Abstract
Internal solitary waves (ISWs) are a common marine internal wave phenomenon in the northern South China Sea that cause significant changes in environmental factors and affect phytoplankton communities. This study investigates the short-term response of phytoplankton communities affected by ISWs based on day–night continuous sampling analysis of the sea area following the passage of an internal wave. The results revealed that, due to the IW-mediated transport of nutrients from deeper to shallower layers, the cell abundance of most small- and medium-volume phytoplankton significantly increased after the passage of ISWs. Using a method based on functional traits, we categorized phytoplankton into four functional groups. Moreover, this study revealed the differences in functional group changes in phytoplankton before and after ISWs. The abundance of mixotrophic phytoplankton in the community decreased, whereas autotrophic and heterotrophic phytoplankton increased.
1. Introduction
Traditionally, the physical mechanisms influencing the structure of marine phytoplankton communities have not been limited to the diapycnal mixing of shallow- and deep-sea waters [1]. To date, many new influencing mechanisms have been found, including the actions of eddies [2], tropical cyclones [3], and propagating planetary waves [4,5]. Internal waves (IW) represent another mechanism that significantly affects the structures of marine phytoplankton communities. As a type of internal wave, internal solitary waves (ISWs) are the most striking; thus, they are the focus of this study. ISWs are solitary waves propagating within a layer of discontinuous density typically formed between two fluid layers. These waves often appear at oceanic ridges, such as the edges of continental shelves, straits, bays, and estuaries, and have large amplitudes and lengths [6]. ISWs transport energy and matter into the ocean, thereby significantly affecting the marine environment, ecology, and engineering. Thus, ISWs are critical for studying the structure of marine phytoplankton communities. However, recent research on internal waves has mainly focused on physics rather than biochemistry [7,8]. Although there have been reports on the enhancement of biological productivity in shallow- and deep-water areas by ISWs, this is primarily based on the concentration of chlorophyll a. Research on changes in the construction of marine phytoplankton communities before and after the passage of ISWs is limited [7,9,10,11,12].
The South China Sea, the third largest marginal sea globally, is situated in a low-latitude area and is part of the tropical deep sea. As a region frequently visited by internal waves, scientists often observe ISWs with significant amplitudes and lengths. These ISWs promote the mixing of the upper and lower layers of seawater, causing nutrient-rich cold water to rise from deep to shallow depths, which has a significant effect on the marine ecological environment in this area. The internal waves in the South China Sea originate from the Luzon Strait, from which they propagate westward to the northern part of the South China Sea [13,14,15]. Internal waves have been widely detected from sea surface roughness in Synthetic Aperture Radar (SAR) images [8]. During their extension into the South China Sea, the internal waves encountered the Dongsha Atoll on the Dongsha Plateau. The depth of the Dongsha Plateau is approximately 600 m; however, it abruptly ends in the south, where the depth increases to over 2000 m. These internal waves can freely propagate in this area and finally dissipate onto the continental shelf on the western side of the South China Sea [8,16]. The natural environment around the Dongsha Atoll provides a unique venue for studying how ISWs enhance marine phytoplankton productivity, particularly the dissipation zone north of the Dongsha Atoll and the transmission zones to the east and south [12]. To further study the effect of internal waves on marine phytoplankton communities, we selected two typical areas in the southwest direction of Dongsha Atoll for our research: Station Q40 at a depth of 668 m and Station Q39 at a depth of 1423 m (see Figure 1). We conducted diurnal time-series observations and analyses at these two stations. Both stations were located in the transmission zones of the ISWs and the ISWs that passed through during this period. By comparing the changes in chlorophyll a, temperature, salinity, nitrogen, phosphates, dissolved oxygen (DO), dissolved organic carbon (DOC), and the structure of marine phytoplankton communities before and after the passage of ISWs, we speculated the specific effect of ISWs on the composition of marine phytoplankton communities and the environment.
Figure 1.
Time-series observation station locations in the northern South China Sea.
In this study, we utilized diurnal time-series observations to investigate the influence of ISWs on the summer phytoplankton community structure in the southwestern sea area of the Dongsha Atoll. We chose to observe the summer (September) community structure for two main reasons: Firstly, the shallower mixed layer and sharp thermocline in this sea area during summer are conducive to the formation of ISWs [17,18]. Secondly, studying the summer allows us to avoid the masking or diluting effect that strengthens the vertical mixing of seawater under prevailing winter monsoons on the influence of ISWs [19]. During the observation period, we successfully captured diurnal changes in the phytoplankton community in the northern South China Sea under the effect of ISWs, as well as their responses to environmental factors. Through this research, we aimed to explore how dynamic environmental processes influence environmental factors and, in turn, affect the spatiotemporal distribution of the phytoplankton community structure in the northern South China Sea.
2. Materials and Methods
2.1. Field Data Collection
The observation period for this study was from 1 September to 4 September 2020, at stations Q40 (115°12.75′ E, 20°5.46′ N) and Q39 (115°16.26′ E, 19°53.44′ N). Observations were made every 3 h at station Q40 and every 4 h at station Q39, with a 24 h observation cycle at each station. Water samples were collected using a SeaBird 911 Plus CTD water sampler (Sea-Bird Electronics Inc., Bellevue, WA, USA), and 2% Lugol’s solution was added on-site for fixation. The temperature, dissolved oxygen data, and salinity were obtained from the CTD. Chlorophyll a data were collected via fluorescence photometry using an F-4500 fluorescence spectrophotometer (HITACHI, Tokyo, Japan). Nutrient data for (NH4+, NO2−, NO3−, PO43−, SiO32−) Dissolved nitrate (NO3−), nitrite (NO2−), ammonia (NH4+), and phosphate (PO43−) were measured by using the SEAL-AA3 Auto Continuous Flow Analytical System. Dissolved inorganic nitrogen (DIN) is the sum of NO3−, NO2− and NH4+. Silicate was measured with a Nutrient Auto Analyzer (Clever chem/anna, DeChem-Tech, Germany) based on silicon molybdenum blue spectrophotometry, and the relative error was less than 0.3% in duplicate measurements. Current data were measured using a 75 kHz Self-contained Acoustic Doppler Current Profiler (ADCP) to determine the specific time of ISWs passage.
