Phytoplankton Diversity and Co-Dependency in a Stratified Oligotrophic Ecosystem in the South Adriatic Sea
Abstract
1. Introduction
2. Materials and Methods
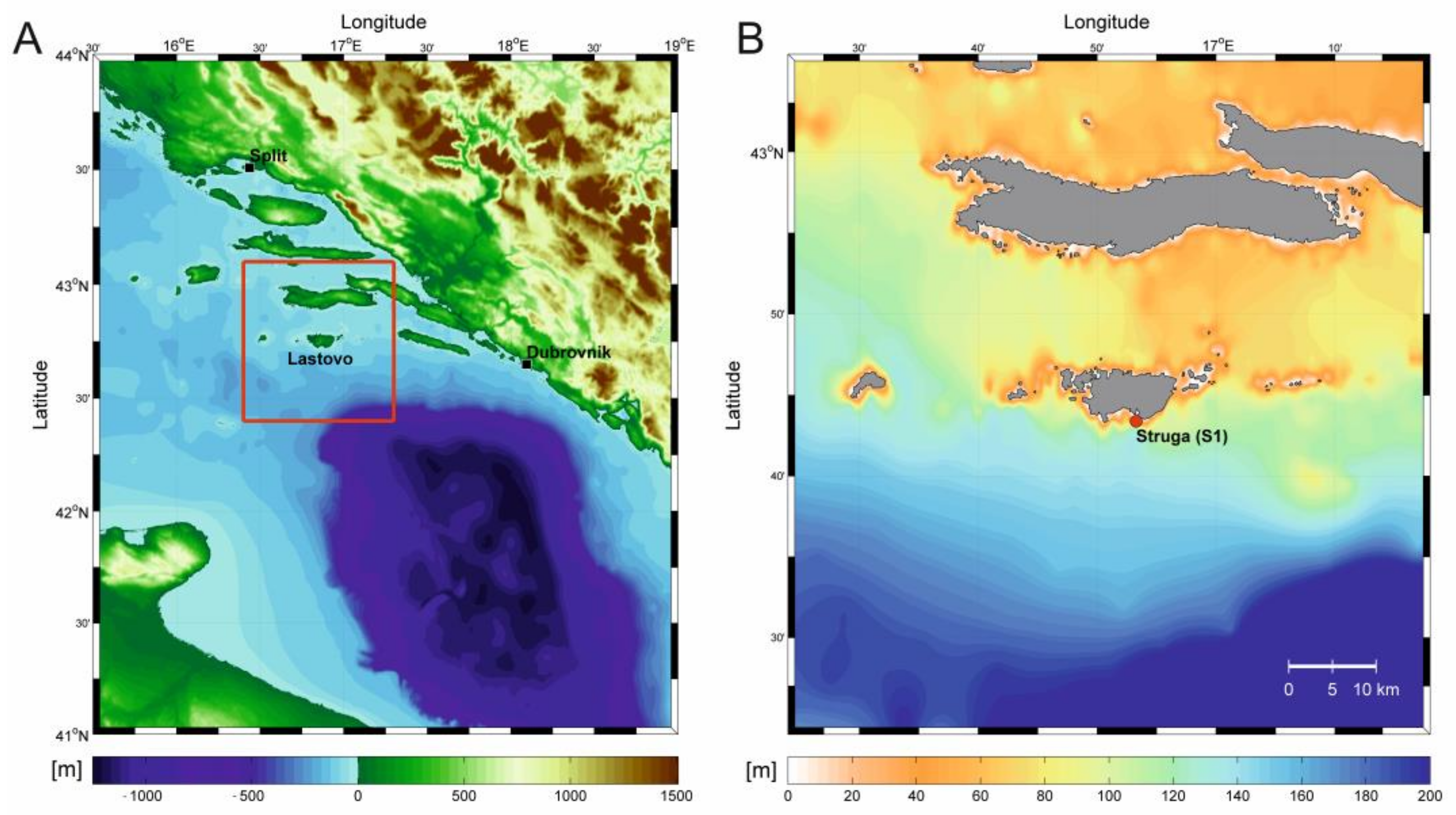
2.1. Study Area
2.2. Sampling
2.3. Plankton Community and Nutrient Analysis
2.4. eDNA Isolation, Amplicon Sequencing and Bioinformatics
2.5. Statistical Analyses
3. Results
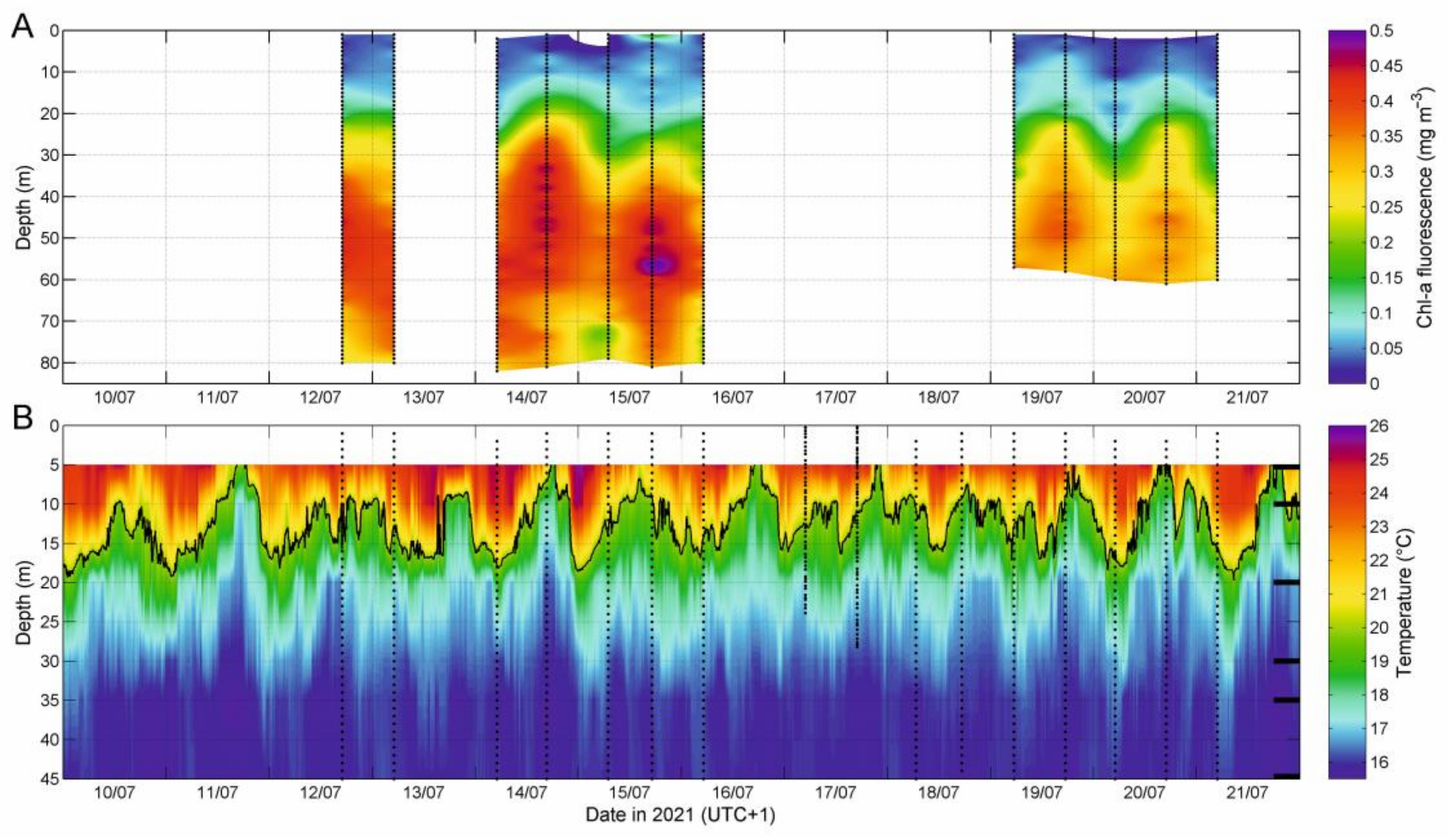
3.1. Water Column Structure
3.2. Phytoplankton Community Diversity
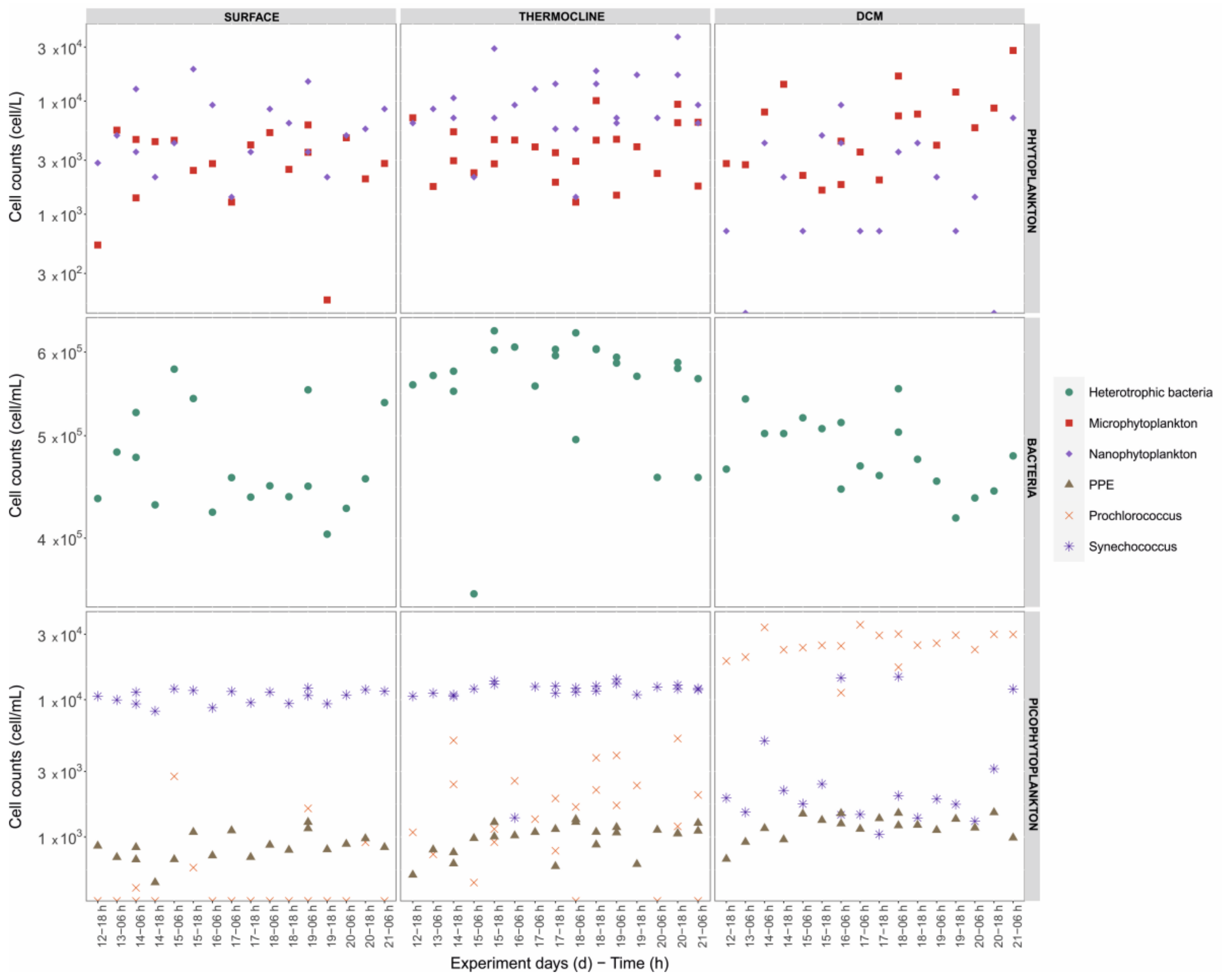
3.2.1. Taxonomic Composition and Abundance
3.2.2. Vertical Distributions and Dominant Taxa
3.3. Picophytoplankton and Bacterioplankton
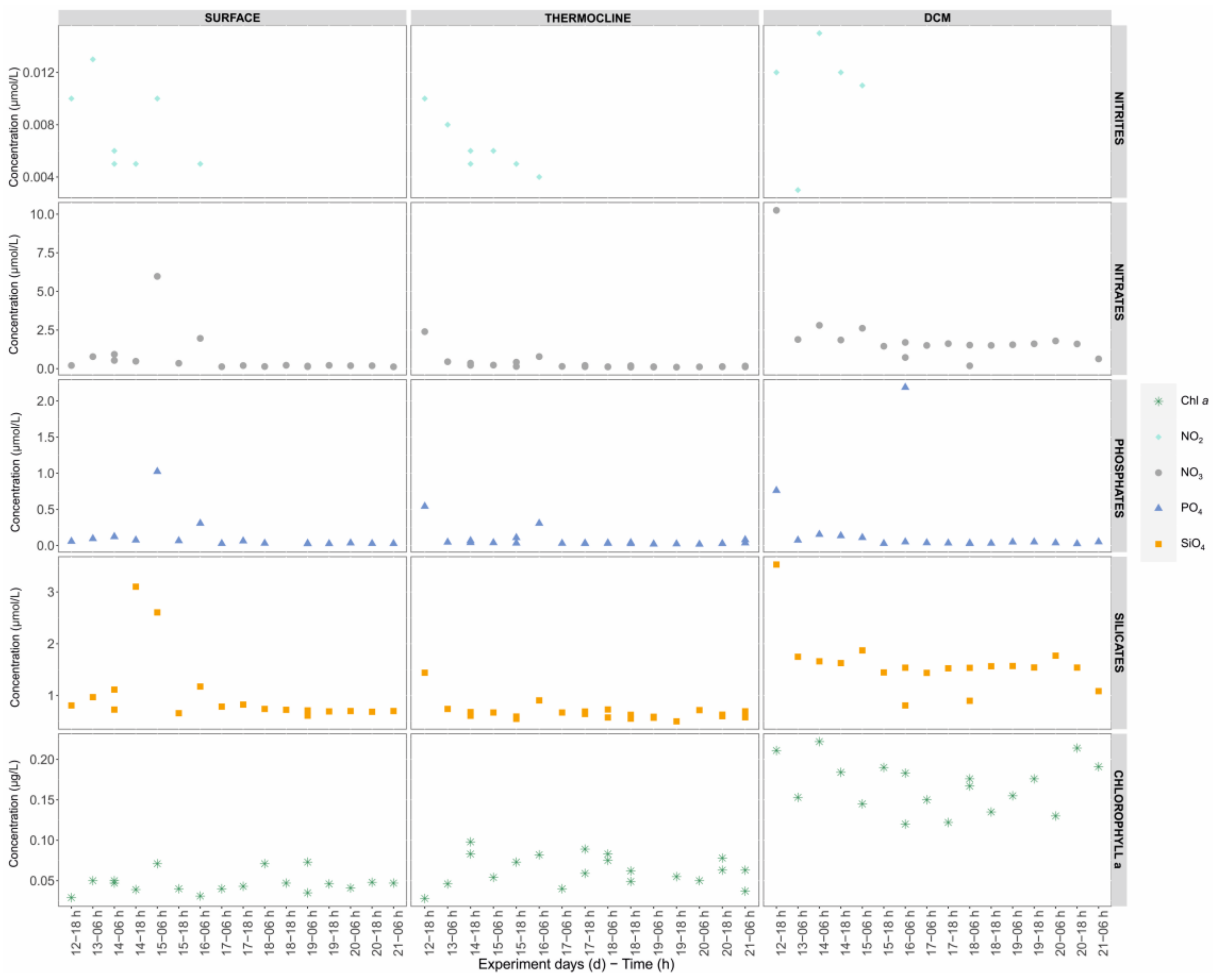
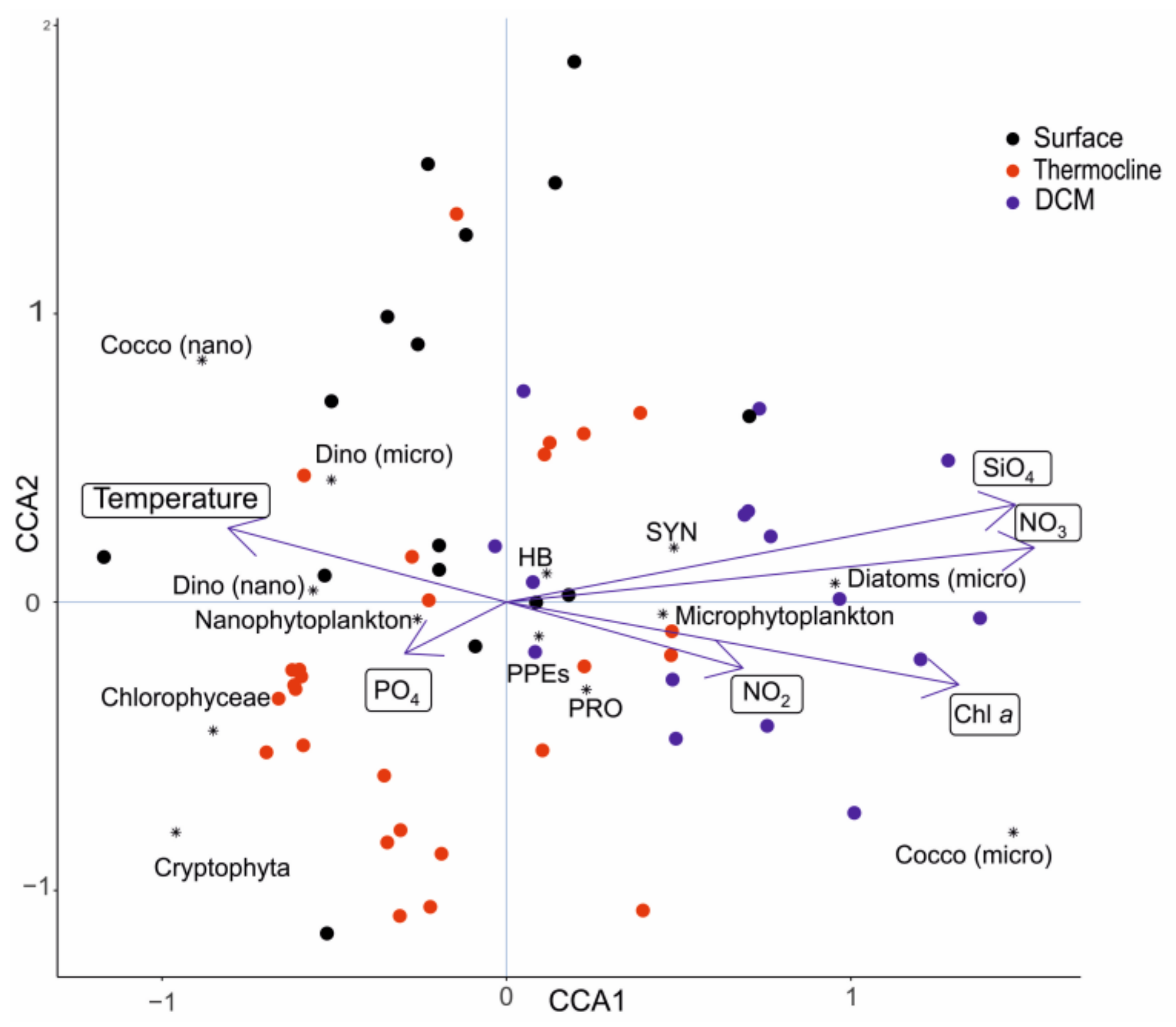
3.4. Correlation between Physico-Chemical Parameters and Plankton Community
3.5. Plankton Community Composition Revealed through HTS
4. Discussion
4.1. Nutrients and Thermal Stratification
