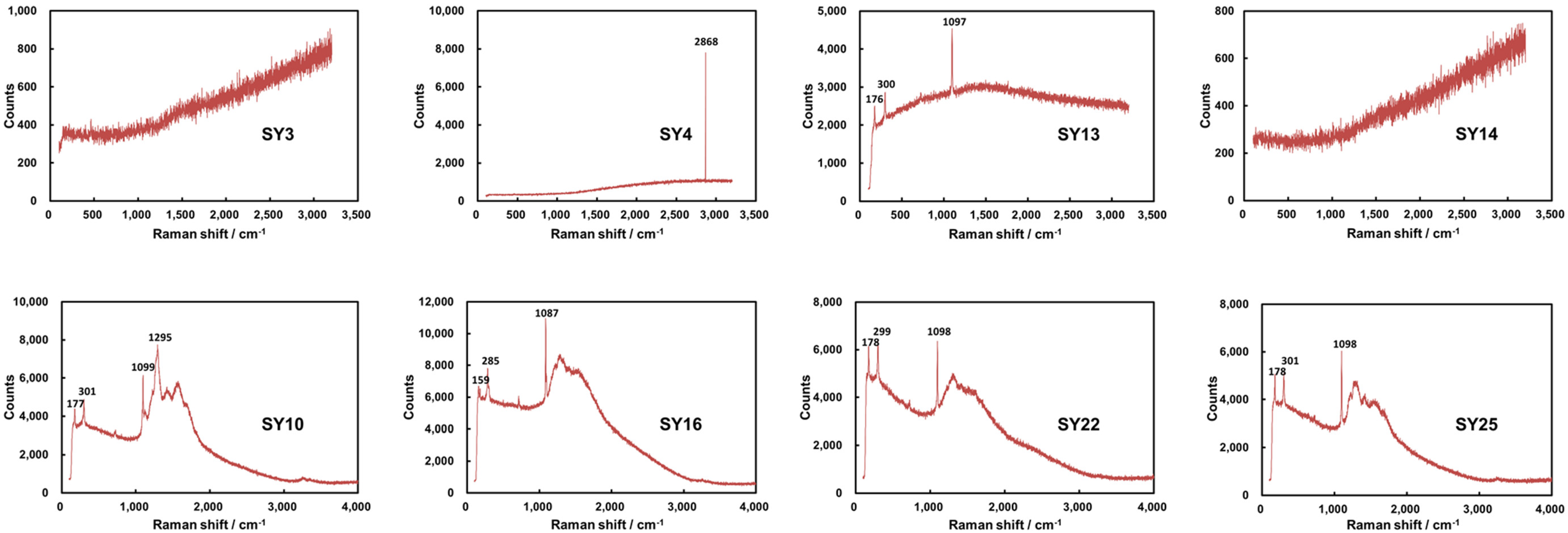
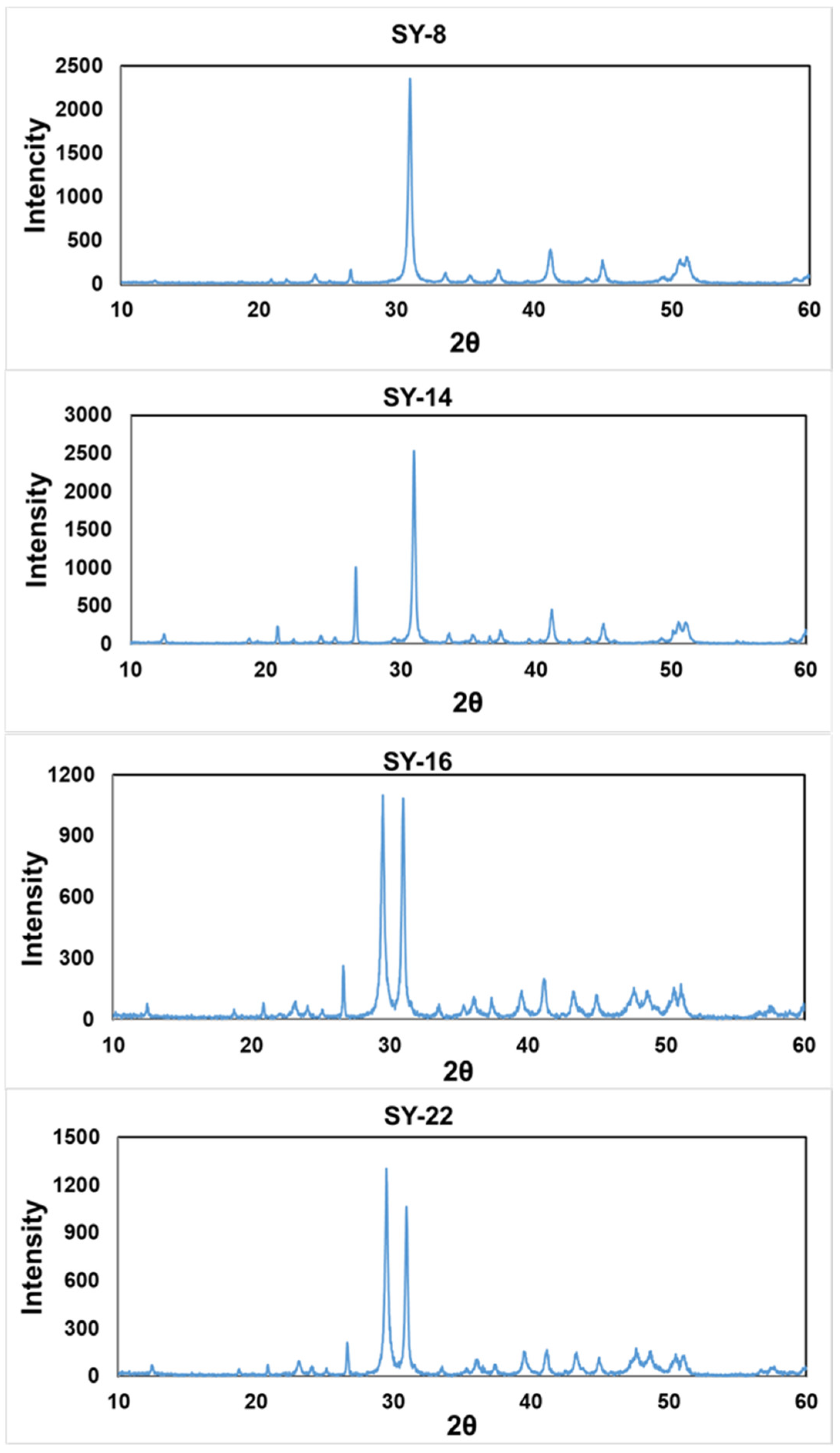
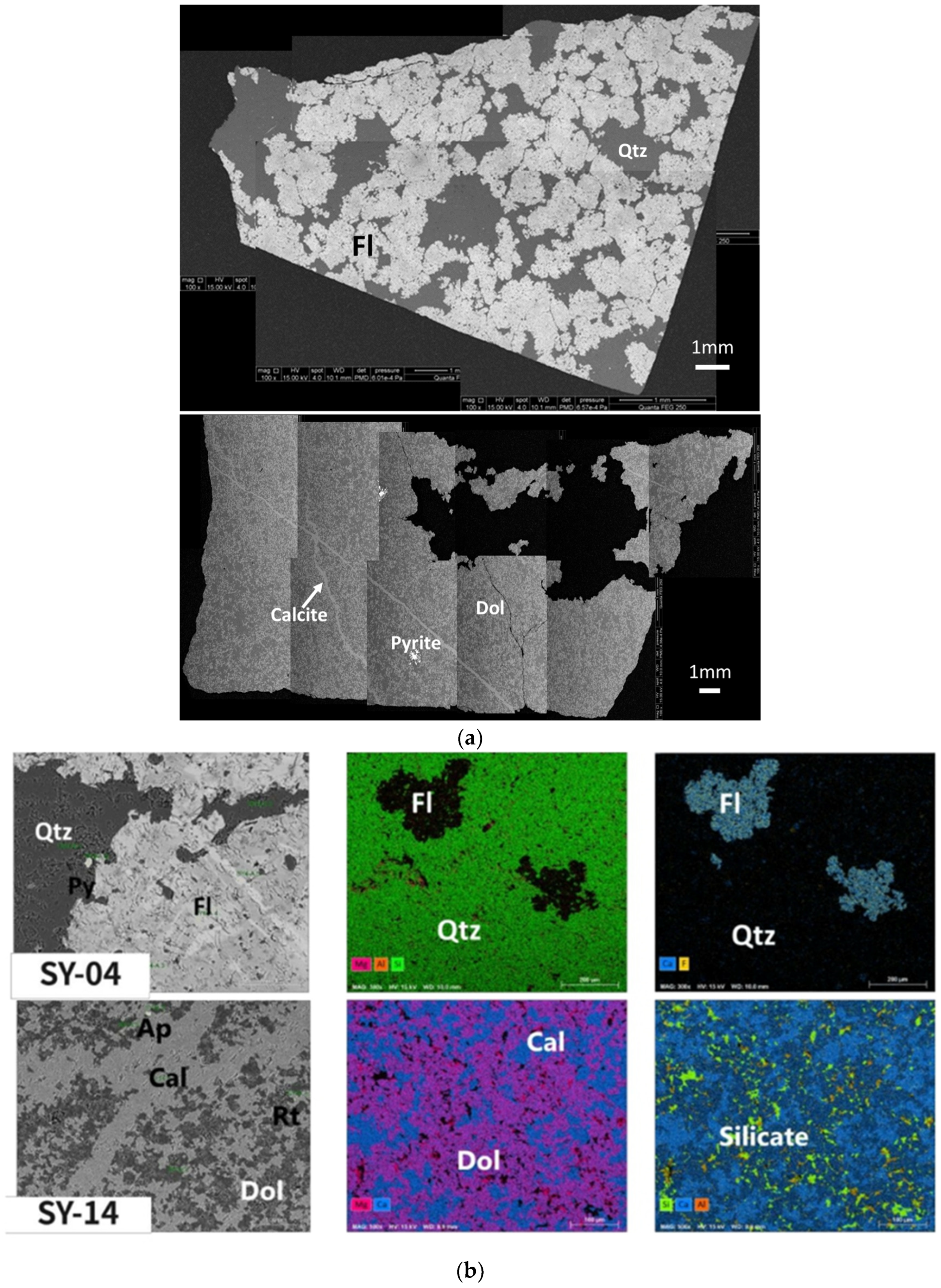
2.2.1. Mineralogical Analysis
2.2.2. Geochemical Analysis
2.2.3. The Sr and Mg Isotopic Analyses
3. Results
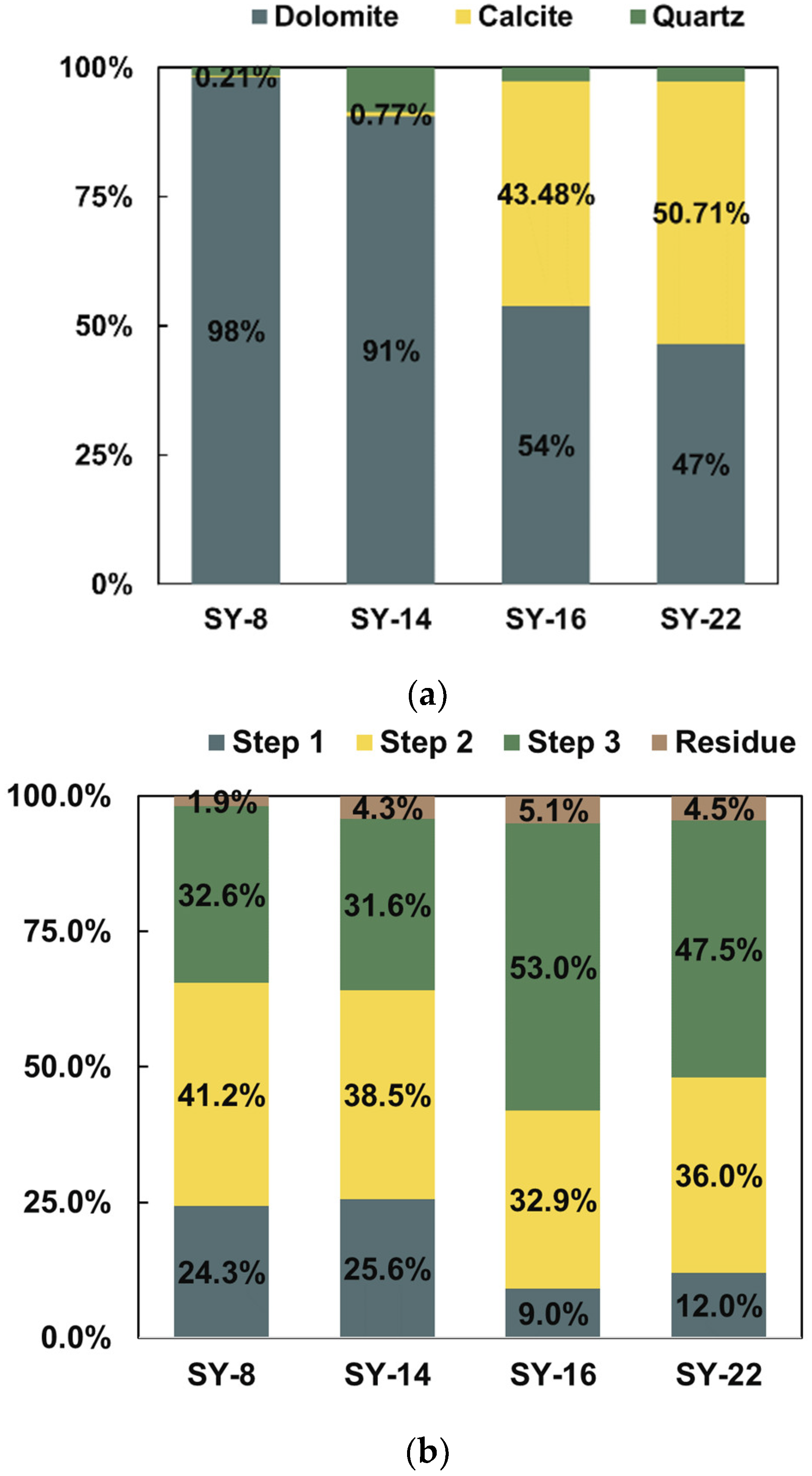
3.1. Mineralogical Compositions in the Cap Dolostone
3.2. Geochemical Results in the Leaching Experiments
4. Discussion
4.1. Evaluation of the New Sequential-Extraction Results
4.2. Separation of Non-Dolomite Phases
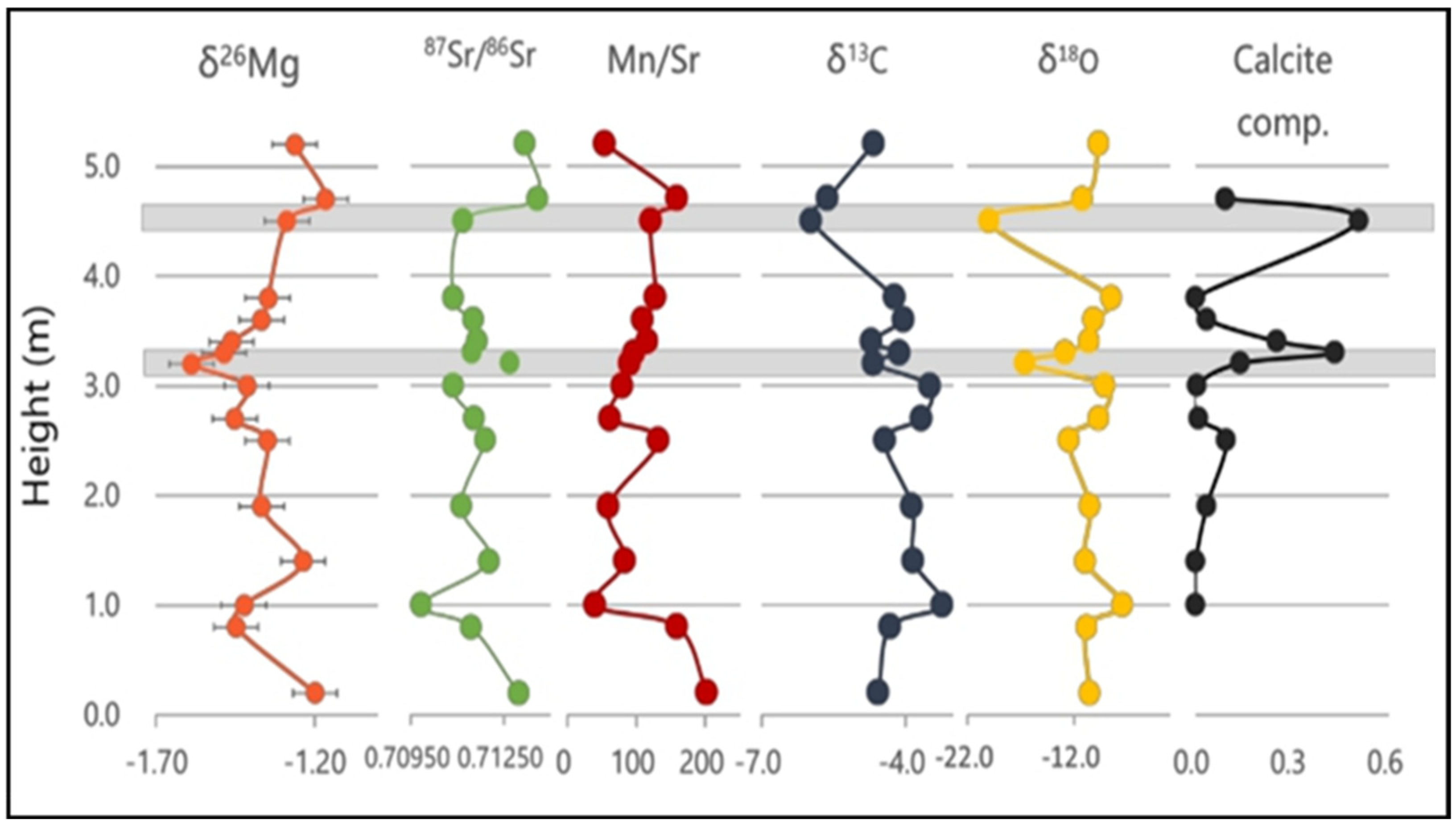
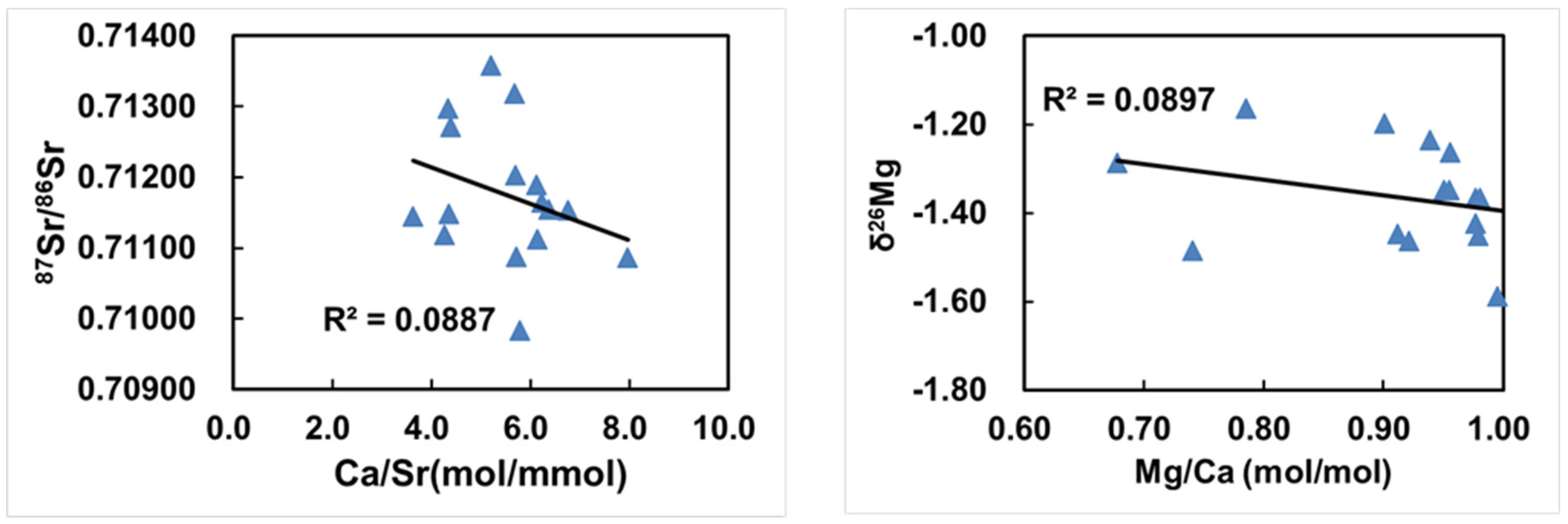
4.3. Diagenesis Artifacts Evaluation
4.4. Implications for the Neoproterozoic Deglaciation
The Mg and Sr Isotopes Co-Variations in Shiyu
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Amount of Sample Dissolved in Each Step | Mg/Ca (Molar Ratio) | Mn/Sr (Molar Ratio) | Fe/(Mg + Ca) × 1000 | Mn/(Ca + Mg) × 1000 | Al/(Mg + Ca) × 1000 | Ti/(Ca + Mg) × 1000 | Sr/(Ca + Mg) × 1,000,000 | Si/(Mg + Ca) × 1000 | 87Sr/86Sr | (2SD) | δ26Mg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8_1 | 22.00 | 0.93 | 71 | 9 | 13 | 0.1 | 0.19 | 296 | 2 | 0.71255 | 0.000055 | −1.46 |
| 8_2 | 37.20 | 0.94 | 80 | 12 | 13 | 1.57 | 0.18 | 267 | 1.1 | 0.7123 | 0.00011 | −1.27 |
| 8_3 | 29.40 | 0.94 | 83 | 17 | 13 | 4.8 | 0.14 | 244 | 3.6 | 0.71203 | 0.000157 | −1.24 |
| 8_R | 11.40 | 1.21 | 74 | 49 | 10 | 106 | 14 | 219 | 0.9 | 0.71393 | 0.000079 | −1.08 |
| 14_1 | 21.60 | 0.83 | 44 | 8 | 13 | 0.19 | 0.19 | 472 | 2.9 | 0.71277 | 0.000042 | −1.42 |
| 14_2 | 29.50 | 0.96 | 77 | 15 | 13 | 5.91 | 0.19 | 275 | 3.5 | 0.71158 | 0.000057 | −1.44 |
| 14_3 | 23.40 | 1.01 | 79 | 26 | 12 | 20.06 | 0.16 | 238 | 9.8 | 0.71088 | 0.000049 | −1.41 |
| 14_R | 25.50 | 2.64 | 96 | 119 | 8 | 231 | 30 | 126 | 1 | 0.71135 | 0.000086 | −1.04 |
| 16_1 | 21.30 | 0.11 | 26 | 2 | 19 | 0 | 0.15 | 1165 | 0.8 | 0.71175 | 0.000063 | −1.81 |
| 16_2 | 39.90 | 0.23 | 46 | 7 | 26 | 1.17 | 0.16 | 896 | 1.2 | 0.71153 | 0.000054 | −1.69 |
| 16_3 | 25.70 | 0.74 | 96 | 21 | 21 | 7.75 | 0.14 | 347 | 4.6 | 0.71149 | 0.000054 | −1.49 |
| 16_R | 13.10 | 1.8 | 144 | 84 | 12 | 185 | 25 | 138 | 0.5 | 0.71691 | 0.000054 | −1.19 |
| 22_1 | 32.70 | 0.08 | 32 | 3 | 20 | 5.91 | 0.14 | 984 | 0.8 | 0.7115 | 0.000058 | −1.49 |
| 22_2 | 41.20 | 0.21 | 58 | 12 | 28 | 1.74 | 0.16 | 782 | 1.4 | 0.71121 | 0.000044 | −1.29 |
| 22_3 | 20.80 | 0.68 | 122 | 34 | 28 | 10.61 | 0.17 | 363 | 6.4 | 0.71119 | 0.000044 | −1.29 |
| 22_R | 5.20 | 1.86 | 202 | 99 | 18 | 226 | 26 | 141 | 1.5 | 0.72600 | 0.000068 | −0.97 |
Appendix B
| Sample | Height (m) | Dolomite (%) | Calcite (%) | Quartz (%) | Mg/Ca * | Sr/Ca * | Mn/Sr * | Fe/(Ca + Mg) | Al/(Ca + Mg) | Si/(Ca + Mg) | Si/Mg | δ13CVPDB | δ18OVPDB | 87Sr/86Sr | (2SD) | δ25Mg | δ26Mg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 0.2 | - | - | - | 0.9 | 0.23 | 202 | 0.034 | 0.023 | 0.015 | 0.042 | −4.56 | −10.52 | 0.712976 | 0.000064 | −0.62 | −1.2 |
