Harmful Algal Blooms: A Prolific Issue in Urban Stormwater Ponds
Abstract
1. Introduction
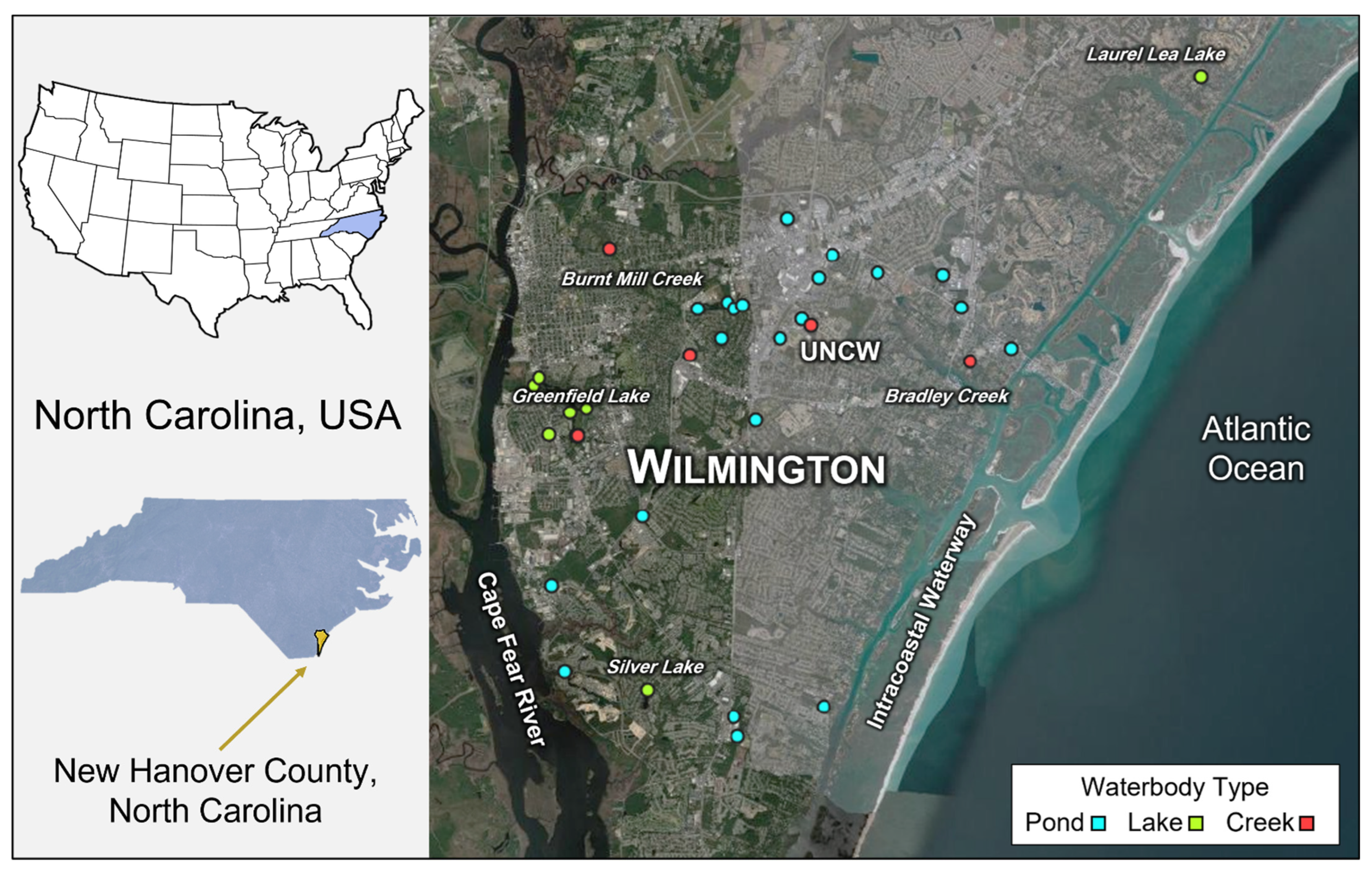
2. Methods
2.1. Sampling and Water Analysis
2.2. Statistical Analyses
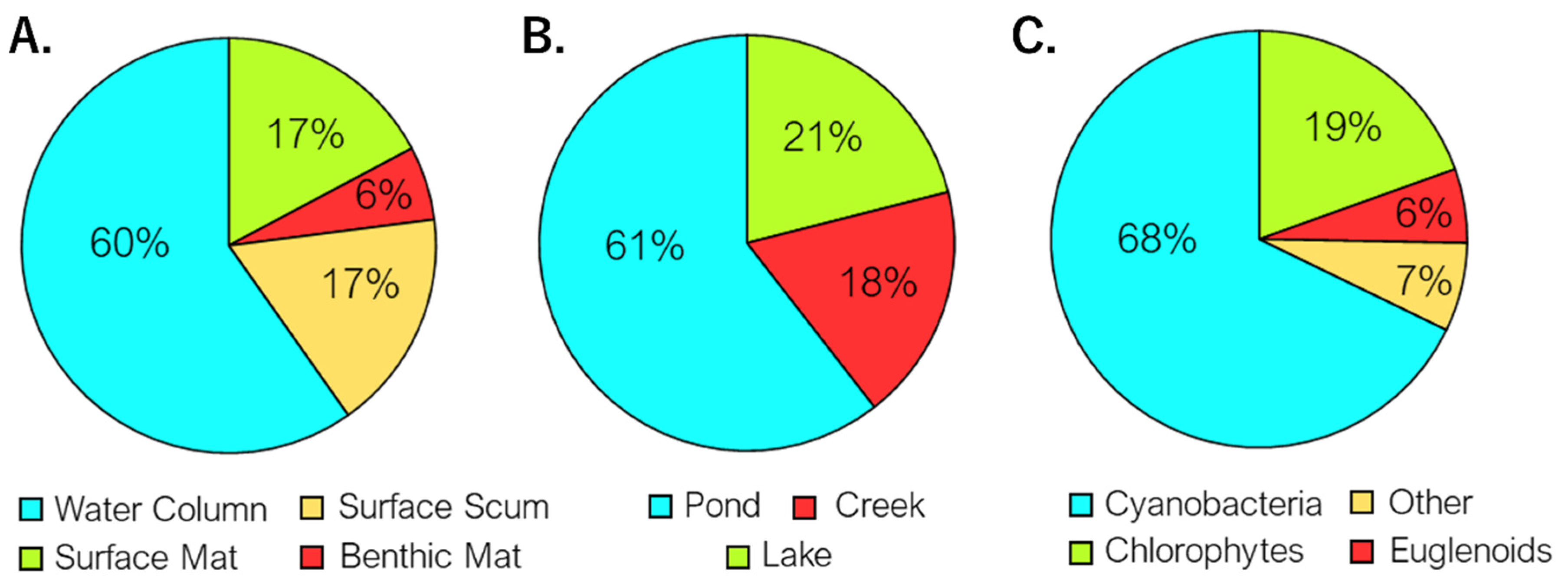
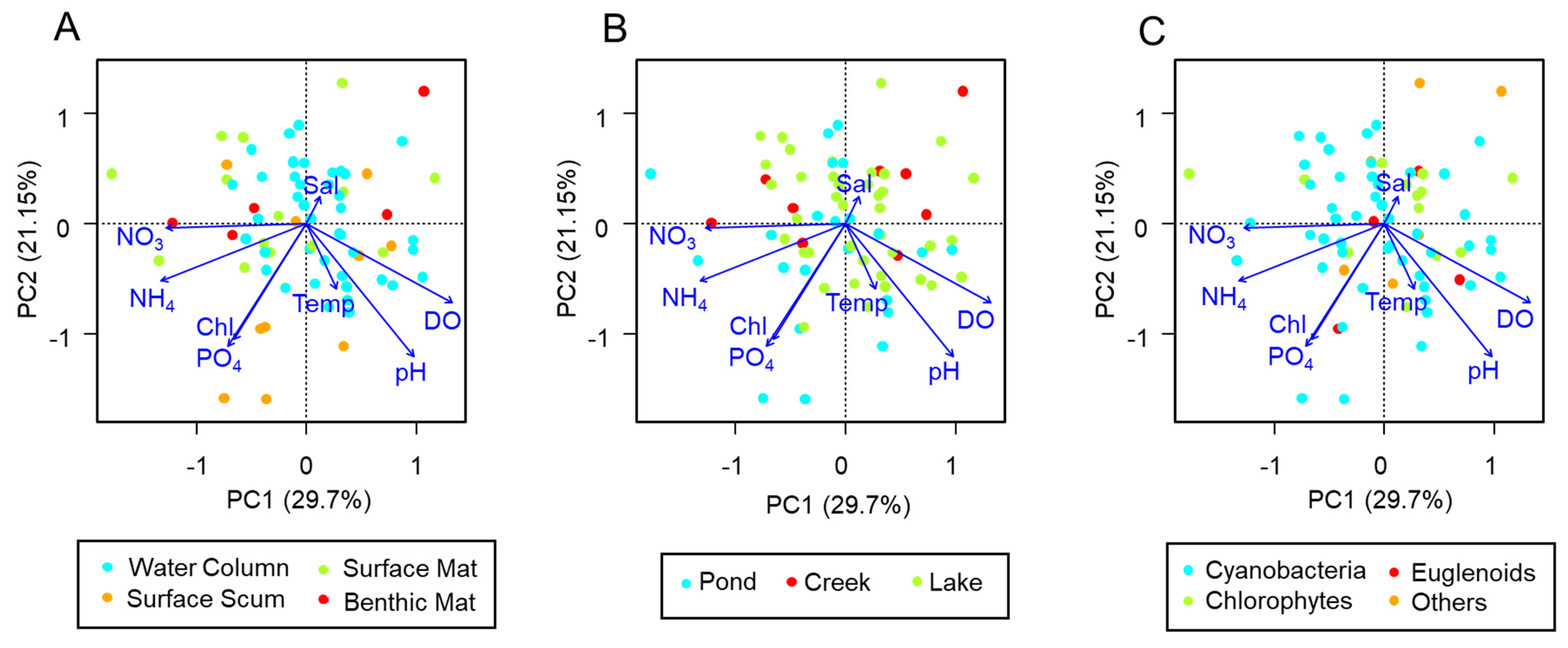
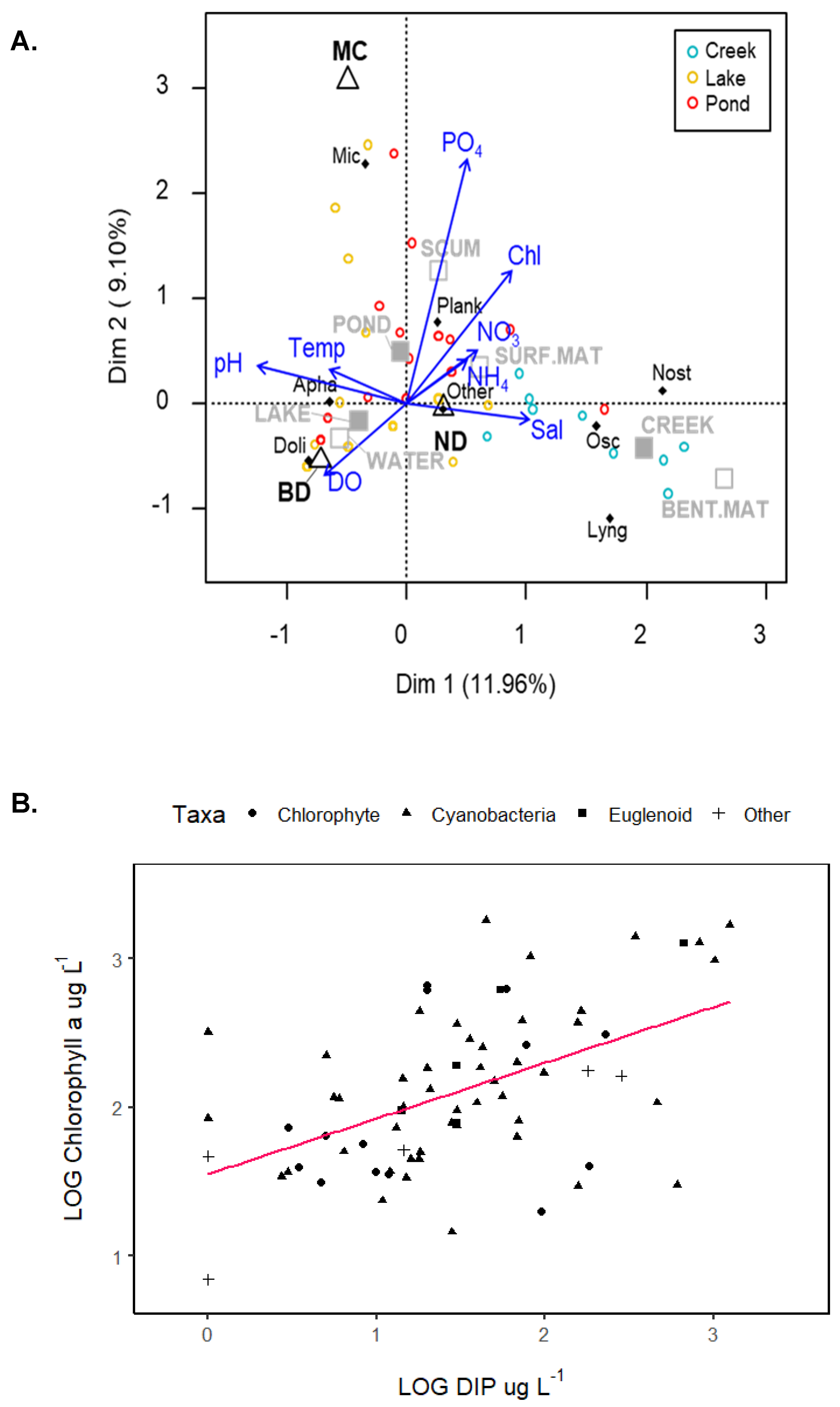
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, D.M.; Burkholder, J.M.; Cochlan, W.P.; Glibert, P.M.; Gobler, C.J.; Heil, C.A.; Kudela, R.M.; Parsons, M.L.; Rensel, J.E.J.; Townsend, D.W.; et al. Harmful Algal Blooms and Eutrophication: Examining Linkages from Selected Coastal Regions of the United States. Harmful Algae 2008, 8, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and Harmful Algal Blooms: A Scientific Consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Beaver, J.R.; Tausz, C.E.; Scotese, K.C.; Pollard, A.I.; Mitchell, R.M. Environmental