The Mechanisms Controlling the CO2 Outgassing of a Karst Spring–River–Lake Continuum: Evidence from Baotuquan Spring Drainage Area, Jinan City, Northern China
Abstract
1. Introduction
2. Materials and Methods
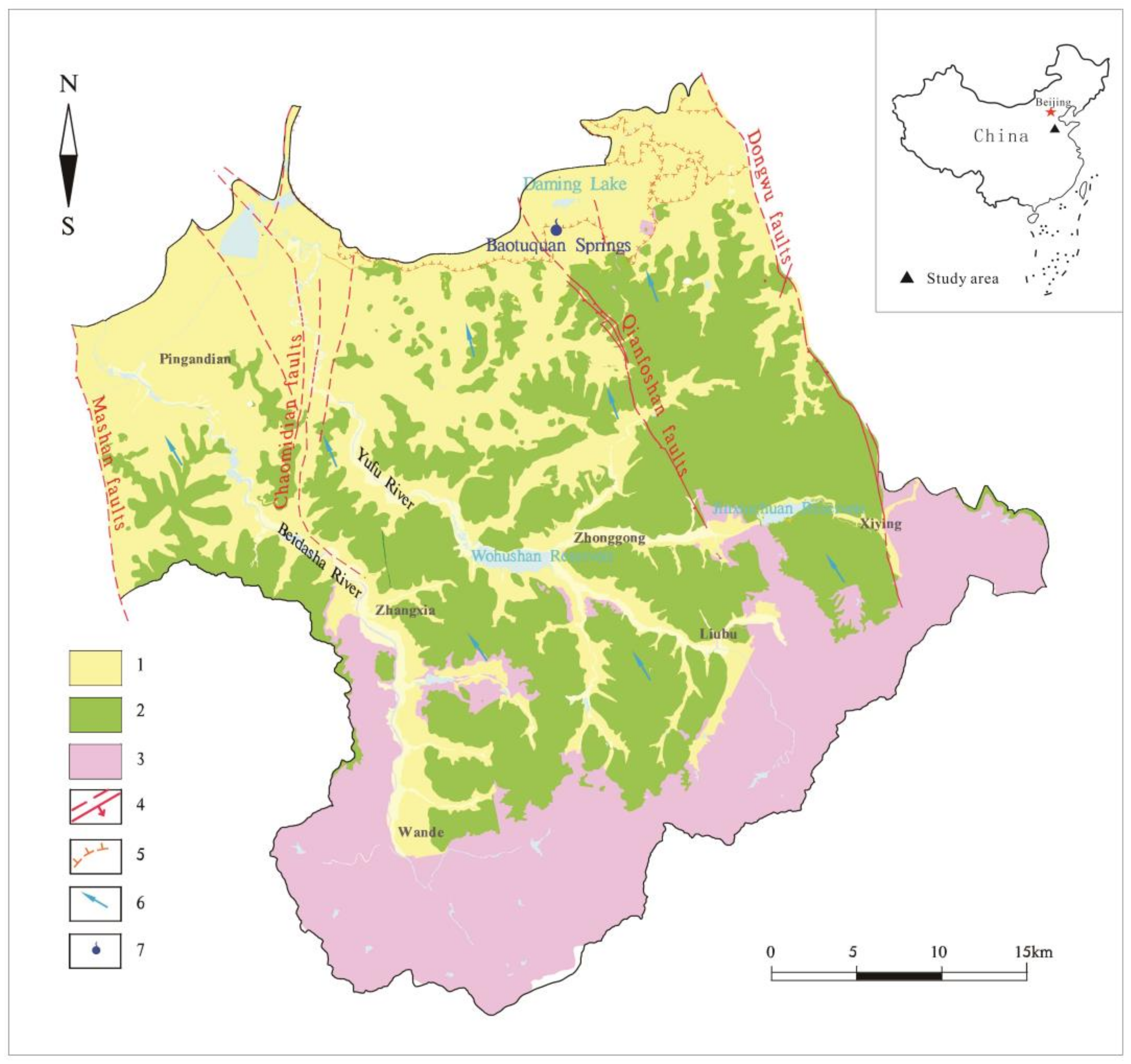
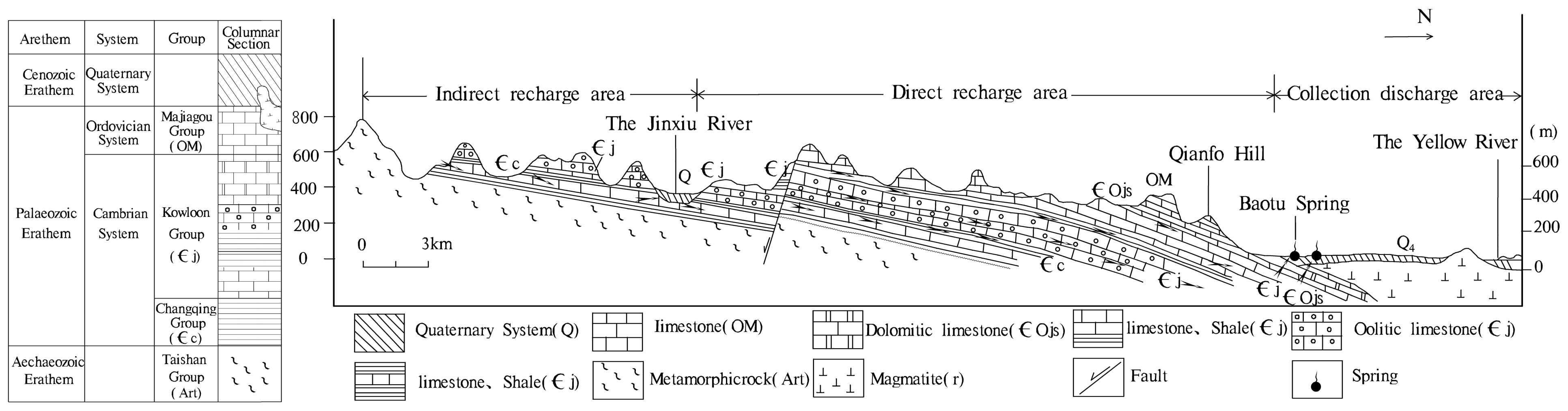
2.1. Study Area
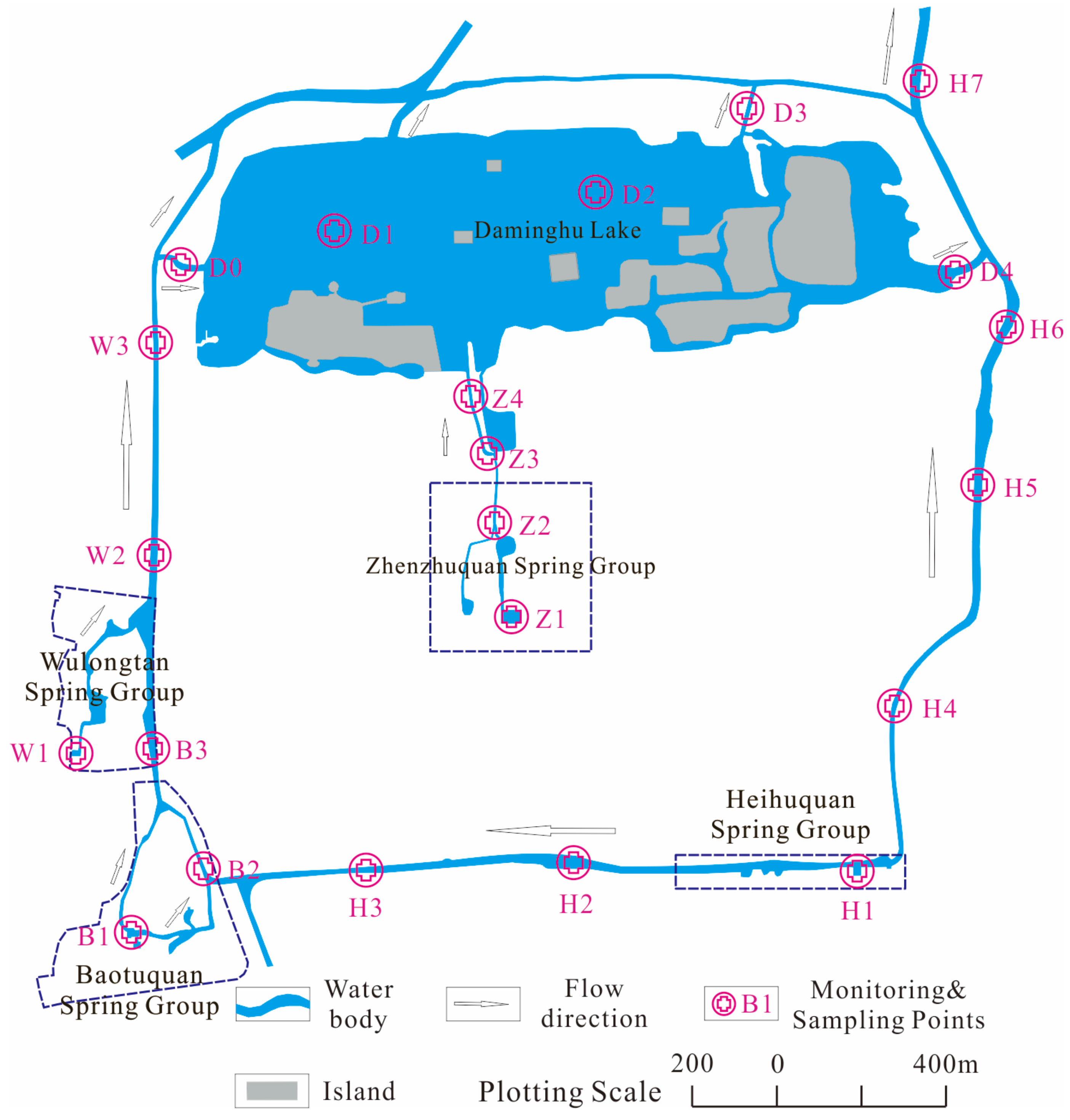
2.2. Sampling and Analysis
2.2.1. Hydrochemical Field Parameters
2.2.2. Discrete Sample Collection and Analysis