2.2. Phytoplankton Data
Phytoplankton samples were analyzed using the Utermöhl method [20]. Samples were concentrated in the laboratory and poured into a Hydro-Bios counting chamber (Hydro-Bios, Kiel, Germany). Cell counts and species identification were performed using a NikonTS100 inverted microscope (Nikon, Tokyo, Japan), and we consulted relevant books for their identification [21,22,23,24].
2.3. Data Analysis
The diversity of the phytoplankton communities was analyzed using the following formula:
The Shannon–Wiener diversity index (H′) [25] was used to calculate the biodiversity index:
Species evenness index (Pielou index) [26]:
Dominance degree of species (species with dominance degree ≥ 0.02 are dominant species) [27]:
where ni is the number of individuals of the i-th species, N is the entire number of species in the collected sample, S is the total number of species in the sample, Pi is the ratio of the number of the i-th species (ni) to the total number (N), fi is the frequency of occurrence of the species.
We divided the phytoplankton community into four functional groups based on the three functional traits of phytoplankton species and analyzed representative phytoplankton species (33 species) with a cell abundance of >260 cells/L. Based on previous studies, the selected traits included cell size (CS) [28], temperature adaptation (TA) [29], and nutrition mode (NM) [30]. CS was divided into four volume categories: <10,000 μm3, 10,000–100,000 μm3, 100,000–1,000,000 μm3, and >1,000,000 μm3. The TA was divided into three types: northern temperate species, eurythermal species, and warm water species. NM was classified into three types: autotrophic, heterotrophic, and mixotrophic (photophagotrophy).
The data were organized into a species × trait data frame (Table A1), and a dissimilarity matrix was calculated using Gower’s distance. Species were clustered based on the similarities and differences in functional traits using Ward’s method. The Elbow method was used to determine the optimal number of functional groups (clusters). Cluster analysis was performed using the Factoextra package in the R statistical software [31,32].
The relationship between the groups of algae after cluster analysis and environmental factors was determined using a generalized additive models (GAM) through the “gam” function in the mgcv package in R [33]. The environmental factors included chlorophyll a, temperature, phosphate, salinity, total nitrogen, and dissolved oxygen. Before analysis, the cell abundances of chlorophyll a and phytoplankton underwent logarithmic transformation (log 10) [34]. The best combination of environmental variables was selected using stepwise regression [35]. The GAM was first run using a method that included all explanatory variables. The optimal model was selected by minimizing the generalized cross-validation (GCV) criterion during the fitting process [33].
3. Results
3.1. Composition of Phytoplankton Species
In this study, 3 phyla, 71 genera, and 186 species (including variants and forms) of phytoplankton were identified (Table A2). Among them, there were 39 genera and 85 species in the phylum Bacillariophyta, accounting for 45.70% of the total species, and the dominant group was the genus Chaetoceros, with 24 species, accounting for 28.24% of the diatom species. The phylum Dinophyta comprises 30 genera and 99 species, accounting for 53.23% of the total number of species. Among these, the genus Oxytoxum had 17 species, accounting for 17.17% of the dinoflagellate species, whereas the genus Gonyaulax had 11 species, accounting for 11.11% of the dinoflagellate species. The phylum Chrysophyta comprised two genera and two species, accounting for 1.07% of the total species.
3.2. Vertical Distribution of Phytoplankton under the Influence of ISWs
In the surveyed area, the phytoplankton cell abundance at the Deep Chlorophyll Maximum (DCM) ranged from 5.33 × 102 to 41.34 × 102 cells/L, with an average of 18.55 × 102 cells/L (Figure 2). Among them, the diatom cell abundance ranged from 2.30 × 102 to 34.73 × 102 cells/L, with an average of 14.98 × 102 cells/L, accounting for 80.78% of the total cell abundance, occupying an absolutely dominant position (Figure 2). The dinoflagellate cell abundance ranged from 0.57 × 102 to 8.84 × 102 cells/L, with an average of 3.32 × 102 cells/L, accounting for 17.92% of the entire cell abundance (Figure 2). The average cell abundance of Chrysophyta was 0.24 × 102 cells/L, accounting for 1.30% of the entire cell abundance.
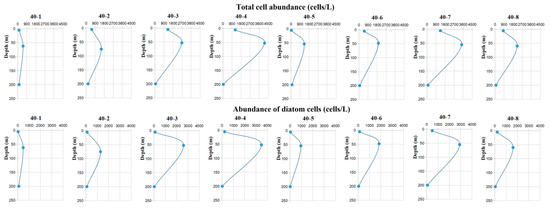
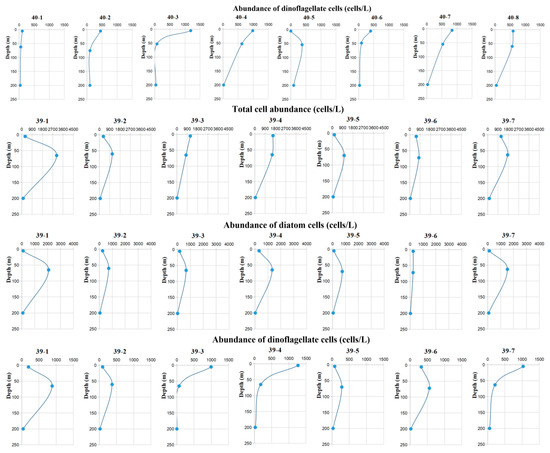
Figure 2.
Changes in total cell abundance, diatom cell abundance, and dinoflagellate cell abundance at Q40 and Q39 stations.
3.2.1. Q40 Station
According to the ADCP observation data, the observation times of Q40-1 and Q40-2 occurred before the arrival of the ISWs, whereas Q40-3, Q40-4, Q40-5, Q40-6, Q40-7, and Q40-8 occurred after the passage of the ISWs. Before ISWs at Q40 station, the total phytoplankton cell abundance in the DCM layer ranged from 5.33 × 102 to 15.07 × 102 cells/L, with an average of 10.20 × 102 cells/L. Among them, diatom cell abundance ranged from 4.76 × 102 to 12.92 × 102 cells/L, with an average of 8.84 × 102 cells/L, accounting for 86.67% of total cell abundance; dinoflagellate cell abundance ranged from 0.57 × 102 to 1.01 × 102 cells/L, with an average of 0.79 × 102 cells/L, accounting for 7.75% of entire cell abundance; and the average cell abundance of Chrysophyta was 0.57 × 102 cells/L, accounting for 5.58% of total cell abundance. After ISWs at Q40 station, the total phytoplankton cell abundance in the DCM layer ranged from 13.23 × 102 to 41.34 × 102 cells/L, with an average of 26.23 × 102 cells/L, a significant increase from before the arrival of ISWs. Among them, diatom cell abundance ranged from 9.48 × 102 to 34.73 × 102 cells/L, with an average of 22.31 × 102 cells/L, accounting for 85.06% of total cell abundance, with diatoms still in an absolutely dominant position; dinoflagellate cell abundance ranged from 0.74 × 102 to 6.26 × 102 cells/L, with an average of 3.78 × 102 cells/L, accounting for 14.41% of entire cell abundance; the average cell abundance of Chrysophyta was 0.14 × 102 cells/L, accounting for 0.53% of total cell abundance.
