Marine Calcareous Biological Ooze Thermoluminescence and Its Application for Paleoclimate Change since the Middle Pleistocene
Abstract
1. Introduction
2. Geographical Setting
2.1. The Ninetyeast Ridge of the Equatorial Northeast Indian Ocean
2.2. The North Atlantic Ice-Rafted Detritus Belt
3. Analysis Methods
3.1. Sample Preparation
3.2. Measurements
4. Results
4.1. TL Measurement Results
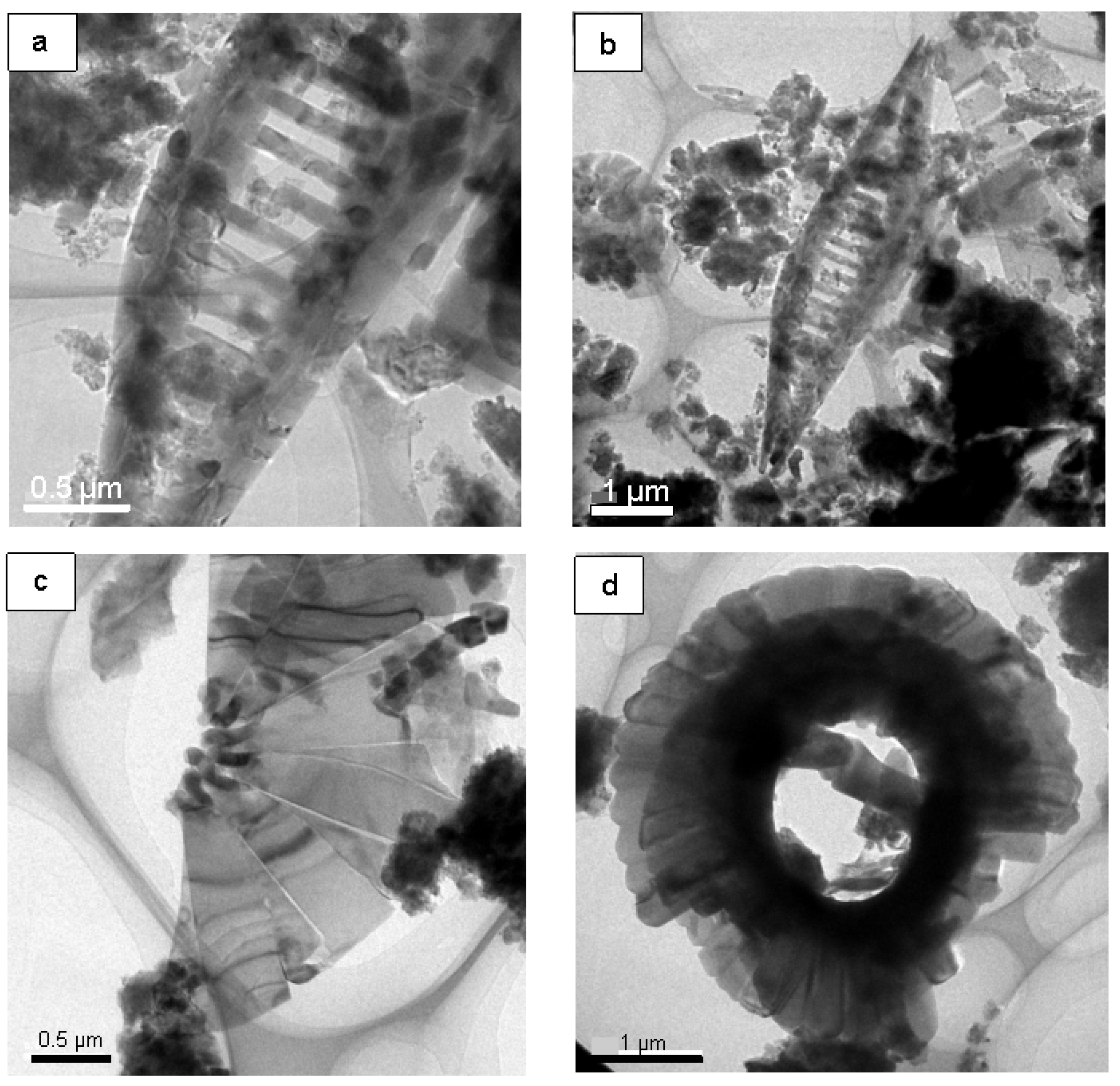
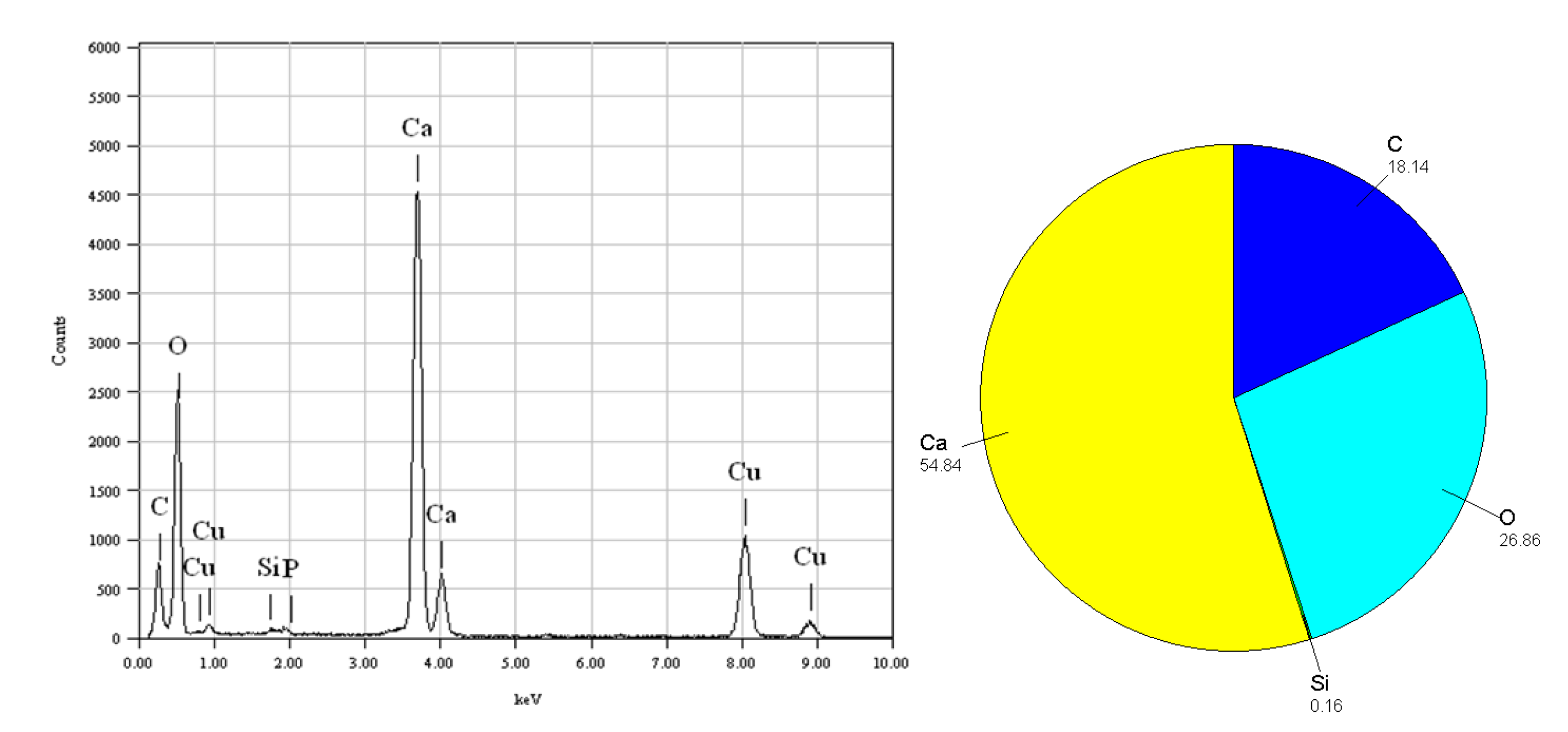
4.2. FE-TEM of Planktonic Foraminifer Shells
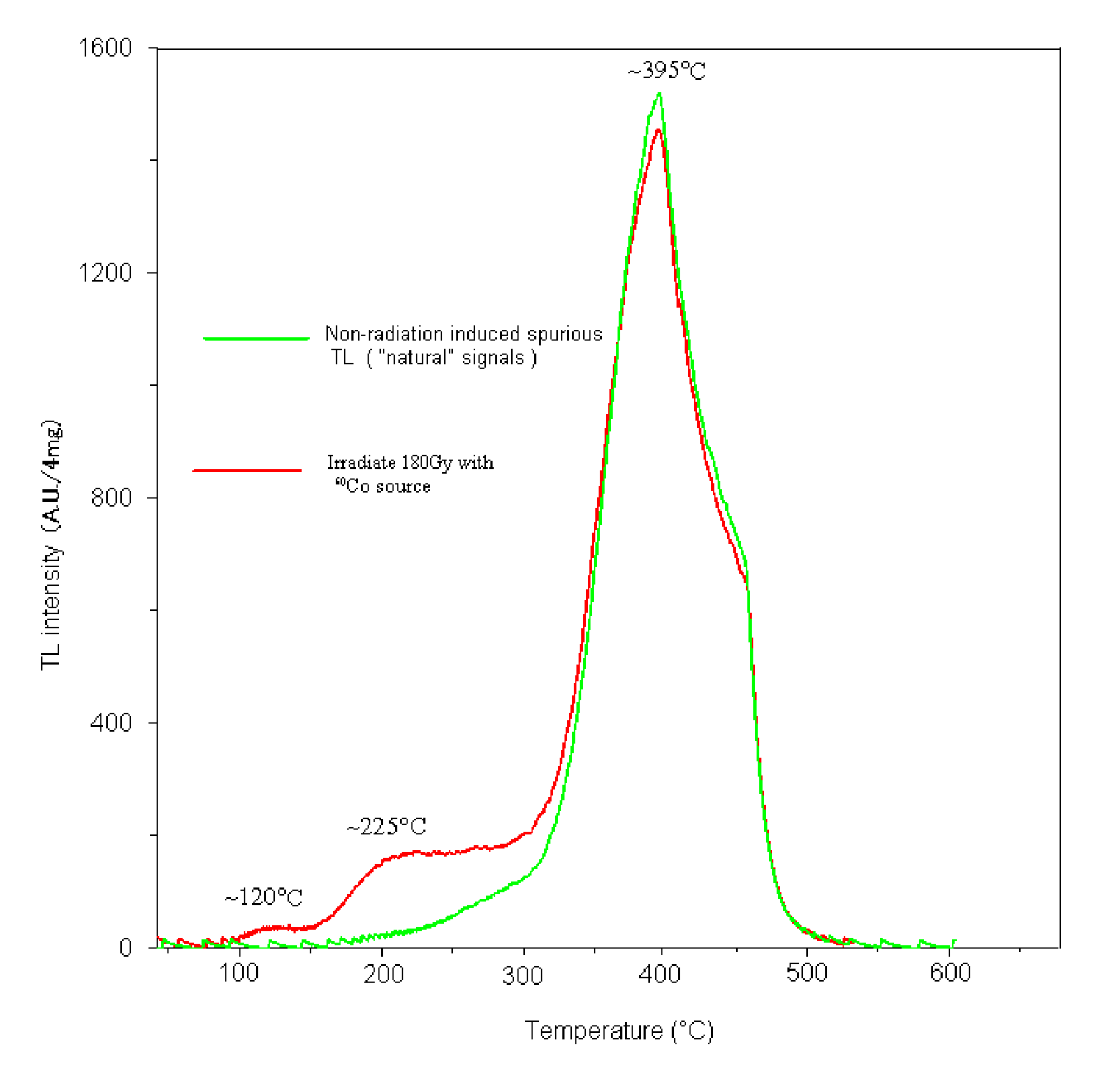
4.3. TL Glow Curve and Thermal Stability of Foraminifer Nannofossil Ooze
4.4. Preservation of TL Signatures
5. Discussion
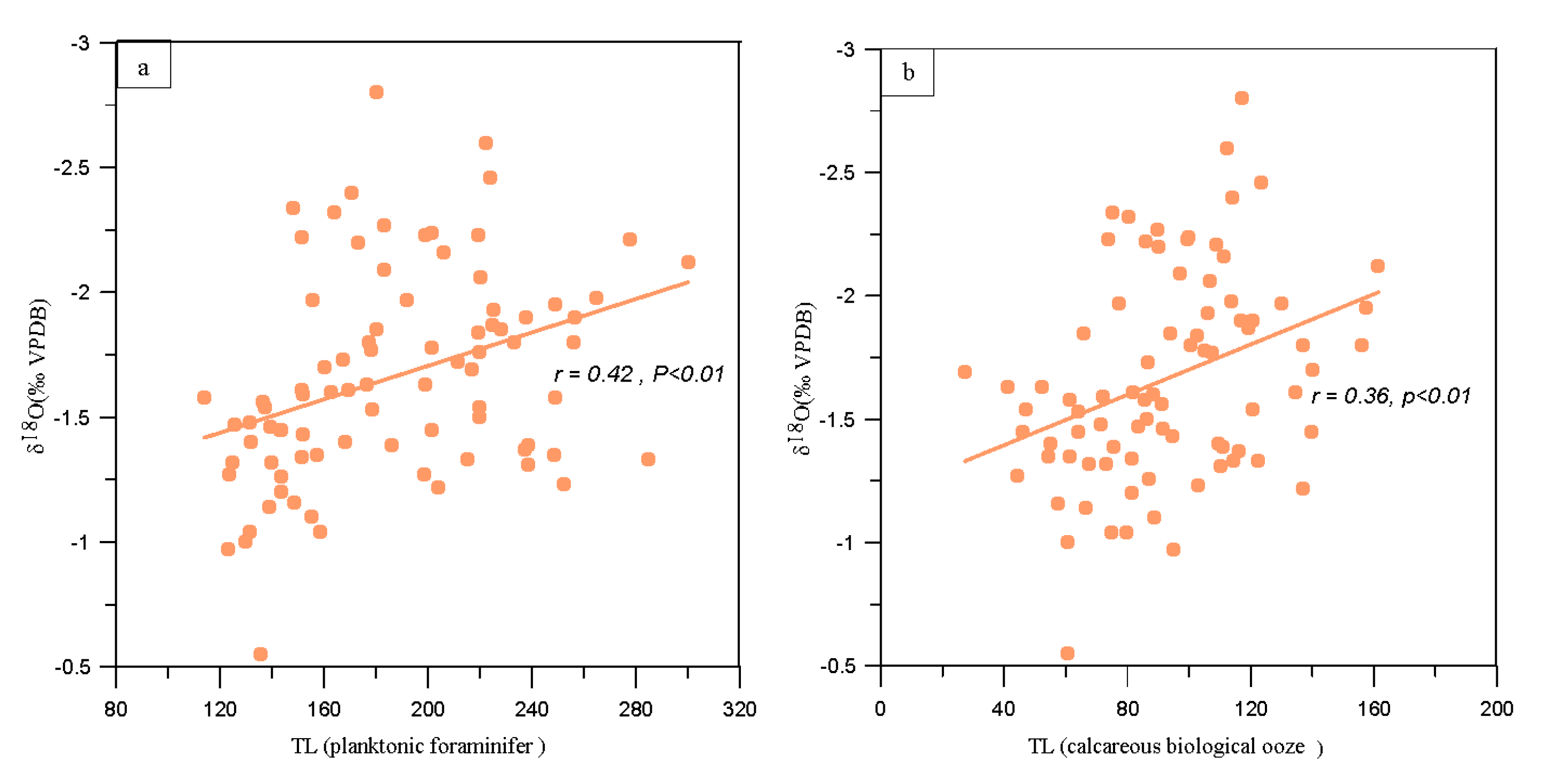
5.1. Relevance between TL and Oxygen Stable Isotope Formation
5.2. Periodic Variations of TL Peak Intensities Associated with Orbital Forcing
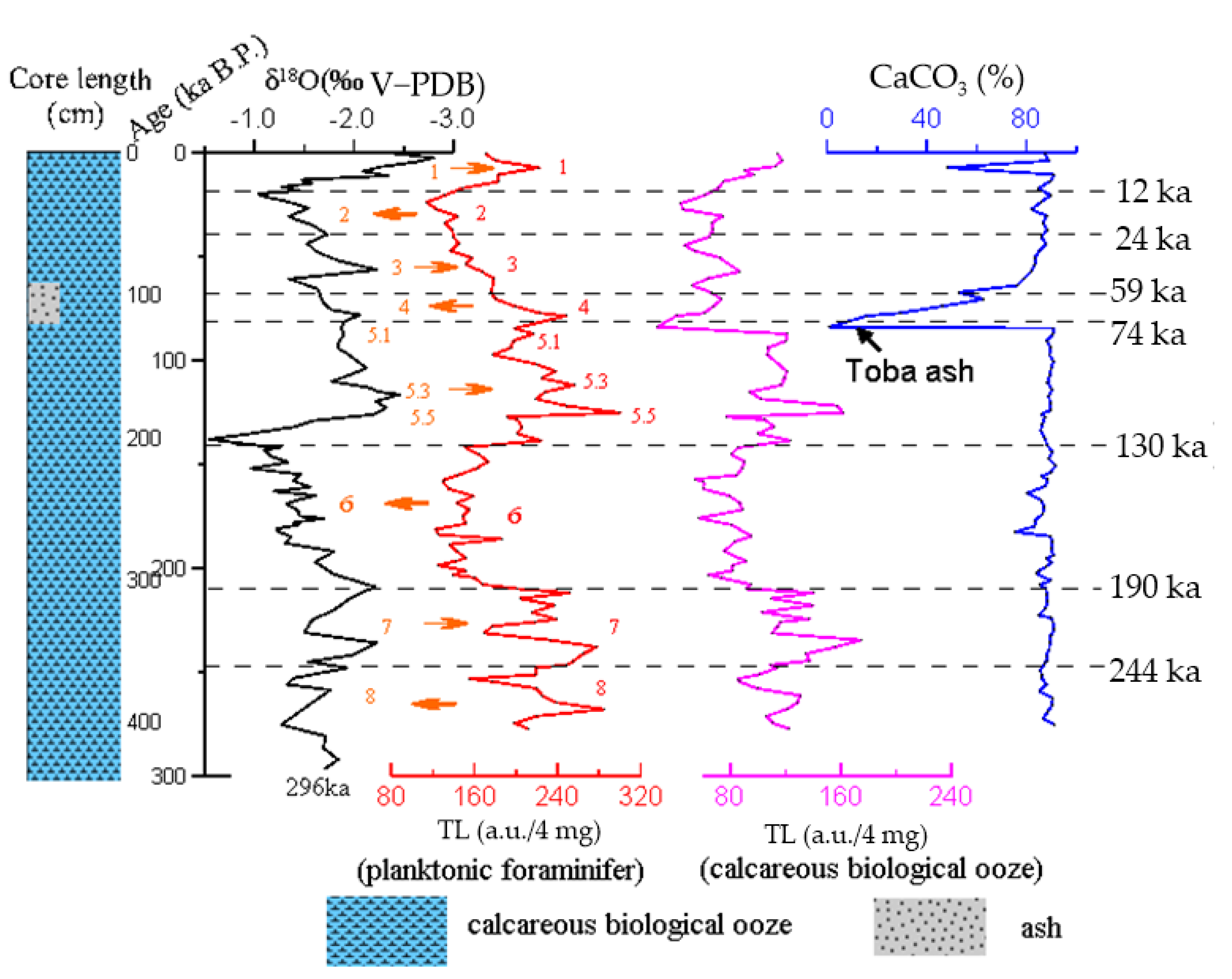
5.3. TL of the Core MD81349 to Paleoclimate Change Response
5.4. TL of the Core U1312B to Paleoclimate Change Response
6. Conclusions
- The TL age profile of calcareous biological ooze reveals eight subglacial cycles and cyclical climate changes since 299 ka B.P. The interglacial period corresponds to enhanced TL. The ice age corresponds to a reduction in TL. The TL of the glacial–interglacial cycle can be compared remotely.
- The analysis results of the TL spectrum show a relationship with the changes in the Earth’s orbital parameters and the corresponding astronomical cycle. Near the equator of the northeast Indian Ocean, it is more significant than the short cycle of 38 ka and 5 ka, while it is more significant than the cycle of 8 ka in the North Atlantic since the middle Pleistocene.
- TL fluctuations have provided us with a relationship between thermoluminescence and the temperature of the formation of calcareous biological ooze.
- Calcareous biological ooze contains TL carrier minerals. The spurious TL intensity of the 395 °C peak from the marine calcareous biological ooze is dose independent, regardless of the irradiation dose.
