Abstract
Coal mining causes surface subsidence, and the accumulated water body is constantly affected by the mining microseism in this process. Understanding the relationship between the subsided water quality and microseism plays a special role in assessing the environmental impact of mining activities. Based on sampling and monitoring, analyzing, and evaluating the Pansan subsided water area of Huainan, the influence of microseism activity on heavy metal elements in subsided water was verified. We found that the microseism effects decreased the contents of Zn, Pb, and Cu in the subsided water by 43.76%, 35.88%, and 28.83%, respectively, and Cd was not detected. The mechanism of heavy metal evolution in the water–sediment system under microseism factors was further explored by simulating experiments with similar materials. The results showed that the mining microseism increases the heavy metal adsorption capacity of suspended solids, and the dissolved heavy metals in water were transformed into suspended heavy metals. The heavy metals of subsided water eventually accumulate in the sediment, and the purpose of controlling heavy metal pollution can be achieved through regular cleaning of the bottom sediment.
1. Introduction
Coal has long dominated China’s primary energy consumption, and this is unlikely to change significantly anytime soon []. Large amounts of underground coal mining led to the creation of voids and eventually ground subsidence. The groundwater depth in the Huainan mining area is shallow, which forms an extensive coal mining subsidence water area []. The subsidence water area is considered to be a special reservoir of freshwater []. Accurate cognition of the control factors for changing the quality of subsidence water is a crucial point for reducing water environmental health risks [,],. Furthermore, the status quo of the water environment in subsidence areas is evaluated objectively and trends are forecasted, which is of crucial scientific value and practical significance for the optimal allocation of water resources and promotes water utilization rate.
There have been many studies on changes in the water environment in coal mining subsidence areas in recent years. Physicochemical indices, nutrient indicators, heavy metal indicators, and biological indicators were employed to reflect the characteristics and changes in the water environment individually or in combination []. The problems of the water environment in a subsidence area have been broadly attributed to external disturbances such as agricultural surface source pollution, coal gangue leachate, and mine water pollution [,]. For instance, Zheng et al. traced the source of sulfate in the Linhuan coal mining subsidence water area and validated that the subsidence area was affected not only by the Huihe River but also by the discharge of local mining wastewater []. Guo et al. demonstrated that underground coal mining leading to subsidence is the main reason for soil moisture, nutrient, and microbial biomass loss, bacterial community structure change, and finally soil degradation []. Chen et al. analyzed the ecological risks caused by the accumulation of potentially harmful elements in six different subsidence water bodies in Huainan and found that Xinji water bodies had the highest health risks []. However, the influence of mining activities and other internal factors on the water quality of subsidence areas has not been reported, and there is still a lack of research on the evolution and mechanism of heavy metals in subsidence water.
In areas with multiple coal seams being repeatedly mined, the surface settlement can last for decades or even longer before it becomes stable []. During this process, rock fracture and mining vibration are inevitable, and the spontaneous or stimulated acoustic emission phenomenon is called microseism [,]. Microseism is primarily mediated by the overburden caving in the goaf, large roof falling, surface subsidence, coal and gas outbursts, underground mining, and other mining activities []. The present research on mine earthquakes focuses on the inducing mechanism, the energy calculation, the microseism monitoring and positioning, and hazard prevention and control [,,,,]. However, the influence of energy disturbance caused by underground mining-induced microseism on the water environment in subsidence areas, especially on the evolution of heavy metals in water, has not been noticed.
Therefore, the main result contents of this paper are as follows: (a) Based on actual monitoring, variance analysis, and health risk assessment, the influence of the microseismic environment on the content of heavy metal pollutants in the subsidence waters was verified. (b) Using similar materials to simulate experiments, the evolution of heavy metal pollutants under microseismic factors was comprehensively understood. (c) The affecting mechanism of microseism on the sedimentation of heavy metal ions in water was discussed.
2. Materials and Methods
2.1. Description of the Study Area
The mining-induced subsidence waterlogged area of Huainan is mostly an enclosed area. The study area is the subsidence waters of Pansan Mine. The subsided water area is 1.6 km2, and the maximum collapse depth is 5.0 m. During the period of sample collection, the 1742 (1) working face of the Pansan Mine was mined below the surface corresponding to the collapsed water areas.
2.2. Collection and Treatment of Samples
The sampling point was precisely positioned by GPS. The specific position of the working face was demarcated according to the upper and lower comparison drawing of the mine shaft and the stope replacement drawing of Pansan Mine. Five sampling points were arranged in collapsed water area (Figure 1). Site 1 was located above the mined-out area of the working face, site 2 was situated above the work zone, and site 3 was located directly above the virgin area of the mine. These local water bodies were affected by microseism. In addition, two sampling points (site 4 and site 5) were set in the non-mining-affected area for sampling for comparative analysis. The sampling point information is listed in Table S1. The overlying water and bottom water were collected separately with a 1 L deepwater sampler at each sampling site because the forms of heavy metals in natural water can be divided into a dissolved state and a suspended state. Raw water was filtered with a 0.45 μm aperture filter membrane, and only the dissolved heavy metal content was detected; these samples were named filtered water samples. However, to analyze all heavy metal content, raw water needs to be treated with nitric acid and perchloric acid, and these samples were called digested samples.