3.2.2. Q39 Station
Due to problems with the ADCP observations, we did not obtain data on ISWs. However, because the distance and observation times between stations Q39 and Q40 were relatively close, we speculate that ISWs passed during the observation period. The total phytoplankton cell abundance in the DCM layer at Q39 station ranged from 7.92 × 102 to 30.41 × 102 cells/L, with an average of 14.35 × 102 cells/L. Among them, diatom cell abundance ranged from 2.30 × 102 to 20.75 × 102 cells/L, with an average of 10.45 × 102 cells/L, accounting for 72.82% of total cell abundance, occupying an absolutely dominant position; dinoflagellate cell abundance ranged from 0.66 × 102 to 8.84 × 102 cells/L, with an average of 3.66 × 102 cells/L, accounting for 25.51% of entire cell abundance; the average cell abundance of Chrysophyta was 0.24 × 102 cells/L, accounting for 1.67% of entire cell abundance.
3.3. Effect of ISWs on Dominant Species
The dominant species at Q40 station before the ISWs were Nitzschia spp., Chaetoceros lorenzianus, Chaetoceros spp., Nitzschia longissima, Skeletonema costatum, Thalassionema nitzschioides, Gyrodinium dominans, Oxytoxum curvatum, Pronoctiluca pelagica, Protoperidinium sp., and Dictyocha speculum. Among them, Nitzschia spp. (14.49%) and T. nitzschioides (13.68%) were the dominant species before the arrival of the ISWs at Q40 station. The average cell abundance of Nitzschia spp. at 5 m, the DCM layer, and 200 m were 49.15 cells/L, 125.4 cells/L, 47.52 cells/L, respectively (Figure 3), and those of T. nitzschioides were 0 cells/L, 209.7 cells/L, 0 cells/L.
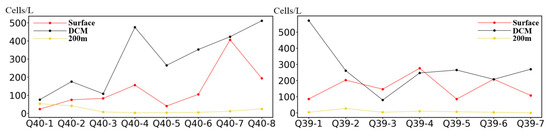
Figure 3.
The abundance changes of Nitzschia spp. in various water layers.
After the passage of ISWs, the dominant species at Q40 station were: Chaetoceros debilis, N. longissima, Nitzschia spp., T. nitzschioides, Thalassiosira spp., Thalassiothrix longissima, G. dominans, and Karenia mikimotoi. Nitzschia spp. (14.80%) were the dominant species after the passage of ISWs at Q40 station. The average cell abundances of Nitzschia spp. at 5 m, the DCM layer, and at 200 m were 163.86 cells/L, 355.88 cells/L, 9.49 cells/L, respectively. The cell abundance of Nitzschia spp. increased significantly compared to that before the arrival of ISWs, but the proportion of cell abundance did not change significantly (Figure 3).
The dominant species at Q39 station were Nitzschia spp., T. nitzschioides, Thalassiosira spp., Scrippsiella trochoidea, Chaetoceros rostratus, Cochlodinium polykrikoides, G. dominans, Gyrodinium instriatum, Oxytoxum crassum, Oxytoxum turbo, and P. pelagica.
The degree of dominance indicates the status and role of a species in a community. In the studied marine area, Nitzschia spp. was the species with absolute advantage, with a high occurrence frequency of up to 100% (Figure 3).
3.4. Changes in Phytoplankton Species
Based on all the dominant phytoplankton species mentioned in Section 3.3, we calculated the proportions of each dominant species at different periods to show the community composition of the dominant species at each period (including 10 diatom species, 10 dinoflagellate species, and 1 chrysophyte species). Nitzschia spp., G. dominans, and T. nitzschioides had relatively high abundances in the DCM layer in all periods, with Nitzschia spp. appearing in all water layers (Figure 4). At Q40 station, C. rostratus and G. instriatum started to appear only after the ISW period. These trends reflect the different responses of phytoplankton community structures to drastic environmental changes.
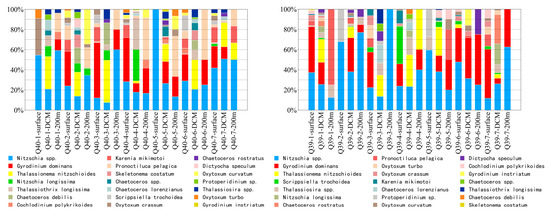
Figure 4.
Percentage Abundance of 21 Dominant Species.
3.5. Diversity and Evenness Indices
The Shannon–Wiener diversity index and Pielou evenness index were used to measure the stability of the phytoplankton community structure.
3.5.1. Q40 Station
Before the ISWs, the Shannon–Wiener diversity index range for the phytoplankton in the 5 m water layer was 3.40–4.28, with an average of 3.84, and the evenness index ranged between 0.81 and 0.89, with an average of 0.85. The diversity index in the DCM water layer ranged from 3.57 to 3.65, with an average of 3.61, while the evenness index ranged from to 0.88–0.91, with an average of 0.90. After the ISWs, the Shannon–Wiener diversity index range for the phytoplankton in the 5 m water layer was 3.56–3.90, with an average of 3.74, and the evenness index ranged between 0.75 and 0.83, with an average of 0.78. The diversity index at the DCM water layer ranged from 3.30 to 4.26, with an average of 3.83, and the evenness index ranged from 0.73 to 0.85, with an average of 0.82.
3.5.2. Q39 Station
The Shannon–Wiener diversity index range for the phytoplankton in the 5 m water layer was 1.36–4.08, with an average of 2.87, and the evenness index ranged between 0.67 and 0.87, with an average of 0.76. The highest average values of the diversity index and evenness index were in the DCM water layer, with the diversity index ranging from 2.77 to 4.27, averaging 3.66, and the evenness index ranging from 0.68 to 0.94, with an average of 0.81.