4.2. Phytoplankton Community in the Oligotrophic Ecosystem
4.2.1. Microphytoplankton
4.2.2. Nanophytoplankton
4.3. Phytoplankton Vertical Distribution
4.4. Picophytoplankton and Heterotrophic Bacteria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Kirchman, D.L. Microbial Ecology of the Oceans; John Wiley & Sons: Hoboken, NJ, USA, 2000; ISBN 978-0-471-29992-9. [Google Scholar]
- Viličić, D. Ecology and Composition of Phytoplankton in the Adriatic Sea; Koeltz Scientific Books: Koenigstein, Germany, 2014; ISBN 978-3-87429-474-4. [Google Scholar]
- Najdek, M.; Paliaga, P.; Šilović, T.; Batistić, M.; Garić, R.; Supić, N.; Ivančić, I.; Ljubimir, S.; Korlević, M.; Jasprica, N.; et al. Picoplankton Community Structure before, during and after Convection Event in the Offshore Waters of the Southern Adriatic Sea. Biogeosciences 2014, 11, 2645–2659. [Google Scholar] [CrossRef]
- Babić, I.; Petrić, I.; Bosak, S.; Mihanović, H.; Dupčić Radić, I.; Ljubešić, Z. Distribution and Diversity of Marine Picocyanobacteria Community: Targeting of Prochlorococcus Ecotypes in Winter Conditions (Southern Adriatic Sea). Mar. Genom. 2017, 36, 3–11. [Google Scholar] [CrossRef]
- Gačić, M.; Civitarese, G.; Miserocchi, S.; Cardin, V.; Crise, A.; Mauri, E. The Open-Ocean Convection in the Southern Adriatic: A Controlling Mechanism of the Spring Phytoplankton Bloom. Cont. Shelf Res. 2002, 22, 1897–1908. [Google Scholar] [CrossRef]
- Batistić, M.; Jasprica, N.; Carić, M.; Čalić, M.; Kovačević, V.; Garić, R.; Njire, J.; Mikuš, J.; Bobanović-Ćolić, S. Biological Evidence of a Winter Convection Event in the South Adriatic: A Phytoplankton Maximum in the Aphotic Zone. Cont. Shelf Res. 2012, 44, 57–71. [Google Scholar] [CrossRef]
- Cerino, F.; Bernardi Aubry, F.; Coppola, J.; La Ferla, R.; Maimone, G.; Socal, G.; Totti, C. Spatial and Temporal Variability of Pico-, Nano- and