Factors Influencing the Quantitative Distribution of Microcystin and Common Potentially Toxigenic Cyanobacteria in U.S. Lakes and Reservoirs. Harmful Algae 2018, 78, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Burkholder, J.M. Causes of Harmful Algal Blooms. In Harmful Algal Blooms; Wiley: Hoboken, NJ, USA, 2018; pp. 1–38. [Google Scholar]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The Effects of Temperature and Nutrients on the Growth and Dynamics of Toxic and Non-Toxic Strains of Microcystis during Cyanobacteria Blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Beaulieu, M.; Pick, F.; Gregory-Eaves, I. Nutrients and Water Temperature Are Significant Predictors of Cyanobacterial Biomass in a 1147 Lakes Data Set. Limnol. Oceanogr. 2013, 58, 1736–1746. [Google Scholar] [CrossRef]
- Walls, J.T.; Wyatt, K.H.; Doll, J.C.; Rubenstein, E.M.; Rober, A.R. Hot and Toxic: Temperature Regulates Microcystin Release from Cyanobacteria. Sci. Total Environ. 2018, 610–611, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Michalak, A.M. Exploring Temperature and Precipitation Impacts on Harmful Algal Blooms across Continental U.S. Lakes. Limnol. Oceanogr. 2019, 65, 992–1009. [Google Scholar] [CrossRef]
- Cotti-Rausch, B.; Majidzadeh, H.; DeVoe, R. Stormwater Ponds in Coastal South Carolina. In 2019 State of Knowledge Full Report; S.C. Sea Grant Consortium: Charleston, SC, USA, 2019. [Google Scholar]
- Drescher, S.; Messersmith, M.; Davis, B.; Sanger, D. State of Knowledge Report: Stormwater Ponds in the Coastal Zone; S.C. DHEC’s Office of Ocean and Coastal Resource Management: Charleston, SC, USA, 2007.