| 5 | 0.8 | - | - | - | 0.91 | 0.28 | 159 | 0.052 | 0.02 | 0.013 | 0.037 | −4.32 | −10.82 | 0.711447 | 0.000059 | −0.72 | −1.45 |
| 6 | 1 | 95.89 | 0.07 | 4.04 | 0.98 | 0.17 | 41 | 0.01 | 0.008 | 0.005 | 0.012 | −3.23 | −7.42 | 0.709828 | 0.000029 | −0.71 | −1.42 |
| 8 | 1.4 | 98.25 | 0.21 | 1.53 | 0.94 | 0.18 | 83 | 0.017 | 0.005 | 0.004 | 0.01 | −3.83 | −10.91 | 0.712031 | 0.000078 | −0.65 | −1.24 |
| 10 | 1.9 | 94.92 | 3.60 | 1.48 | 0.98 | 0.16 | 60 | 0.014 | 0.009 | 0.005 | 0.014 | −3.86 | −10.52 | 0.711126 | 0.000036 | −0.7 | −1.37 |
| 12 | 2.5 | 89.02 | 9.67 | 1.31 | 0.95 | 0.16 | 132 | 0.025 | 0.007 | 0.005 | 0.013 | −4.43 | −12.49 | 0.711896 | 0.000017 | −0.69 | −1.35 |
| 13 | 2.7 | 95.49 | 1.04 | 3.47 | 0.98 | 0.16 | 62 | 0.01 | 0.009 | 0.005 | 0.014 | −3.67 | −9.69 | 0.711547 | 0.000024 | −0.78 | −1.45 |
| 14 | 3 | 90.67 | 0.77 | 8.56 | 1.01 | 0.18 | 79 | 0.026 | 0.02 | 0.01 | 0.026 | −3.5 | −9.07 | 0.710875 | 0.000025 | −0.74 | −1.41 |
| 15 | 3.2 | 76.40 | 13.97 | 9.64 | 1 | 0.23 | 90 | 0.023 | 0.019 | 0.011 | 0.03 | −4.67 | −16.55 | 0.71271 | 0.000054 | −0.84 | −1.59 |
| 16 | 3.3 | 53.91 | 43.48 | 2.61 | 0.74 | 0.23 | 96 | 0.021 | 0.008 | 0.005 | 0.015 | −4.13 | −12.77 | 0.711487 | 0.000027 | −0.81 | −1.49 |
| 17 | 3.4 | 71.26 | 25.27 | 3.47 | 0.92 | 0.16 | 116 | 0.017 | 0.011 | 0.007 | 0.019 | −4.7 | −10.58 | 0.711649 | 0.000028 | −0.77 | −1.46 |
| 19 | 3.6 | 89.10 | 3.63 | 7.27 | 0.98 | 0.15 | 110 | 0.019 | 0.014 | 0.009 | 0.025 | −4.04 | −10.17 | 0.711529 | 0.000034 | −0.71 | −1.37 |
| 20 | 3.8 | 95.36 | 0.18 | 4.46 | 0.95 | 0.13 | 128 | 0.019 | 0.01 | 0.005 | 0.015 | −4.23 | −8.51 | 0.710868 | 0.000039 | −0.71 | −1.35 |
| 22 | 4.5 | 46.62 | 50.71 | 2.67 | 0.68 | 0.23 | 122 | 0.034 | 0.011 | 0.006 | 0.022 | −5.97 | −19.99 | 0.711193 | 0.000022 | −0.66 | −1.29 |
| 23 | 4.7 | 85.74 | 9.30 | 4.96 | 0.79 | 0.19 | 159 | 0.042 | 0.009 | 0.005 | 0.014 | −5.62 | −11.21 | 0.713586 | 0.000027 | −0.65 | −1.16 |
| 24 | 5.2 | - | - | - | 0.96 | 0.18 | 55 | 0.015 | 0.008 | 0.003 | 0.009 | −4.66 | −9.65 | 0.713191 | 0.000042 | −0.62 | −1.26 |
| Mean | □ | 83.30 | 12.50 | 4.30 | 0.92 | 0.19 | 106 | 0.024 | 0.012 | 0.007 | 0.02 | −4.34 | −11.3 | 0.71175 | 0.000038 | −0.71 | −1.37 |
Appendix C
References
- Hoffman, P.F.; Schrag, D.P. The snowball Earth hypothesis: Testing the limits of global change. Terra 2002, 14, 129–155. [Google Scholar] [CrossRef] [Green Version]
- Kirschvink, J.L. Late Proterozoic low-latitude global glaciation: The snowball Earth. Proterozoic Biosph. 1992, 52, 51–52. [Google Scholar]
