Highly Effective Removal of Ciprofloxacin Antibiotic from Water by Magnetic Metal–Organic Framework
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent and Materials
2.2. Apparatus
2.3. Synthesis of Fe3O4 Nanorods
2.4. Synthesis of Cu3(BTC)2 and Zn3(BTC)2
2.5. Synthesis of Fe3O4/Cu3(BTC)2 and Fe3O4/Zn3(BTC)2
2.6. Adsorption Experiments
2.7. Chromatographic Conditions for CIP
2.8. Recyclability Experiments
3. Results and Discussion
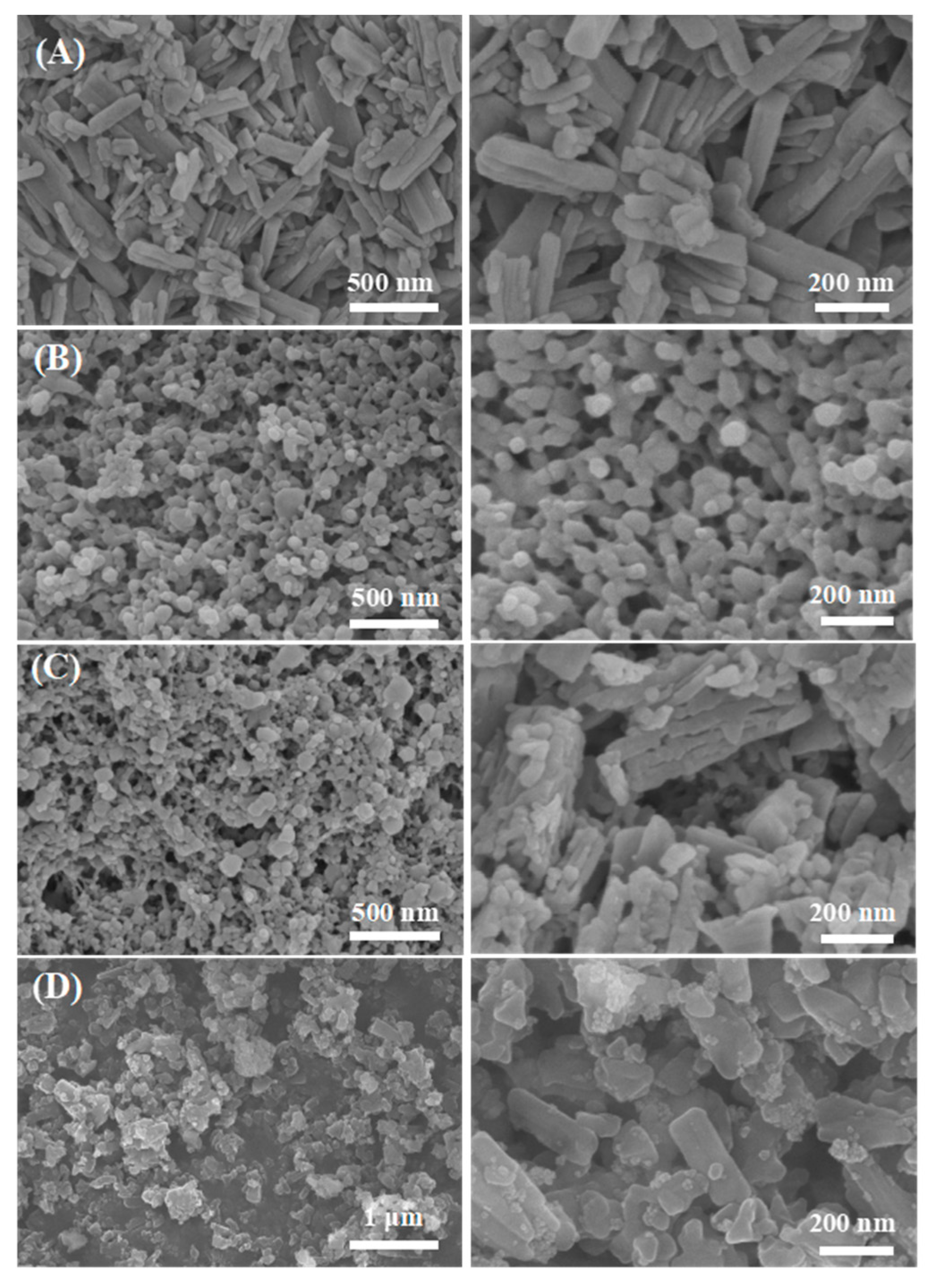
3.1. Characterization of Cu3(BTC)2, Fe3O4/Cu3(BTC)2, and Fe3O4/Zn3(BTC)2
3.2. Effect of Oscillation Time on CIP Adsorption
3.3. Effect of pH on CIP Adsorption
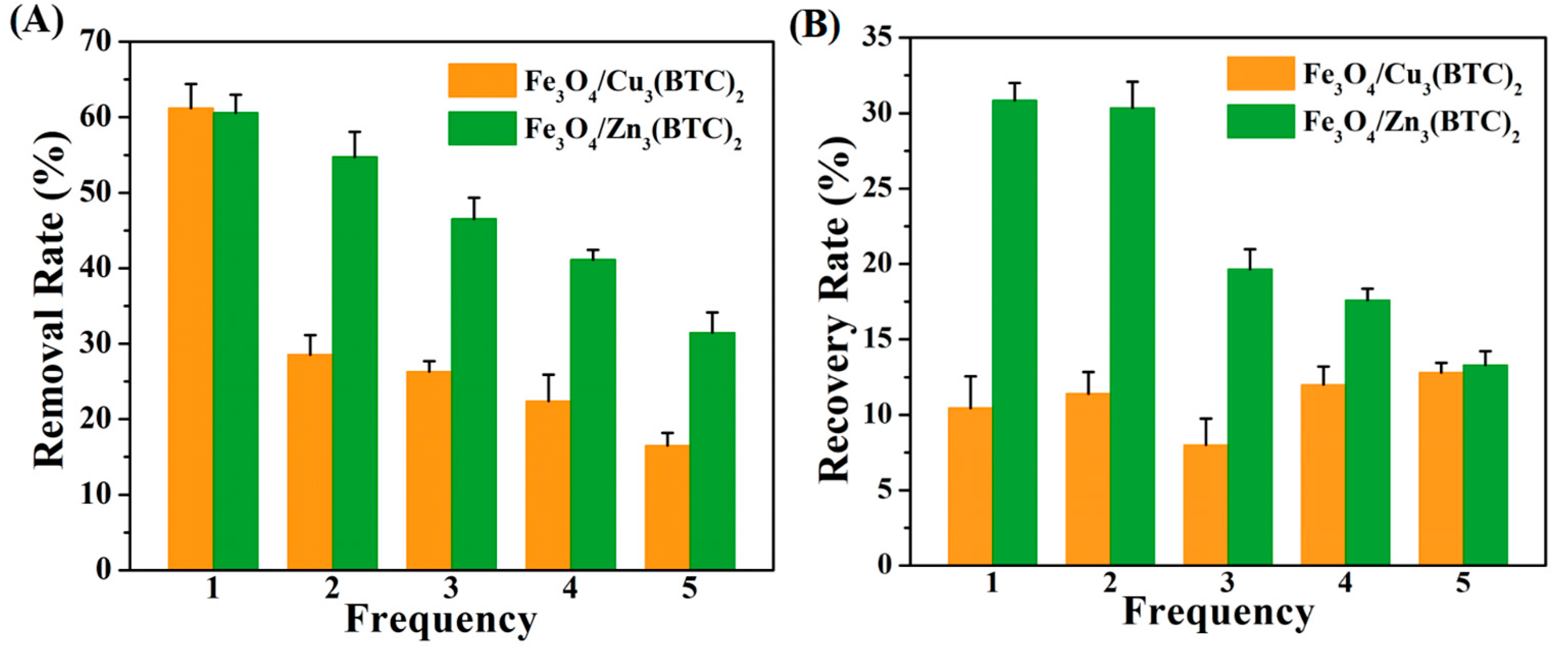
3.4. Regeneration and Reusability of Fe3O4/Zn3(BTC)2 MMOF
3.5. CIP-Adsorption Kinetics and Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.Q.; Zhang, H.; Alvarez, P.J.J. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin. China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, L.; Pitarch, E.; Fonseca, E.; B’anez, M.I.; Botero, A.M.; Claros, J.; Pastor, L.; Hernandez’, F. Investigation of pharmaceuticals in a conventional wastewater treatment plant: Removal efficiency, seasonal variation and impact of a nearby hospital. J. Environ. Chem. Eng. 2021, 9, 105548. [Google Scholar] [CrossRef]
- Palacio, D.A.; Rivas, B.L.; Urbano, B.F. Ultrafiltration membranes with three watersoluble polyelectrolyte copolymers to remove ciprofloxacin from aqueous systems. Chem. Eng. J. 2018, 351, 85–93. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E.; Kompare, B. Removal of pharmaceutical residues in a pilot wastewater treatment plant. Anal. Bioanal. Chem. 2007, 387, 1379–1387. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Mompelat, S.; Bot, B.L.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Janjani, H. Antibiotics adsorption from aqueous solutions using carbon nanotubes: A systematic review. Toxin Rev. 2020, 39, 87–98. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Zhao, J.H.; Yang, C. Efficient removal of ciprofloxacin by peroxymonosulfate/Mn3O4-MnO2 catalytic oxidation system. Chem. Eng. J. 2017, 327, 481–489. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Xie, B.; Han, J.; Zhan, S.; Tian, Y. Efficient mineralization of ciprofloxacin using a 3D CexZr1-xO2/RGO composite cathode. Environ. Sci. Nano 2017, 4, 425–436. [Google Scholar] [CrossRef]