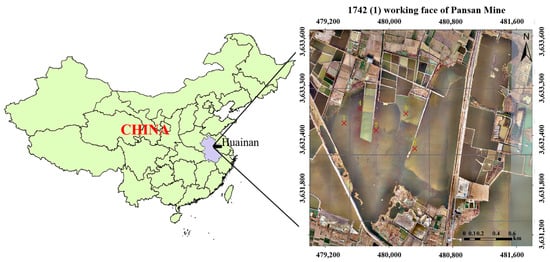
Figure 1.
Map of the investigated area and location of sampling sites.
The turbidity and pH of samples were measured in the field with a turbidimeter (NEP-5000-LINK, Australia) and pH meter (PHS-2C, China). And then, heavy metal concentrations in water samples were tested by inductively coupled plasma atomic emission spectrometry (ICP-AES, PS-6, BAIRD). The hydrochemistry background values of shallow groundwater in the study area are presented in Table S2. The average of the three repeated tests was reported as data for quality assurance. The error range of the measured value was controlled within 5%.
2.3. Statistical Analysis and Health Risk Appraisal
One-way variance analysis (ANOVA) was used to analyze the concentration differences of different heavy metals in the microseism-affected and non-affected areas. Regression analysis was performed using SPSS19.0 software. The health risk of dissolved heavy metals in subsidence water was divided into carcinogenic risk and non-carcinogenic risk, as recommended by the United States Environmental Protection Agency (USEPA) []. The carcinogenic risk and non-carcinogenic risk of direct skin contact with pollutants in adults were calculated as follows [,]:
where CRi is the carcinogenic risk of heavy metals; HQi is the hazard quotient describing the non-carcinogenic risk posed by heavy metals; SFi is the slope factor of dermal exposure to carcinogen. RfDi is the reference dose. ADD is the average daily dose of heavy metals. It is expressed according to Equation (3) [].
where Ci is the concentration of heavy metals (ug/L); BW expresses the body weight; SA means the surface area of the skin; qi represents the carcinogenic intensity coefficient of skin penetration of heavy metal elements in water; EF indicates the exposure frequency in days year; ET shows the exposure time; ED denotes the exposure duration in the year; and AT is the average time in days. The specific values of various parameters required for calculation were obtained from the literature [] and are listed in Table S2. The total hazard index (HI) of non-carcinogenic risks caused by various pollutants is defined as follows []:
When HI < 1, negative health effects from non-carcinogenic substances in the dermis exposure pathway can be ignored; otherwise, there will be adverse effects on health [].
In this study, four heavy metals in the subsidence water were determined, among which Cd was carcinogenic and Cu, Pb, and Zn were non-carcinogenic. In terms of cancer risk, referring to the classification standard of USEPA, the allowable health risk limit of a single element is 1 × 10−6 a−1, and that of multiple elements is 1 × 10−4 a−1 []. According to the standard of drinking water in China (GB5749-2006), the risk value of each toxic substance should be less than or equal to 1 × 10−3 a−1 [].
2.4. Design and Fabrication of the Microseism Similarity Model Experiment
A similarity model experiment can artificially control and alter test conditions to determine the effects of single or multiple factors []. The result is clear and intuitive, with a short cycle and quick effect. And it can supplement problems that on-site water quality monitoring and computer simulation cannot solve []. Therefore, a similar material simulation experiment was introduced to explore the relationship between microseism and heavy metal response in subsided waters.
The occurrence condition of a coal seam in the 14021 (3) working face of Panyi Mine was taken as the prototype of the similarity model. The lithology of the bedrock above the coal seam of the working face is mainly mudstone, sandstone, and sandy mudstone, which belongs to the medium-hard type and is 65 m thick. The quaternary loose layer above the bedrock is about 300 m thick and is the interactive sediment of the clay and sand layer, among which the sand layer is rich in water. This surface adopts the method of mining, longwall backward mining, and all caving methods to manage the roof.
A three-dimensional model test bench was used for the similar simulation test; its specifications were 2 m × 1 m × 1.3 m (length × width × height). The similarity ratio of the model was determined as 1:300 according to the geometric similarity principle. The dynamic similarity principle requires that all forces between model and prototype remain similar []. The rock properties of coal measure strata are mostly sedimentary rocks. Generally, the mechanical characteristics of sedimentary rocks can be satisfied by using similar materials composed of aggregates and binders []. Considering the convenience of the fabrication technology and material source, river sand was selected as aggregate, lime and gypsum were used as the binder, and mica powder was used as the stratified material. Different types of rock formations were modeled by varying the composition of aggregate and cementing materials. Matters needing attention for materials include the selection of slaked lime and calcined plaster, as well as the sand particle size of 0.15 mm–0.5 mm []. The simulated material consumption of each layer is calculated according to formula (5) [,]:
where G―the weight of each layer of material in the model; l―model length; b―model width; h―simulation layer thickness; γp―volume weight of the simulated rock layer. The prototype parameters and simulation parameters of rock properties of the 14021 (3) working faces are shown in Table S4.
G = l × b × h × γp
Kinematic similarity theory stipulates that the time required for each corresponding point in the model and prototype to complete the motion along the geometrically similar orbit conforms to a certain proportion []. According to Newton’s law and the derivation of the similarity criterion of rock strata movement, the time ratio can be obtained from the square root of the similar model scale []. The time similarity ratio is about 1:18. In addition, the water samples of the similar model experiment were collected every half an hour.