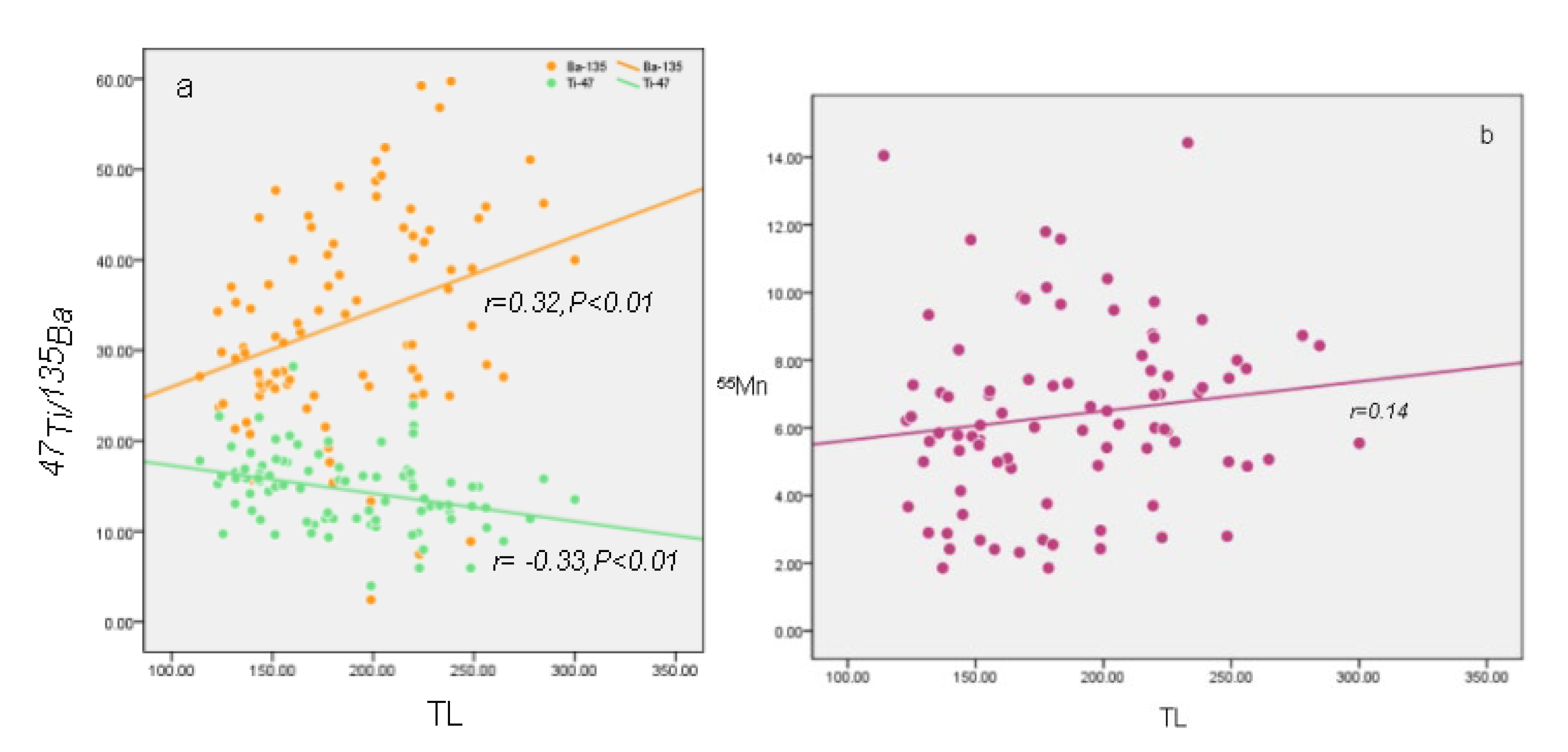
- The impurity ions (e.g., Ba2+ and Mn2+) doped in carbonate act as activators of TL, while Ti2+ acts as a suppressant.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huntley, D.J.; Johnson, H.P. Thermoluminescence as a potential means of dating siliceous ocean sediments. Can. J. Earth Sci. 1976, 13, 593–596. [Google Scholar] [CrossRef]
- Wintle, A.G.; Huntley, D.J. Thermoluminescence dating of a deep-sea sediment core. Nature 1979, 279, 710–712. [Google Scholar] [CrossRef]
- Wintle, A.G.; Huntley, D.J. Thermoluminescence dating of ocean sediments. Can. J. Earth Sci. 1980, 17, 348–360. [Google Scholar] [CrossRef]
- Yassin, A.; Abdel, R. Thermoluminescence dosimetry using natural calcite. J. Taibah Univ. Sci. 2016, 10, 286–295. [Google Scholar]
- Chen, G.F.; Hu, C.Y.; Li, N.; Yi, Z.H. Thermoluminescence in response to the mass extinction event in Penglaitan Section in Laibin, Guangxi. Sci. China Earth Sci. 2013, 56, 1350–1356. [Google Scholar] [CrossRef]
- Joseph, J.T.; Merlin, L.M.; Eduardo, G.Y.; Adam, C.C. Thermoluminescent microparticle thermal history sensors. Microsyst. Nanoeng. 2016, 2, 16037. [Google Scholar] [CrossRef]
- Noriyuki, T.; Atsushi, S.; Hiroshi, I.; Katsuyuki, H.; Takayuki, H. Thermoluminescence of coral skeletons: A high-sensitivity proxy of diagenetic alteration of aragonite. Sci. Rep. 2017, 7, 17969. [Google Scholar] [CrossRef]
- Qiu, Z.P.; Song, H.J.; Hu, C.Y.; Wignall, P.B.; Song, H.Y. Carbonate Thermoluminescence and its implication for marine productivity change during the Permian-Triassic transition. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 526, 72–79. [Google Scholar] [CrossRef]
- Johnson, N.M. An empirical isothermal decay law for the thermoluminescence of calcite. J. Geophys. Res. 1965, 70, 4653–4662. [Google Scholar] [CrossRef]
- Vaz, J.E.; Zeller, E.J. Thermoluminescence of calcite from high gamma radiation doses. Am. Mineral. 1966, 51, 1156–1166. [Google Scholar]