- Bojer, C.; Schöbel, J.; Martin, T.; Ertl, M.; Schmalz, H.; Breua, J. Clinical wastewater treatment: Photochemical removal of an anionic antibiotic (ciprofloxacin) by mesostructured high aspect ratio ZnO nanotubes. Appl. Catal. B Environ. 2017, 204, 561–565. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Deepa, J.R. Nano-zinc oxide incorporated graphene oxide/nanocellulose composite for the adsorption and photo catalytic degradation of ciprofloxacin hydrochloride from aqueous solutions. J. Colloid Interface Sci. 2017, 490, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Dwivedi, A.D.; Lee, W.N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano 2018, 5, 27–47. [Google Scholar] [CrossRef]
- Tian, L.; Pang, X.L.; Xu, H.; Liu, D.S.; Lu, X.H.; Li, J.; Wang, J.; Li, Z. Cation-anion dual doping modifying electronic structure of hollow CoP nanoboxes for enhanced water oxidation electrocatalysis. Inorg. Chem. 2022, 42, 16944–16951. [Google Scholar] [CrossRef]
- Song, M.; Lu, X.H.; Du, M.L.; Chen, Z.Y.; Zhu, C.; Xu, H.; Cheng, W.J.; Zhuang, W.C.; Li, Z.; Tian, L. Electronic and architecture engineering of hammer-shaped Ir-NiMoO4-ZIF for effective oxygen evolution. CrystEngComm 2022, 24, 5995–6000. [Google Scholar] [CrossRef]
- Tian, L.; Chen, Z.Y.; Wang, T.J.; Cao, M.; Lu, X.H.; Cheng, W.J.; He, C.C.; Wang, J.; Li, Z. Mo doping and Se vacancy engineering for boosting electrocatalytic water oxidation by regulating the electronic structure of self-supported Co9Se8@NiSe. Nanoscale 2023, 15, 259–265. [Google Scholar] [CrossRef]
- Tian, L.; Chen, H.Y.; Lu, X.H.; Liu, D.S.; Cheng, W.J.; Liu, Y.Y.; Li, J.; Li, Z. Local photothermal and photoelectric effect synergistically boost hollow CeO2/CoS2 heterostructure electrocatalytic oxygen evolution reaction. J. Colloid Interface Sci. 2022, 628, 663–672. [Google Scholar] [CrossRef]
- Yu, F.; Sun, S.; Han, S.; Zheng, J.; Ma, J. Adsorption removal of ciprofloxacin by multiwalled carbon nanotubes with different oxygen contents from aqueous solutions. Chem. Eng. J. 2016, 285, 588–595. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Ma, J. Enhanced adsorption and removal of ciprofloxacin on regenerable long TiO2 nanotube/graphene oxide hydrogel adsorbents. J. Nanomater. 2015, 8, 675862. [Google Scholar]