Microphytoplankton in the Offshore Waters of the Southern Adriatic Sea (Mediterranean Sea). Cont. Shelf Res. 2012, 44, 94–105. [Google Scholar] [CrossRef]
- Ljubimir, S.; Jasprica, N.; Čalić, M.; Hrustić, E.; Dupčić Radić, I.; Car, A.; Batistić, M. Interannual (2009–2013) Variability of Winter-Spring Phytoplankton in the Open South Adriatic Sea: Effects of Deep Convection and Lateral Advection. Cont. Shelf Res. 2017, 143, 311–321. [Google Scholar] [CrossRef]
- Vilibić, I.; Matijević, S.; Šepić, J.; Kušpilić, G. Changes in the Adriatic Oceanographic Properties Induced by the Eastern Mediterranean Transient. Biogeosciences 2012, 9, 2085–2097. [Google Scholar] [CrossRef]
- Lučić, D.; Ljubešić, Z.; Babić, I.; Bosak, S.; Cetinić, I.; Vilibić, I.; Mihanović, H.; Hure, M.; Njire, J.; Lučić, P.; et al. Unusual Winter Zooplankton Bloom in the Open Southern Adriatic Sea. Turk. J. Zool. 2017, 41, 1024–1035. [Google Scholar] [CrossRef]
