Enhancing Biocontrol of Harmful Algae Blooms: Seasonal Variation in Allelopathic Capacity of Myriophyllum aquaticum
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Microcystis aeruginosa
2.2. Collection of Macrophytes
2.3. Preparation and Obtaining of Aqueous Extracts
2.4. Analysis of Total Phenolic Compounds of Plant Extracts
2.5. Bioassay
2.6. Cyanobacteria Growth
2.7. Evaluation of Cellular Viability
2.8. Determination of Photosynthetic Pigments
2.9. Intracellular Microcystin-LR Quantification (MCLR)
2.10. Data Analysis
3. Results
3.1. Content Total of Phenolic Compounds on Aqueous Extract
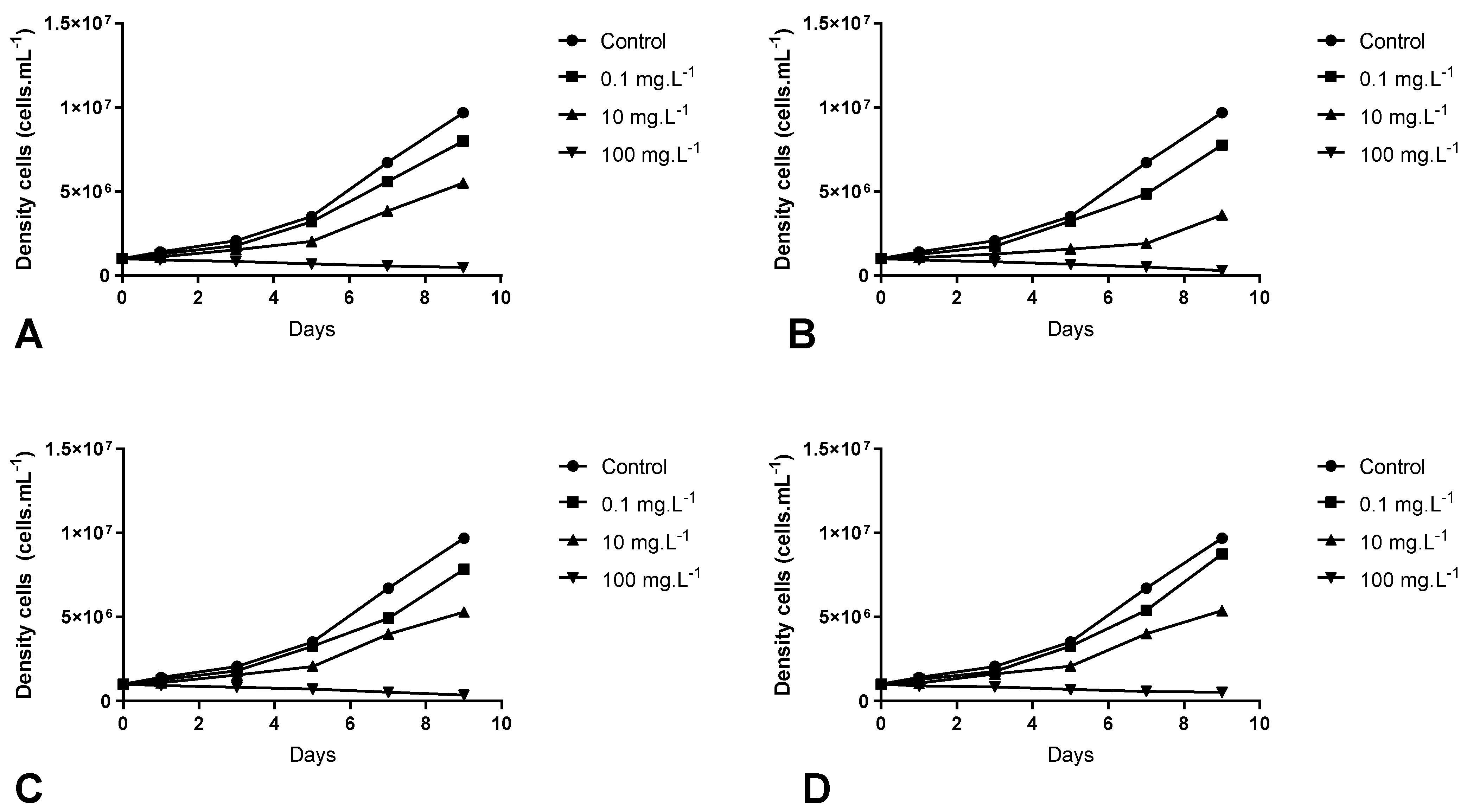
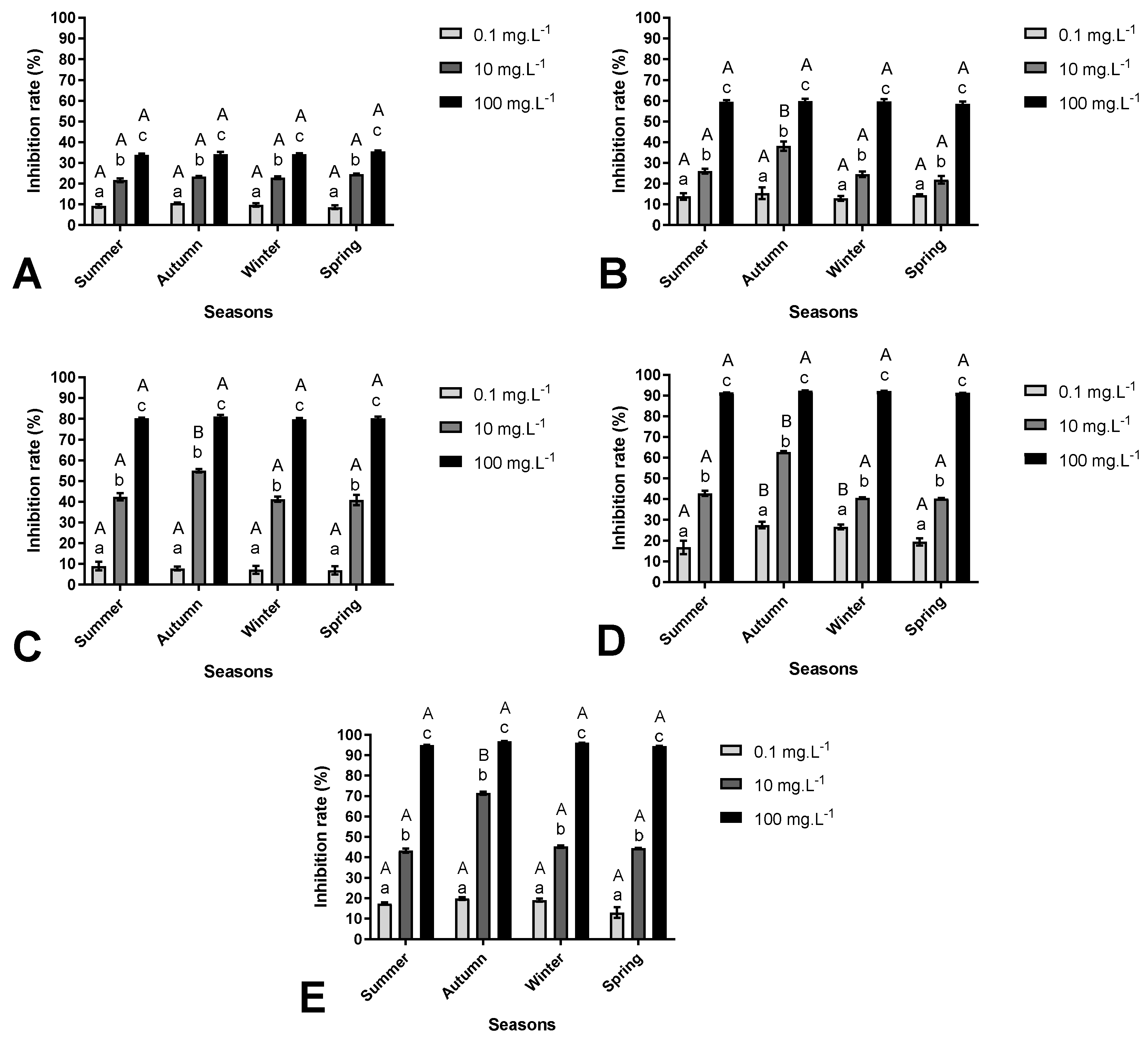
3.2. Inhibitory Effects of Aqueous Extract of M. aquaticum on M. aeruginosa Growth
3.3. Cellular Viability of M. aeruginosa after Exposure to Aqueous Extracts
3.4. Effects on Photosynthetic Pigments from M. aeruginosa
3.5. Concentration and Removal Efficiency of Intracellular MCLR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tanvir, R.U.; Hu, Z.; Zhang, Y.; Lu, J. Cyanobacterial Community Succession and Associated Cyanotoxin Production in Hypereutrophic and Eutrophic Freshwaters. Environ. Pollut. 2021, 290, 118056. [Google Scholar] [CrossRef]
- Ramya, M.; Umamaheswari, A.; Elumalai, S. Global Health Concern of Cyanotoxins in Surface Water and Its Various Detection Methods. Curr. Bot. 2020, 11, 65–74. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The Rise of Harmful Cyanobacteria Blooms: The Potential Roles of Eutrophication and Climate Change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Li, B.; Yin, Y.; Kang, L.; Feng, L.; Liu, Y.; Du, Z.; Tian, Y.; Zhang, L. A Review: Application of Allelochemicals in Water Ecological Restoration—Algal Inhibition. Chemosphere 2021, 267, 128869. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kumari, N.; Häder, D.-P.; Sinha, R.P. Cyanobacterial Blooms and Their Implications in the Changing Environment. Adv. Environ. Eng. Res. 2022, 3, 1–41. [Google Scholar] [CrossRef]
- Calado, S.L.d.M.; Vicentini, M.; Santos, G.S.; Pelanda, A.; Santos, H.; Coral, L.A.; Magalhães, V.d.F.; Mela, M.; Cestari, M.M.; Silva de Assis, H.C. Sublethal Effects of Microcystin-LR in the Exposure and Depuration Time in a Neotropical Fish: Multibiomarker Approach. Ecotoxicol. Environ. Saf. 2019, 183, 109527. [Google Scholar] [CrossRef]