3.6. Community Structure Cluster Analysis
Four phytoplankton functional groups were identified based on these three functional traits (Figure 5; Table 1).
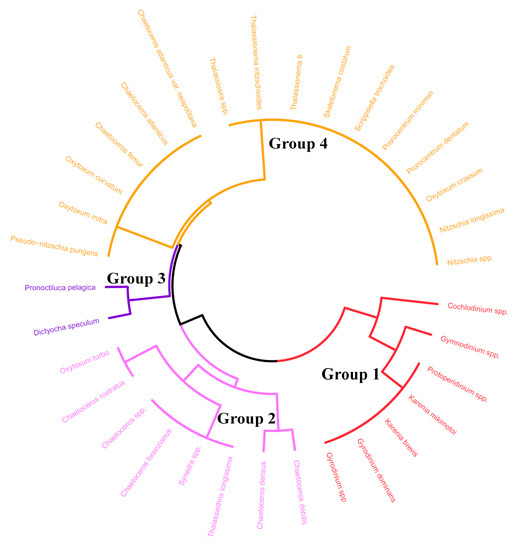
Figure 5.
Dendrogram of functional groups of phytoplankton identified on the basis of functional traits (via agglomerative hierarchical clustering).

Table 1.
The functional characteristics of four identified phytoplankton functional groups.
Group 1 includes small-to medium-volume phytoplankton, eurythermal species, and heterotrophic dinoflagellates (Table 1). At the Q40 station, the average cell abundance of this group was 26.14 cells/L before the ISWs and 80.90 cells/L after the ISWs. The cell abundance in Group 1 was significantly lower before ISWs treatment than after ISWs treatment.
Group 2 included small-to medium-volume phytoplankton, species ranging from temperate to eurythermal to warm water, and autotrophic diatoms and dinoflagellates (Table 1). At the Q40 station, the average cell abundance of this group was 25.21 cells/L before the ISWs and 54.18 cells/L after the ISWs. The cell abundance in Group 2 was significantly lower before ISWs treatment than after ISWs treatment.
Group 3 included small-volume phytoplankton, species ranging from eurythermal to warm water, and mixotrophic chrysophytes and dinoflagellates (Table 1). At the Q40 station, the average cell abundance of this group was 49.59 cells/L before the ISWs and 26.09 cells/L after the ISWs. The cell abundance in Group 3 was significantly higher before ISWs treatment than after ISWs treatment.
Small-volume phytoplankton, species ranging from eurythermal to warm water, and autotrophic diatoms and dinoflagellates (Table 1) were assigned to Group 4. At the Q40 station, the average cell abundance of this group was 46.54 cells/L before the ISWs and 86.81 cells/L after the ISWs. The cell abundance in Group 4 was significantly lower before ISWs treatment than after ISWs treatment.
3.7. Correlation between Phytoplankton and Environmental Factors
Our Generalized Additive Model (GAM) annalysis revealed relationships between the four functional groups and environmental variables (Figure 6 and Table 2). Temperature exhibited a negative correlation with Groups 3 and 4. Group 1 showed a positive correlation below 22 °C but became negatively correlated above 22 °C. Group 2 showed a positive correlation below 25 °C but became negatively correlated above 25 °C. The concentration of chlorophyll a showed a negative correlation with group 1 but exhibited a positive correlation with group 4. Although the influence of phosphate on the four functional groups was minimal, the total nitrogen concentration showed a negative correlation with all four groups. DOC showed a positive correlation with all functional groups, whereas dissolved oxygen first showed a positive correlation with Group 3 and then became negatively correlated.
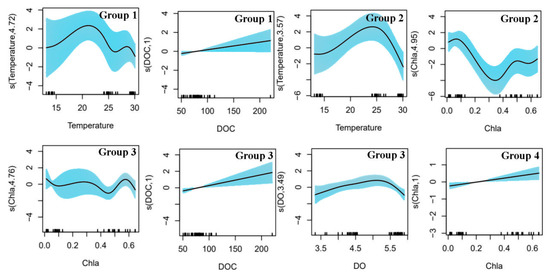
Figure 6.
Relationships between abundance of functional groups and environmental factors (temperature, chlorophyll a, dissolved organic carbon, dissolved oxygen) in the area. The shaded areas are the 95% confidence intervals.

Table 2.
Generalized additive model results for phytoplankton and environmental factors among the four transects.
4. Discussion
4.1. Effect of ISWs on Phytoplankton and Dominant Species
The ISWs in the studied sea area are of the sinking type. When ISWs pass through this area, they mix the upper and lower water layers. This mixing process transports water bodies from deeper layers, which contain higher nutrient concentrations, to shallower layers. This provides more abundant nutrients for phytoplankton in the shallower layers, thereby stimulating their growth and reproduction [36]. As we observed, after the ISWs, the DCM layer rose to varying degrees at different time points (Figure 2). Furthermore, the adaptability of different phytoplankton species, particularly the dominant species, to environmental conditions varies. For instance, after the passage of ISWs, the average cell abundance of Nitzschia spp. increased in all water layers; however, its average proportion did not change significantly. This is because Nitzschia spp. have better adaptability to environmental changes [37] and can maintain a relatively high growth rate under new environmental conditions caused by ISWs. However, other species adapt differently to environmental changes; therefore, the proliferation efficiency of each species after ISWs is not balanced. Moreover, changes in cell abundance caused by ISWs are also affected by the interactions between species. For example, different species of phytoplankton compete for nutrients [38], and species that can use newly supplied nutrients more quickly or effectively will have greater growth after treatment with ISWs; thus, they have a stronger competitive advantage.
4.2. Response of Four Functional Groups to Environmental Factor Changes under the Effect of ISWs
According to the GAM analysis, temperature has a positive correlation with the heterotrophic dinoflagellate population (Group 1), which consists of species from the north temperate zone (positive correlation above 22 °C and a negative correlation below 22 °C); it also has a positive correlation with the autotrophic diatoms and dinoflagellates (Group 2) consisting of species from the north temperate zone—wide-temperature zone—warm water zone before 24 °C, and then becomes negatively correlated. This is because the physiological and metabolic activities of some dinoflagellates are enhanced within suitable temperature ranges, thereby increasing their growth and reproduction [39]. However, when the temperature exceeds this range, it can damage the physiological and metabolic activities of dinoflagellate cells, thereby affecting their survival. In addition, heterotrophic dinoflagellates rely on the ingestion of organic matter to obtain energy, and high temperatures can affect their ability to capture organic matter, further affecting their survival [40].