2.3. CO2 Fluxes across the Water–Air Interface
3. Results
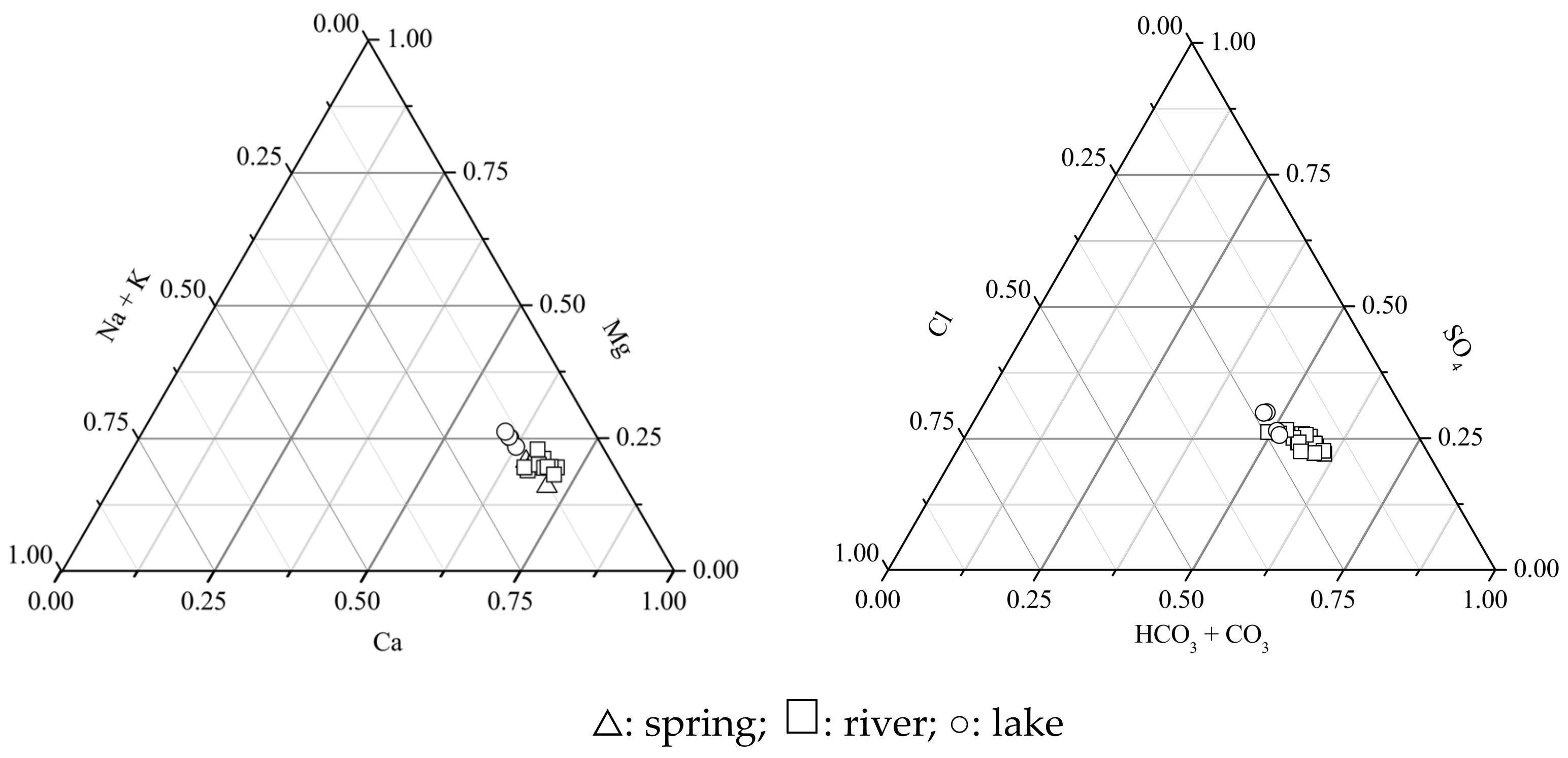
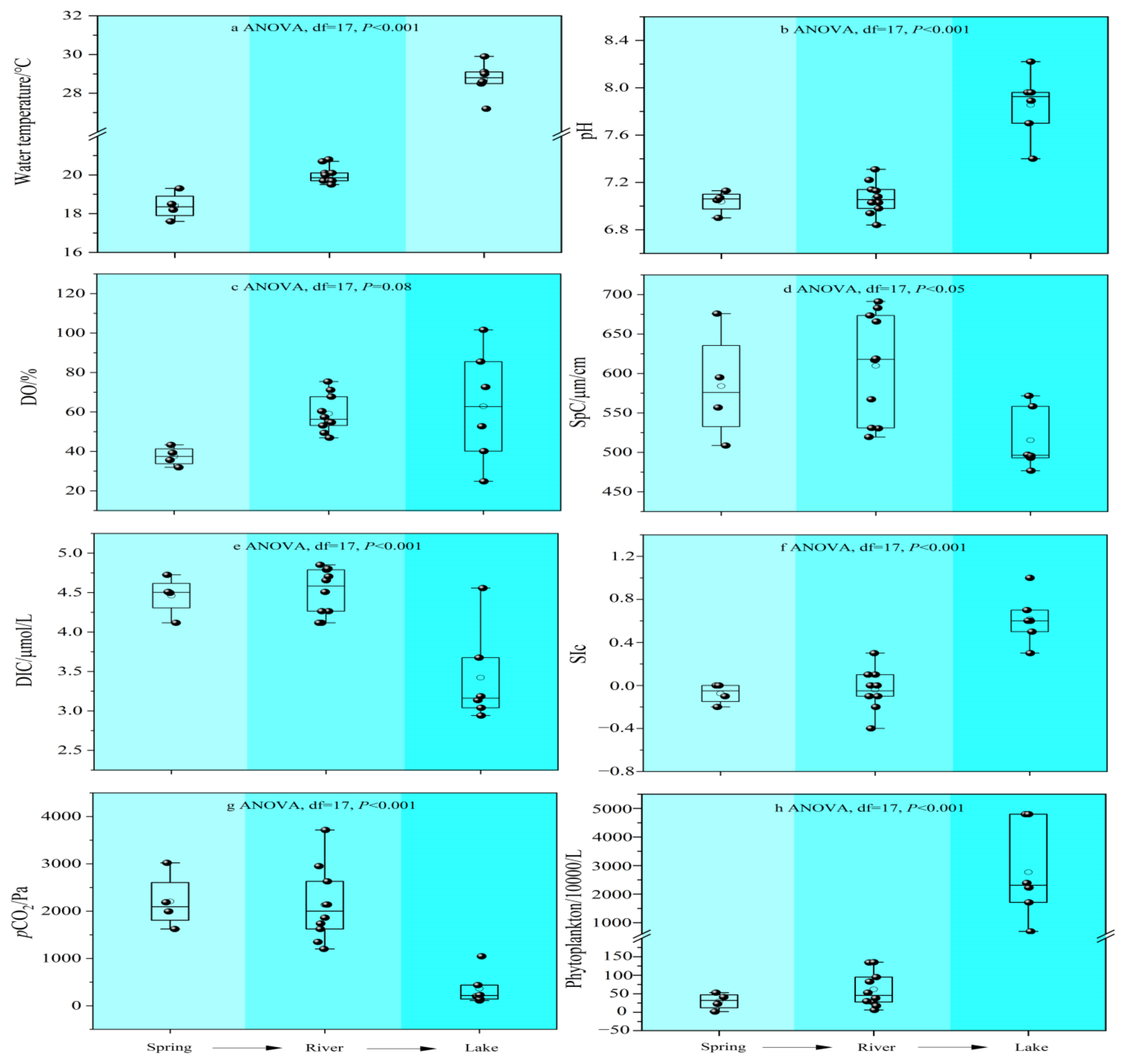
3.1. Hydrochemical Composition
3.2. Phytoplankton
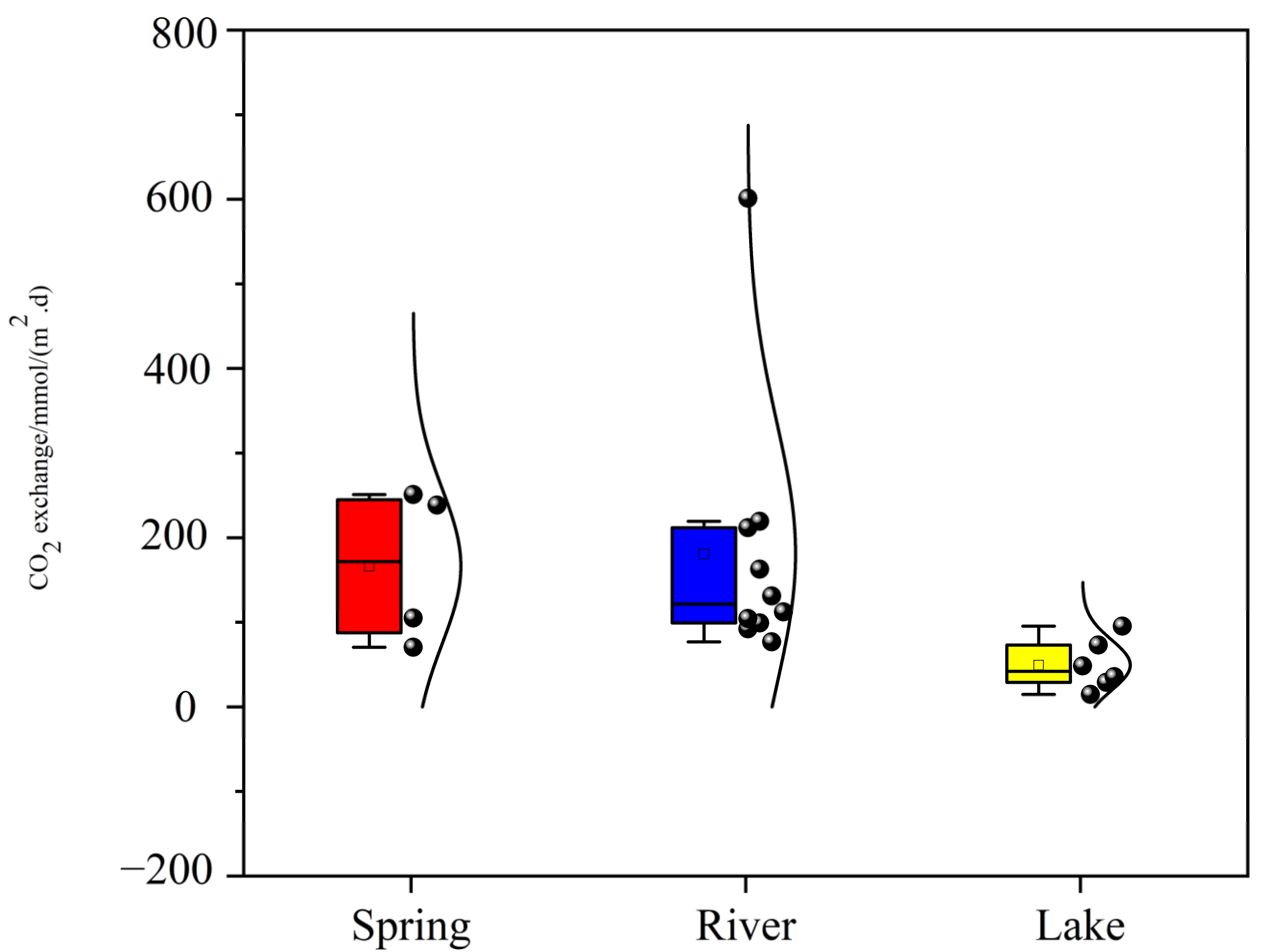
3.3. CO2 Degassing Fluxes at the Water–Air Interface
4. Discussion
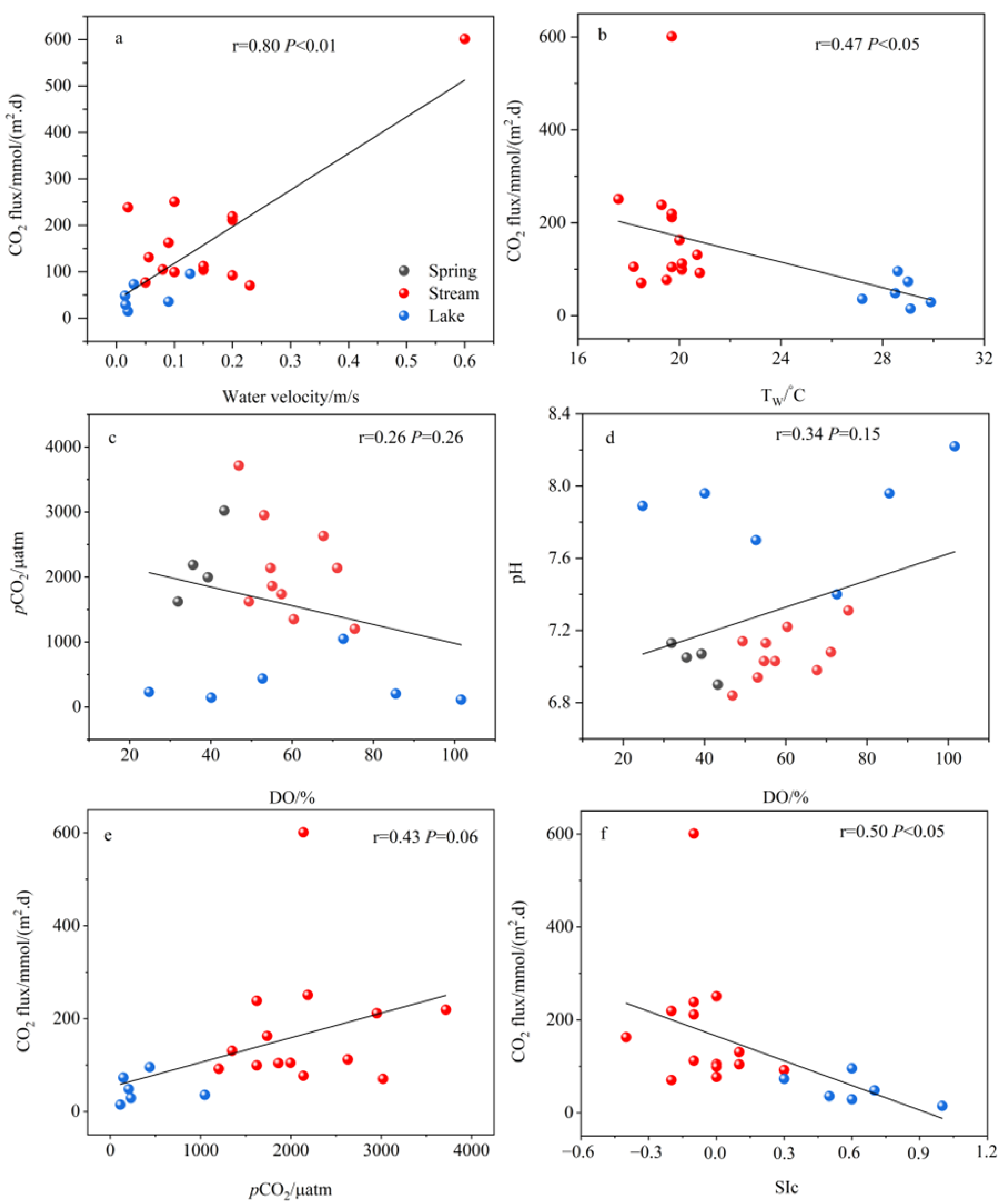
4.1. Controls on CO2 Fluxes
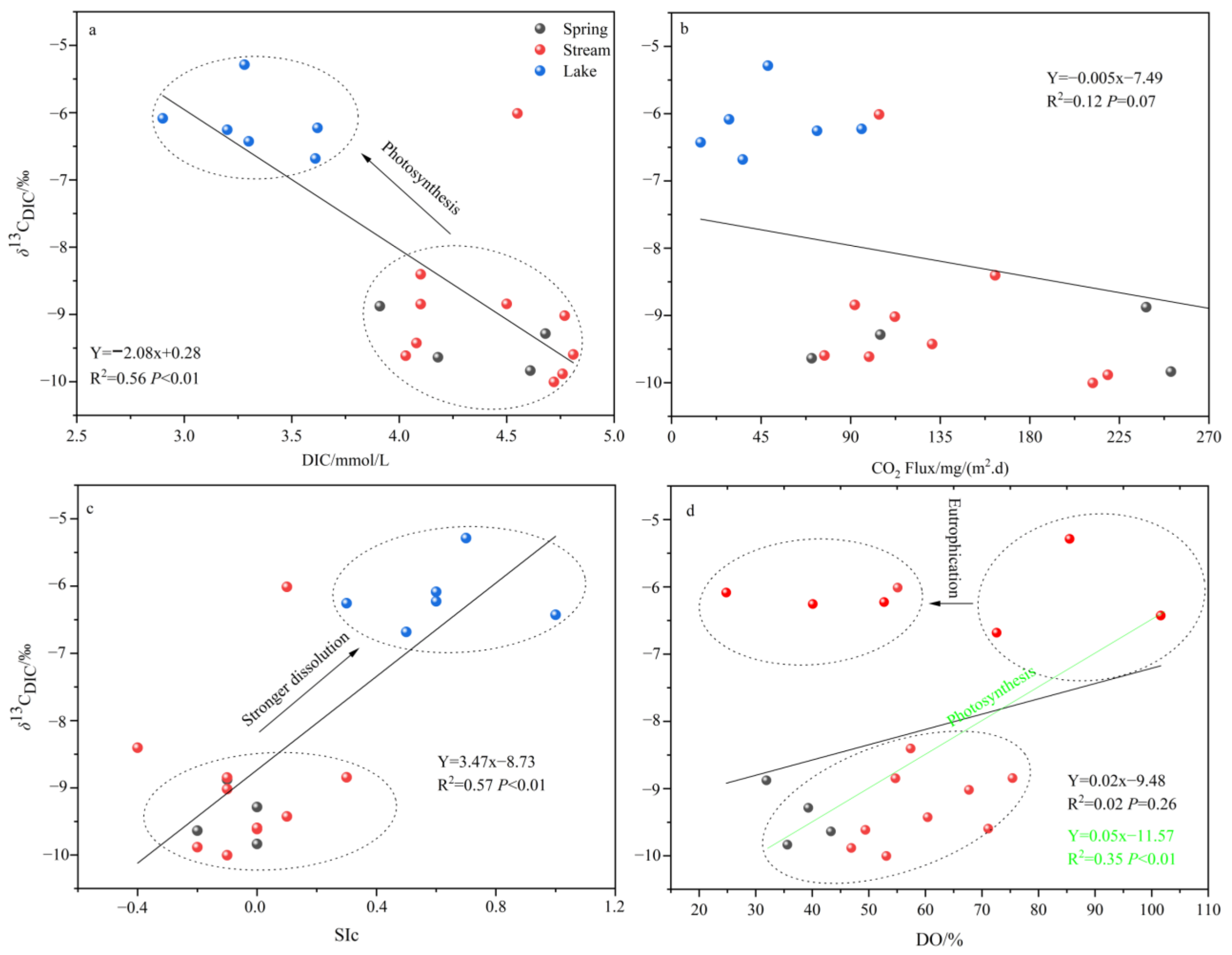
4.2. Processes Affecting the Decline of CO2 Fluxes in Lakes
4.3. Implications
5. Conclusions
- (1)
- The water chemistry parameters such as pH, DIC, SIc, pCO2, and planktonic biomass changed little between the two because of the rapid input to the river after the spring outcrop, but they all changed dramatically after entering the lake. Due to the presence of a significant pCO2 concentration gradient, the spring emerges from the dew point with high CO2 off-gassing. And as the spring enters the river, the degree of influence of flow velocity also increases significantly, and the river CO2 degassing volume under the joint control of both shows a slightly increasing trend. However, as the river water joins the lake, the CO2 degassing volume shows a significant decreasing trend.
- (2)
- Both the decrease in flow velocity and the increase in temperature in the lake promoted the survival and reproduction of phytoplankton, making the phytoplankton biomass in the lake as high as 3383.79 × 104 per L, which provided favorable conditions for the photosynthesis of aquatic plants. In addition, the significantly decreasing DIC concentration and increasing δ13CDIC values of the lake also indicated the important role of aquatic plant photosynthesis in limiting CO2 degassing in the lake. Of course, the summer eutrophication of the lake, influenced by domestic sewage, also increased the intensity of aquatic plant photosynthesis, but at the same time, it constrained the dissolved oxygen (DO) concentration of the lake water.