A certain amount of river sand, slaked lime, calcined plaster, and water was added into the blender quickly and stirred evenly, and the quality was calculated based on the ratio of similar materials in each rock layer. The material was poured into a pre-installed mold, smoothed out with a scraper, and compacted to maintain the volume weight. During the shaping process, mica powder was scattered between the rock layers as the bedding plane. The model was laid from bottom to top, and the height was consistent with the simulated height of the rock layer. After the rock layer was shaped, fine sand with a thickness of 21 cm was laid above the model to simulate unconsolidated formation. Finally, a cistern with a size of 2 m × 1 m × 0.9 m and a thickness of 2 cm of Perspex was placed above the simulated unconsolidated formation to maintain full contact. After allowing the material to dry naturally for a week, mining of the simulated coal seam was carried out. The vibration source could be placed in the model test system when the mine goaf was formed. Figure 2 is a photo of the similar material model where the vibration source was placed.

Figure 2.
Similar material model with microseism device.
2.5. Similarity Model Experimental Process
The similarity model experiment was used to explore the influence of vibration generated by underground coal mining on the water quality of the subsidence waters. A small vibration pump was employed as a vibration source. The field microseism monitoring in the western mining area of Panyi Mine is introduced in the support information, and the results show that there are obvious vibration pulse signals in the mining area, while the coal gob area signals are weak and regular (Figure S1). The frequency range of microseism in the mining area is 15 Hz to 80 Hz, which has similarities with previous studies []. Referring to the above research, the average value was selected as the frequency of the vibration pump in the model, namely 50 Hz.
Due to the low content of heavy metals in the subsided water area, the simulation test was divided into two groups to make the reaction phenomenon obvious. Group I: The simulated water sample of 200 L in the cistern was from subsided water area above the working face 14021 (3) of Panyi Mine. Group II: The cistern contains not only 200 L of raw water, but also water in which 10 mL solutions of Cu, Cd, Pb, and Zn with a concentration of 1000 mg/L were added. Sediment with a thickness of 5 cm was uniformly laid at the bottom of the cistern, which was collected from the same place as the water. After settling occurred for two days, the vibration simulation test was carried out when the water and sediment were fully contracted and stabilized. The first sample was taken after significant changes in turbidity.
3. Results and Discussion
3.1. Distribution Characteristics of Turbidity in the Subsided Water Area
The upper water and bottom water of each sampling point were collected; the upper water was labeled as A, and the bottom water was labeled as B. There are clear differences in the vertical distribution of turbidity in the subsided water area. Upper water has significantly better turbidity than bottom water, mainly distributed in the range of 3.15 FTU to 6.71 FTU, while bottom water turbidity is mainly distributed in the range of 3.78 FTU to 10.08 FTU (Table S5). The water depth of shallow lakes is no more than 5 m, and there is no obvious stratification of water temperature in summer. Relevant studies show that under the action of winds above 4 m/s, shallow lakes present a suspended matter content in the bottom water that is significantly higher than that in the upper water [,]. The water sample was collected in June. The average wind speed in June in Huainan was 2.6 m/s, and the maximum depth of sampling points in the collapsed waters was 3.5 m. In addition, the sampling was carried out on sunny and windless days, and the water surface was free from waves. The collapsed waters still have obvious stratification phenomena in the study area. This abnormal phenomenon suggests that there are external factors such as underground microseism which affect the water environment.
3.2. Influence of Microseism on Heavy Metals in the Subsided Water
The content of heavy metals is an important factor affecting the safety of the water environment. Four heavy metals, Cu, Cd, Pb, and Zn, which are common in water, were selected as detection objects to perform a scientific assessment of heavy metal pollution in subsidence waters. Heavy metal pollution in upper and bottom water at each sampling point is shown in Table S5. The total concentrations of heavy metals in upper water and bottom water at each sampling point were significantly different. The heavy metal content in the upper water is lower than that in the bottom water, which is analogous to the stratification of suspended matter concentration in water. This suggests that the heavy metal content and suspended matter in the water go hand in hand. To extract the suspended heavy metals, which are adsorbed on the suspended matter of the water body, the water sample was digested. Total dissolved and suspended heavy metal content were detected in the digested water sample. Due to the large particle size of the suspended matter in the water body, it cannot pass through the 0.45 μm aperture filter membrane, so only the dissolved heavy metal content is detected in the filtered water sample. The results showed that the heavy metal content in the filtered water is much smaller than that in the digested water, indicating that only a few of the heavy metals exist in the dissolved state. The heavy metal content in water is higher than the background value, and there is certainly potential pollution.
As presented in Figure 3, a single-factor analysis of variance was conducted for the contents of heavy metals (Cu, Cd, Pb, and Zn) in the microseism-affected area and the contrast area. The heavy metal contents after digestion in the contrast area were remarkably higher than those in the microseism-affected area. Compared to the contrast area, the contents of Zn, Pb, and Cu in the microseism-affected area were 6.13, 25.84, and 6.37 ug/L, which were reduced by 43.76%, 35.88%, and 28.83%, respectively, while Cd was not detected. ANOVA also showed that the differences in the contents of the four heavy metals were significant between the two areas (p < 0.05), and the difference in Pb content was the largest. In general, the results demonstrate that the microseismic environment in the study area could dramatically reduce the content of heavy metals in water bodies.