In the oligotrophic northern South China Sea, the cell abundances of medium- and large-sized phytoplankton were far lower than that of small-sized phytoplankton, and chlorophyll a was significantly positively correlated with small-sized autotrophic phytoplankton (Group 4). When water temperatures are high, small phytoplankton usually have higher growth rates, which allow them to rapidly utilize resources in nutrient-poor areas, thereby gaining an advantage in competition [41,42]. Moreover, a high-temperature environment is more suitable for small phytoplankton because it has a higher surface-area-to-volume ratio, which enables them to absorb light and nutrients [43].
DOC is positively correlated with most medium-sized dinoflagellates (Group 1) and significantly positively correlated with small dinoflagellates and chrysophytes (Group 3). Compared with diatoms, medium- and small-sized dinoflagellates have higher productivity in the ocean [44], faster growth rates, and stronger adaptability [39]. Column mixing caused by ISWs not only brings surface water to the deep layers but also brings nutrient-rich deep water to the upper layers. This provides dinoflagellates and chrysophytes with more nutrients, increasing their productivity and biomass and thus increasing the concentration of DOC.
Dissolved oxygen is initially positively correlated and then negatively correlated with small mixotrophic dinoflagellates and chrysophytes (Group 3). This phenomenon is related to the balance between respiration and photosynthesis in the phytoplankton. Dinoflagellates and chrysophytes perform photosynthesis during the day, absorbing carbon dioxide and releasing oxygen, thus increasing the concentration of dissolved oxygen and resulting in a positive correlation. However, at night or in environments with insufficient light, these phytoplankton perform respiration, consume oxygen, and produce carbon dioxide. Thus, the concentration of dissolved oxygen decreases, exhibiting a negative correlation [45]. Moreover, because they are mixotrophic, they switch to heterotrophic nutrition when light is insufficient [30], further consuming dissolved oxygen.
5. Conclusions
ISWs significantly alter the phytoplankton community structure. Downwelling ISWs passing through marine areas cause water column mixing, bringing nutrient-rich deep water to the surface, providing phytoplankton with more nutrients, stimulating their growth and reproduction, and significantly increasing the abundances of most medium- and small-sized diatoms and dinoflagellates. Furthermore, according to our GAM analysis, under the effect of ISWs, changes in environmental factors such as temperature, chlorophyll a, DOC, and dissolved oxygen have different effects on different functional groups of phytoplankton, resulting in a decrease in the abundance of mixotrophic phytoplankton and an increase in the abundance of autotrophic and heterotrophic phytoplankton in the phytoplankton community.
Author Contributions
Conceptualization, Z.G.; methodology, Z.G., R.G.; investigation, L.Z.; data curation, Y.L.; writing—original draft preparation, Z.G.; writing—review and editing, Z.G., S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. 31750001). The data and sample collection for this study were based on the shared voyage of the National Natural Science Foundation of China (Voyage number: NORC2019-05).
Data Availability Statement
Not applicable.
Acknowledgments
“Dong Fang Hong 3” research vessel, for which we would like to express our gratitude.
Conflicts of Interest
We state that there were no conflict of interest related to this study. All funding information and people who provided help during this study have been mentioned in the manuscript.
Appendix A

Table A1.
Species × trait data frame.
Table A1.
Species × trait data frame.
| Taxa | Volume (μm3) | Nutritional Type | Ecological Group |
|---|---|---|---|
| Group1 | |||
| Cochlodinium spp. | 100,000–1,000,000 | HN | BT |
| Gymnodinium spp. | <10,000 | HN | BT |
| Protoperidinium spp. | 10,000–100,000 | HN | BT |
| Karenia mikimotoi | 10,000–100,000 | HN | BT |
| Karenia brevis | 10,000–100,000 | HN | BT |
| Gyrodinium dominans | 10,000–100,000 | HN | BT |
| Gyrodinium spp. | 10,000–100,000 | HN | BT |
| Group2 | |||
| Chaetoceros debilis | <10,000 | AN | NT |
| Chaetoceros densus | 10,000–100,000 | AN | NT |
| Thalassiothrix longissima | 10,000–100,000 | AN | BT |
| Synedra spp. | 10,000–100,000 | AN | BT |
| Chaetoceros lorenzianus | 10,000–100,000 | AN | BT |
| Chaetoceros spp. | 10,000–100,000 | AN | BT |
| Chaetoceros rostratus | 10,000–100,000 | AN | BT |
| Oxytoxum turbo | 10,000–100,000 | AN | WW |
| Group3 | |||
| Dictyocha speculum | <10,000 | MN | BT |
| Pronoctiluca pelagica | <10,000 | MN | WW |
| Group4 | |||
| Pseudo-nitzschia pungens | <10,000 | AN | WW |
| Oxytoxum mitra | <10,000 | AN | WW |
| Oxytoxum curvatum | <10,000 | AN | WW |
| Chaetoceros femur | <10,000 | AN | WW |
| Chaetoceros atlanticus | <10,000 | AN | WW |
| Chaetoceros atlanticus var. neapolitana | <10,000 | AN | WW |
| Thalassiosira spp. | <10,000 | AN | BT |
| Thalassionema nitzschioides | <10,000 | AN | BT |
| Thalassionema frauenfeldii | <10,000 | AN | BT |
| Skeletonema costatum | <10,000 | AN | BT |
| Scrippsiella trochoidea | <10,000 | AN | BT |
| Prorocentrum minimun | <10,000 | AN | BT |
| Prorocentrum dentatum | <10,000 | AN | BT |
| Oxytoxum crassum | <10,000 | AN | BT |
| Nitzschia longissima | <10,000 | AN | BT |
| Nitzschia spp. | <10,000 | AN | BT |

Table A2.
Species list of the phytoplankton assemblage.
Table A2.