- Xu, B.; Xiao, S.; Zou, H.; Chen, Y.; Li, Z.X.; Song, B.; Liu, D.; Zhou, C.; Yuan, X. SHRIMP zircon U-Pb age constraints on Neoproterozoic Quruqtagh diamictites in NW China. Precambrian Res. 2009, 168, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, P.F.; Kaufman, A.J.; Halverson, G.P.; Schrag, D.P. A Neoproterozoic Snowball Earth. Science 1998, 281, 1342–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melezhik, V.A.; Fallick, A.E.; Rychanchik, D.V.; Kuznetsov, A.B. Palaeoproterozoic evaporites in Fennoscandia: Implications for seawater sulphate, the rise of atmospheric oxygen and local amplification of the δ13C excursion. Terra Nova 2005, 17, 141–148. [Google Scholar] [CrossRef]
- Alene, M.; Jenkin, G.R.T.; Leng, M.J.; Darbyshire DP, F. The Tambien Group, Ethiopia: An early Cryogenian (ca. 800–735 Ma) Neoproterozoic sequence in the Arabian–Nubian Shield. Precambrian Res. 2006, 147, 79–99. [Google Scholar] [CrossRef]
- Gammon, P.R.; McKirdy, D.M.; Smith, H.D. The paragenetic history of a Marinoan cap carbonate. Sediment. Geol. 2012, 243–244, 1–16. [Google Scholar] [CrossRef]
- Ramme, L.; Marotzke, J. Climate and ocean circulation in the aftermath of a Marinoan snowball Earth. Clim. Past 2022, 18, 759–774. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin, Germany, 2004; 244p. [Google Scholar]
- Jiang, G.; Kennedy, M.J.; Christie-Blick, N. Stable isotopic evidence for methane seeps in Neoproterozoic postglacial cap carbonates. Nature 2003, 426, 822–826. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, M.J.; Christie-Blick, N.; Prave, A.R. Carbon isotopic composition of Neoproterozoic glacial carbonates as a test of paleoceonographic models for snowball Earth phenomena. Geology 2001, 29, 1135–1138. [Google Scholar] [CrossRef]
- Svensen, H.; Sverre Planke, A.M.-S.; Jamtveit, B.; Myklebust, R.; Eidem, T.R.; Rey, S.S. Release of methane from a volcanic basin as a mechanism for initial Eocene global warming. Nature 2004, 429, 3–6. [Google Scholar] [CrossRef]
- Zheng, Y.-F.; Wu, R.-X.; Wu, Y.-B.; Zhang, S.-B.; Yuan, H.; Wu, F.-Y. Rift melting of juvenile arc-derived crust: Geochemical evidence from Neoproterozoic volcanic and granitic rocks in the Jiangnan Orogen, South China. Precambrian Res. 2008, 163, 351–383. [Google Scholar] [CrossRef]
- Jacobsen, S.B. Gas hydrates and deglaciations. Nature 2001, 412, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Shi, X.; Zhang, S. Methane seeps, methane hydrate destabilization, and the late Neoproterozoic postglacial cap carbonates. Chin. Sci. Bull. 2006, 51, 1152–1173. [Google Scholar]
- Kennedy, M.; Mrofka, D.; von der Borch, C. Snowball Earth termination by destabilization of equatorial permafrost methane clathrate. Nature 2008, 453, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Shields, G.A. Neoproterozoic cap carbonates: A critical appraisal of existing models and the plumeworld hypothesis. Terra Nova 2005, 17, 299–310. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Raub, T.D.; Macdonald, F.A.; Evans, D.A.D. Neoproterozoic cap-dolostone deposition in stratified glacial meltwater plume. Earth Planet. Sci. Lett. 2014, 404, 22–32. [Google Scholar] [CrossRef]