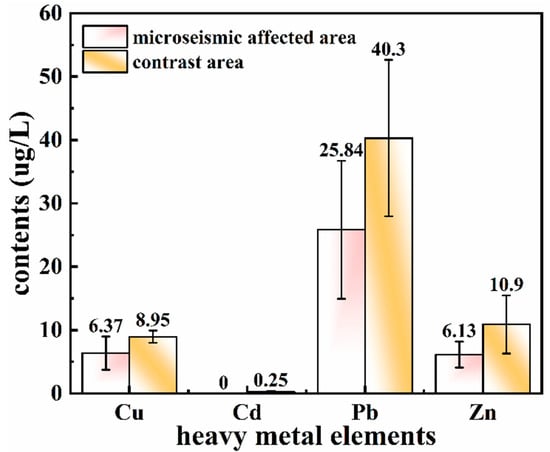
Figure 3.
Comparison of the contents of heavy metals in the microseism-affected area and the contrast area.
The health risk assessment results of dissolved heavy metals in subsidence waters via dermal exposure pathways are shown in Table 1. Cd was only detected at sampling sites 4 and 5. And Cd has a very low carcinogenic risk, just 1.19 × 10−7, well below negligible levels. For non-carcinogenic elements, Pb has the highest hazard quotients, followed by Cu, which is close to the maximum risk allowed by Chinese drinking water standards. However, the hazard indices of the three non-carcinogenic elements were all below 1, indicating that there was no possibility of health effects []. In addition, the hazard index was the uppermost at site 4, and the hazard index of site 2 was the smallest. On the whole, the hazard index value in the microseism-affected area is lower than that in the non-affected area. The decrease in the heavy metal hazard index is related to the influence of the microseismic environment. Therefore, it is necessary to pay continuous attention to the health risk of dissolved heavy metals in Huainan subsidence waters and implement appropriate management measures to further reduce the risk value and ensure water safety.

Table 1.
Health risk values of dissolved heavy metals in subsidence waters of Pansan Mine.
3.3. Heavy Metal Variation in Microseismic Simulation Experiment
3.3.1. Evolution of Heavy Metals in Water
The purpose of setting experimental group Ⅱ was to amplify the change in heavy metals in the raw water of experimental group Ⅰ. The overall change trends of the monitoring results of pH, turbidity, and heavy metals in the two groups were similar. Figure 4a shows that the turbidity showed decreases with the increase in vibration time, which indicated that suspended particulate in water precipitated. Also, the pH value of the water was slightly reduced by the vibration (Figure 4b). Turbidity refers to the degree to which suspended matter in water obstructs light transmission, and its value is related to the amount and particle size of suspended matter and other factors []. In the two groups of simulation experiments, the factors affecting water turbidity were the same. Therefore, after a period of vibration, the turbidity values of the two groups of experimental water bodies will coincide.
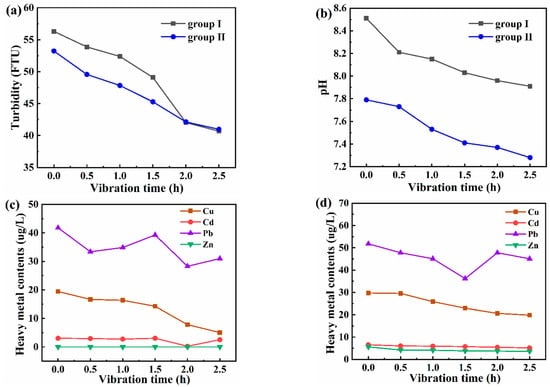
Figure 4.
(a) The pH value change curves; (b) the turbidity change curves. The change curves of heavy metals in the (c) filtered water sample and (d) digested water sample in group II.
Organic matter, colloids, and biofilm are suspended matter in subsided water, but suspended particles are the most abundant. Suspended particles are composed of clay minerals such as montmorillonite, illite, and kaolinite, which are inorganic ion exchangers with complex aluminosilicate structures and can exchange cations and anions at the interface. The hydroxyl groups and interlayer metals on the suspended particles undergo ion exchange with heavy metals, which are adsorbed on the suspended particles, a process that can be considered as a surface complexation reaction []. The vibration process can promote the complexation reaction and reduce the heavy metal content in water (Figure 4c,d). The adsorption of suspended particulate plays a very important role in removing trace metal pollutants from water and changing the distribution of trace metal pollutants between solid and water phases [,]. For example, Lu et al. verified that a low pH value, high suspended particulate concentration, and high organic matter content can enhance the adsorption of Cu []. With the continuous increase in vibration time, the heavy metal content gradually tends to be stable and is restricted by the particle concentration effect. On the other hand, the heavy metal content of digested water was much higher than that of filtered water, indicating that most heavy metal ions were adsorbed on the surface of suspended particles, which was consistent with the actual sampling results.
Due to the low content of heavy metals in the subsided water area, the response to vibration time differs greatly from the pH value and turbidity index. The quantitative correlation between the factors (such as turbidity, pH, and heavy metal content after digestion) and vibration time was obtained utilizing unitary linear regression. The validity of the equation was tested using related coefficients. The results of the regression analysis results are shown in Table 2. Turbidity and pH indices showed an excellent correlation with vibration time. Moreover, Pearson correlation analysis was performed for the relationships between pH and turbidity. There was a certain positive relationship between the two, with the correlation coefficient r = 0.967 (p = 0.002 < 0.01), indicating that the microseismic environment has a great influence on the precipitation and aging of suspended matter and then can change the pH value. At the significant level of 0.05, there is a good correlation between the vibration time and the contents of Cu, Cd, and Zn, and the regression equation has a linear relationship. On the contrary, the regression equation fitting between Pb content and vibration time is poorly fitted, and the significance is greater than 0.05; that is to say, the change in vibration time has no significant effect on the change in Pb content.

