Differences of Nitrogen Transformation Pathways and Their Functional Microorganisms in Water and Sediment of a Seasonally Frozen Lake, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Study Lake
2.2. Sample Collection and Treatment
2.3. Physical and Chemical Analyses of Samples
2.4. DNA Extraction, Library Construction and Metagenomics Sequencing
2.5. Sequence Processing and Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
3.1. Physical and Chemical Properties of Samples
3.2. Detection Frequency of Nitrogen-Related Genes
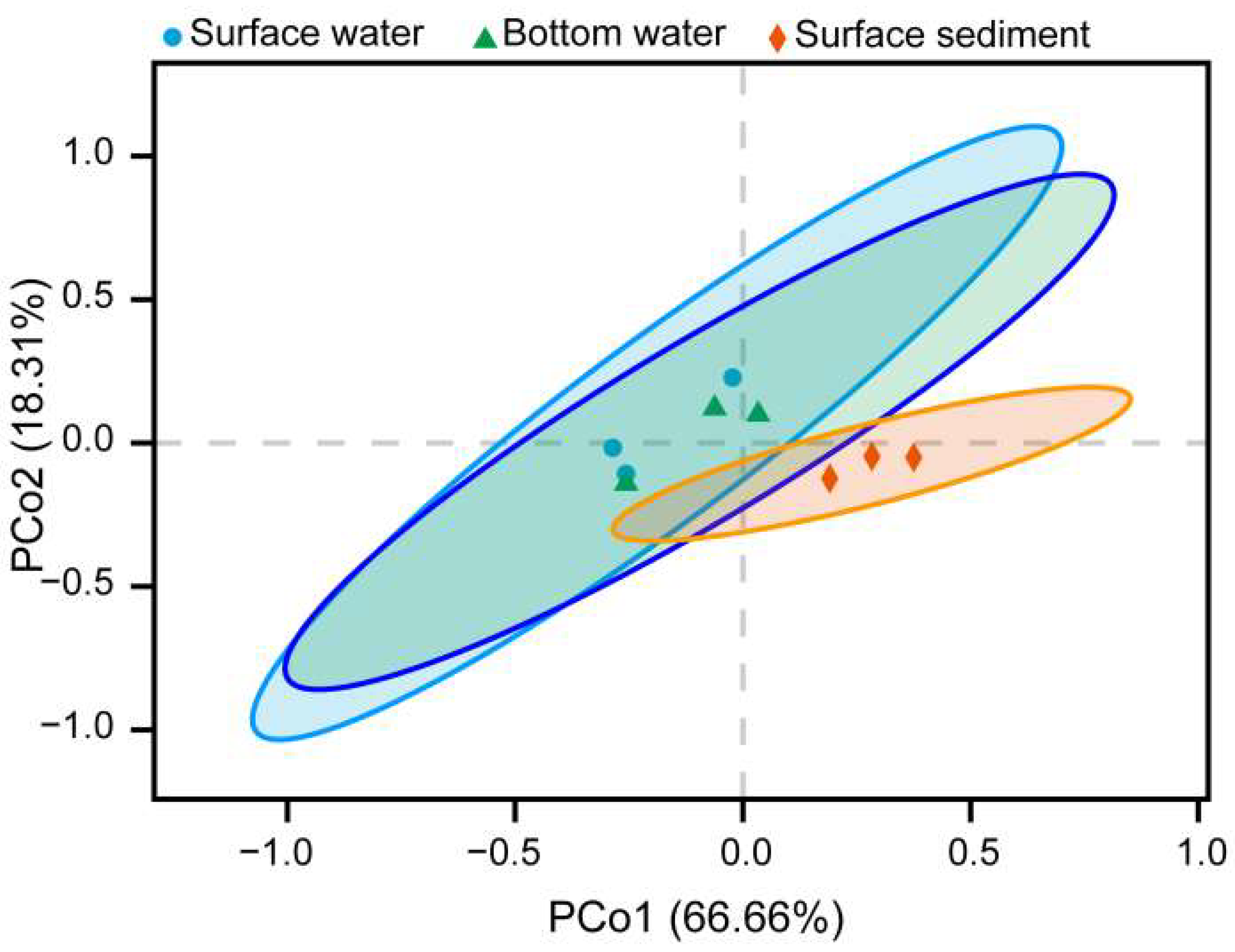
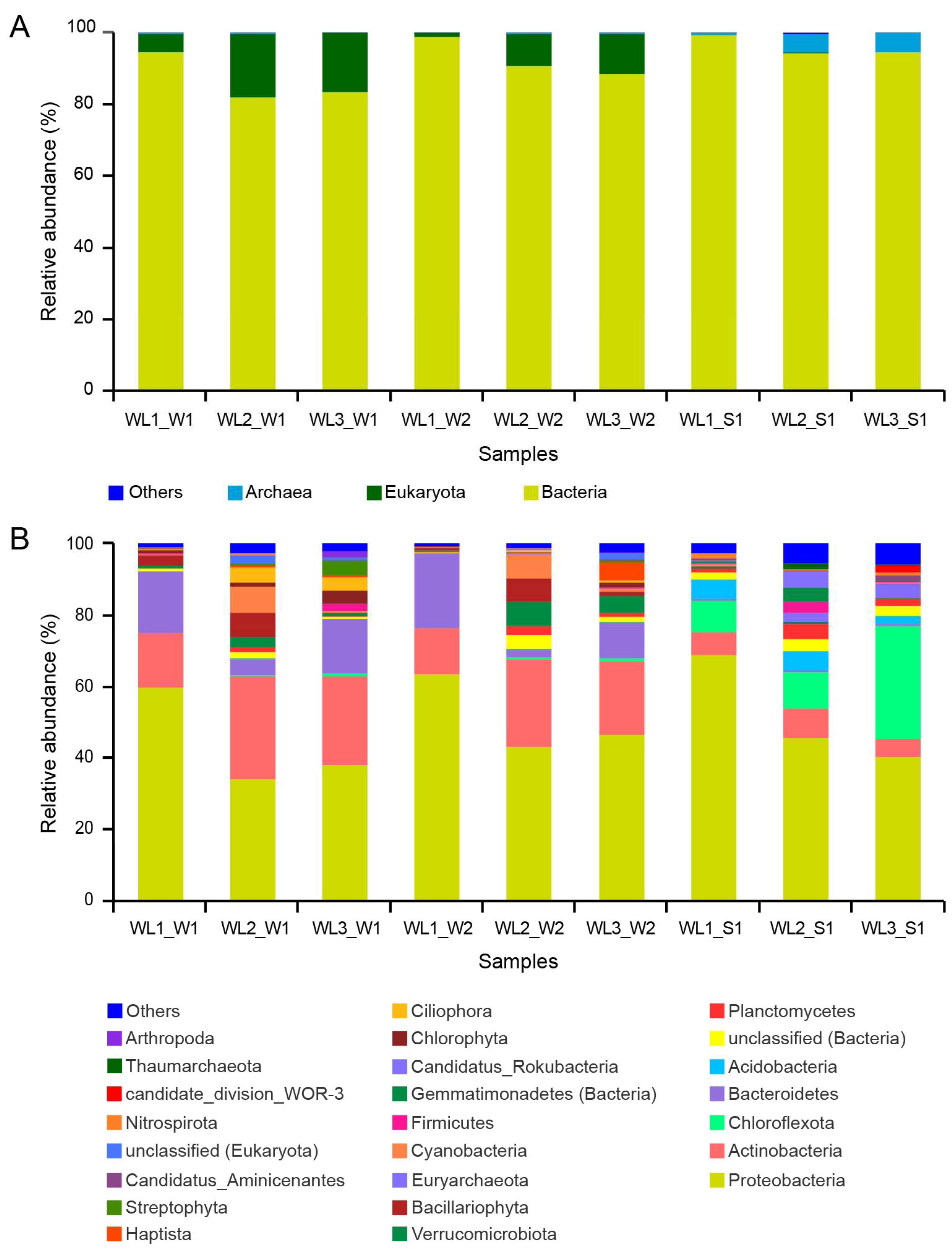
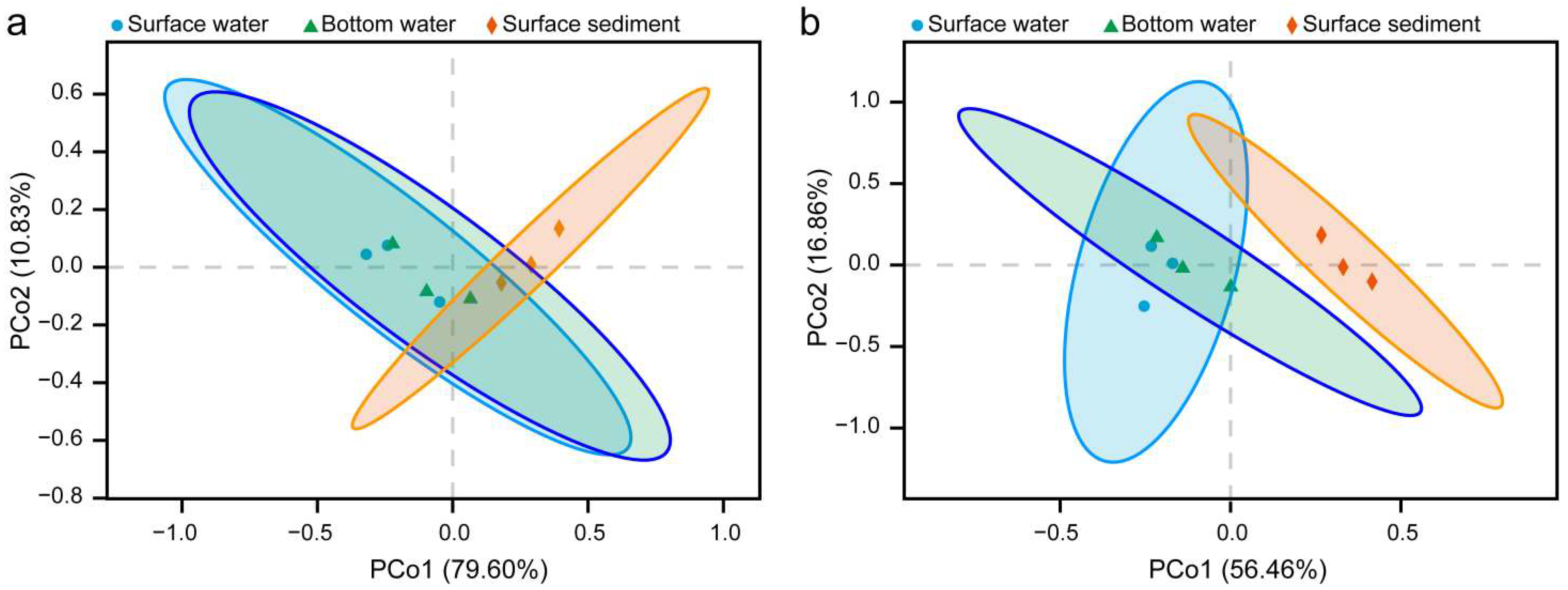
3.3. Composition and Comparison of Nitrogen-Processing Microbiome
4. Discussion
4.1. Characterization of Nitrogen Transformation Pathways
4.2. Nitrogen Transformation Pathways and Their Differences
4.3. Nitrogen Transformation Functional Microorganisms and Their Differences
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Ecology. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Parsons, C.; Stueken, E.E.; Rosen, C.J.; Mateos, K.; Anderson, R.E. Radiation of nitrogen-metabolizing enzymes across the tree of life tracks environmental transitions in Earth history. Geobiology 2021, 19, 18–34. [Google Scholar] [CrossRef]
- Qin, B.; Yang, L.; Chen, F.; Zhu, G.; Zhang, L.; Chen, Y. Mechanism and control of lake eutrophication. Chin. Sci. Bull. 2006, 51, 2401–2412. [Google Scholar] [CrossRef]
- Lin, S.S.; Shen, S.L.; Zhou, A.; Lyu, H.M. Assessment and management of lake eutrophication: A case study in Lake Erhai, China. Sci. Total. Environ. 2021, 751, 141618. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y.; Klotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, R94–R98. [Google Scholar] [CrossRef]
- Ollivier, J.; Towe, S.; Bannert, A.; Hai, B.; Kastl, E.M.; Meyer, A.; Su, M.X.; Kleineidam, K.; Schloter, M. Nitrogen turnover in soil and global change. FEMS Microbiol. Ecol. 2011, 78, 3–16. [Google Scholar] [CrossRef]
- Karl, D.M.; Michaels, A.F. Nitrogen Cycle. In Encyclopedia of Ocean Sciences, 2nd ed.; Steele, J.H., Ed.; Academic Press: Oxford, UK, 2001; pp. 32–39. ISBN 978-0-12-374473-9. [Google Scholar]