- Takahashi, T.; Umehara, A.; Tsutsumi, H. Diffusion of Microcystins (Cyanobacteria Hepatotoxins) from the Reservoir of Isahaya Bay, Japan, into the Marine and Surrounding Ecosystems as a Result of Large-Scale Drainage. Mar. Pollut. Bull. 2014, 89, 250–258. [Google Scholar] [CrossRef]
- Rodrigues, N.B.; Pitol, D.L.; Tocchini de Figueiredo, F.A.; Tenfen das Chagas Lima, A.C.; Burdick Henry, T.; Mardegan Issa, J.P.; de Aragão Umbuzeiro, G.; Pereira, B.F. Microcystin-LR at Sublethal Concentrations Induce Rapid Morphology of Liver and Muscle Tissues in the Fish Species Astyanax altiparanae (Lambari). Toxicon 2022, 211, 70–78. [Google Scholar] [CrossRef]
- Tazart, Z.; Douma, M.; Tebaa, L.; Loudiki, M. Use of Macrophytes Allelopathy in the Biocontrol of Harmful Microcystis aeruginosa Blooms. Water Sci. Technol. Water Supply 2019, 19, 245–253. [Google Scholar] [CrossRef]
- Mathur, P.; Sanyal, D.; Callahan, D.L.; Conlan, X.A.; Pfeffer, F.M. Treatment Technologies to Mitigate the Harmful Effects of Recalcitrant Fluoroquinolone Antibiotics on the Environment and Human Health. Environ. Pollut. 2021, 291, 118233. [Google Scholar] [CrossRef]
- Tazart, Z.; Manganelli, M.; Scardala, S.; Buratti, F.M.; Di Gregorio, F.N.; Douma, M.; Mouhri, K.; Testai, E.; Loudiki, M. Remediation Strategies to Control Toxic Cyanobacterial Blooms: Effects of Macrophyte Aqueous Extracts on Microcystis aeruginosa (Growth, Toxin Production and Oxidative Stress Response) and on Bacterial Ectoenzymatic Activities. Microorganisms 2021, 9, 1782. [Google Scholar] [CrossRef]
- Swe, T.; Lombardo, P.; Ballot, A.; Thrane, J.E.; Sample, J.; Eriksen, T.E.; Mjelde, M. The Importance of Aquatic Macrophytes in a Eutrophic Tropical Shallow Lake. Limnologica 2021, 90, 125910. [Google Scholar] [CrossRef]
- Kakade, A.; Salama, E.S.; Han, H.; Zheng, Y.; Kulshrestha, S.; Jalalah, M.; Harraz, F.A.; Alsareii, S.A.; Li, X. World Eutrophic Pollution of Lake and River: Biotreatment Potential and Future Perspectives. Environ. Technol. Innov. 2021, 23, 101604. [Google Scholar] [CrossRef]
- Triest, L.; Stiers, I.; Van Onsem, S. Biomanipulation as a Nature-Based Solution to Reduce Cyanobacterial Blooms. Aquat. Ecol. 2016, 50, 461–483. [Google Scholar] [CrossRef]