- Faure, G. Principles of Isotope Geology; John Wiley and Sons Inc.: New York, NY, USA, 1986; 589p, ISBN 0471629863. [Google Scholar]
- Palmer, M.R.; Elderfield, H. Sr isotope composition of sea water over the past 75 Myr. Nature 1985, 314, 526–528. [Google Scholar] [CrossRef]
- Elderfield, H. Strontium isotope stratigraphy. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1986, 57, 71–90. [Google Scholar] [CrossRef]
- Hess, J.; Bender, M.L.; Schilling, J.-G. Evolution of the Ratio of Strontium-87 to Strontium-86 in Seawater from Cretaceous to Present. Science 1986, 231, 979–984. [Google Scholar] [CrossRef]
- Palmer, M.R.; Edmond, J.M. The strontium isotope budget of the modern ocean. Earth Planet. Sci. Lett. 1989, 92, 11–26. [Google Scholar] [CrossRef]
- Rosman, K.J.R.; Taylor, P.D.P. Isotopic compositions of the elements 1997 (Technical Report). Pure Appl. Chem. 1998, 70, 217–235. [Google Scholar] [CrossRef]
- Gray, C.M.; Compston, W. Excess 26Mg in the Allende Meteorite. Nature 1974, 251, 495–497. [Google Scholar] [CrossRef]
- Li, D.; Shields-Zhou, G.A.; Ling, H.F.; Thirlwall, M. Dissolution methods for strontium isotope stratigraphy: Guidelines for the use of bulk carbonate and phosphorite rocks. Chem. Geol. 2011, 290, 133–144. [Google Scholar] [CrossRef]
- Liu, S.A.; Teng, F.Z.; He, Y.; Ke, S.; Li, S. Investigation of magnesium isotope fractionation during granite differentiation: Implication for Mg isotopic composition of the continental crust. Earth Planet. Sci. Lett. 2010, 297, 646–654. [Google Scholar] [CrossRef]
- Teng, F.Z.; Wadhwa, M.; Helz, R.T. Investigation of magnesium isotope fractionation during basalt differentiation: Implications for a chondritic composition of the terrestrial mantle. Earth Planet. Sci. Lett. 2007, 261, 84–92. [Google Scholar] [CrossRef]
- Teng, F.Z.; Li, W.Y.; Ke, S.; Marty, B.; Dauphas, N.; Huang, S.; Pourmand, A. Magnesium isotopic composition of the Earth and chondrites. Geochim. Cosmochim. Acta 2010, 74, 4150–4166. [Google Scholar] [CrossRef]
- Yang, W.; Teng, F.Z.; Zhang, H.F. Chondritic magnesium isotopic composition of the terrestrial mantle: A case study of peridotite xenoliths from the North China craton. Earth Planet. Sci. Lett. 2009, 288, 475–482. [Google Scholar] [CrossRef]
- Teng, F.Z.; Li, W.Y.; Rudnick, R.L.; Gardner, L.R. Contrasting lithium and magnesium isotope fractionation during continental weathering. Earth Planet. Sci. Lett. 2010, 300, 63–71. [Google Scholar] [CrossRef]