- Batlle-Aguilar, J.; Brovelli, A.; Porporato, A.; Barry, D.A. Modelling soil carbon and nitrogen cycles during land use change. Sustain. Agric. 2011, 2, 499–527. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Krysenko, S.; Wohlleben, W. Polyamine and Ethanolamine Metabolism in Bacteria as an Important Component of Nitrogen Assimilation for Survival and Pathogenicity. Med. Sci. 2022, 10, 40. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, N.-S.; Fu, J.-J.; Huang, B.-C.; Jin, R.-C. Role and application of quorum sensing in anaerobic ammonium oxidation (anammox) process: A review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 626–648. [Google Scholar] [CrossRef]
- van Kessel, M.A.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.J.; Kartal, B.; Jetten, M.S.; Lucker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef]
- Hochman, A.; Nissany, A.; Amizur, M. Nitrate reduction and assimilation by a moderately halophilic, halotolerant bacterium Ba1. Biochim. Biophys. Acta (BBA) Gen. Subj. 1988, 965, 82–89. [Google Scholar] [CrossRef]
- Li, Q.; Bu, C.; Ahmad, H.A.; Guimbaud, C.; Gao, B.; Qiao, Z.; Ding, S.; Ni, S.Q. The distribution of dissimilatory nitrate reduction to ammonium bacteria in multistage constructed wetland of Jining, Shandong, China. Environ. Sci. Pollut. Res. Int. 2021, 28, 4749–4761. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, X.; Li, J.; Wu, X.; Feng, H.; Dong, W. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci. Total. Environ. 2021, 801, 149319. [Google Scholar] [CrossRef]
- Thompson, A.W.; Foster, R.A.; Krupke, A.; Carter, B.J.; Musat, N.; Vaulot, D.; Kuypers, M.M.; Zehr, J.P. Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science 2012, 337, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Zhang, T.; Yan, Q. Characterization and quantification of ammonia-oxidizing archaea (AOA) and bacteria (AOB) in a nitrogen-removing reactor using T-RFLP and qPCR. Appl. Microbiol. Biotechnol. 2010, 87, 1167–1176. [Google Scholar] [CrossRef]
- Romero-Rodríguez, A.; Maldonado-Carmona, N.; Ruiz-Villafán, B.; Koirala, N.; Rocha, D.; Sánchez, S. Interplay between carbon, nitrogen and phosphate utilization in the control of secondary metabolite production in Streptomyces. Antonie Van Leeuwenhoek 2018, 111, 761–781. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Kamp, A.; Hogslund, S.; Risgaard-Petersen, N.; Stief, P. Nitrate Storage and Dissimilatory Nitrate Reduction by Eukaryotic Microbes. Front. Microbiol. 2015, 6, 1492. [Google Scholar] [CrossRef]