- Lewitus, A.J.; Brock, L.M.; Burke, M.K.; DeMattio, K.A.; Wilde, S.B. Lagoonal Stormwater Detention Ponds as Promoters of Harmful Algal Blooms and Eutrophication along the South Carolina Coast. Harmful Algae 2008, 8, 60–65. [Google Scholar] [CrossRef]
- Siegel, A.; Cotti-Rausch, B.; Greenfield, D.I.; Pinckney, J.L. Nutrient Controls of Planktonic Cyanobacteria Biomass in Coastal Stormwater Detention Ponds. Mar. Ecol. Prog. Ser. 2011, 434, 15–27. [Google Scholar] [CrossRef]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.A.; Johnson, M.-V.V.; Morton, S.L.; Perkins, D.A.K.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are Harmful Algal Blooms Becoming the Greatest Inland Water Quality Threat to Public Health and Aquatic Ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef]
- Zurawell, R.W.; Chen, H.; Burke, J.M.; Prepas, E.E. Hepatotoxic Cyanobacteria: A Review of the Biological Importance of Microcystins in Freshwater Environments. J. Toxicol. Environ. Health Part B Crit. Rev. 2005, 8, 1–37. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Madamwar, D.; Incharoensakdi, A. Bloom Dynamics of Cyanobacteria and Their Toxins: Environmental Health Impacts and Mitigation Strategies. Front. Microbiol. 2015, 6, 1254. [Google Scholar] [CrossRef] [PubMed]
- Nowruzi, B.; Porzani, S.J. Toxic Compounds Produced by Cyanobacteria Belonging to Several Species of the Order Nostocales: A Review. J. Appl. Toxicol. 2021, 41, 510–548. [Google Scholar] [CrossRef] [PubMed]
- Loftin, K.A.; Graham, J.L.; Hilborn, E.D.; Lehmann, S.C.; Meyer, M.T.; Dietze, J.E.; Griffith, C.B. Cyanotoxins in Inland Lakes of the United States: Occurrence and Potential Recreational Health Risks in the EPA National Lakes Assessment 2007. Harmful Algae 2016, 56, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.Y.; Liang, S.; Lee, J. Toxin-Producing Cyanobacteria in Freshwater: A Review of the Problems, Impact on Drinking Water Safety, and Efforts for Protecting Public Health. J. Microbiol. 2013, 51, 1–10. [Google Scholar] [CrossRef]
- Bullerjahn, G.S.; McKay, R.M.; Davis, T.W.; Baker, D.B.; Boyer, G.L.; D’Anglada, L.V.; Doucette, G.J.; Ho, J.C.; Irwin, E.G.; Kling, C.L.; et al. Global Solutions to Regional Problems: Collecting Global Expertise to Address the Problem of Harmful Cyanobacterial Blooms. A Lake Erie Case Study. Harmful Algae 2016, 54, 223–238. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Wessel, R.A.; Lester, M.; Pim, J.G.; Cassani, J.R.; Cox, P.A. Toxin Analysis of Freshwater Cyanobacterial and Marine Harmful Algal Blooms on the West Coast of Florida and Implications for Estuarine Environments. Neurotox. Res. 2021, 39, 27–35. [Google Scholar] [CrossRef]
- Schaefer, A.M.; Yrastorza, L.; Stockley, N.; Harvey, K.; Harris, N.; Grady, R.; Sullivan, J.; McFarland, M.; Reif, J.S. Exposure to Microcystin among Coastal Residents during a Cyanobacteria Bloom in Florida. Harmful Algae 2020, 92, 101769. [Google Scholar] [CrossRef]
- Melaram, R.; Lopez-Dueñas, B. Detection and Occurrence of Microcystins and Nodularins in Lake Manatee and Lake Washington-Two Floridian Drinking Water Systems. Front. Water 2022, 4, 899572. [Google Scholar] [CrossRef]
- Heil, C.A.; Muni-Morgan, A.L. Florida’s Harmful Algal Bloom (HAB) Problem: Escalating Risks to Human, Environmental and Economic Health With Climate Change. Front. Ecol. Evol. 2021, 9, 646080. [Google Scholar] [CrossRef]
- Olokotum, M.; Mitroi, V.; Troussellier, M.; Semyalo, R.; Bernard, C.; Montuelle, B.; Okello, W.; Quiblier, C.; Humbert, J.F. A Review of the Socioecological Causes and Consequences of Cyanobacterial Blooms in Lake Victoria. Harmful Algae 2020, 96, 101829. [Google Scholar] [CrossRef]
- Chernova, E.; Sidelev, S.; Russkikh, I.; Korneva, L.; Solovyova, V.; Mineeva, N.; Stepanova, I.; Zhakovskaya, Z. Spatial Distribution of Cyanotoxins and Ratios of Microcystin to Biomass Indicators in the Reservoirs of the Volga, Kama and Don Rivers, the European Part of Russia. Limnologica 2020, 84, 125819. [Google Scholar] [CrossRef]
- Li, J.; Hansson, L.A.; Persson, K.M. Nutrient Control to Prevent the Occurrence of Cyanobacterial Blooms in a Eutrophic Lake in Southern Sweden, Used for Drinking Water Supply. Water 2018, 10, 919. [Google Scholar] [CrossRef]
- Qin, B.; Zhu, G.; Gao, G.; Zhang, Y.; Li, W.; Paerl, H.W.; Carmichael, W.W. A Drinking Water Crisis in Lake Taihu, China: Linkage to Climatic Variability and Lake Management. Environ. Manag. 2010, 45, 105–112. [Google Scholar] [CrossRef]
- Facey, J.A.; Michie, L.E.; King, J.J.; Hitchcock, J.N.; Apte, S.C.; Mitrovic, S.M. Severe Cyanobacterial Blooms in an Australian Lake; Causes and Factors Controlling Succession Patterns. Harmful Algae 2022, 117, 102284. [Google Scholar] [CrossRef]
- Moreira, M.G.A.L.; Hinegk, L.; Salvadore, A.; Zolezzi, G.; Hölker, F.; Domecq, S.R.A.M.; Bocci, M.; Carrer, S.; De Nat, L.; Escribá, J.; et al. Eutrophication, Research and Management History of the Shallow Ypacaraí Lake (Paraguay). Sustainability 2018, 10, 2426. [Google Scholar] [CrossRef]
- Gurbuz, F.; Uzunmehmetoğlu, O.Y.; Diler, Ö.; Metcalf, J.S.; Codd, G.A. Occurrence of Microcystins in Water, Bloom, Sediment and Fish from a Public Water Supply. Sci. Total Environ. 2016, 562, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, Y.; Mohammadipanah, F.; Te, S.H.; You, L.; Gin, K.Y.-H. Biodiversity, Phylogeny and Toxin Production Profile of Cyanobacterial Strains Isolated from Lake Latyan in Iran. Harmful Algae 2021, 106, 102054. [Google Scholar] [CrossRef]
- Catherine, Q.; Susanna, W.; Isidora, E.S.; Mark, H.; Aurélie, V.; Jean-François, H. A Review of Current Knowledge on Toxic Benthic Freshwater Cyanobacteria—Ecology, Toxin Production and Risk Management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Hudon, C.; De Sève, M.; Cattaneo, A. Increasing Occurrence of the Benthic Filamentous Cyanobacterium Lyngbya Wollei: A Symptom of Freshwater Ecosystem Degradation. Freshw. Sci. 2014, 33, 606–618. [Google Scholar] [CrossRef]
- McAllister, T.G.; Wood, S.A.; Hawes, I. The Rise of Toxic Benthic Phormidium Proliferations: A Review of Their Taxonomy, Distribution, Toxin Content and Factors Regulating Prevalence and Increased Severity. Harmful Algae 2016, 55, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Toxic Algae Found in Wilmington Pond after Death of Dogs. WWAY News. 27 August 2019. Available online: https://www.wwaytv3.com/toxic-algae-found-in-wilmington-pond-after-death-of-dogs/ (accessed on 1 June 2023).