- (3)
- The CO2 degassing processes at the water–gas interface of karst springs, rivers, and lakes show significant differences under the influence of different physical, chemical, and biological interactions. However, by comparing the CO2 degassing processes at the water–gas interface of karst springs, rivers, and lakes, it was found that karst lakes are the most active places for the biogeochemical processes of carbon elements, which are not only influenced by the complex effects of river inputs, human activities, and the carbon cycle of the lakes themselves but are also important places for the possible formation of carbon sinks, which deserve careful verification and in-depth study.
- (4)
- The results of this study highlight the significance of the dynamic response of CO2 degassing processes at the water–gas interface to different inland karst water bodies, i.e., spring–river–lake systems. It is an ideal study area to study the response of karst river carbon fluxes to the artificially constructed river–lake system.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 172–185. [Google Scholar] [CrossRef]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Ciais, P.; Sabine, C.; Bala, G.; Bopp, L.; Brovkin, V.; Canadell, J.; Chhabra, A.; DeFries, R.; Galloway, J.; Heimann, M.; et al. Carbon and Other Biogeochemical Cycles. Climate Change. The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 465–570. [Google Scholar]
- Park, P.K.; Gordon, L.I.; Hager, S.W.; Cissell, M.C. Carbon Dioxide Partial Pressure in the Columbia River. Science 1969, 166, 867–868. [Google Scholar] [CrossRef]
- Kling, G.W.; Kipphut, G.W.; Miller, M.C. The flux of CO2 and CH4 from lakes and rivers in arctic Alaska. Hydrobiologia 1992, 240, 23–36. [Google Scholar] [CrossRef]
- Cole, J.J.; Caraco, N.F.; Kling, G.W.; Kratz, T.K. Carbon dioxide supersaturation in the surface waters of lakes. Science 1994, 265, 1568–1570. [Google Scholar] [CrossRef]
- Raymond, P.A.; Caraco, N.F.; Cole, J.J. Carbon Dioxide Concentration and Atmospheric Flux in the Hudson River. Estuaries 1997, 20, 381–390. [Google Scholar] [CrossRef]
- Frankignoulle, M.; Abril, G.; Borges, A.; Bourge, I.; Canon, C.; Delille, B.; Libert, E.; Théate, J.-M. Carbon Dioxide Emission from European Estuaries. Science 1998, 282, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Hope, D.; Palmer, S.M.; Billett, M.F.; Dawson, J.J.C. Variations in dissolved CO2 and CH4 in a first-order stream and catchment: An investigation of soil-stream linkages. Hydrol. Process. 2004, 18, 3255–3275. [Google Scholar] [CrossRef]
- Yao, G.; Gao, Q.; Wang, Z.; Huang, X.; He, T.; Zhang, Y.; Jiao, S.; Ding, J. Dynamics of CO2 partial pressure and CO2 outgassing in the lower reaches of the Xijiang River, a subtropical monsoon river in China. Sci. Total. Environ. 2007, 376, 255–266. [Google Scholar] [CrossRef]
- Lynch, J.K.; Beatty, C.M.; Seidel, M.P.; Jungst, L.J.; DeGrandpre, M.D. Controls of riverine CO2over an annual cycle determined using direct, high temporal resolution pCO2 measurements. J. Geophys. Res. Atmos. 2010, 115, G03016. [Google Scholar] [CrossRef]
- Wang, S.; Yeager, K.M.; Wan, G.; Liu, C.; Wang, Y.; Lü, Y. Carbon export and HCO3− fate in carbonate catchments: A case study in the karst plateau of southwestern China. Appl. Geochem. 2012, 27, 64–72. [Google Scholar] [CrossRef]
- Li, S.; Lu, X.X.; Bush, R.T. CO2 partial pressure and CO2 emission in the Lower Mekong River. J. Hydrol. 2013, 504, 40–56. [Google Scholar] [CrossRef]
- Pu, J.; Li, J.; Khadka, M.B.; Martin, J.B.; Zhang, T.; Yu, S.; Yuan, D. In-stream metabolism and atmospheric carbon sequestration in a groundwater-fed karst stream. Sci. Total. Environ. 2017, 579, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.S.; Wang, Y.; Zhang, J.; Xu, H.; Wei, X. Human impact on the historical change of CO2degassing flux in River Changjiang. Geochem. Trans. 2007, 8, 7. [Google Scholar] [CrossRef]
- Ran, L.; Lu, X.X.; Yang, H.; Li, L.; Yu, R.; Sun, H.; Han, J. CO2outgassing from the Yellow River network and its implications for riverine carbon cycle. J. Geophys. Res. Biogeosci. 2015, 120, 1334–1347. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Yuan, X.; Chen, H.; Peng, C.; Zhu, Q.; Yue, J.; Ren, H.; Deng, W.; Liu, H. pCO2 and CO2 fluxes of the metropolitan river network in relation to the urbanization of Chongqing, China. J. Geophys. Res. Biogeosci. 2017, 122, 470–486. [Google Scholar] [CrossRef]
- Banks, D.; Odling, N.E.; Skarphagen, H.; Rohr-Torp, E. Permeability and stress in crystalline rocks. Terra Nova 1996, 8, 223–235. [Google Scholar] [CrossRef]
- Stober; Bucher. Origin of salinity of deep groundwater in crystalline rocks. Terra Nova 1999, 11, 181–185. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Kortelainen, P.; Sobek, S.; Müller, R.; Rantakari, M. Carbon Dioxide in Boreal Surface Waters: A Comparison of Lakes and Streams. Ecosystems 2012, 15, 1295–1307. [Google Scholar] [CrossRef]
- Peter, H.; Singer, G.A.; Preiler, C.; Chifflard, P.; Steniczka, G.; Battin, T.J. Scales and drivers of temporal pCO2 dynamics in an Alpine stream. J. Geophys. Res. Biogeosci. 2014, 119, 1078–1091. [Google Scholar] [CrossRef]