- Tipper, E.T.; Galy, A.; Bickle, M.J. Riverine evidence for a fractionated reservoir of Ca and Mg on the continents: Implications for the oceanic Ca cycle. Earth Planet. Sci. Lett. 2006, 247, 267–279. [Google Scholar] [CrossRef]
- Tipper, E.T.; Galy, A.; Bickle, M.J. Calcium and magnesium isotope systematics in rivers draining the Himalaya-Tibetan-Plateau region: Lithological or fractionation control? Geochim. Cosmochim. Acta 2008, 72, 1057–1075. [Google Scholar] [CrossRef]
- Galy, A.; Bar-Matthews, M.; Halicz, L.; O’Nions, R. Mg isotopic composition of carbonate: Insight from speleothem formation. Earth Planet. Sci. Lett. 2002, 201, 105–115. [Google Scholar] [CrossRef]
- Gao, T.; Ke, S.; Teng, F.Z.; Chen, S.; He, Y.; Li, S.G. Magnesium isotope fractionation during dolostone weathering. Chem. Geol. 2016, 445, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Hu, W.; Wang, X.; Lu, Y.; Wang, L.; Liao, Z.; Li, W. Resetting of Mg isotopes between calcite and dolomite during burial metamorphism: Outlook of Mg isotopes as geothermometer and seawater proxy. Geochim. Cosmochim. Acta 2017, 208, 24–40. [Google Scholar] [CrossRef] [Green Version]
- Li, W.Y.; Teng, F.Z.; Ke, S.; Rudnick, R.L.; Gao, S.; Wu, F.Y.; Chappell, B.W. Heterogeneous magnesium isotopic composition of the upper continental crust. Geochim. Cosmochim. Acta 2010, 74, 6867–6884. [Google Scholar] [CrossRef]
- Shen, B. The Mg isotopic systematics of granitoids incontinental arcs and implications for the roleof chemical weathering in crust formation. Proc. Natl. Acad. Sci. USA 2009, 106, 20652–20657. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Lundstrom, C.C.; Glessner, J.; Ianno, A.; Boudreau, A.; Li, J.; Ferré, E.C.; Marshak, S.; DeFrates, J. Chemical and isotopic fractionation of wet andesite in a temperature gradient: Experiments and models suggesting a new mechanism of magma differentiation. Geochim. Cosmochim. Acta 2009, 73, 729–749. [Google Scholar] [CrossRef]
- Huang, F.; Chakraborty, P.; Lundstrom, C.C.; Holmden, C.; Glessner, J.J.G.; Kieffer, S.W.; Lesher, C.E. Isotope fractionation in silicate melts by thermal diffusion. Nature 2010, 464, 396–400. [Google Scholar] [CrossRef]
- Richter, F.M.; Watson, E.B.; Mendybaev, R.A.; Teng, F.-Z.; Janney, P.E. Magnesium isotope fractionation in silicate melts by chemical and thermal diffusion. Geochim. Cosmochim. Acta 2008, 72, 206–220. [Google Scholar] [CrossRef]
- Richter, F.M.; Dauphas, N.; Teng, F.Z. Non-traditional fractionation of nontraditional isotopes: Evaporation, chemical diffusion and Soret diffusion. Chem. Geol. 2009, 258, 92–103. [Google Scholar] [CrossRef]
- Geske, A.; Zorlu, J.; Richter, D.K.; Buhl, D.; Niedermayr, A.; Immenhauser, A. Impact of diagenesis and low grade metamorphosis on isotope (δ26Mg, δ13C, δ18O and 87Sr/86Sr) and elemental (Ca, Mg, Mn, Fe and Sr) signatures of Triassic sabkha dolomites. Chem. Geol. 2012, 332–333, 45–64. [Google Scholar] [CrossRef]