- Woehle, C.; Roy, A.S.; Glock, N.; Wein, T.; Weissenbach, J.; Rosenstiel, P.; Hiebenthal, C.; Michels, J.; Schonfeld, J.; Dagan, T. A Novel Eukaryotic Denitrification Pathway in Foraminifera. Curr. Biol. 2018, 28, 2536–2543.e2535. [Google Scholar] [CrossRef]
- Walsh, S.E.; Vavrus, S.J.; Foley, J.A.; Fisher, V.A.; Wynne, R.H.; Lenters, J.D. Global patterns of lake ice phenology and climate: Model simulations and observations. J. Geophys. Res. Atmos. 1998, 103, 28825–28837. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Livingstone, D.M.; Meili, M.; Jensen, O.; Benson, B.; Magnuson, J.J. Large geographical differences in the sensitivity of ice-covered lakes and rivers in the Northern Hemisphere to temperature changes. Glob. Chang. Biol. 2011, 17, 268–275. [Google Scholar] [CrossRef]
- Verpoorter, C.; Kutser, T.; Seekell, D.A.; Tranvik, L.J. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 2014, 41, 6396–6402. [Google Scholar] [CrossRef]
- Salonen, K.; Leppäranta, M.; Viljanen, M.; Gulati, R.D. Perspectives in winter limnology: Closing the annual cycle of freezing lakes. Aquat. Ecol. 2009, 43, 609–616. [Google Scholar] [CrossRef]
- Powers, S.M.; Baulch, H.M.; Hampton, S.E.; Labou, S.G.; Lottig, N.R.; Stanley, E.H. Nitrification contributes to winter oxygen depletion in seasonally frozen forested lakes. Biogeochemistry 2017, 136, 119–129. [Google Scholar] [CrossRef]
- Üveges, V.; Tapolczai, K.; Krienitz, L.; Padisák, J. Photosynthetic characteristics and physiological plasticity of an Aphanizomenon flos-aquae (Cyanobacteria, Nostocaceae) winter bloom in a deep oligo-mesotrophic lake (Lake Stechlin, Germany). Hydrobiologia 2012, 698, 263–272. [Google Scholar] [CrossRef]
- Vanderploeg, H.A.; Bolsenga, S.J.; Fahnenstiel, G.L.; Liebig, J.R.; Gardner, W.S. Plankton ecology in an ice-covered bay of Lake Michigan: Utilization of a winter phytoplankton bloom by reproducing copepods. Hydrobiologia 1992, 243, 175–183. [Google Scholar] [CrossRef]
- Twiss, M.; McKay, R.; Bourbonniere, R.; Bullerjahn, G.; Carrick, H.; Smith, R.; Winter, J.; D’souza, N.; Furey, P.; Lashaway, A. Diatoms abound in ice-covered Lake Erie: An investigation of offshore winter limnology in Lake Erie over the period 2007 to 2010. J. Great Lakes Res. 2012, 38, 18–30. [Google Scholar] [CrossRef]