Species list of the phytoplankton assemblage.
| Dinophyta | Protoperidinium simulum (Paulsen) Balech, 1974 |
| Alexandrium minutum Halim, 1960 | Protoperidinium spp. |
| Alexandrium spp. | Pyrocystis noctiluca Murray ex Haeckel |
| Alexandrium tamarense (Lebour) Balech, 1985 | Pyrophacus steinii (Schiller) Wall & Dale |
| Amphisolenia schroederi Kofoid | Scrippsiella trochoidea (Stein) Balech ex Loeblich III |
| Blepharocysta splendor-maris (Ehrenberg) Ehrenberg | Bacillariophyta |
| Ceratocorys bipes (Cleve) Kofoid, 1910 | Actinoptychus hexagonus Grunow in Schmidt, 1874 |
| Ceratocorys horrida Stein, 1883 | Actinoptychus senarius (Ehr.) Ehrenberg |
| Cochlodinium brandtii Wulff | Arachnoidiscus ehrenbergii Bailey ex Ehrenberg |
| Cochlodinium polykrikoides Margelef | Asteromphalus flabellatus (Brébisson) Greville, 1859 |
| Cochlodinium spp. | Bacteriastrum comosum Pavillard |
| Corythodinium belgicae (Meunier) F.J.R.Taylor, 1976 | Bacteriastrum furcatum Shadbolt, 1854 |
| Corythodinium carinatum (Gaarder) F.J.R.Taylor, 1976 | Bacteriastrum hyalinum Lauder |
| Corythodinium constrictum (Stein) Taylor | Bacteriastrum mediterraneum Pavillard |
| Corythodinium curvicaudatum (Kofoid) F.J.R.Taylor, 1976 | Biddulphia pellucida Castracane, 1886 |
| Corythodinium latum (Gaarder) F.J.R.Taylor, 1976 | Cerataulina bergonii Ostenfeld, 1903 |
| Corythodinium reticulatum (Stein) Taylor, 1976 | Chaetoceros affinis Lauder |
| Corythodinium spp. | Chaetoceros atlanticus Cleve |
| Corythodinium tesselatum (Stein) Loeblich Jr.& Loeblich III | Chaetoceros atlanticus var. neapolitana (Schröder) Hustedt |
| Dinophysis acuminata Claparède et Lachmann | Chaetoceros buceros Karsten |
| Dinophysis favus (Kofoid & Michener) Balech | Chaetoceros castracanei Karsten |
| Dinophysis oviformis Chen & Ni, 1988 | Chaetoceros coarctatus Lauder |
| Dinophysis parva Schiller | Chaetoceros constrictus Gran |
| Dinophysis spp. | Chaetoceros danicus Cleve |
| Diplopsalopsis bomba (Stein ex Jorgensen) Dodge & Toriumi | Chaetoceros debilis Cleve |
| Dolichodinium lineatum (Kofoid & Michener) Kofoid & Adamson, 1933 | Chaetoceros densus (Cleve) Cleve, 1899 |
| Gonyaulax cochlea Meunier, 1919 | Chaetoceros denticulatus Lauder |
| Gonyaulax kofoidii Pavillard, 1909 | Chaetoceros distans Cleve |
| Gonyaulax macroporus Mangin, 1922 | Chaetoceros femur Schütt |
| Gonyaulax minuta Kofoid & Michener, 1911 | Chaetoceros hirundinellus Qian |
| Gonyaulax monospina Rampi, 1951 | Chaetoceros lauderi Ralfs |
| Gonyaulax ovalis Schiller, 1929 | Chaetoceros lorenzianus Grunow |
| Gonyaulax pacifica Kofoid, 1907 | Chaetoceros messanensis Castracane |
| Gonyaulax polygramma Stein, 1883 | Chaetoceros paradoxus Cleve |
| Gonyaulax spinifera (Claparede & Lachmann) Diesing, 1866 | Chaetoceros pelagicus Cleve |
| Gonyaulax spp. | Chaetoceros peruvianus Brightwell |
| Gonyaulax turbynei Murray & Whitting, 1899 | Chaetoceros pseudodichaeta Ikari |
| Gymnodinium spp. | Chaetoceros rostratus Lauder |
| Gyrodinium dominans Hulbert | Chaetoceros saltans Cleve |
| Gyrodinium falcatum Kofoid & Swezy, 1921 | Chaetoceros spp. |
| Gyrodinium instriatum Freudenthal et Lee | Corethron criophilum Castracane |
| Gyrodinium spirale (Bergh) Kofoid et Swezy | Coscinodiscus asteromphalus Ehrenberg |
| Gyrodinium spp. | Coscinodiscus granii Grough |
| Heterocapsa triquetra (Ehrenberg) Stein, 1883 | Coscinodiscus jonesianus (Greville) Ostenfeld |
| Heterodinium agassizii Kofoid | Coscinodiscus radiatus Ehrenberg |
| Heterodinium milneri (Murray & Whitting) Kofoid, 1906 | Coscinodiscus spp. |
| Heterodinium whittingiae Kofoid, 1906 | Cylindrotheca closterium (Ehrenberg) Reimann & J.C. Lewin, 1964 |
| Histioneis gregoryi Böhm | Diploneis bombus Ehrenberg |
| Karenia brevis (Davis) G.Hansen & Moestrup | Eucampia cornuta (Cleve) Grunow |
| Karenia mikimotoi Hansen & Moestrup | Eunotogramma debile Grunow in Van Heurck, 1883 |
| Lingulodinium polyedrum (Stein) Dodge | Fragilaria spp. |
| Lissodinium spp. | Fragilariopsis doliolus (Wallich) Medlin & Sims |
| Lissodinium taylorii Carbonell-Moore, 1993 | Guinardia flaccida (Castracane) Peragallo |
| Neoceratium boehmii (Graham et Bronikovsky) | Guinardia striata (Stolterfoth) Hasle et al. |
| Neoceratium horridum (Gran) Gómez, Moreira & López-Garcia | Helicotheca tamesis (Shrubsole) Ricard |
| Neoceratium kofoidii (Jörgensen) Gómez, Moreira & López-Garcia | Hemiaulus hauckii Grunow ex Van Heurck, 1882 |
| Neoceratium longipes (Bailey) Gómez, Moreira & López-Garcia | Hemiaulus membranacus Cleve |
| Neoceratium setaceum (Jörgensen) Gómez, Moreira & López-Garcia | Hemiaulus sinensis Greville |
| Neoceratium teres (Kofoid) Gómez, Moreira & López-Garcia | Leptocylindrus danicus Cleve |
| Ornithocercus thumii (Schmidt) Kofoid & Skogsberg | Leptocylindrus mediterraneus (H. Peragallo) Hasle |
| Oxytoxum crassum Schiller | Mastogloia rostrata (Wallich) Hustedt |
| Oxytoxum curvatum (Kofoid) Kofoid, 1911 | Navicula membranacea Cleve, 1897 |
| Oxytoxum depressum Schiller, 1937 | Navicula spp. |
| Oxytoxum elongatum Wood, 1963 | Nitzschia longissima (Brébisson) Ralfs, 1861 |