- Kong, X.; Liu, Y.; Pi, J.; Li, W.; Shang, J. Low-cost magnetic herbal biochar: Characterization and application for antibiotic removal. Environ. Sci. Pollut. Res. 2017, 24, 6679–6687. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Fan, Y.; Huang, Y. Facile low temperature hydrothermal synthesis of magnetic mesoporous carbon nanocomposite for adsorption removal of ciprofloxacin antibiotic. Ind. Eng. Chem. Res. 2013, 52, 2604–2612. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.M.; O’Keeffe, M.; Reedijk, J. Terminology of metal-organic frameworks and coordination polymers. Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Chakraborty, A. Highly efficient adsorption desalination employing protonated-amino-functionalized MOFs. Desalination 2022, 541, 116045. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, P.; Zhou, H.C.; Sharma, V.K. Water-stable metalorganic frameworks for aqueous removal of heavy metals and radionuclides: A review. Chemosphere 2018, 209, 783. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Li, H.; Li, S.; Cao, X. Adsorption mechanisms of ibuprofen and naproxen to UiO-66 and UiO-66-NH2: Batch experiment and DFT calculation. Chem. Eng. J. 2019, 360, 645–653. [Google Scholar] [CrossRef]
- Guo, X.; Kang, C.; Huang, H.; Chang, Y.; Zhong, C. Exploration of functional MOFs for efficient removal of fluoroquinolone antibiotics from water. Microporous Mesoporous Mater. 2019, 286, 84–91. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Umar, A.; Khan, M.A. Removal of fluoroquinolone drug, levofloxacin, from aqueous phase over iron based MOFs, MIL-100(Fe). J. Solid State Chem. 2020, 281, 121029. [Google Scholar] [CrossRef]
- Wu, G.; Ma, J.; Li, S.; Guan, J.; Jiang, B.; Wang, L.; Li, J.; Chen, L. Magnetic copper-based metal organic framework as an effective and recyclable adsorbent for removal of two fluoroquinolone antibiotics from aqueous solutions. J. Colloid Interface Sci. 2018, 528, 360–371. [Google Scholar] [CrossRef]