- Bertilsson, S.B.A.; Carey, C.C.; Fey, S.B.; Grossart, H.P.; Grubisic, L.M.; Jones, I.D.; Kirillin, G.; Lennon, J.T.; Shade, A.; Smyth, R.L. The under-ice microbiome of seasonally frozen lakes. Limnol. Oceanogr. 2013, 58, 1998–2012. [Google Scholar] [CrossRef]
- Quan, D.; Shi, X.; Zhao, S.; Zhang, S.; Liu, J. Eutrophication of Lake Ulansuhai in 2006–2017 and its main impact factors. J. Lake Sci. 2019, 31, 1259–1267. [Google Scholar] [CrossRef]
- He, Y.; Sun, D.; Wu, J.; Sun, Y. Factors controlling the past ~150-year ecological dynamics of Lake Wuliangsu in the upper reaches of the Yellow River, China. Holocene 2015, 25, 1394–1401. [Google Scholar] [CrossRef]
- Sun, H.; Lu, X.; Yu, R.; Yang, J.; Liu, X.; Cao, Z.; Zhang, Z.; Li, M.; Geng, Y. Eutrophication decreased CO2 but increased CH4 emissions from lake: A case study of a shallow Lake Ulansuhai. Water Res. 2021, 201, 117363. [Google Scholar] [CrossRef]
- Li, G.; Zhang, S.; Shi, X.; Zhan, L.; Zhao, S.; Sun, B.; Liu, Y.; Tian, Z.; Li, Z.; Arvola, L.; et al. Spatiotemporal variability and diffusive emissions of greenhouse gas in a shallow eutrophic lake in Inner Mongolia, China. Ecol. Indic. 2022, 145, 109578. [Google Scholar] [CrossRef]
- Yang, T.; Hei, P.; Song, J.; Zhang, J.; Zhu, Z.; Zhang, Y.; Yang, J.; Liu, C.; Jin, J.; Quan, J. Nitrogen variations during the ice-on season in the eutrophic lakes. Environ. Pollut. 2019, 247, 1089–1099. [Google Scholar] [CrossRef]
- Chen, X.; Yang, L.; Xiao, L.; Miao, A.; Xi, B. Nitrogen removal by denitrification during cyanobacterial bloom in Lake Taihu. J. Freshw. Ecol. 2012, 27, 243–258. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, L.; Gong, D.; Lu, J. Effects of sterilization treatments on the analysis of TOC in water samples. J. Environ. Sci. 2010, 22, 789–795. [Google Scholar] [CrossRef]
- Hu, L.; Shi, X.; Yu, Z.; Lin, T.; Wang, H.; Ma, D.; Guo, Z.; Yang, Z. Distribution of sedimentary organic matter in estuarine–inner shelf regions of the East China Sea: Implications for hydrodynamic forces and anthropogenic impact. Mar. Chem. 2012, 142–144, 29–40. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Park, J.; Takagi, T. MetaGene: Prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006, 34, 5623–5630. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Morales, S.E.; Cosart, T.; Holben, W.E. Bacterial gene abundances as indicators of greenhouse gas emission in soils. ISME J. 2010, 4, 799–808. [Google Scholar] [CrossRef]