Table 2.
Regression analysis results of vibration time and monitoring data in group II.
The migration capacity of heavy metals in collapsed water is related to their contents and chemical forms. Heavy metals may migrate either through flow with water or by adsorption on suspended solids. Cu, Cd, and Zn mainly exist in a suspended state in raw water of the subsidence area, and fine tailing particles are used as transport carriers. For the most part, however, Pb exists in dissolved form, meaning that it was more likely to migrate with water flow and have a high level of potential harm.
3.3.2. Evolution of Heavy Metals in Sediment
The thickness of sediment affects the adsorption and desorption of heavy metals. Generally, a thin sediment thickness has little effect on the desorption of Cu and Pb but has a great influence on the desorption of Cd []. Therefore, 5 cm thick sediment was laid at the bottom of the model water during model design and production. In the microseismic simulation test, the sediment was sampled and monitored to analyze the content of heavy metal elements. Figure 5 shows that the heavy metal content in sediment increases with the extension of vibration time. This proves once again that microseismic environments promote heavy metal precipitation in water. However, the heavy metals in the sediment will be released into the water again with the changes in various environmental factors in the water, forming secondary pollution in the water. Related studies have demonstrated that the pH value of water is the most important influencing factor.
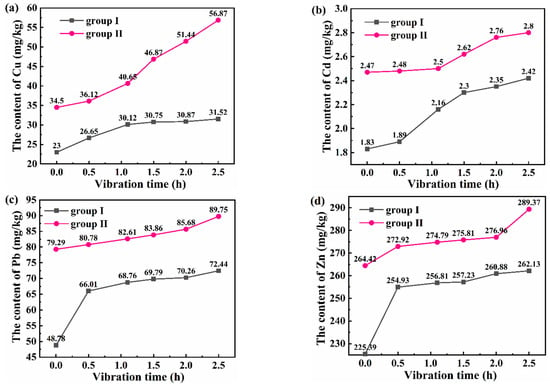
Figure 5.
The change curves of (a) Cu, (b) Cd, (c) Pb, and (d) Zn contents in the simulated sediment.
To investigate the release of heavy metals from sediment of collapsed waters at different pH values, the release rates of Cu, Zn, Pb, and Cd were simulated under the condition of pH = 4, 6, 8, and 10. As shown in Figure 6, the four heavy metals in the sediment are released at four pH values. Compared with neutral and alkaline environments, the release rate is larger in an acidic environment. When the pH value is low, the fractions of water-soluble and exchangeable metals in the sediment increase, which is conducive to the release of heavy metals []. When the pH value is high, the main fraction of heavy metals fraction is acid-soluble (carbonate and manganese oxides) and the residual is insoluble, causing heavy metals to be adsorbed in the sediment and difficult to release []. The above simulation results show that the microseism environment reduces the pH value of the subsided water, so the release rate of heavy metals in the sediment increases. However, the microseism factor makes the suspended solid gradually precipitate after absorbing a large amount of heavy metals, and the adsorption amount of heavy metals in the water is far greater than the release amount of heavy metals in the sediment; eventually, both tend to be stable.
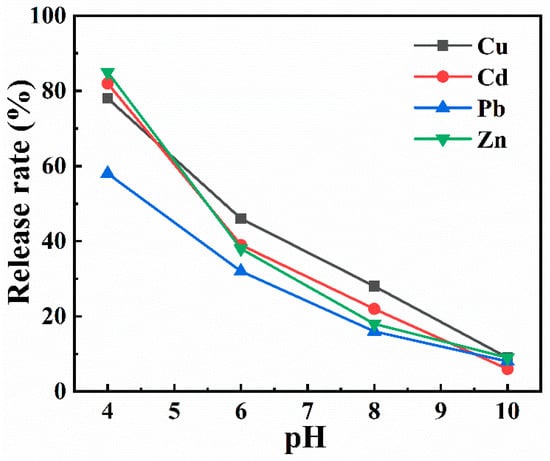
Figure 6.
Effect of different pH values on four heavy metals released from sediments.
3.4. Mechanism of Microseismic Action on Heavy Metals
Wave motion is the most common motion phenomenon in a natural water body. Water particles are in the situation of simple harmonic oscillation near their equilibrium position under wave action. For a subsidence water area with a shallow depth, the release of microseismic energy leads to the generation of wave motion. Water particles in shallow water move elliptically. The horizontal radius of the ellipse remains constant while the vertical radius decreases with increasing distance. The furthest water particles only make the horizontal round-trip movement. Therefore, the wave motion gives rise to a different distribution of suspended matter in collapsed water. In addition, since microseism causes the water particles to be in an uninterrupted state of simple harmonic vibration, the probability of particle collision increases. According to molecular collision theory, the vibration increases the number of activated molecules capable of effective collisions, which makes chemical reactions and adsorption more likely to occur between molecules. The heavy metal ions in the collapsed water area are more likely to combine with OH- and form more hydroxide precipitation. This is one of the reasons why heavy metal ions in the simulation experiment decrease and tend to be stable.