- Zhu, X.; Dao, G.; Tao, Y.; Zhan, X.; Hu, H. A Review on Control of Harmful Algal Blooms by Plant-Derived Allelochemicals. J. Hazard. Mater. 2021, 401, 123403. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Han, Z.; Ye, J.; Liu, Z. The Influence of Aquatic Macrophytes on Microcystis aeruginosa Growth. Ecol. Eng. 2012, 42, 130–133. [Google Scholar] [CrossRef]
- Thomaz, S.M. Ecosystem Services Provided by Freshwater Macrophytes. Hydrobiologia 2023, 850, 2757–2777. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, L.; Yang, X.; Zhang, S.; Xia, W.; Li, C. Allelopathic Control of Freshwater Phytoplankton by the Submerged Macrophyte Najas Minor All. Acta Ecol. Sin. 2014, 34, 351–355. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, X.; Lin, F.; Grossart, H.-P.; Nie, Z.; Sun, L.; Xu, C.; Shi, J. Continuous-Release Beads of Natural Allelochemicals for the Long-Term Control of Cyanobacterial Growth: Preparation, Release Dynamics and Inhibitory Effects. Water Res. 2016, 95, 113–123. [Google Scholar] [CrossRef]
- Kitamura, R.; Silva, A.; Pagioro, T.; Martins, L. Bioatividade alelopática de extratos metanólicos de Myriophyllum aquaticum (Vell.) Verdc. sobre Microcystis aeruginosa Kutzing. Encicl. Biosf. 2021, 18, 530–543. [Google Scholar] [CrossRef]
- Nakai, S.; Zou, G.; Okuda, T.; Nishijima, W.; Hosomi, M.; Okada, M. Polyphenols and Fatty Acids Responsible for Anti-Cyanobacterial Allelopathic Effects of Submerged Macrophyte Myriophyllum spicatum. Water Sci. Technol. 2012, 66, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, F.; Luo, P.; Li, Z.; Zheng, L.; Wang, H.; Zou, D.; Wu, J. Allelopathic Effects of Myriophyllum aquaticum on Two Cyanobacteria of Anabaena Flos-Aquae and Microcystis aeruginosa. Bull. Environ. Contam. Toxicol. 2017, 98, 556–561. [Google Scholar] [CrossRef]
- Nakai, S. Myriophyllum spicatum-Released Allelopathic Polyphenols Inhibiting Growth of Blue-Green Algae Microcystis aeruginosa. Water Res. 2000, 34, 3026–3032. [Google Scholar] [CrossRef]
- Kitamura, R.S.A.; da Silva, A.R.S.; Pagioro, T.A.; Martins, L.R.R. Use of Myriophyllum aquaticum to Inhibit Microcystis aeruginosa Growth and Remove Microcystin-LR. Rev. Bras.Ciênc. Ambient. 2022, 57, 434–441. [Google Scholar] [CrossRef]