- Gao, G.; Xing, Y.; Liu, T.; Wang, J.; Hou, X. UiO-66(Zr) as sorbent for porous membrane protected micro-solid-phase extraction androgens and progestogens in environmental water samples coupled with LC-MS/MS analysis: The application of experimental and molecular simulation method. Microchem. J. 2019, 146, 126–133. [Google Scholar] [CrossRef]
- Muhammet, Ş.A.; Erena, H.A.; Harun, Ç. Production of microporous Cu-doped BTC (Cu-BTC) metal-organic framework composite materials, superior adsorbents for the removal of methylene blue. J. Environ. Chem. Eng. 2020, 8, 104247. [Google Scholar]
- Abdullah, M.; Aldawsari, I.H.A. Activated carbon/MOFs composite: AC/NH2-MIL-101(Cr), synthesis and application in high performance adsorption of p-nitrophenol. J. Saudi Chem. Soc. 2020, 24, 693–703. [Google Scholar]
- Liu, X.; Zhou, Y.; Zhang, J.; Lin, T.; Lin, L.; Zeng, G. Iron containing metal-organic frameworks: Structure, synthesis, and applications in environmental remediation. ACS Appl. Mater. Interfaces 2017, 9, 20255–20275. [Google Scholar] [CrossRef] [PubMed]
- Capsoni, D.; Guerra, G.; Puscalau, C.; Maraschi, F.; Bruni, G.; Monteforte, F.; Profumo, A.; Sturini, M. Zinc based metal-organic frameworks as ofloxacin adsorbents in polluted waters: ZIF-8 vs. Zn3(BTC)2. Int. J. Environ. Res. Public Health 2021, 18, 1433. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, R.; Kardani, F.; Ramezani, Z. Preparation and characterization of magnetic metal-organic framework nanocomposite as solid-phase microextraction fibers coupled with high-performance liquid chromatography for determination of non-steroidal anti-inflammatory drugs in biological fluids and tablet formulation samples. Microchem. J. 2018, 144, 270–284. [Google Scholar]
- Li, X.; Dong, W.; Zhang, C.; Guo, W.; Wang, C.; Li, Y.; Wang, H. Leaf-Like Fe/C composite assembled by iron veins interpenetrated into amorphous carbon lamina for high-performance microwave absorption. Compos. A 2021, 140, 106202. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Emam, H.E.; Rocha, J.; Silva, A.M.S. Cu-BTC metal-organic framework natural fabric composites for fuel purification. Fuel Process. Technol. 2017, 159, 306–312. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B.; Fulekar, M.H. A study on the influence of metal (Fe, Bi, and Ag) doping on structural, optical, and antimicrobial activity of ZnO nanostructures. Adv Compos Hybrid Mater. 2020, 3, 551–569. [Google Scholar] [CrossRef]