- Dos Santos, P.C.; Fang, Z.; Mason, S.W.; Setubal, J.C.; Dixon, R. Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. BMC Genom. 2012, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Albright, M.B.; Timalsina, B.; Martiny, J.B.; Dunbar, J. Comparative genomics of nitrogen cycling pathways in bacteria and archaea. Microb. Ecol. 2019, 77, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.E.; Schimel, J.P.; Firestone, M.K. Short-term partitioning of ammonium and nitrate between plants and microbes in an annual grassland. Soil Biol. Biochem. 1989, 21, 409–415. [Google Scholar] [CrossRef]
- Newell, S.E.; Pritchard, K.R.; Foster, S.Q.; Fulweiler, R.W. Molecular evidence for sediment nitrogen fixation in a temperate New England estuary. PeerJ 2016, 4, e1615. [Google Scholar] [CrossRef]
- Tempest, D.W.; Meers, J.L.; Brown, C.M. Synthesis of glutamate in Aerobacter aerogenes by a hitherto unknown route. Biochem. J. 1970, 117, 405–407. [Google Scholar] [CrossRef]
- Souza, R.C.; Hungria, M.; Cantão, M.E.; Vasconcelos, A.T.R.; Nogueira, M.A.; Vicente, V.A. Metagenomic analysis reveals microbial functional redundancies and specificities in a soil under different tillage and crop-management regimes. Appl. Soil Ecol. 2015, 86, 106–112. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, M.; Yang, Y.; Xie, Y.; Ren, Z.; Peng, W.; Du, X. Taxonomic structure and potential nitrogen metabolism of microbial assemblage in a large hypereutrophic steppe lake. Environ. Sci. Pollut. Res. Int. 2019, 26, 21151–21160. [Google Scholar] [CrossRef]
- Fernandez-Valiente, E.; Quesada, A.; Howard-Williams, C.; Hawes, I. N2-Fixation in Cyanobacterial Mats from Ponds on the McMurdo Ice Shelf, Antarctica. Microb. Ecol. 2001, 42, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Priscu, J.C. Microbial Phototrophic, Heterotrophic, and Diazotrophic Activities Associated with Aggregates in the Permanent Ice Cover of Lake Bonney, Antarctica. Microb. Ecol. 1998, 36, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, E.; Baulch, H.M. Winter nitrification in ice-covered lakes. PLoS ONE 2019, 14, e0224864. [Google Scholar] [CrossRef]