- Medlin, W.L. Color center growth curves in calcite. J. Phys. Chem. Solids 1967, 28, 1725–1733. [Google Scholar] [CrossRef]
- Bothner, M.H.; Johnson, N.M. Natural thermoluminescence dosimetry in Late Pleistocene pelagic sediments. J. Geophys. Res. 1969, 74, 5331–5338. [Google Scholar] [CrossRef]
- Christodoulides, C.; Fremlin, J.H. Thermoluminescence of biological materials. Nature 1971, 232, 257–258. [Google Scholar] [CrossRef]
- Nambi, K.S.V. Thermoluminescence investigations of natural calcite crystals of differing genesis. Thermochim. Acta 1978, 27, 61–67. [Google Scholar] [CrossRef]
- Sunta, C.M. A Review of thermoluminescence of calcium fluoride, calcium sulphate and calcium carbonate. Radiat. Prot. Dosim. 1984, 8, 25–44. [Google Scholar] [CrossRef]
- Calderon, T.; Aguilar, M.; Jaquel, F.; Coy-Yll, R. Thermoluminescence from natural calcites. J. Phys. C Solid State Phys. 1984, 17, 2027–2038. [Google Scholar] [CrossRef]
- Carmichael, L.A.; Sanderson, D.C.W.; Riain, S.N. Thermoluminescence measurement of calcite shells. Radiat. Meas. 1994, 23, 455–463. [Google Scholar] [CrossRef]
- Ninagawa, K.; Takahashi, N.; Wada, T.; Yamamoto, I.; Yamashita, N.; Yamashita, Y. Thermo-luminescence measurements of a calcite shell for dating. Quat. Sci. Rev. 1988, 7, 367–371. [Google Scholar] [CrossRef]
- Duller, G.A.T.; Penkman, K.E.H.; Wintle, A.G. Assessing the potential for using biogenic calcites as dosemeters for luminescence dating. Radiat. Meas. 2009, 44, 429–433. [Google Scholar] [CrossRef]
- Stirling, R.J.; Duller, G.A.T.; Roberts, H.M. Developing a single-aliquot protocol for measuring equivalent dose in biogenic carbonates. Radiat. Meas. 2012, 47, 725–731. [Google Scholar] [CrossRef]
- Liu, H.S.; Fang, N.Q.; Hou, S.L.; Chen, Y.X. Natural thermolum inescence of fossilforaminiferls as a potential proxy for deep sea temperature changes. Acta Oceanol. Sin. 2008, 27, 30–34. [Google Scholar]
- Chen, M.T.; Farrell, J. Planktonic foraminifer faunal variations in the northeastern Indian Ocean: A high-resolution record of the past 800,000 years from site 758. Proc. Ocean. Drill. Program Sci. Results 1990, 121, 125–126. [Google Scholar]