Another factor may be that the microseism reduces the activation energy between anions and heavy metal ions and increases the possibility of forming precipitation. In this study, a simulation experiment was established to monitor the precipitation reaction rate of ZnS under and without the influence of vibration. The reaction device is shown in Figure 7. Then, the activation energies of the two cases were calculated. The specific calculation formulas and steps are described in the supporting information. The monitoring data of pH values and reaction time at reaction temperatures of 25 °C and 30 °C without a vibration source are shown in Table 3. A vibrator was added to the reaction solution with a vibration frequency of 50 Hz. This is to correspond to the microseismic similarity simulation experiment. The monitoring data of pH values and reaction time under vibration conditions are shown in Table 4. According to the calculation results obtained from the simulation experiment, the activation energy of ZnS precipitation generated by the reaction of Zn2+ and S2− is 83.42 KJ/mol under normal conditions. But under the influence of vibration, the activation energy of the reaction is 71.14 KJ/mol. The results of the activation energy simulation experiments demonstrate that the vibration facilitates the precipitation reaction of heavy metals and anions.
Figure 7.
Reaction activation energy simulation test device diagram.

Table 3.
Calculation data of activation energy in simulation experiment without vibration.

Table 4.
Calculation data of activation energy in simulation experiment under vibration condition.
4. Conclusions
In the process of coal mining, subsidence waters are constantly affected by mining pollution sources, but the monitoring found that the microseism induced by coal mining activities (frequency range in 15–80 Hz) reduced the heavy metal content of the subsidence water, and only a small part of Cu, Cd, Pb, and Zn exists in the dissolved state. To explore the causes of the above phenomena, the occurrence conditions of a coal seam in the working face 14021 (3) of Panyi Mine were taken as the prototype for a microseism simulation experiment. The results showed that the microseism effect caused fluctuations in the subsidence water, resulting in different distributions of suspended matter in the overlying and bottom layers. Microseism primarily affects three aspects: Firstly, a large number of heavy metal ions are absorbed by suspended solids in water and then precipitated. Secondly, the adsorption quantity of heavy metals in water is greater than the release quantity of heavy metals from sediment. In addition, microseism reduces the activation energy of the reaction between heavy metal ions and anions, which makes heavy metal ions precipitate out of water easily. The research results provide a new idea for influencing factors of water quality in subsidence areas, which could be of certain reference value for optimizing water resource allocation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15142624/s1. Supplementary data for this article can be found [,].
Author Contributions
Conceptualization, L.X.; methodology, L.X.; validation, K.Z.; formal analysis, J.W.; investigation, L.X.; data curation, L.X.; writing—original draft preparation, J.W.; writing—review and editing, K.Z.; visualization, J.W.; supervision, K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (41472323), the Department of Science and Technology of Anhui Province (2022h11020024), Science and Technology Bureau of Huainan City (2021130), State Key Laboratory of Mining Response and Disaster Prevention and Control in Deep Coal Mines (SKLMRDPC21KF19), Anhui University of Science and Technology (QNYB2021-02 and 2023yjrc43), Anhui Construction Engineering Group (SG2025Q11), and Huaibei Mining (Group) Co., Ltd. (2022101 and 2023067).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, X.; Jiang, X.-W.; Chen, Y.-F.; Tian, H.; Xu, N.-X. The influences of mining subsidence on the ecological environment and public infrastructure: A case study at the Haolaigou Iron Ore Mine in Baotou, China. Environ. Earth Sci. 2009, 59, 803–810. [Google Scholar] [CrossRef]
- Dong, S.; Samsonov, S.; Yin, H.; Yao, S.; Xu, C. Spatio-temporal analysis of ground subsidence due to underground coal mining in Huainan coalfield, China. Environ. Earth Sci. 2014, 73, 5523–5534. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, C.; Wang, J.; Sun, Q.; He, X.; Cao, G.; Zhao, Y.; Yan, L.; Gong, B. Using storage of coal-mining subsidence area for minimizing flood. J. Hydrol. 2019, 572, 571–581. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Yang, Y.; Ansari, U.; Han, Y.; Li, X.; Cheng, Y. Preliminary experimental investigation on long-term fracture conductivity for evaluating the feasibility and efficiency of fracturing operation in offshore hydrate-bearing sediments. Ocean. Eng. 2023, 281, 114949. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, D.; Yin, J.; Zhou, X.; Li, Y.; Chi, P.; Han, Y.; Ansari, U.; Cheng, Y. Sediment Instability Caused by Gas Production from Hydrate-bearing Sediment in Northern South China Sea by Horizontal Wellbore: Evolution and Mechanism. Nat. Resour. Res. 2023, 32, 1595–1620. [Google Scholar] [CrossRef]