- Song, S.; Li, C.; Shi, X.; Zhao, S.; Tian, W.; Li, Z.; Bai, Y.; Cao, X.; Wang, Q.; Huotari, J.; et al. Under-ice metabolism in a shallow lake in a cold and arid climate. Freshw. Biol. 2019, 64, 1710–1720. [Google Scholar] [CrossRef]
- Jetten, M.S.; Strous, M.; van de Pas-Schoonen, K.T.; Schalk, J.; van Dongen, U.G.; van de Graaf, A.A.; Logemann, S.; Muyzer, G.; van Loosdrecht, M.C.; Kuenen, J.G. The anaerobic oxidation of ammonium. FEMS Microbiol. Rev. 1998, 22, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.-H. Sustainable nitrogen elimination biotechnologies: A review. Process. Biochem. 2006, 41, 1709–1721. [Google Scholar] [CrossRef]
- El Hassan, G.A.; Zablotowicz, R.M.; Focht, D.D. Kinetics of denitrifying growth by fast-growing cowpea rhizobia. Appl. Environ. Microbiol. 1985, 49, 517–521. [Google Scholar] [CrossRef]
- Murray, R.E.; Parsons, L.L.; Smith, M.S. Aerobic and anaerobic growth of rifampin-resistant denitrifying bacteria in soil. Appl. Environ. Microbiol. 1990, 56, 323–328. [Google Scholar] [CrossRef]
- Kumar, M.; Lin, J.G. Co-existence of anammox and denitrification for simultaneous nitrogen and carbon removal—Strategies and issues. J. Hazard. Mater. 2010, 178, 1–9. [Google Scholar] [CrossRef]
- Severin, I.; Confurius-Guns, V.; Stal, L.J. Effect of salinity on nitrogenase activity and composition of the active diazotrophic community in intertidal microbial mats. Arch. Microbiol. 2012, 194, 483–491. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L. Spatial-seasonal variations of nitrogen fixation of water column in Taihu Lake. Aeta Scentiae Circunstantine 2016, 36, 1129–1136. [Google Scholar] [CrossRef]
- Bellenger, J.P.; Darnajoux, R.; Zhang, X.; Kraepiel, A.M.L. Biological nitrogen fixation by alternative nitrogenases in terrestrial ecosystems: A review. Biogeochemistry 2020, 149, 53–73. [Google Scholar] [CrossRef]
- Tian, L.; Yan, Z.; Wang, C.; Xu, S.; Jiang, H. Habitat heterogeneity induces regional differences in sediment nitrogen fixation in eutrophic freshwater lake. Sci. Total Environ. 2021, 772, 145594. [Google Scholar] [CrossRef]