- Farrell, I.W.; Janecek, T.R. Late Neogene paleoceangraphy and paleoclimatology of the northeast Indian Ocean (site758). Proc. Ocean. Drill. Program Sci. Results 1990, 121, 297–350. [Google Scholar]
- Gerard, C.B.; Rusty, L. Iceberg dischardes into the North Atlantic on millennial time scales during the last glaciation. Science 1995, 267, 1005–1010. [Google Scholar]
- Dansgaard, W.; Johnsen, S.J.; Clausen, H.B.; Dahl-Jensen, D.; Gundestrup, N.S.; Hammer, C.U.; Hvidberg, C.S.; Steffensen, J.P.; Sveinbj Örnsdottir, A.E.; Jouzel, J.; et al. Evidence for general instability of past climate from a 250-kyr ice-core record. Nature 1993, 364, 218–220. [Google Scholar] [CrossRef]
- Stein, R.; Kanamatsu, T.; Alvarez Zarikian, C.A.; Higgins, S.; Zhai, Q. North Atlantic paleocenography: The last five million years. EOS 2006, 87, 129–133. [Google Scholar] [CrossRef]
- Channell, J.E.T.; Kanamatsu, T.; Sato, T.; Stein, R.; Alvarez Zarikian, C.A.; Malone, M.J. Expedition 303/306 Scientists. In Proceedings of the Integrated Ocean Drilling Program; Integrated Ocean Drilling Program Management International, Inc., College Station, TX, USA, 2006; Volume 303/306. [Google Scholar] [CrossRef]
- Roque, C.; Guibert, P.; Vartanian, E.; Bechtel, F.; Schvoerer, M. Thermoluminescence—Dating of calcite: Study of heated limestone fragments from Upper Paleolithic layers at Combe Saunière, Dordogne, France. Quat. Sci. Rev. 2001, 20, 935–938. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, C.; Hu, C.Y.; Xie, S.C. Spurious thermoluminescence characteristics of the Ediacaran Doushantuo formation (ca. 635–551 Ma) and its implications for marine dissolved organic carbon reservoir. J. Earth Sci. 2015, 26, 883–892. [Google Scholar] [CrossRef]
- Pagonis, V.; Maniatis, Y.; Michael, C.; Bassiakos, Y. Spurious and regenerated thermoluminescence in calcite powder samples. Radiat. Meas. 1997, 27, 37–42. [Google Scholar] [CrossRef]