- Mahsa, A.; Bahareh, B.; Rahman, H. Unusual synthesis of nanostructured Zn-MOF by bipolar electrochemistry in ionic liquid-based electrolyte: Intrinsic alkaline phosphatase-like activity. J. Electroanal. Chem. 2022, 914, 116306. [Google Scholar]
- Li, Z.J.; Ma, M.Y.; Zhang, Z.K.; Zhou, L.L.; Yun, J.M.; Liu, R.J. Efficiently removal of ciprofloxacin from aqueous solution by MIL-101(Cr)-HSO3: The enhanced electrostatic interaction. J. Porous Mater. 2020, 27, 189–204. [Google Scholar] [CrossRef]
- Li, J.G.; Beuerman, R.; Verma, C. The effect of molecular shape on oligomerization of hydrophobic drugs: Molecular simulations of ciprofloxacin and nutlin. J. Chem. Phys. 2018, 148, 104902. [Google Scholar] [CrossRef]











| Adsorbent | SBET (m2/g) | Pore Volume (cm3/g) | Average Particle Size (nm) |
|---|---|---|---|
| Cu3(BTC)2 | 1054.36 | 0.97 | 3.68 |
| Zn3(BTC)2 | 13.81 | 0.03 | 9.60 |
| Fe3O4/Cu3(BTC)2 | 619.13 | 0.53 | 3.45 |
| Fe3O4/Zn3(BTC)2 | 27.35 | 0.13 | 18.31 |
| Adsorbent | qexp (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 | r2 | qe | k2 | r2 | ||
| Fe3O4/Zn3(BTC)2 | 5.19 | 4.89 | 0.205 | 0.952 | 5.12 | 0.065 | 0.971 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Chang, H.; Wei, W.; Yu, H.; Chen, Z.; Cheng, X.; Chen, D.; Jin, Y.; Han, D.; Xu, W. Highly Effective Removal of Ciprofloxacin Antibiotic from Water by Magnetic Metal–Organic Framework. Water 2023, 15, 2531. https://doi.org/10.3390/w15142531
Yu B, Chang H, Wei W, Yu H, Chen Z, Cheng X, Chen D, Jin Y, Han D, Xu W. Highly Effective Removal of Ciprofloxacin Antibiotic from Water by Magnetic Metal–Organic Framework. Water. 2023; 15(14):2531. https://doi.org/10.3390/w15142531
Chicago/Turabian StyleYu, Binbin, Hongchao Chang, Wenwan Wei, Hua Yu, Zhangxin Chen, Xiaoye Cheng, Dan Chen, Yanxian Jin, Deman Han, and Wei Xu. 2023. "Highly Effective Removal of Ciprofloxacin Antibiotic from Water by Magnetic Metal–Organic Framework" Water 15, no. 14: 2531. https://doi.org/10.3390/w15142531