- He, Y.; Zhou, Q.H.; Liu, B.Y.; Cheng, L.; Tian, Y.; Zhang, Y.Y.; Wu, Z. Bin Programmed Cell Death in the Cyanobacterium Microcystis aeruginosa Induced by Allelopathic Effect of Submerged Macrophyte Myriophyllum spicatum in Co-Culture System. J. Appl. Phycol. 2016, 28, 2805–2814. [Google Scholar] [CrossRef]
- Bauer, N.; Blaschke, U.; Beutler, E.; Gross, E.M.; Jenett-Siems, K.; Siems, K.; Hilt, S. Seasonal and Interannual Dynamics of Polyphenols in Myriophyllum verticillatum and Their Allelopathic Activity on Anabaena variabilis. Aquat. Bot. 2009, 91, 110–116. [Google Scholar] [CrossRef]
- Santonja, M.; le Rouzic, B.; Thiébaut, G. Seasonal Dependence and Functional Implications of Macrophyte–Phytoplankton Allelopathic Interactions. Freshw. Biol. 2018, 63, 1161–1172. [Google Scholar] [CrossRef]
- Gorham, P.R.; McLachlan, J.; Hammer, U.T.; Kim, W.K. Isolation and Culture of Toxic Strains of Anabaena Flos-Aquae (Lyngb.) de Bréb. SIL Proc. 1922–2010 1964, 15, 796–804. [Google Scholar] [CrossRef]
- De Almeida, A.R.; Passig, F.H.; Pagioro, T.A.; Do Nascimento, P.T.H.; De Carvalho, K.Q. Remoção de Microcistina-LR Da Microcystis aeruginosa Utilizando Bagaço de Cana-de-Açúcar in Natura e Carvão Ativado. Rev. Ambiente Água 2016, 11, 188–197. [Google Scholar] [CrossRef]
- Amerine, M.A.; Ough, C.S. Análisis de Vinos y Mostos; Acribia: Zaragoza, Spain, 1976. [Google Scholar]
- Cheng, W.; Xuexiu, C.; Hongjuan, D.; Difu, L.; Junyan, L. Allelopathic Inhibitory Effect of Myriophyllum aquaticum (Vell.) Verdc. on Microcystis aeruginosa and Its Physiological Mechanism. Acta Ecol. Sin. 2008, 28, 2595–2603. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 603, 591–592. [Google Scholar] [CrossRef]
- Rizzo, R.F.; dos Santos, B.d.N.C.; de Castro, G.F.P.d.S.; Passos, T.S.; Nascimento, M.d.A.; Guerra, H.D.; da Silva, C.G.; Dias, D.d.S.; Domingues, J.R.; de Lima-Araújo, K.G. Production of Phycobiliproteins by Arthrospira Platensis under Different Lightconditions for Application in Food Products. Food Sci. Technol. 2015, 35, 247–252. [Google Scholar] [CrossRef]
- Chapman, D.J.; Kremer, B.P. Experimental Phycology: A Laboratory Manual; CUP Archiv: Cambridge, UK, 1988. [Google Scholar]