- Liu, J.; Fang, N.; Wang, F.; Yang, F.; Ding, X. Features of ice-rafted debris (IRD) at IODP site U1312 and their palaeoenvironmental implications during the last 2.6 Myr. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 511, 364–378. [Google Scholar] [CrossRef]
- Lisiecki, L.E.; Raymo, M.E. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography 2005, 20, PA1003. [Google Scholar] [CrossRef]
- Medlin, W.L. Thermoluminescence of sedimentary rocks. In Proceedings of the 6th World Petroleum Congress, Frankfurt am Main, Germany, 19–26 June 1963; Volume 1, pp. 63–77. [Google Scholar]
- Ponnusamy, V.P.; Ramasamy, V.; Anandalakshmi, K. Effect of preheating in biogenic shells-thermostimulated luminescence and FTIR study. Indian J. Pure Appl. Phys. 2006, 44, 13–19. [Google Scholar]
- Zhang, J.J.; Wang, L.B. Thermoluminescence dating of calcite—Alpha effectiveness and measurement protocols. J. Lumin. 2020, 223, 117205–117213. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, J.J.; Wang, L.B.; Zhao, H.; Li, S.H. Equivalent dose estimation of calcite using isothermal thermoluminescence signals. Quat. Geochronol. 2022, 70, 101310. [Google Scholar] [CrossRef]
- Biswas, R.H.; Herman, F.; King, G.E.; Braun, J. Thermoluminescence of feldspar as a multi-thermochronometer to constrain the temporal variation of rock exhumation in the recent past. Earth Planet. Sci. Lett. 2018, 495, 56–68. [Google Scholar] [CrossRef]
- Biswas, R.H.; Frédéric, H.; Georgina, E.K.; Benjamin, L.; Ashok, K.S. Surface paleothermometry using low-temperature thermoluminescence of feldspar. Clim. Past 2020, 16, 2075–2093. [Google Scholar] [CrossRef]
- Engin, B.; Güven, O. Thermoluminescence dating of Denizli travertines from the southwestern part of Turkey. Appl. Radiat. Isot. 1997, 48, 1257–1264. [Google Scholar] [CrossRef]
- Aitken, M.J. Thermoluminescence Dating; Academic Press Inc.: London, UK, 1985. [Google Scholar]