- Welschmeyer, N.A. Fluorometric Analysis of Chlorophyll a in the Presence of Chlorophyll b and Pheopigments. Limnol. Oceanogr. 1994, 39, 1985–1992. [Google Scholar] [CrossRef]
- APHA. 4500-P J. Persulfate Method for Simultaneous Determination of Total Nitrogen and Total Phosphorous; Eaton, A.D., Clesceri, L.S., Rice, E.W., Greenberg, A.E., Eds.; American Public Health Association, American Water Works Association, Water Environmental Federation: Washington, DC, USA, 2005. [Google Scholar]
- Zimmermann, C.F.; Keefe, C.W. Method 365.5 Determination of Orthophosphate in Estuarine and Coastal Waters by Automated Colorimetric Analysis; U.S. Environmental Protection Agency: Washington, DC, USA, 1997; pp. 1–9.
- Nienaber, M.A.; Steinitz-Kannan, M. A Guide to Cyanobacteria; The University Press of Kentucky: Lexington, KY, USA, 2018; ISBN 9780813175614. [Google Scholar]
- Rattner, B.A.; Wazniak, C.E.; Lankton, J.S.; McGowan, P.C.; Drovetski, S.V.; Egerton, T.A. Review of Harmful Algal Bloom Effects on Birds with Implications for Avian Wildlife in the Chesapeake Bay Region. Harmful Algae 2022, 120, 102319. [Google Scholar] [CrossRef]
- Li, X.; Dreher, T.W.; Li, R. An Overview of Diversity, Occurrence, Genetics and Toxin Production of Bloom-Forming Dolichospermum (Anabaena) Species. Harmful Algae 2016, 54, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A Review of Microcystin Detections in Estuarine and Marine Waters: Environmental Implications and Human Health Risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Occurrence and Toxicity of Microcystin Congeners Other than MC-LR and MC-RR: A Review. Food Chem. Toxicol. 2019, 125, 106–132. [Google Scholar] [CrossRef]
- Janssen, E.M.L. Cyanobacterial Peptides beyond Microcystins—A Review on Co-Occurrence, Toxicity, and Challenges for Risk Assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef]
- Scarlett, K.R.; Kim, S.; Lovin, L.M.; Chatterjee, S.; Scott, J.T.; Brooks, B.W. Global Scanning of Cylindrospermopsin: Critical Review and Analysis of Aquatic Occurrence, Bioaccumulation, Toxicity and Health Hazards. Sci. Total Environ. 2020, 738, 139807. [Google Scholar] [CrossRef]
- Chen, G.; Wang, L.; Wang, M.; Hu, T. Comprehensive Insights into the Occurrence and Toxicological Issues of Nodularins. Mar. Pollut. Bull. 2021, 162, 111884. [Google Scholar] [CrossRef]
- Christensen, V.G.; Khan, E. Freshwater Neurotoxins and Concerns for Human, Animal, and Ecosystem Health: A Review of Anatoxin-a and Saxitoxin. Sci. Total Environ. 2020, 736, 139515. [Google Scholar] [CrossRef]
- Kulabhusan, P.K.; Campbell, K. Recent Trends in the Detection of Freshwater Cyanotoxins with a Critical Note on Their Occurrence in Asia. Trends Environ. Anal. Chem. 2021, 32, e00150. [Google Scholar] [CrossRef]
- Massey, I.Y.; Wu, P.; Wei, J.; Luo, J.; Ding, P.; Wei, H.; Yang, F. A Mini-Review on Detection Methods of Microcystins. Toxins 2020, 12, 641. [Google Scholar] [CrossRef]
- Tanvir, R.U.; Hu, Z.; Zhang, Y.; Lu, J. Cyanobacterial Community Succession and Associated Cyanotoxin Production in Hypereutrophic and Eutrophic Freshwaters. Environ. Pollut. 2021, 290, 118056. [Google Scholar] [CrossRef]
- Kim, S.G.; Rhee, S.K.; Ahn, C.Y.; Ko, S.R.; Choi, G.G.; Bae, J.W.; Park, Y.H.; Oh, H.M. Determination of Cyanobacterial Diversity during Algal Blooms in Daechung Reservoir, Korea, on the Basis of CpcBA Intergenic Spacer Region Analysis. Appl. Environ. Microbiol. 2006, 72, 3252–3258. [Google Scholar] [CrossRef] [PubMed]
- Sabart, M.; Crenn, K.; Perrière, F.; Abila, A.; Leremboure, M.; Colombet, J.; Jousse, C.; Latour, D. Co-Occurrence of Microcystin and Anatoxin-a in the Freshwater Lake Aydat (France): Analytical and Molecular Approaches during a Three-Year Survey. Harmful Algae 2015, 48, 12–20. [Google Scholar] [CrossRef]
- Ruiz, M.; Galanti, L.; Ruibal, A.L.; Rodriguez, M.I.; Wunderlin, D.A.; Amé, M.V. First Report of Microcystins and Anatoxin-a Co-Occurrence in San Roque Reservoir (Córdoba, Argentina). Water. Air. Soil Pollut. 2013, 224, 1593. [Google Scholar] [CrossRef]