- Huang, K.-J.; Teng, F.-Z.; Shen, B.; Xiao, S.; Lang, X.; Ma, H.-R.; Peng, Y. Episode of intense chemical weathering during the termination of the 635 Ma Marinoan glaciation. Proc. Natl. Acad. Sci. USA 2016, 113, 201607712. [Google Scholar] [CrossRef]
- Halverson, G.P.; Dudás F, Ö.; Maloof, A.C.; Bowring, S.A. Evolution of the 87Sr/86Sr composition of Neoproterozoic seawater. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 256, 103–129. [Google Scholar] [CrossRef]
- Bailey, T.R.; Mcarthur, J.M.; Prince, H.; Thirlwall, M.F. Dissolution methods for strontium isotope stratigraphy: Whole rock analysis. Chem. Geol. 2000, 167, 313–319. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, C.; Guan, C.; Yuan, X.; Chen, Z.; Wan, B. An integrated carbon, oxygen, and strontium isotopic studies of the Lantian Formation in South China with implications for the Shuram anomaly. Chem. Geol. 2014, 373, 10–26. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Zheng, Y.F.; Chen, F. Trace element and strontium isotope constraints on sedimentary environment of Ediacaran carbonates in southern Anhui, South China. Chem. Geol. 2009, 265, 345–362. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Raub, T.D. Geochemical constraints on the origin of Marinoan Cap-dolostones from Nuccaleena Formation, South Australia. Chem. Geol. 2013, 351, 95–104. [Google Scholar] [CrossRef]
- Ling, H.-F.; Feng, H.-Z.; Pan, J.-Y.; Jiang, S.-Y.; Chen, Y.-Q.; Chen, X. Carbon isotope variation through the Neoproterozoic Doushantuo and Dengying Formations, South China: Implications for chemostratigraphy and paleoenvironmental change. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 254, 158–174. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, Z.; Xiao, S.; Zhou, C.; Hua, H. An early Ediacaran assemblage of macroscopic and morphologically differentiated eukaryotes. Nature 2011, 470, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; You, C.F.; Huang, K.F.; Chung, C.H. Precise determination of triple Sr isotopes (δ87Sr and δ88Sr) using MC-ICP-MS. Talanta 2012, 88, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wombacher, F.; Eisenhauer, A.; Heuserac, A.; Weyer, S. Separation of Mg, Ca and Fe from geological reference materials for stable isotope ratio analyses by MC-ICP-MS and double-spike TIMS. J. Anal. At. Spectrom. 2009, 24, 627–636. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Z.; Cheng, H.; Zhang, Z.; Frost, R.L. A Raman spectroscopic comparison of calcite and dolomite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 158–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.Y.; Zheng, Y.F. Stable isotope evidence for involvement of de-glacial meltwater in Ediacaran carbonates in South China. Chem. Geol. 2010, 271, 86–100. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y. Geochemistry of vein and wallrock carbonates from the Ediacaran system in South China: Insights into the origins of depositional and post depositional fluids. Chem. Geol. 2015, 404, 71–87. [Google Scholar] [CrossRef]