- Castagnoli, G.C.; Bonono, G.; Provenzale, A. On the thermoluminescence profile of an Ionian sea sediment: Evidence of 137, 118, 12.1, and 10.8y cycles in the last two millennia. Nuovo C. C 1988, 11, 1–12. [Google Scholar] [CrossRef]
- Castagnoli, G.C.; Bonono, G.; Provenzale, A.; Serio, M. On The solar origin of the thermoluminescence profile of the GT14 core. Sol. Phys. 1990, 127, 357–377. [Google Scholar] [CrossRef]
- Rosenthal, Y.; Boyle, E.A.; Labeyrie, L.; Oppo, D. Glacial enrichments of authigenic Cd and U in Subantarctic sediments: A climatic control on the elements’ oceanic budget? Paleoceanography 1995, 10, 395–413. [Google Scholar] [CrossRef]
- Zeller, E.J.; Wary, J.L.; Daniels, F. Factors in age determination of carbonate sediments by thermoluminescence. Bull. Am. Assoc. Pet. Geol. 1957, 41, 121–129. [Google Scholar]
- Zeller, E.J.; Wary, J.L.; Daniels, F. Thermoluminescence induced by pressure and by crystallization. J. Chem. Phys. 1955, 23, 2187. [Google Scholar] [CrossRef]
- Townsend, P.D.; Luff, B.J.; Wood, R.A. Mn2+ transitions in the thermoluminescence emission spectra of calcite. Radiat. Meas. 1994, 23, 433–440. [Google Scholar] [CrossRef]
- Kononova, V.A.; Tarashchan, A.N. Thermoluminescence of carbonates from carbonatites. Int. Geol. Rev. 1970, 12, 272–280. [Google Scholar] [CrossRef]
- Martinson, D.G.; Pisias, N.G.; Hays, J.D.; John, I.; Moore, T.C.; Shackleton, N.J. Age dating and the orbital theory of the ice ages: Development of a high resolution 0-300000 years chronostratigraphy. Quat. Res. 1987, 27, 1–29. [Google Scholar] [CrossRef]
- Shemesh, A.; Charlis, C.D.; Fairbanks, R.G. Oxygen isotopes in biogenic silica: Global changes in ocean temperature and isotopic composition. Science 1992, 256, 1434–1436. [Google Scholar] [CrossRef]
- Berger, A.; Crucifix, M.; Hodell, D.A.; Past Interglacials Working Group of Pages. Interglacials of the last 800,000 years. Rev. Geophys. 2016, 54, 162–219. [Google Scholar] [CrossRef]
- Johnson, N.M. Thermoluminescence in biogenic calcium carbonate. J. Sediment. Res. 1960, 30, 305–313. [Google Scholar]