- Torres, M.d.A.; Micheletto, J.; de Liz, M.V.; Pagioro, T.A.; Rocha Martins, L.R.; Martins de Freitas, A. Microcystis aeruginosa Inactivation and Microcystin-LR Degradation by the Photo-Fenton Process at the Initial near-Neutral PH. Photochem. Photobiol. Sci. 2020, 19, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Yang, D.; Joo, S.; Park, S. Allelopathic Inhibition Effects of Myriophyllum spicatum on Growths of Bloom-Forming Cyanobacteria and Other Phytoplankton Species in Coexistence Experiments. J. Plant Biol. 2021, 64, 501–510. [Google Scholar] [CrossRef]
- Riedl, J.; Kluender, C.; Sans-Piché, F.; Heilmeier, H.; Altenburger, R.; Schmitt-Jansen, M. Spatial and Temporal Variation in Metabolic Fingerprints of Field-Growing Myriophyllum spicatum. Aquat. Bot. 2012, 102, 34–43. [Google Scholar] [CrossRef]
- Wersal, R.M.; Cheshier, J.C.; Madsen, J.D.; Gerard, P.D. Phenology, Starch Allocation, and Environmental Effects on Myriophyllum aquaticum. Aquat. Bot. 2011, 95, 194–199. [Google Scholar] [CrossRef]
- Hilt, S.; Gross, E.M. Can Allelopathically Active Submerged Macrophytes Stabilise Clear-Water States in Shallow Lakes? Basic Appl. Ecol. 2008, 9, 422–432. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, P.; Zeng, G.; Cai, X.; Liu, S.; Yin, Y.; Hu, X.; Hu, X.; Tan, X. Growth Inhibition and Oxidative Damage of Microcystis aeruginosa Induced by Crude Extract of Sagittaria trifolia Tubers. J. Environ. Sci. 2016, 43, 40–47. [Google Scholar] [CrossRef]
- Gross, E.M.; Meyer, H.; Schilling, G. Release and ecological impact of algicidal hydrolysable polyphenols in Myriophyllum spicatum. Phytochemistry 1996, 41, 133–138. [Google Scholar] [CrossRef]
- MacColl, R.; Guard-Friar, D. Phycobiliproteins, 1st ed.; CRC Press: New York, NY, USA, 2018; Volume 1. [Google Scholar]
- Leu, E.; Krieger-Liszkay, A.; Goussias, C.; Gross, E.M. Polyphenolic Allelochemicals from the Aquatic Angiosperm Myriophyllum spicatum Inhibit Photosystem II. Plant Physiol. 2002, 130, 2011–2018. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Wang, J.; Gao, Y.; Wu, Z. Study on the Mechanism of Allelopathic Influence on Cyanobacteria and Chlorophytes by Submerged Macrophyte (Myriophyllum spicatum) and Its Secretion. Aquat. Toxicol. 2010, 98, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, P.; Guo, N. Effects of Nonylphenol on the Growth and Microcystin Production of Microcystis Strains. Environ. Res. 2007, 103, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, H.; Chen, J.; Ye, J. Effects of Allelochemical Extracted from Water Lettuce (Pistia stratiotes Linn.) on the Growth, Microcystin Production and Release of Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2013, 20, 8192–8201. [Google Scholar] [CrossRef]