- Wen, B.; Evans DA, D.; Li, Y.; Wang, Z.; Liu, C. Newly discovered Neoproterozoic diamictite and cap carbonate (DCC) couplet in Tarim Craton, NW China: Stratigraphy, geochemistry, and paleoenvironment. Precambrian Res. 2015, 271, 278–294. [Google Scholar] [CrossRef]
- Jacobsen, S.B.; Kaufman, A.J. The Sr, C and O isotopic evolution of Neoproterozoic seawater-comment. Chem. Geol. 1999, 181, 193–195. [Google Scholar]
- Kaufman, A.J.; Jacobsen, S.B.; Knoll, A.H. The Vendian record of Sr and C isotopic variations in seawater: Implications for tectonics and paleoclimate. Earth Planet. Sci. Lett. 1993, 120, 409–430. [Google Scholar] [CrossRef]
- Lumsden, D.N.; Lloyd, R.V. Mn(II) partitioning between calcium and magnesium sites in studies of dolomite origin. Geochim. Cosmochim. Acta 1984, 48, 1861–1865. [Google Scholar] [CrossRef]
- Xiao, S.; Bao, H.; Wang, H.; Kaufman, A.J.; Zhou, C.; Li, G.; Yuan, X.; Ling, H. The Neoproterozoic Quruqtagh Group in eastern Chinese Tianshan: Evidence for a post Marinoan glaciation. Precambrian Res. 2004, 130, 1–26. [Google Scholar] [CrossRef]
- Sawaki, Y.; Ohno, T.; Tahata, M.; Komiya, T.; Hirata, T.; Maruyama, S.; Windley, B.F.; Han, J.; Shu, D.; Li, Y. The Ediacaran radiogenic Sr isotope excursion in the Doushantuo Formation in the Three Gorges area, South China. Precambrian Res. 2010, 176, 46–64. [Google Scholar] [CrossRef]
- Tipper, E.T.; Galy, A.; Gaillardet, J.; Bickle, M.J.; Elderfield, H.; Carder, E.A. The magnesium isotope budget of the modern ocean: Constraints from riverine magnesium isotope ratios. Earth Planet. Sci. Lett. 2006, 250, 241–253. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, W.; Li, Y.; Xu, Z. Magnesium isotopic composition of rivers draining karst-dominated regions in Southwest China. Chem. Geol. 2022, 606, 121002. [Google Scholar] [CrossRef]





| Reference | Concentrarion of Acetic Acid (v/v) | Mineral Phase | Remarks |
|---|---|---|---|
| This study | 0.25%, 1.0% and 1.5% | Dolomite | 3-step-extraction |
| Bailey [46] | 20% | Calcite | Dissolve 10% sample in every step |
| Ling et al. [50] | 1 M (~5%) | Dolomite | 50 °C for 15 h |
| Zhao et al. [48] | 0.5 M (~2.5%) | Dolomite | Room temp. for 4 h |
| 3.5 M (~17.5%) | 60 °C for 24 h | ||
| Li et al. [26] | 0.2%/1% | Dolomite | First step for ~30–40% sample |
| 0.2%/2.5% | Second step for ~30–40% sample | ||
| Liu et al. [49] | 0.25%–10% | Dolomite | 15-step extraction |
| Wang et al. [47] | 1 M (~5%) | Dolomite | For trace elements |