- Esme, I.; Dilek, T.; Mehmet BEr Muhammed, H. Classification of thermoluminescence features of CaCO3 with long short-term memory model. Luminescence 2021, 36, 1684–1689. [Google Scholar] [CrossRef]
- Ronca, L.B.; Zeller, E.J. Thermoluminescence as a function of climate and temperature. Am. J. Sci. 1965, 263, 416–428. [Google Scholar] [CrossRef]
- Carla, T.; Silvia, A.; Sara, R.; Gianna, V.; Salvatore, M. Data Descriptor: A foraminiferal δ18O record covering the last 2200 years. Sci. Data 2016, 3, 160042. [Google Scholar] [CrossRef]
- Hays, J.D.; Imbrie, J.; Shackleton, N.J. Variations in the Earth orbit pacemaker of the ice age. Science 1976, 194, 1121–1132. [Google Scholar] [CrossRef]
- Ruddiman, W.F.; Raymo, M.; Mcintrye, A. Matayama 41,000 year cycles: North Atlantic Ocean and Northern hemisphere ice sheets. Earth Planet Sci. Lett. 1986, 80, 117–129. [Google Scholar] [CrossRef]
- Jouzel, J.; Lorius, C.; Pettl, J.R.; Genthon, C.; Barkov, N.I.; Kotlyakov, V.M.; Petrov, V.M. Vostok ice core: A continuous isotope temperature record over the last climate cycle (160,000 years). Nature 1987, 329, 403–408. [Google Scholar] [CrossRef]
- Shackleton, N.J. The 100,000-Year Ice-age cycle identified and found to lag temperature, carbon dioxide, and orbital eccentricity. Science 2000, 289, 1897–1902. [Google Scholar] [CrossRef]
- Casstagnoli, G.C.; Bonino, G.; Attolini, M.R.; Galli, M. The 11y cycle in the thermoluminescence profile of sea sediments. Nuovo C. C 1984, 7, 69–73. [Google Scholar] [CrossRef]
- Casstagnoli, G.C.; Bonino, G.; Attolini, M.R.; Beer, J. Solar cycles in the last centuries in 10Be and δ18O in polar ice and in thermoluminescence signals of a sea sediment. Nuovo C. C 1984, 7, 235–244. [Google Scholar] [CrossRef]
- Casstagnoli, G.C.; Bonino, G. Comparison of thermoluminescence profiles in recent sea cores. Nucl. Tracks Radiat. Meas. 1985, 10, 759–761. [Google Scholar] [CrossRef]
- Casstagnoli, G.C.; Bonino, G.; Attolini, M.R.; Nanni, T. The Schwabe cycle in the thermoluminescence profiles of an Ionian sea core. Nuovo C. C 1987, 10, 315–322. [Google Scholar] [CrossRef]