- Lehner, B.; Liermann, R.; Revenga, C.; Vorosmarty, C.; Fekete, B.; Crouzet, P.; Döll, P.; Endejan, M.; Frenken, K.; Magome, J.; et al. High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front. Ecol. Environ. 2011, 9, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the world’s free-flowing rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.V.; Stanford, J.A. The Serial Discontinuity Concept of Lotic Ecosystem; Fontaine, T.D., Bartell, S.M., Eds.; Dynamics of Lotic Ecosystems; Ann Arbor Science Publishers: Ann Arbor, MI, USA, 1983; pp. 29–42. [Google Scholar]
- Barros, N.; Cole, J.J.; Tranvik, L.J.; Prairie, Y.T.; Bastviken, D.; Huszar, V.L.; del Giorgio, P.; Roland, F. Carbon emission from hydroelectric reservoirs linked to reservoir age and latitude. Nat. Geosci. 2011, 4, 593–596. [Google Scholar] [CrossRef]
- Milanović, P. Dams and Reservoirs in Karst. In Karst Management; Van Beynen, P.E., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 47–73. [Google Scholar]
- Manoutsoglou, E.; Lazos, I.; Steiakakis, E.; Vafeidis, A. The Geomorphological and Geological Structure of the Samaria Gorge, Crete, Greece—Geological Models Comprehensive Review and the Link with the Geomorphological Evolution. Appl. Sci. 2022, 12, 10670. [Google Scholar] [CrossRef]
- Gong, R.B.; Han, Z.S.; Wang, T.F.; Li, C.M.; Zheng, B.D.; Zhou, X.V.; Wang, Z.H.; Zhang, B.R. Hydrogeological Survey Report of Water Supply Near Ji’nan; Hydrogeology Engineering Geology Brigade of Shandong Geological Bureau: Ji’nan, China, 1959. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA; The American Water Works Association and the Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Atekwana, E.A.; Krishnamurthy, R.V. Seasonal variations of dissolved inorganic carbon and δ13C of surface waters: Application of a modified gas evolution technique. J. Hydrol. 1998, 205, 265–278. [Google Scholar] [CrossRef]
- Wigley, T.M.L. WATSPEC: A Computer Program for Determining the Equilibrium Speciation of Aqueous Solutions; Geo-Abstracts for the journal of British Geomorphological Research Group; British Society for Geomorphology: London, UK, 1977; pp. 1–49. [Google Scholar]
- Hu, H.J.; Wei, Y.X. Freshwater Algae in China: System, Ecology and Classification; Science Press: Beijing, China, 2006. [Google Scholar]
- Liu, W.; Pu, J.B.; Zhang, C. A Portable Greenhouse Gas Collection Equipment in Water and Land Dual-Use. China Patent ZL 201420363633.4, 11 May 2014. (In Chinese). [Google Scholar]
- UNESCO/IHAGHG. Greenhouse gas emissions related to freshwater reservoirs. In World Bank Report; The World Bank: Washington, DC, USA, 2010; pp. 64–127. [Google Scholar]
- Zheng, L.; Tang, H.; Wang, Z.; Wang, Z. Phytoplankton investigation and water quality evaluation in the landscape water of Daminghu Lake in Jiannan. J. Shandong Norm. Univ. 2017, 32, 135–138. (In Chinese) [Google Scholar]
- Zhang, T.; Li, J.; Pu, J.; Martin, J.B.; Khadk, M.B.; Wu, F.; Li, L.; Jiang, F.; Huang, S.; Yuan, D. River sequesters atmospheric carbon and limits the CO2 degassing in karst area, southwest China. Sci. Total. Environ. 2017, 609, 92–101. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Pu, J.; Yuan, D. Carbon dioxide exchanges and their controlling factors in Guijiang River, SW China. J. Hydrol. 2019, 578, 124073. [Google Scholar] [CrossRef]
- Drysdale, R.; Lucas, S.; Carthew, K. The influence of diurnal temperatures on the hydrochemistry of a tufa-depositing stream. Hydrol. Process. 2003, 17, 3421–3441. [Google Scholar] [CrossRef]
- Alin, S.R.; de Fátima, F.L.; Rasera, M.; Salimon, C.I.; Richey, J.E.; Holtgrieve, G.W.; Krusche, A.V.; Snidvongs, A. Physical controls on carbon dioxide transfer velocity and flux in low-gradient river systems and implications for regional carbon budgets. J. Geophys. Res. Atmos. 2011, 116, G01009. [Google Scholar] [CrossRef]
- Hall, R.O.; Ulseth, A.J. Gas exchange in streams and rivers. WIREs Water 2020, 7, e1391. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Pu, J.; Wu, F. Physical and chemical control on CO2 gas transfer velocities from a low-gradient subtropical stream. Water Res. 2021, 204, 117564. [Google Scholar] [CrossRef] [PubMed]
- Raymond, P.A.; Zappa, C.J.; Butman, D.; Bott, T.L.; Potter, J.; Mulholland, P.; Laursen, A.E.; McDowell, W.H.; Newbold, D. Scaling the gas transfer velocity and hydraulic geometry in streams and small rivers. Limnol. Oceanogr. Fluids Environ. 2012, 2, 41–53. [Google Scholar] [CrossRef]
- Barth, J.A.; Veizer, J. Carbon cycle in St. Lawrence aquatic ecosystems at Cornwall (Ontario), Canada: Seasonal and spatial variations. Chem. Geol. 1999, 159, 107–128. [Google Scholar] [CrossRef]
- Meybeck, M. Riverine transport of atmospheric carbon: Sources, global typology and budget. Water Air Soil Pollut. 1993, 70, 443–463. [Google Scholar] [CrossRef]
- Ludwig, W.; Amiotte Suchet, P.; Probst, J.L. River discharges of carbon to the world’s oceans: Determining local inputs of alkalinity and of dissolved and particulate organic carbon. Sci. Terre Et Planètes (Comptes rendus de l’Académie des sciences) 1996, 323, 1007–1014. [Google Scholar]
- De Montety, V.; Martin, J.B.; Cohen, M.; Foster, C.; Kurz, M. Influence of diel biogeochemical cycles on carbonate equilibrium in a karst river. Chem. Geol. 2011, 283, 31–43. [Google Scholar] [CrossRef]