- Harland, F.; Wood, S.A.; Broady, P.; Williamson, W.; Gaw, S. Changes in Saxitoxin-Production through Growth Phases in the Metaphytic Cyanobacterium Scytonema Cf. Crispum. Toxicon. 2015, 103, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, J.D.; Westrick, J.A.; Furr, E.; Birbeck, J.A.; Reitz, L.A.; Stanislawczyk, K.; Li, W.; Weber, P.K.; Bridgeman, T.B.; Davis, T.W.; et al. Quantification of Microcystin Production and Biodegradation Rates in the Western Basin of Lake Erie. Limnol. Oceanogr. 2022, 67, 1470–1483. [Google Scholar] [CrossRef]
- Cottingham, K.L.; Ewing, H.A.; Greer, M.L.; Carey, C.C.; Weathers, K.C. Cyanobacteria as Biological Drivers of Lake Nitrogen and Phosphorus Cycling. Ecosphere 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Mallin, M.A.; Cahoon, L.B. The Hidden Impacts of Phosphorus Pollution to Streams and Rivers. Bioscience 2020, 70, 315–329. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M.; Landsberg, J.H.; Lefebvre, K.A.; Provoost, P.; Richlen, M.L.; et al. Marine Harmful Algal Blooms (HABs) in the United States: History, Current Status and Future Trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef]
- Glibert, P.M.; Allen, J.I.; Bouwman, A.F.; Brown, C.W.; Flynn, K.J.; Lewitus, A.J.; Madden, C.J. Modeling of HABs and Eutrophication: Status, Advances, Challenges. J. Mar. Syst. 2010, 83, 262–275. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The Rise of Harmful Cyanobacteria Blooms: The Potential Roles of Eutrophication and Climate Change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Reed, M.L.; DiTullio, G.R.; Kacenas, S.E.; Greenfield, D.I. Effects of Nitrogen and Dissolved Organic Carbon on Microplankton Abundances in Four Coastal South Carolina (USA) Systems. Aquat. Microb. Ecol. 2015, 76, 1–14. [Google Scholar] [CrossRef]
- Mallin, M.A.; McIver, M.R.; Wambach, E.J.; Robuck, A.R. Algal Blooms, Circulators, Waterfowl, and Eutrophic Greenfield Lake, North Carolina. Lake Reserv. Manag. 2016, 32, 168–181. [Google Scholar] [CrossRef]
- Yan, X.; Xu, X.; Wang, M.; Wang, G.; Wu, S.; Li, Z.; Sun, H.; Shi, A.; Yang, Y. Climate Warming and Cyanobacteria Blooms: Looks at Their Relationships from a New Perspective. Water Res. 2017, 125, 449–457. [Google Scholar] [CrossRef]
- Kramer, B.J.; Jankowiak, J.G.; Nanjappa, D.; Harke, M.J.; Gobler, C.J. Nitrogen and Phosphorus Significantly Alter Growth, Nitrogen Fixation, Anatoxin-a Content, and the Transcriptome of the Bloom-Forming Cyanobacterium, Dolichospermum. Front. Microbiol. 2022, 13, 955032. [Google Scholar] [CrossRef]
- Hupfer, M.; Lewandowski, J. Oxygen Controls the Phosphorus Release from Lake Sediments—A Long-Lasting Paradigm in Limnology. Int. Rev. Hydrobiol. 2008, 93, 415–432. [Google Scholar] [CrossRef]
- González-Madina, L.; Levrini, P.; de Tezanos Pinto, P.; Burwood, M.; Crisci, C.; Cardozo, A.; Lagomarsino, J.J.; Pacheco, J.P.; Fosalba, C.; Méndez, G.; et al. Blooms of Toxic Raphidiopsis Raciborskii in Laguna Del Sauce (Uruguay): Environmental Drivers and Impacts. Hydrobiologia 2022, 849, 4041–4058. [Google Scholar] [CrossRef]
- Gibbs, M.M.; Roygard, J.; Patterson, M.; Brown, L.; Brown, D. Factors Influencing Cyanobacteria Blooms: Review of the Historical Monitoring Data to Assess Management Options for Lake Horowhenua. N. Z. J. Mar. Freshw. Res. 2022, 1–27. [Google Scholar] [CrossRef]
- Gagrani, V.; Diemer, J.A.; Karl, J.J.; Allan, C.J. Assessing the Hydrologic and Water Quality Benefits of a Network of Stormwater Control Measures in a SE U.S. Piedmont Watershed. J. Am. Water Resour. Assoc. 2014, 50, 128–142. [Google Scholar] [CrossRef]
- Sanger, D.; Blair, A.; DiDonato, G.; Washburn, T.; Jones, S.; Riekerk, G.; Wirth, E.; Stewart, J.; White, D.; Vandiver, L.; et al. Impacts of Coastal Development on the Ecology of Tidal Creek Ecosystems of the US Southeast Including Consequences to Humans. Estuaries Coasts 2015, 38, 49–56. [Google Scholar] [CrossRef]
- Mallin, M.A.; Burkholder, J.M.; Cahoon, L.B.; Grogan, A.E.; Sanger, D.M.; Smith, E. An Environmental Assessment of the North and South Carolina Coasts, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780128050682. [Google Scholar]
- Brabec, E.; Schulte, S.; Richards, P.L. Impervious Surfaces and Water Quality: A Review of Current Literature and Its Implications for Watershed Planning Impervious Surfaces and Water Quality: A Review of Current Literature and Its Implications. J. Plan. Lit. 2002, 16, 499–514. [Google Scholar] [CrossRef]