- Gilsbach, L.; Schütte, P.; Franken, G. Applying water risk assessment methods in mining: Current challenges and opportunities. Water Resour. Ind. 2019, 22, 100118. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, Z.; Liu, G. Distribution and potential ecological risk of heavy metals accumulated in subsidence lakes formed in the Huainan Coalfield, China. Environ. Forensics 2017, 18, 251–257. [Google Scholar] [CrossRef]
- Vishwakarma, A.K.; Mishra, V.N.; Rai, R.; Shrivastva, B.K. Quantitative assessment of the effect of mining subsidence on the health of native floras using remote sensing techniques. Results Geophys. Sci. 2021, 8, 100031. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, X.; Dong, X.; Wei, X.; Jiang, C.; Tang, Q. Using delta(34)S-SO4 and delta(18)O-SO4 to trace the sources of sulfate in different types of surface water from the Linhuan coal-mining subsidence area of Huaibei, China. Ecotoxicol. Environ. Saf. 2019, 181, 231–240. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Huang, H.; Yang, F. Deciphering soil bacterial community structure in subsidence area caused by underground coal mining in arid and semiarid area. Appl. Soil Ecol. 2021, 163, 103916. [Google Scholar] [CrossRef]
- Chen, G.; Wang, X.; Wang, R.; Liu, G. Health risk assessment of potentially harmful elements in subsidence water bodies using a Monte Carlo approach: An example from the Huainan coal mining area, China. Ecotoxicol. Environ. Saf. 2019, 171, 737–745. [Google Scholar] [CrossRef]
- Loupasakis, C.; Angelitsa, V.; Rozos, D.; Spanou, N. Mining geohazards—Land subsidence caused by the dewatering of opencast coal mines: The case study of the Amyntaio coal mine, Florina, Greece. Nat. Hazards 2013, 70, 675–691. [Google Scholar] [CrossRef]
- Song, Z.; Song, G.; Tang, W.; Zhao, Y.; Yan, D.; Zhang, W. Spatial and temporal distribution of Mo in the overlying water of a reservoir downstream from mining area. J. Env. Sci. (China) 2021, 102, 256–262. [Google Scholar] [CrossRef]
- Wang, H.; Ge, M. Acoustic emission/microseismic source location analysis for a limestone mine exhibiting high horizontal stresses. Int. J. Rock Mech. Min. Sci. 2008, 45, 720–728. [Google Scholar] [CrossRef]
- Barthwal, H.; van der Baan, M. Microseismicity observed in an underground mine: Source mechanisms and possible causes. Geomech. Energy Environ. 2020, 22, 100167. [Google Scholar] [CrossRef]
- Cheng, J.; Song, G.; Sun, X.; Wen, L.; Li, F. Research Developments and Prospects on Microseismic Source Location in Mines. Engineering 2018, 4, 653–660. [Google Scholar] [CrossRef]
- Chen, D.; Martin Mai, P. Automatic identification model of micro-earthquakes and blasting events in Laohutai coal mine based on the measurement of source parameter difference. Measurement 2021, 184, 109883. [Google Scholar] [CrossRef]
- Chen, D.; Wang, E.-Y.; Li, N. Study on the rupture properties and automatic identification model of micro-earthquakes and blasting events in a coal mine. Soil Dyn. Earthq. Eng. 2021, 146, 106759. [Google Scholar] [CrossRef]
- Ghosh, G.K.; Sivakumar, C. Application of underground microseismic monitoring for ground failure and secure longwall coal mining operation: A case study in an Indian mine. J. Appl. Geophys. 2018, 150, 21–39. [Google Scholar] [CrossRef]
- Leake, M.R.; Conrad, W.J.; Westman, E.C.; Ghaychi Afrouz, S.; Molka, R.J. Microseismic monitoring and analysis of induced seismicity source mechanisms in a retreating room and pillar coal mine in the Eastern United States. Undergr. Space 2017, 2, 115–124. [Google Scholar] [CrossRef]
- Wang, N.; Han, J.; Wei, Y.; Li, G.; Sun, Y. Potential Ecological Risk and Health Risk Assessment of Heavy Metals and Metalloid in Soil around Xunyang Mining Areas. Sustainability 2019, 11, 4828. [Google Scholar] [CrossRef]
- Gao, S.; Li, C.; Jia, C.; Zhang, H.; Guan, Q.; Wu, X.; Wang, J.; Lv, M. Health risk assessment of groundwater nitrate contamination: A case study of a typical karst hydrogeological unit in East China. Environ. Sci. Pollut. Res. Int. 2020, 27, 9274–9287. [Google Scholar] [CrossRef]
- Liu, M.; Han, Z.; Yang, Y. Accumulation, temporal variation, source apportionment and risk assessment of heavy metals in agricultural soils from the middle reaches of Fenhe River basin, North China. RSC Adv. 2019, 9, 21893–21902. [Google Scholar] [CrossRef]
- Saha, N.; Rahman, M.S.; Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017, 185, 70–78. [Google Scholar] [CrossRef]
- Yan, J.; Qu, Z.; Li, F.; Li, H. Heavy metals in the water environment of Yangtze River Economic Belt: Status, fuzzy environmental risk assessment and management. Urban Clim. 2021, 40, 100981. [Google Scholar] [CrossRef]
- Jehan, S.; Ullah, I.; Khan, S.; Muhammad, S.; Khattak, S.A.; Khan, T. Evaluation of the Swat River, Northern Pakistan, water quality using multivariate statistical techniques and water quality index (WQI) model. Environ. Sci. Pollut. Res. Int. 2020, 27, 38545–38558. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, G. Pollution evaluation, human health effect and tracing source of trace elements on road dust of Dhanbad, a highly polluted industrial coal belt of India. Environ. Geochem. Health 2021, 43, 2081–2103. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Huang, Y.; Zhang, W.; Shi, Z.; Yu, D.; Chen, Y.; Liu, C.; Wang, R. Effect of different industrial activities on soil heavy metal pollution, ecological risk, and health risk. Environ. Monit. Assess. 2021, 193, 20. [Google Scholar] [CrossRef]