| 0.1 M (~0.5%), 4 h | Wash out first 30%, then take supernatant |
| Sample | Height (m) | Raman | XRD | ||
|---|---|---|---|---|---|
| Dolomite (%) | Calcite (%) | Quartz (%) | |||
| SY-26 | 5.5 | - | - | - | - |
| SY-25 | 5.3 | Carbonate | - | - | - |
| SY-24 | 5.2 | Carbonate | - | - | - |
| SY-23 | 4.7 | Carbonate | 85.74 | 9.3 | 4.96 |
| SY-22 | 4.5 | Carbonate | 46.62 | 50.71 | 2.67 |
| SY-21 | 4 | Carbonate | - | - | - |
| SY-20 | 3.8 | Carbonate | 95.36 | 0.18 | 4.46 |
| SY-19 | 3.6 | - | 89.1 | 3.63 | 7.27 |
| SY-17 | 3.4 | Carbonate | 71.26 | 25.27 | 3.47 |
| SY-16 | 3.3 | Carbonate | 53.91 | 43.48 | 2.61 |
| SY-15 | 3.2 | Carbonate | 76.4 | 13.97 | 9.64 |
| SY-14 | 3 | Carbonate | 90.67 | 0.77 | 8.56 |
| SY-13 | 2.7 | Carbonate | 95.49 | 1.04 | 3.47 |
| SY-12 | 2.5 | Carbonate | 89.02 | 9.67 | 1.313 |
| SY-11 | 2.2 | - | 94.92 | 3.6 | 1.478 |
| SY-10 | 1.9 | Carbonate | 98.25 | 0.21 | 1.534 |
| SY-9 | 1.7 | - | - | - | - |
| SY-8 | 1.4 | Carbonate | - | - | - |
| SY-7 | 1.3 | Carbonate | - | - | - |
| SY-6 | 1 | Carbonate | 95.89 | 0.07 | 4.04 |
| SY-5 | 0.8 | Carbonate | - | - | - |
| SY-4 | 0.6 | Non-carbonate | - | - | - |
| SY-3 | 0.4 | Non-carbonate | - | - | - |
| SY-2 | 0.2 | Carbonate | - | - | - |
| SY-1 | 0.1 | Non-carbonate | - | - | - |
| Step | Reagent | Reaction Condition | Target Phase |
|---|---|---|---|
| Wash | 6 mL Mili-Q water | 10 min supersonic bath | Water soluble phase and ion-exchange site |
| Step 1 | 6 mL 0.25% HAc | 15 min supersonic bath | Calcite and altered dolomite |
| Step 2 | 6 mL 1% HAc | 15 min supersonic bath | (Calcite) and dolomite |
| Step 3 | 6 mL 1.5% HAc | 14 min supersonic bath | Dolomite |
| Residue | 3 mL HNO3 and 3 mL HF | 48 h at 100 °C | Silicate phase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-S.; You, C.-F.; Chung, C.-H.; Huang, K.-F.; Zhou, C. Mg and Sr Isotopes in Cap Dolostone: Implications for Oceanic Mixing after a Neoproterozoic Snowball Earth Event. Water 2023, 15, 2688. https://doi.org/10.3390/w15152688
Lin S-S, You C-F, Chung C-H, Huang K-F, Zhou C. Mg and Sr Isotopes in Cap Dolostone: Implications for Oceanic Mixing after a Neoproterozoic Snowball Earth Event. Water. 2023; 15(15):2688. https://doi.org/10.3390/w15152688
Chicago/Turabian StyleLin, Shiau-Shiun, Chen-Feng You, Chuan-Hsiung Chung, Kuo-Fang Huang, and Chuanming Zhou. 2023. "Mg and Sr Isotopes in Cap Dolostone: Implications for Oceanic Mixing after a Neoproterozoic Snowball Earth Event" Water 15, no. 15: 2688. https://doi.org/10.3390/w15152688
APA StyleLin, S.-S., You, C.-F., Chung, C.-H., Huang, K.-F., & Zhou, C. (2023). Mg and Sr Isotopes in Cap Dolostone: Implications for Oceanic Mixing after a Neoproterozoic Snowball Earth Event. Water, 15(15), 2688. https://doi.org/10.3390/w15152688





_Huang.png)