- Halm, H.; Musat, N.; Lam, P.; Langlois, R.; Musat, F.; Peduzzi, S.; Lavik, G.; Schubert, C.J.; Sinha, B.; LaRoche, J.; et al. Co-occurrence of denitrification and nitrogen fixation in a meromictic lake, Lake Cadagno (Switzerland). Environ. Microbiol. 2009, 11, 1945–1958. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, Y.; Yan, X.; Ji, G. Cold Temperature Effects on Long-Term Nitrogen Transformation Pathway in a Tidal Flow Constructed Wetland. Environ. Sci. Technol. 2015, 49, 13550–13557. [Google Scholar] [CrossRef]
- Fang, X.; Stefan, H.G. Dynamics of heat exchange between sediment and water in a lake. Water Resour. Res. 1996, 32, 1719–1727. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Li, B.B.; Yang, Z.C.; Cheng, Y.Y.; Liu, D.F.; Yu, H.Q. Mediation of functional gene and bacterial community profiles in the sediments of eutrophic Chaohu Lake by total nitrogen and season. Environ. Pollut. 2019, 250, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Lin, L.; Huang, H.; Chen, J. Differentiation of Nitrogen and Microbial Community in the Sediments from Lake Erhai, Yunnan–Kweichow Plateau, China. Geomicrobiol. J. 2020, 37, 818–825. [Google Scholar] [CrossRef]
- Shang, Y.; Wu, X.; Wang, X.; Wei, Q.; Ma, S.; Sun, G.; Zhang, H.; Wang, L.; Dou, H.; Zhang, H. Factors affecting seasonal variation of microbial community structure in Hulun Lake, China. Sci. Total Environ. 2022, 805, 150294. [Google Scholar] [CrossRef]
- Deng, M.; Hou, J.; Song, K.; Chen, J.; Gou, J.; Li, D.; He, X. Community metagenomic assembly reveals microbes that contribute to the vertical stratification of nitrogen cycling in an aquaculture pond. Aquaculture 2020, 520, 734911. [Google Scholar] [CrossRef]
- Lew, S.; Koblížek, M.; Lew, M.; Medová, H.; Glińska-Lewczuk, K.; Owsianny, P.M. Seasonal changes of microbial communities in two shallow peat bog lakes. Folia Microbiol. 2015, 60, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Yang, Y.; Wu, Z.; Feng, Q.; Xie, S.; Liu, Y. Spatiotemporal variation of planktonic and sediment bacterial assemblages in two plateau freshwater lakes at different trophic status. Appl. Microbiol. Biotechnol. 2016, 100, 4161–4175. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chao, J.; Gong, Y.; Wang, Y.; Wilhelm, S.W.; Gao, G. Spatiotemporal dynamics of bacterial community composition in large shallow eutrophic Lake Taihu: High overlap between free-living and particle-attached assemblages. Limnol. Oceanogr. 2017, 62, 1366–1382. [Google Scholar] [CrossRef]