- Öequist, M.G.; Wallin, M.; Seibert, J.; Bishop, K.; Laudon, H. Dissolved Inorganic Carbon Export Across the Soil/Stream Interface and Its Fate in a Boreal Headwater Stream. Environ. Sci. Technol. 2009, 43, 7364–7369. [Google Scholar] [CrossRef]
- Grömping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2006, 17, 139–147. [Google Scholar] [CrossRef]
- Wang, S.; Wan, G.; Liu, C.; Yang, W.; Zhu, Z.; Xiao, H.; Tao, F. Geochemical changes of CO2 and its atmospheric CO2 source sink effect in lakes of Yunnan-Guizhou Plateau. Quat. Res. 2003, 23, 581. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, C.; Wang, F.; Wang, B.; Wang, S.; Liu, F. Spatiotemporal characteristics and diffusion flux of partial pressure of dissolved carbon dioxide (pCO2) in Hongjiadu reservoir. Chin. J. Ecol. 2008, 27, 1193–1199. [Google Scholar]
- Pu, J.; Li, J.; Zhang, T.; Martin, J.B.; Yuan, D. Varying thermal structure controls the dynamics of CO2 emissions from a subtropical reservoir, south China. Water Res. 2020, 178, 115831. [Google Scholar] [CrossRef]
- Telmer, K.; Veizer, J. Carbon fluxes, pCO2 and substrate weathering in a large northern river basin, Canada: Carbon isotope perspectives. Chem. Geol. 1999, 159, 61–86. [Google Scholar] [CrossRef]
- Cerling, T.E.; Solomon, D.; Quade, J.; Bowman, J.R. On the isotopic composition of carbon in soil carbon dioxide. Geochim. Cosmochim. Acta 1991, 55, 3403–3405. [Google Scholar] [CrossRef]
- Palmer, S.M.; Hope, D.; Billett, M.F.; Dawson, J.J.; Bryant, C.L. Sources of organic and inorganic carbon in a headwater stream: Evidence from carbon isotope studies. Biogeochemistry 2001, 52, 321–338. [Google Scholar] [CrossRef]
- Mook, W.G.; de Vries, J.J. Environmental isotopes in the hydrological cycle principles and applications. In Technical Documents in Hydrology1; UNESCO; IAEA: Paris, France, 2000; Volume 4, No. 39. [Google Scholar]
- Doctor, D.H.; Kendall, C.; Sebestyen, S.D.; Shanley, J.B.; Ohte, N.; Boyer, E.W. Carbon isotope fractionation of dissolved inorganic carbon (DIC) due to outgassing of carbon dioxide from a headwater stream. Hydrol. Process. 2008, 22, 2410–2423. [Google Scholar] [CrossRef]
- Hellings, L.; Dehairs, F.; Van Damme, S.; Baeyens, W. Dissolved inorganic carbon in a highly polluted estuary (the Scheldt). Limnol. Oceanogr. 2001, 46, 1406–1414. [Google Scholar] [CrossRef]
- Davidson, E.A.; Figueiredo, R.O.; Markewitz, D.; Aufdenkampe, A.K. Dissolved CO2 in small catchment streams of eastern Amazonia: A minor pathway of terrestrial carbon loss. J. Geophys. Res. Atmos. 2010, 115, 470–479. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, J.-J.; Pu, J.; Xiao, Q.; Zhang, T.; Li, J. Flux and influencing factors of CO2 outgassing in a karst spring-fed creek: Implications for carbonate weathering-related carbon sink assessment. J. Hydrol. 2020, 596, 125710. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Macpherson, G.; Yang, R.; Chen, B.; Sun, H. Diurnal hydrochemical variations in a karst spring and two ponds, Maolan Karst Experimental Site, China: Biological pump effects. J. Hydrol. 2015, 522, 407–417. [Google Scholar] [CrossRef]
- Lu, W.; Wang, S.; Yeager, K.M.; Liu, F.; Huang, Q.; Yang, Y.; Xiang, P.; Lü, Y.; Liu, C.-Q. Importance of Considered Organic Versus Inorganic Source of Carbon to Lakes for Calculating Net Effect on Landscape C Budgets. J. Geophys. Res. Biogeosci. 2018, 123, 1302–1317. [Google Scholar] [CrossRef]
- Peng, X.; Liu, C.-Q.; Wang, B.; Zhao, Y.-C. The impact of damming on geochemical behavior of dissolved inorganic carbon in a karst river. Chin. Sci. Bull. 2014, 59, 2348–2355. [Google Scholar] [CrossRef]
- Qiu, J. Chinese dam may be a methane menace. Nature 2009, 29, 1038. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]

| Coefficient | Intercept | R2 | Lmg b | df | ||
|---|---|---|---|---|---|---|
| pCO2 | Water Velocity | pCO2 | Water Velocity | |||
| 0.07 | 0.77 *** | 0.0 | 0.65 | 9.3% | 55.2% | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhang, T.; Liu, H.; Ma, P.; Teng, Y.; Guan, Q.; Yu, L.; Liu, C.; Li, Y.; Li, C.; et al. The Mechanisms Controlling the CO2 Outgassing of a Karst Spring–River–Lake Continuum: Evidence from Baotuquan Spring Drainage Area, Jinan City, Northern China. Water 2023, 15, 2567. https://doi.org/10.3390/w15142567
Liu W, Zhang T, Liu H, Ma P, Teng Y, Guan Q, Yu L, Liu C, Li Y, Li C, et al. The Mechanisms Controlling the CO2 Outgassing of a Karst Spring–River–Lake Continuum: Evidence from Baotuquan Spring Drainage Area, Jinan City, Northern China. Water. 2023; 15(14):2567. https://doi.org/10.3390/w15142567
Chicago/Turabian StyleLiu, Wen, Tao Zhang, Haoran Liu, Pengfei Ma, Yue Teng, Qin Guan, Lingqin Yu, Chunwei Liu, Yiping Li, Chuanlei Li, and et al. 2023. "The Mechanisms Controlling the CO2 Outgassing of a Karst Spring–River–Lake Continuum: Evidence from Baotuquan Spring Drainage Area, Jinan City, Northern China" Water 15, no. 14: 2567. https://doi.org/10.3390/w15142567
APA StyleLiu, W., Zhang, T., Liu, H., Ma, P., Teng, Y., Guan, Q., Yu, L., Liu, C., Li, Y., Li, C., Li, C., & Pu, J. (2023). The Mechanisms Controlling the CO2 Outgassing of a Karst Spring–River–Lake Continuum: Evidence from Baotuquan Spring Drainage Area, Jinan City, Northern China. Water, 15(14), 2567. https://doi.org/10.3390/w15142567