- Xie, G.; Hu, X.; Du, Y.; Jin, Q.; Liu, Y.; Tang, C.; Hu, X.; Li, G.; Chen, Z.; Zhou, D.; et al. Light-Driven Breakdown of Microcystin-LR in Water: A Critical Review. Chem. Eng. J. 2021, 417, 129244. [Google Scholar] [CrossRef]
- Robertson, P.K.J.; Lawton, L.A.; Cornish, B.J.P.A. The Involvement of Phycocyanin Pigment in the Photodecomposition of the Cyanobacterial Toxin, Microcystin-LR. J. Porphyr. Phthalocyanines 1999, 3, 544–551. [Google Scholar] [CrossRef]

| Season | Total Phenolic Compounds (mg·mL−1) | One-Way ANOVA | ||
|---|---|---|---|---|
| D.F | F | p | ||
| Summer | 47.40 ± 2.31 a | 3 | 43.09 | <0.0001 |
| Autumn | 97.40 ± 2.31 b | |||
| Winter | 84.73 ± 4.81 b | |||
| Spring | 62.73 ± 3.52 a | |||
| D.F | Cells Growth (Cells·mL−1) | Extract Effects | |
|---|---|---|---|
| Two-way ANOVA F-values | |||
| Extracts | 2 | 48.18 *** | - |
| Season | 3 | 59.77 *** | - |
| Extracts vs. season | 9 | 26.92 *** | - |
| Seasons | Extracts (mg·L−1) | - | |
| Summer | Control (0) | 1.06 × 107 ± 2.30 × 105 aA | - |
| 0.1 | 7.85 × 106 ± 9.77 × 104 bA | Algistatic | |
| 10 | 5.59 × 106 ± 1.76 × 105 cA | Algistatic | |
| 100 | 8.00 × 103 ± 2.00 × 103 dA | Algicide | |
| Autumn | Control (0) | 1.06 × 107 ± 2.30 × 105 aA | - |
| 0.1 | 6.63 × 106 ± 1.29 × 105 bB | Algistatic | |
| 10 | 4.63 × 106 ± 1.33 × 105 cB | Algistatic | |
| 100 | 1.33 × 103 ± 6.66 × 102 dB | Algicide | |
| Winter | Control (0) | 1.06 × 107 ± 2.30 × 105 aA | - |
| 0.1 | 7.23 × 106 ± 5.88 × 105 bA | Algistatic | |
| 10 | 5.88 × 106 ± 1.66 × 105 cA | Algistatic | |
| 100 | 6.00 × 103 ± 3.46 × 102 dA | Algicide | |
| Spring | Control (0) | 1.06 × 107 ± 2.30 × 105 aA | - |
| 0.1 | 7.84 × 106 ± 6.66 × 104 bA | Algistatic | |
| 10 | 5.81 × 106 ± 1.11 × 105 cA | Algistatic | |
| 100 | 2.53 × 106 ± 7.42 × 104 dC | Algicide | |
| Comparison of means (p ≤ 0.05) | |||
| Seasons | Summer | 6.01 × 106 ± 2.25 × 106 A | - |
| Autumn | 5.47 × 106 ± 2.20 × 106 B | - | |
| Winter | 5.94 × 106 ± 2.22 × 106 B | - | |
| Spring | 6.13 × 106 ± 2.19 × 106 A | - | |
| D.F | Microcystin-LR Concentration (ng·L−1) | Intracellular Microcystin-LR Removal (%) | |