- Williams, B.A.; Watson, J.E.M.; Beyer, H.L.; Klein, C.J.; Montgomery, J.; Runting, R.K.; Roberson, L.A.; Halpern, B.S.; Grantham, H.S.; Kuempel, C.D.; et al. Global Rarity of Intact Coastal Regions. Conserv. Biol. 2022, 36, e13874. [Google Scholar] [CrossRef]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-Physiological Adaptations That Favour Freshwater Cyanobacteria in a Changing Climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef]
- Jovanović, J.; Popović, S.; Simić, G.S.; Jovanović, V.; Predojević, D.; Jovanović, D.; Karadžić, V. Freshwater Cyanobacteria in Waters Intended for Human Consumption in Serbia: Two Decades of Changes in Diversity. Arch. Biol. Sci. 2022, 74, 217–226. [Google Scholar] [CrossRef]
- Cordeiro-Araújo, M.K.; Lorenzi, A.S.; Chia, M.A.; Mota, E.C.; do Carmo Bittencourt-Oliveira, M. Insights into the Impact of Increasing Temperature, Light Intensity, and UV-B Exposure on the Circadian Rhythm of Microcystin Production and Release, and the Expression of Mcy Genes in the Cyanobacterium Microcystis Aeruginosa. J. Appl. Phycol. 2022, 34, 231–242. [Google Scholar] [CrossRef]
- Kramer, B.J.; Gobler, C.J. Simulated Heat Waves Promote the Growth but Suppress the N2 Fixation Rates of Dolichospermum Spp. and Cyanobacterial Communities in Temperate Lakes. Ecol. Indic. 2023, 147, 109983. [Google Scholar] [CrossRef]
- Kramer, B.J.; Hem, R.; Gobler, C.J. Elevated CO2 Significantly Increases N2 Fixation, Growth Rates, and Alters Microcystin, Anatoxin, and Saxitoxin Cell Quotas in Strains of the Bloom-Forming Cyanobacteria, Dolichospermum. Harmful Algae 2022, 120, 102354. [Google Scholar] [CrossRef]
- Wahl, T.; Jain, S.; Bender, J.; Meyers, S.D.; Luther, M.E. Increasing Risk of Compound Flooding from Storm Surge and Rainfall for Major US Cities. Nat. Clim. Chang. 2015, 5, 1093–1097. [Google Scholar] [CrossRef]
- Manda, A.K.; Klein, W.A. Adaptation Strategies to Address Rising Water Tables in Coastal Environments Under Future Climate and Sea-Level Rise Scenarios. In Coastal Zone Management; Elsevier: Amsterdam, The Netherlands, 2019; pp. 403–409. [Google Scholar]
- Houliez, E.; Briand, E.; Malo, F.; Rovillon, G.A.; Hervé, F.; Robert, E.; Marchand, L.; Zykwinska, A.; Caruana, A.M.N. Physiological Changes Induced by Sodium Chloride Stress in Aphanizomenon gracile, Cylindrospermopsis raciborskii and Dolichospermum sp. Harmful Algae 2021, 103, 102028. [Google Scholar] [CrossRef]
- Duval, C.; Thomazeau, S.; Drelin, Y.; Yéprémian, C.; Bouvy, M.; Couloux, A.; Troussellier, M.; Rousseau, F.; Bernard, C. Phylogeny and Salt-Tolerance of Freshwater Nostocales Strains: Contribution to Their Systematics and Evolution. Harmful Algae 2018, 73, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Marzec, H.; Żeglińska, L.; Pliński, M. The Effect of Salinity on the Growth, Toxin Production, and Morphology of Nodularia Spumigena Isolated from the Gulf of Gdańsk, Southern Baltic Sea. J. Appl. Phycol. 2005, 17, 171–179. [Google Scholar] [CrossRef]
- Tonk, L.; Bosch, K.; Visser, P.M.; Huisman, J. Salt Tolerance of the Harmful Cyanobacterium Microcystis Aeruginosa. Aquat. Microb. Ecol. 2007, 46, 117–123. [Google Scholar] [CrossRef]
- Bormans, M.; Amzil, Z.; Mineaud, E.; Brient, L.; Savar, V.; Robert, E.; Lance, E. Demonstrated Transfer of Cyanobacteria and Cyanotoxins along a Freshwater-Marine Continuum in France. Harmful Algae 2019, 87, 101639. [Google Scholar] [CrossRef] [PubMed]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, Eutrophication and Harmful Algal Blooms along the Freshwater to Marine Continuum. WIREs Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Herren, L.W.; Paule, A.L. Septic Systems Contribute to Nutrient Pollution and Harmful Algal Blooms in the St. Lucie Estuary, Southeast Florida, USA. Harmful Algae 2017, 70, 1–22. [Google Scholar] [CrossRef]
- Wood, S.M.; Kremp, A.; Savela, H.; Akter, S.; Vartti, V.-P.; Saarni, S.; Suikkanen, S. Cyanobacterial Akinete Distribution, Viability, and Cyanotoxin Records in Sediment Archives From the Northern Baltic Sea. Front. Microbiol. 2021, 12, 681881. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Dickey, D.A.; Kinder, C.A.; Reed, R.E.; Mallin, M.A.; McIver, M.R.; Cahoon, L.B.; Melia, G.; Brownie, C.; Smith, J.; et al. Comprehensive Trend Analysis of Nutrients and Related Variables in a Large Eutrophic Estuary: A Decadal Study of Anthropogenic and Climatic Influences. Limnol. Oceanogr. 2006, 51, 463–487. [Google Scholar] [CrossRef]