- Pejman, A.; Nabi Bidhendi, G.; Ardestani, M.; Saeedi, M.; Baghvand, A. Fractionation of heavy metals in sediments and assessment of their availability risk: A case study in the northwestern of Persian Gulf. Mar. Pollut. Bull. 2017, 114, 881–887. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Zhang, G.; Zhao, Y.; Zhu, P.; Ma, X.; Li, X. Study on the coupling evolution of air and temperature field in coal mine goafs based on the similarity simulation experiments. Fuel 2021, 283, 118905. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C. Similarity simulation of bolt support in a coal roadway in a tectonic stress field. Min. Sci. Technol. (China) 2010, 20, 718–722. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Shang, Z.; Ren, T.; Chen, P.; Wang, Z.; Shi, Z.; Lv, P. Experimental study on the preparation method of coal-like materials based on similarity of material properties and drilling parameters. Powder Technol. 2022, 395, 26–42. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, G.; Jia, Z.; Zheng, C.; Wang, W. Similarity simulation of mining-crack-evolution characteristics of overburden strata in deep coal mining with large dip. J. Pet. Sci. Eng. 2018, 165, 477–487. [Google Scholar] [CrossRef]
- Guang-li, G.; Jian-feng, Z.; Xie-xing, M.; Qiang, W.; Xian-ni, Z. Similar material and numerical simulation of strata movement laws with long wall fully mechanized gangue backfilling. Procedia Earth Planet. Sci. 2009, 1, 1089–1094. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Bai, Y.; Li, S. Similarity theory for the physical simulation of natural gas hydrate reservoir development. Min. Sci. Technol. (China) 2010, 20, 782–788. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, S.; Wen, G.; Dai, L.; Wang, B. Coal-like material for coal and gas outburst simulation tests. Int. J. Rock Mech. Min. Sci. 2015, 74, 151–156. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Danilov, F.I. Application of dimensional analysis and similarity theory for simulation of electrode kinetics described by the Marcus–Hush–Chidsey formalism. J. Electroanal. Chem. 2012, 669, 50–54. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Liang, R.; Liang, B.; Zhang, T. Virtual-joint based motion similarity criteria for human–robot kinematics mapping. Robot. Auton. Syst. 2020, 125, 103412. [Google Scholar] [CrossRef]
- Liu, J.-p.; Feng, X.-t.; Li, Y.-h.; Xu, S.-d.; Sheng, Y. Studies on temporal and spatial variation of microseismic activities in a deep metal mine. Int. J. Rock Mech. Min. Sci. 2013, 60, 171–179. [Google Scholar] [CrossRef]
- Tang, C.; Li, Y.; He, C.; Acharya, K. Dynamic behavior of sediment resuspension and nutrients release in the shallow and wind-exposed Meiliang Bay of Lake Taihu. Sci. Total Environ. 2020, 708, 135131. [Google Scholar] [CrossRef]
- Jalil, A.; Li, Y.; Zhang, K.; Gao, X.; Wang, W.; Khan, H.O.S.; Pan, B.; Ali, S.; Acharya, K. Wind-induced hydrodynamic changes impact on sediment resuspension for large, shallow Lake Taihu, China. Int. J. Sediment Res. 2019, 34, 205–215. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Miljojkovic, D.; Trepsic, I.; Milovancevic, M. Assessment of physical and chemical indicators on water turbidity. Phys. A Stat. Mech. Appl. 2019, 527, 121171. [Google Scholar] [CrossRef]
- Ji, H.; Ding, H.; Tang, L.; Li, C.; Gao, Y.; Briki, M. Chemical composition and transportation characteristic of trace metals in suspended particulate matter collected upstream of a metropolitan drinking water source, Beijing. J. Geochem. Explor. 2016, 169, 123–136. [Google Scholar] [CrossRef]
- Helali, M.A.; Zaaboub, N.; Oueslati, W.; Added, A.; Aleya, L. Suspended particulate matter fluxes along with their associated metals, organic matter and carbonates in a coastal Mediterranean area affected by mining activities. Mar. Pollut. Bull. 2016, 104, 171–181. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Yang, K. Assessment and sources of heavy metals in suspended particulate matter in a tropical catchment, northeast Thailand. J. Clean. Prod. 2020, 265, 121898. [Google Scholar] [CrossRef]
- Lu, Y.-F.; Allen, H.E. Partitioning of copper onto suspended particulate matter in river waters. Sci. Total Environ. 2001, 277, 119–132. [Google Scholar] [CrossRef]
- Fan, J.-Y.; He, X.-Y.; Wang, D.-Z. Experimental study on the effects of sediment size and porosity on contaminant adsorption/desorption and interfacial diffusion characteristics. J. Hydrodyn. 2013, 25, 20–26. [Google Scholar] [CrossRef]
- Miranda, L.S.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Adsorption-desorption behavior of heavy metals in aquatic environments: Influence of sediment, water and metal ionic properties. J. Hazard. Mater. 2022, 421, 126743. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, Y.; Zhang, H.; Huang, C.; Pei, Y. Fraction spatial distributions and ecological risk assessment of heavy metals in the sediments of Baiyangdian Lake. Ecotoxicol. Environ. Saf. 2019, 174, 417–428. [Google Scholar] [CrossRef]
- Kongsune, P.; Rattanapan, S.; Chanajaree, R. The removal of Pb2+ from aqueous solution using mangosteen peel activated carbon: Isotherm, kinetic, thermodynamic and binding energy calculation. Groundw. Sustain. Dev. 2021, 12, 100524. [Google Scholar] [CrossRef]
- Yang, P.; Liu, C.; Guo, Q.; Liu., Y. Variation of activation energy determined by a modified Arrhenius approach: Roles of dynamic recrystallization on the hot deformation of Ni-based superalloy. J. Mater. Sci. Technol. 2021, 72, 162–171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).