|---|---|---|---|
| Two-way ANOVA F-values | |||
| Extracts | 2 | 1700 *** | 2130 *** |
| Season | 3 | 263.3 *** | 517.9 *** |
| Extracts vs. season | 9 | 122.3 *** | 132.6 *** |
| Seasons | Extracts (mg·L−1) | ||
| Summer | Control (0) | 30.93± 0.69 aA | - |
| 0.1 | 30.98 ± 0.76 aA | 0.18 ± 0.05 aA | |
| 10 | 7.58 ± 0.31 bA | 75.51 ± 1.44 bAB | |
| 100 | 4.21 ± 0.68 cA | 85.98 ± 3.90 dA | |
| Autumn | Control (0) | 30.93 ± 0.69 aA | - |
| 0.1 | 28.19 ± 1.00 bB | 6.68 ± 1.23 aB | |
| 10 | 6.42 ± 0.30 cB | 79.28 ± 0.54 bA | |
| 100 | 4.93 ± 0.83 dA | 89.03 ± 0.97 cA | |
| Winter | Control (0) | 30.93± 0.69 aA | - |
| 0.1 | 30.86 ± 1.18 aA | 0.40 ± 0.27 aA | |
| 10 | 6.58 ± 0.18 bAB | 78.75 ± 0.12 bAB | |
| 100 | 4.47 ± 0.27 cA | 85.54 ± 1.03 cA | |
| Spring | Control (0) | 30.93± 0.69 aA | - |
| 0.1 | 29.21 ± 1.41 aA | 2.89 ± 0.43 aA | |
| 10 | 7.29 ± 0.48 bA | 75.10 ± 1.65 bB | |
| 100 | 6.78± 0.04 bB | 78.11 ± 0.36 bB | |
| Comparison of means (p ≤ 0.05) | |||
| Seasons | Summer | 18.44± 7.27 A | 53.83± 17.80 AC |
| Autumn | 17.82± 7.02 A | 58.33± 18.76 B | |
| Winter | 18.23± 7.34 A | 54.90 ± 18.46 AB | |
| Spring | 18.07 ± 6.41 A | 52.02 ± 20.46 C | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitamura, R.S.A.; da Silva, A.R.S.; Pagioro, T.A.; Martins, L.R.R. Enhancing Biocontrol of Harmful Algae Blooms: Seasonal Variation in Allelopathic Capacity of Myriophyllum aquaticum. Water 2023, 15, 2344. https://doi.org/10.3390/w15132344
Kitamura RSA, da Silva ARS, Pagioro TA, Martins LRR. Enhancing Biocontrol of Harmful Algae Blooms: Seasonal Variation in Allelopathic Capacity of Myriophyllum aquaticum. Water. 2023; 15(13):2344. https://doi.org/10.3390/w15132344
Chicago/Turabian StyleKitamura, Rafael Shinji Akiyama, Ana Roberta Soares da Silva, Thomaz Aurelio Pagioro, and Lúcia Regina Rocha Martins. 2023. "Enhancing Biocontrol of Harmful Algae Blooms: Seasonal Variation in Allelopathic Capacity of Myriophyllum aquaticum" Water 15, no. 13: 2344. https://doi.org/10.3390/w15132344
APA StyleKitamura, R. S. A., da Silva, A. R. S., Pagioro, T. A., & Martins, L. R. R. (2023). Enhancing Biocontrol of Harmful Algae Blooms: Seasonal Variation in Allelopathic Capacity of Myriophyllum aquaticum. Water, 15(13), 2344. https://doi.org/10.3390/w15132344