- McGill, B.J.; Etienne, R.S.; Gray, J.S.; Alonso, D.; Anderson, M.J.; Benecha, H.K.; Dornelas, M.; Enquist, B.J.; Green, J.L.; He, F.; et al. Species abundance distributions: Moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 2007, 10, 995–1015. [Google Scholar] [CrossRef]
- Lynn, D.H. Ciliophora. In Handbook of the Protists; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 679–730. ISBN 978-3-319-28149-0. [Google Scholar]
- Jumas-Bilak, E.; Roudiere, L.; Marchandin, H. Description of ‘Synergistetes’ phyl. nov. and emended description of the phylum ‘Deferribacteres’ and of the family Syntrophomonadaceae, phylum ‘Firmicutes’. Int. J. Syst. Evol. Microbiol. 2009, 59, 1028–1035. [Google Scholar] [CrossRef]
- Tamames, J.; Abellan, J.J.; Pignatelli, M.; Camacho, A.; Moya, A. Environmental distribution of prokaryotic taxa. BMC Microbiol. 2010, 10, 85. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Zhang, S.; Lu, J.; Shi, X.; Zhao, S.; Sun, B.; Wang, Y.; Li, G.; Cui, Z.; Pan, X.; et al. Differences of Nitrogen Transformation Pathways and Their Functional Microorganisms in Water and Sediment of a Seasonally Frozen Lake, China. Water 2023, 15, 2332. https://doi.org/10.3390/w15132332
Tian Z, Zhang S, Lu J, Shi X, Zhao S, Sun B, Wang Y, Li G, Cui Z, Pan X, et al. Differences of Nitrogen Transformation Pathways and Their Functional Microorganisms in Water and Sediment of a Seasonally Frozen Lake, China. Water. 2023; 15(13):2332. https://doi.org/10.3390/w15132332
Chicago/Turabian StyleTian, Zhiqiang, Sheng Zhang, Junping Lu, Xiaohong Shi, Shengnan Zhao, Biao Sun, Yanjun Wang, Guohua Li, Zhimou Cui, Xueru Pan, and et al. 2023. "Differences of Nitrogen Transformation Pathways and Their Functional Microorganisms in Water and Sediment of a Seasonally Frozen Lake, China" Water 15, no. 13: 2332. https://doi.org/10.3390/w15132332
APA StyleTian, Z., Zhang, S., Lu, J., Shi, X., Zhao, S., Sun, B., Wang, Y., Li, G., Cui, Z., Pan, X., Li, G., & Zhang, Z. (2023). Differences of Nitrogen Transformation Pathways and Their Functional Microorganisms in Water and Sediment of a Seasonally Frozen Lake, China. Water, 15(13), 2332. https://doi.org/10.3390/w15132332