- Preece, E.P.; Moore, B.C.; Hardy, F.J. Transfer of Microcystin from Freshwater Lakes to Puget Sound, WA and Toxin Accumulation in Marine Mussels (Mytilus Trossulus). Ecotoxicol. Environ. Saf. 2015, 122, 98–105. [Google Scholar] [CrossRef]
- Gibble, C.M.; Peacock, M.B.; Kudela, R.M. Evidence of Freshwater Algal Toxins in Marine Shellfish: Implications for Human and Aquatic Health. Harmful Algae 2016, 59, 59–66. [Google Scholar] [CrossRef]
- Bukaveckas, P.A.; Franklin, R.; Tassone, S.; Trache, B.; Egerton, T. Cyanobacteria and Cyanotoxins at the River-Estuarine Transition. Harmful Algae 2018, 76, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Staedler, M.; Miller, W.A.; Toy-Choutka, S.; Dominik, C.; et al. Evidence for a Novel Marine Harmful Algal Bloom: Cyanotoxin (Microcystin) Transfer from Land to Sea Otters. PLoS ONE 2010, 5, e12576. [Google Scholar] [CrossRef]
- Caly, L.F.; Rodríguez, D.C.; Peñuela, G.A. Monitoring of Cyanobacteria and Cyanotoxins in a Colombian Tropical Reservoir. Environ. Sci. Pollut. Res. 2022, 29, 52775–52787. [Google Scholar] [CrossRef] [PubMed]
- Grentell, J.; Adhikary, R.K.; Lal, A. Cyanobacteria, Water Quality and Public Health Implications: A Systematic Scoping Review. Australas. J. Water Resour. 2022, 27, 1–13. [Google Scholar] [CrossRef]
- Tito, J.C.R.; Luna, L.M.G.; Noppe, W.N.; Hubert, I.A. First Report on Microcystin-LR Occurrence in Water Reservoirs of Eastern Cuba, and Environmental Trigger Factors. Toxins 2022, 14, 209. [Google Scholar] [CrossRef]
- Aguilera, A.; Almanza, V.; Haakonsson, S.; Palacio, H.; Benitez Rodas, G.A.; Barros, M.U.G.; Capelo-Neto, J.; Urrutia, R.; Aubriot, L.; Bonilla, S. Cyanobacterial Bloom Monitoring and Assessment in Latin America. Harmful Algae 2023, 125, 102429. [Google Scholar] [CrossRef]
- Isaacs, J.D.; Strangman, W.K.; Barbera, A.E.; Mallin, M.A.; McIver, M.R.; Wright, J.L.C. Microcystins and Two New Micropeptin Cyanopeptides Produced by Unprecedented Microcystis Aeruginosa Blooms in North Carolina’s Cape Fear River. Harmful Algae 2014, 31, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Iraola, N.D.; Mallin, M.A.; Cahoon, L.B.; Gamble, D.W.; Zamora, P.B. Nutrient Dynamics in a Eutrophic Blackwater Urban Lake. Lake Reserv. Manag. 2022, 38, 28–46. [Google Scholar] [CrossRef]
- North Carolina Water Resources Division. 2024 303(d) Listing and Delisting Methodology; North Carolina Department of Environmental Quality: Raleigh, NC, USA, 2023.



| Genera | Identified Species in This Study | Potential Toxin Production |
|---|---|---|
| Anabaena | ATX, CYN, MC, STX, Guanitoxin | |
| Anabaenopsis | ATX, MC, STX | |
| Aphanizomenon | A. flos-aqua | ATX, CYN, MC, STX, Nodularins |
| Cuspidothrix * | C. issatschenkoi | ATX, STX |
| Chrysoporum | CYN | |
| Cylindrospermum | ATX | |
| Dolichospermum * | D. affine, D. circinales, D. crassum, D. cf. flos-aqua, D. perturbatum, D. planctonicum, D. smithii, D. spiroides | ATX, CYN, MC, STX, Guanitoxin |
| Lyngbya | CYN, MC, STX, Lyngbyatoxin | |
| Microcystis * | M. aeruginosa, M. flos-aquae, M. ichthioblable, M. novacekii, M. protocystis | ATX, MC |
| Nodularia | Nodularins | |
| Oscillatoria | ATX, CYN, MC, Lyngbyatoxin | |
| Planktothrix | P. agarhii | ATX, CYN, MC, STX, Lyngbyatoxin |
| Phormidium | ATX, MC | |
| Sphaerospermopsis | S. reniformis, S. aphanizomenoides | ATX, CYN, Guanitoxin |
| Synechococcus | ATX, CYN, MC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grogan, A.E.; Alves-de-Souza, C.; Cahoon, L.B.; Mallin, M.A. Harmful Algal Blooms: A Prolific Issue in Urban Stormwater Ponds. Water 2023, 15, 2436. https://doi.org/10.3390/w15132436
Grogan AE, Alves-de-Souza C, Cahoon LB, Mallin MA. Harmful Algal Blooms: A Prolific Issue in Urban Stormwater Ponds. Water. 2023; 15(13):2436. https://doi.org/10.3390/w15132436
Chicago/Turabian StyleGrogan, Amy E., Catharina Alves-de-Souza, Lawrence B. Cahoon, and Michael A. Mallin. 2023. "Harmful Algal Blooms: A Prolific Issue in Urban Stormwater Ponds" Water 15, no. 13: 2436. https://doi.org/10.3390/w15132436
APA StyleGrogan, A. E., Alves-de-Souza, C., Cahoon, L. B., & Mallin, M. A. (2023). Harmful Algal Blooms: A Prolific Issue in Urban Stormwater Ponds. Water, 15(13), 2436. https://doi.org/10.3390/w15132436