| Oxytoxum laticeps Schiller | Nitzschia lorenziana Grunow |
| Oxytoxum longiceps Schiller | Nitzschia panduriformis Hustedt in Schmidt et al., 1921 |
| Oxytoxum milneri Murray & Whitting, 1899 | Nitzschia spp. |
| Oxytoxum mitra Stein, 1883 | Odontella sinensis (Greville) Grunow |
| Oxytoxum mucronatum Hope, 1954 | Palmeria hardmaniana Greville |
| Oxytoxum parvum Schiller, 1937 | Paralia sulcata (Ehrenberg) Cleve, 1873 |
| Oxytoxum sceptrum (Stein) Schröder | Pinnularia spp. |
| Oxytoxum scolopax Stein | Planktoniella formosa Qian & Wang |
| Oxytoxum sphaeroideum Stein | Planktoniella sol Qian et Wang |
| Oxytoxum spp. | Pleurosigma acutum Norman |
| Oxytoxum turbo Kofoid | Pleurosigma pelagicum Peragallo |
| Oxytoxum variabilis Schiller | Pleurosigma spp. |
| Palaeophalacroma unicinctum Schiller, 1928 | Pseudo-nitzschia pungens (Grunow ex Cleve) Hasle |
| Podolampas bipes Stein | Rhabodonema adriaticum Kützing |
| Podolampas palmipes Stein | Rhizosolenia alata f. indica (Peragallo) Ostenfeld |
| Pronoctiluca pelagica Fabre-Domerqne | Rhizosolenia bergonii Peragallo |
| Pronoctiluca spinifera (Lohmann) Schiller, 1932 | Rhizosolenia sinensis Qian |
| Prorocentrum compressum (Ostenfeld) Abé | Rhizosolenia styliformis Brightwell |
| Prorocentrum dentatum Stein | Skeletonema costatum (Greville) Cleve |
| Prorocentrum lenticulatum (Matzenauer) Taylor | Synedra spp. |
| Prorocentrum lima (Ehrenberg) Dodge | Thalassionema frauenfeldii (Grunow) Hallegraeff |
| Prorocentrum minimun (Pavillard) Schiller | Thalassionema nitzschioides Grunow |
| Prorocentrum sigmoides Böhm | Thalassiosira eccentrica (Ehrenberg) Cleve, 1904 |
| Prorocentrum spp. | Thalassiosira spp. |
| Prorocentrum triestinum Schiller | Thalassiothrix longissima Cleve et Grunow |
| Protoceratium areolatum Kofoid | Triceratium affine Grunow |
| Protoperidinium conicum (Gran) Balech | Triceratium favus Ehrenberg |
| Protoperidinium excentricum (Paulsen) Balech, 1974 | Dictyochophyceae |
| Protoperidinium orientale (Matzenauer) Balech | Dictyocha fibula Ehrenberg |
| Protoperidinium ovum (Schiller) Balech, 1974 | Dictyocha speculum Ehrenberg |
| Protoperidinium pyrum (Balech) Balech |
References
- McGillicuddy, D.J.; Robinson, A.R. Eddy-induced nutrient supply and new productivity in the Sargasso Sea. Deep Sea Res. (Part I) 1997, 44, 1427–1450. [Google Scholar] [CrossRef]
- McGillicuddy, D.J.; Robinson, A.R.; Siegel, D.A.; Jannasch, H.W.; Johnson, R.; Dickey, T.D.; McNeil, J.; Michaels, A.F.; Knap, A.H. Influence of mesoscale eddies on new productivity in the Sargasso Sea. Nature 1998, 394, 263–265. [Google Scholar] [CrossRef]
- Lin, I.; Liu, W.T.; Wu, C.C.; Wong, G.T.F.; Hu, C.; Chen, Z.; Liang, W.D.; Yang, Y.; Liu, K.K. New evidence for enhanced ocean primary productivity triggered by tropical cyclone. Geophys. Res. Lett. 2003, 30, 1718. [Google Scholar] [CrossRef]
- Cipollini, P.; Cromwell, C.; Challenor, P.G.; Raffaglio, S. Rossby waves detected in global ocean colour data. Geophys. Res. Lett. 2001, 28, 323–326. [Google Scholar] [CrossRef]
- Uz, B.M.; Yoder, J.A.; Osychny, V. Pumping nutrients to ocean surface waters by the action of propagating planetary waves. Nature 2001, 409, 597–600. [Google Scholar] [CrossRef]
- Muench, K.; Lipscomb, M.; Lee, M.; Kuehl, G. Homologous cysteine-containing sequences in tryptophanyl-tRNA synthetases from Escherichia coli and human placentas. Science 1975, 187, 1089–1091. [Google Scholar] [CrossRef]
- Holligan, P.M.; Pingree, R.D.; Mardell, G.T. Oceanic solitons, nutrient pulses and phytoplankton growth. Nature 1985, 314, 348–350. [Google Scholar] [CrossRef]
- Liu, C.T.; Pinkel, R.; Hsu, M.K.; Klymak, J.M.; Chien, H.W.; Villanoy, C. Nonlinear internal waves from the Luzon Strait. Eos Trans. AGU 2006, 87, 449–451. [Google Scholar] [CrossRef]
- da Silva, J.C.B.; New, A.L.; Srokosz, M.A.; Smyth, T.J. On the observability of internal tidal waves in remotely-sensed ocean colour data. Geophys. Res. Lett. 2002, 29, 1569. [Google Scholar] [CrossRef]
- Wang, Y.H.; Dai, C.F.; Chen, Y.Y. Physical and ecological processes of internal waves on an isolated reef ecosystem in the South China Sea. Geophys. Res. Lett. 2007, 34, L18609. [Google Scholar] [CrossRef]
- Wilson, C. Chlorophyll anomalies along the critical latitude at 30°N in the NE Pacific. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Pan, X.; Wong, G.T.; Shiah, F.K.; Ho, T.Y. Enhancement of biological productivity by internal waves: Observations in the summertime in the northern South China Sea. J. Oceanogr. 2012, 68, 427–437. [Google Scholar] [CrossRef]
- Chang, M.H.; Lien, R.C.; Tang, T.Y.; D’Asaro, E.A.; Yang, Y.J. Energy flux of nonlinear internal waves in northern South China Sea. Geophys. Res. Lett. 2006, 33. Available online: https://agupubs.onlinelibrary.wiley.com/doi/full/10.1029/2005GL025196 (accessed on 25 May 2023). [CrossRef]
- Chao, S.Y.; Ko, D.S.; Lien, R.C.; Shaw, P.T. Assessing the west ridge of Luzon Strait as an internal wave mediator. J. Oceanogr. 2007, 63, 897–911. [Google Scholar] [CrossRef]