- Castagnoli, G.C.; Bonono, G.; Della, M.P.; Taricco, C. Record of thermoluminescence in sea sediments in the last millennia. Nuovo C. C 1997, 20, 1–8. [Google Scholar]
- Castagnoli, G.C.; Bonono, G.; Della, M.P.; Procopio, S.; Taricco, C. On the solar origin of the 200y Suess wiggles: Evidence from thermoluminescence in sea sediments. Nuovo C. C 1998, 21, 237–241. [Google Scholar]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Rose, W.I.; Chesner, C.A. Dispersal of ash in the great Toba eruption, 75ka. Geology 1987, 15, 913–917. [Google Scholar] [CrossRef]
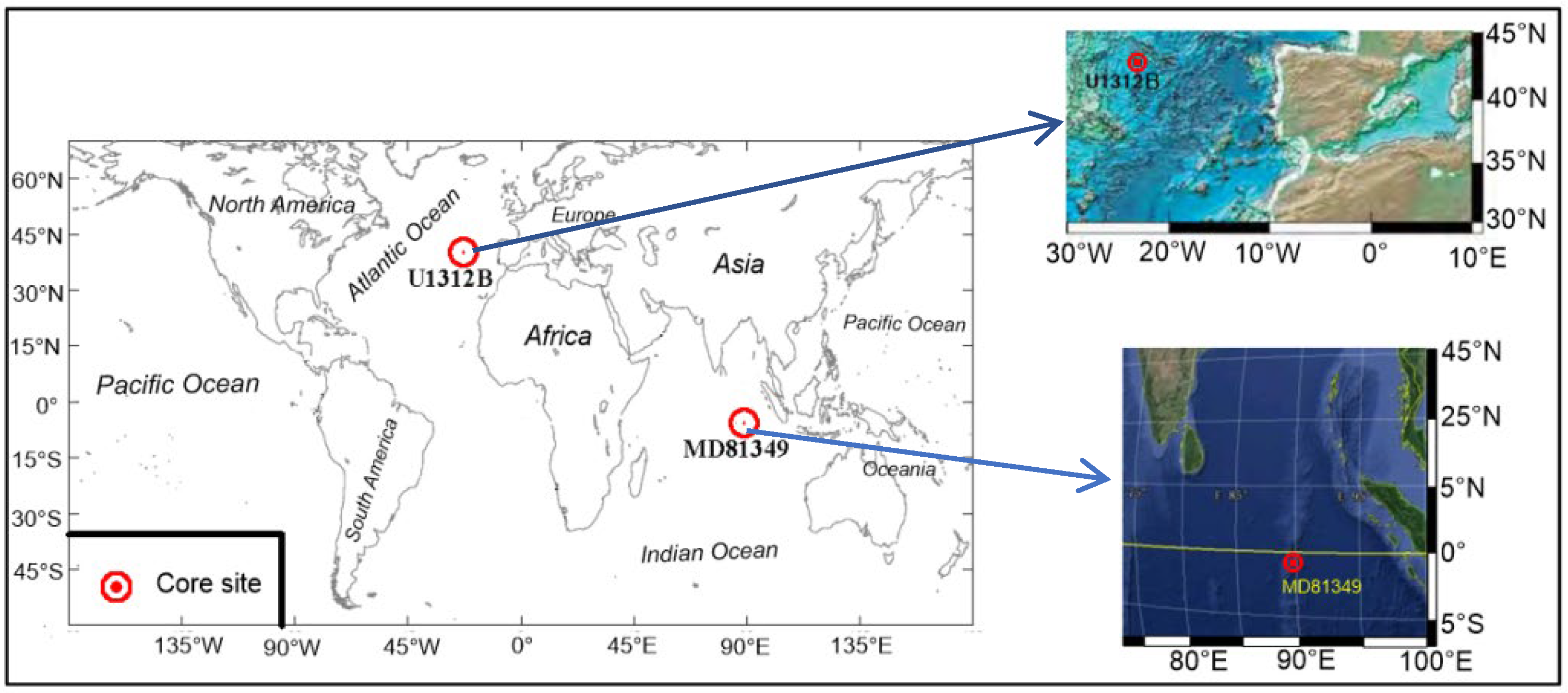




| Regions | Core | Seawater Depth(m) | Core Length(m) | Latitude | Longitude | Age | Core DrillingTime |
|---|---|---|---|---|---|---|---|
| Northeast Indian Ocean | MD81349 | 2505 | 4.30 | 1°01′0 S | 89°22′0 E | 296 ka B.P. | 1981 |
| North Atlantic Ocean | IODP306-U1312B (1H-2H)CC * | 3533.6 | 6.60 | 42°50.2150′ N | 23°5.2652′ W | 299 ka B.P. | 2005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Liu, H.; Hou, S.; Wang, N.; Fang, N. Marine Calcareous Biological Ooze Thermoluminescence and Its Application for Paleoclimate Change since the Middle Pleistocene. Water 2023, 15, 2618. https://doi.org/10.3390/w15142618
Zhang P, Liu H, Hou S, Wang N, Fang N. Marine Calcareous Biological Ooze Thermoluminescence and Its Application for Paleoclimate Change since the Middle Pleistocene. Water. 2023; 15(14):2618. https://doi.org/10.3390/w15142618
Chicago/Turabian StyleZhang, Ping, Haisheng Liu, Shengli Hou, Nanping Wang, and Nianqiao Fang. 2023. "Marine Calcareous Biological Ooze Thermoluminescence and Its Application for Paleoclimate Change since the Middle Pleistocene" Water 15, no. 14: 2618. https://doi.org/10.3390/w15142618
APA StyleZhang, P., Liu, H., Hou, S., Wang, N., & Fang, N. (2023). Marine Calcareous Biological Ooze Thermoluminescence and Its Application for Paleoclimate Change since the Middle Pleistocene. Water, 15(14), 2618. https://doi.org/10.3390/w15142618