- Hsu, M.K.; Liu, A.K.; Liu, C. A study of internal waves in the China Seas and Yellow Sea using SAR. Cont. Shelf Res. 2000, 20, 389–410. [Google Scholar] [CrossRef]
- Zhang, Z.; Fringer, O.B.; Ramp, S.R. Three dimensional, nonhydrostatic numerical simulation of nonlinear internal wave generation and propagation in the South China Sea. J. Geophys. Res. 2011, 116, C05022. [Google Scholar] [CrossRef]
- Qu, T.; Du, Y.; Gan, J.; Wang, D. Mean seasonal cycle of isothermal depth in the South China Sea. J. Geophys. Res. 2007, 112, C02020. [Google Scholar] [CrossRef]
- Shaw, P.T.; Ko, D.S.; Chao, S.Y. Internal solitary waves induced by flow over a ridge: With applications to the northern South China Sea. J. Geophys. Res. 2009, 114, C02019. [Google Scholar] [CrossRef]
- Tseng, C.M.; Wong, G.T.F.; Lin, I.I.; Wu, C.R.; Liu, K.K. A unique seasonal pattern in phytoplankton biomass in low-latitude waters in the South China Sea. Geophys. Res. Lett. 2005, 32, L08608. [Google Scholar] [CrossRef]
- CEN (European Committee for Standardization). Water Quality—Guidance Standard on the Enumeration of Phytoplankton Using Inverted Microscopy (Utermohl Technique); CEN: Brussels, Belgium, 2006. [Google Scholar]
- Yang, S.M.; Dong, S.G. Atlas of Common Planktonic Diatoms in China Sea Area; Ocean University of China Press: Qingdao, China, 2006; pp. 1–267. [Google Scholar]
- Yang, S.M.; Li, R.X.; Dong, S.G. Dinoflagellates I (Prorocentrales, Dinophysiaoes) in Chinese Waters; Ocean Press: Beijing, China, 2014; pp. 1–156. [Google Scholar]
- Yang, S.M.; Li, R.X.; Dong, S.G. Dinoflagellates II (Goneaulacales) in Chinese Waters; Ocean Press: Beijing, China, 2016; pp. 1–248. [Google Scholar]
- Yang, S.M.; Li, R.X.; Dong, S.G. Dinoflagellates III (Peridiniales) in Chinese Waters; Ocean Press: Beijing, China, 2019; pp. 1–211. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; pp. 1–125. [Google Scholar]
- Pielou, E.G. An introduction to mathematical ecology. J. Ecol. 1970, 58, 1–286. [Google Scholar]
- Lampitt, R.S.; Wishner, K.F.; Turley, C.M.; Angel, M.V. Marine snow studies in the Northeast Atlantic Ocean: Distribution, composition and role as a food source for migrating plankton. Mar. Biol. 1993, 116, 689–702. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A. Trait-Based Community Ecology of Phytoplankton. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [Google Scholar] [CrossRef]
- Righetti, D.; Vogt, M.; Gruber, N.; Psomas, A.; Zimmermann, N.E. Global pattern of phytoplankton diversity driven by temperature and environmental variability. Sci. Adv. 2019, 5, eaau6253. [Google Scholar] [CrossRef] [PubMed]
- Stoecker, D.K.; Hansen, P.J.; Caron, D.A.; Mitra, A. Mixotrophy in the Marine Plankton. Annu. Rev. Mar. Sci. 2017, 9, 311–335. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R package version 1.0.7; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=factoextra (accessed on 1 March 2023).
- Krzton, W.; Kosiba, J. Variations in zooplankton functional groups density in freshwater ecosystems exposed to cyanobacterial blooms. Sci. Total Environ. 2020, 730, 139044. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman & Hall/CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Campbell, J.W. The lognormal distribution as a model for bio-optical variability in the sea. J. Geophys. Res. Oceans 1995, 100, 13237–13254. [Google Scholar] [CrossRef]
- Marra, G.; Wood, S.N. Practical variable selection for generalized additive models. Comput. Stat. Data. Anal. 2011, 55, 2372–2387. [Google Scholar] [CrossRef]
- Cai, S.; Xie, J.; He, J. An Overview of Internal Solitary Waves in the South China Sea. Surv. Geophys. 2012, 33, 927–943. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Comparisons of nitrate uptake, storage, and reduction in marine diatoms and flagellates. J. Phycol. 2000, 36, 903–913. [Google Scholar] [CrossRef]
- Margalef, R. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol. Acta 1978, 1, 493–509. [Google Scholar]
- Thomas, M.K.; Kremer, C.T.; Klausmeier, C.A.; Litchman, E. A global pattern of thermal adaptation in marine phytoplankton. Science 2012, 338, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Berman-Frank, I.; Bopp, L.; Boyd, P.W.; Galbraith, E.D.; Geider, R.J.; Guieu, C.; Jaccard, S.L.; et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Schofield, O.M.; Falkowski, P.G. The role of functional traits and trade-offs in structuring phytoplankton communities: Scaling from cellular to ecosystem level. Ecol. Lett. 2007, 10, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Marañón, E.; Cermeño, P.; Rodríguez, J.; Zubkov, M.V.; Harris, R.P. Scaling of phytoplankton photosynthesis and cell size in the ocean. Limnol. Oceanogr. 2007, 52, 2190–2198. [Google Scholar] [CrossRef]
- Marañón, E. Cell size as a key determinant of phytoplankton metabolism and community structure. Annu. Rev. Mar. Sci. 2015, 7, 241–264. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Freing, A.; Wallace DW, R.; Bange, H.W. Global oceanic production of nitrous oxide. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1245–1255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).