- Hure, M.; Mihanović, H.; Lučić, D.; Ljubešić, Z.; Kružić, P. Mesozooplankton Spatial Distribution and Community Structure in the South Adriatic Sea during Two Winters (2015, 2016). Mar. Ecol. 2018, 39, e12488. [Google Scholar] [CrossRef]
- Mihanović, H.; Orlić, M.; Pasarić, Z. Diurnal Thermocline Oscillations Driven by Tidal Flow around an Island in the Middle Adriatic. J. Mar. Syst. 2009, 78, S157–S168. [Google Scholar] [CrossRef]
- Mihanović, H.; Beg Paklar, G.; Orlić, M. Resonant Excitation of Island-Trapped Waves in a Shallow, Seasonally Stratified Sea. Cont. Shelf Res. 2014, 77, 24–37. [Google Scholar] [CrossRef]
- Hogg, N.G. Observations of Internal Kelvin Waves Trapped Round Bermuda. J. Phys. Oceanogr. 1980, 10, 1353–1376. [Google Scholar] [CrossRef]
- Brink, K.H. Island-Trapped Waves, with Application to Observations off Bermuda. Dyn. Atmos. Ocean. 1999, 29, 93–118. [Google Scholar] [CrossRef]
- Luther, D.S. Trapped Waves around the Hawaiian Islands. In Proceedings of the 3rd ‘Aha Huliko’ a Hawaiian Winter Workshop, Hawaiian Ocean Experiment, Honolulu, HI, USA, 21–24 January 1985; pp. 261–301. [Google Scholar]
- Merrifield, M.A.; Yang, L.; Luther, D.S. Numerical Simulations of a Storm-Generated Island-Trapped Wave Event at the Hawaiian Islands. J. Geophys. Res. Ocean. 2002, 107, 33-1–33-10. [Google Scholar] [CrossRef]
- Pizarro, O.; Shaffer, G. Wind-Driven, Coastal-Trapped Waves off the Island of Gotland, Baltic Sea. J. Phys. Oceanogr. 1998, 28, 2117–2129. [Google Scholar] [CrossRef]
- Lazure, P.; Le Cann, B.; Bezaud, M. Large Diurnal Bottom Temperature Oscillations around the Saint Pierre and Miquelon Archipelago. Sci. Rep. 2018, 8, 13882. [Google Scholar] [CrossRef] [PubMed]
- Mihanović, H.; Orlić, M.; Pasarić, Z. Diurnal Internal Tides Detected in the Adriatic. Ann. Geophys. 2006, 24, 2773–2780. [Google Scholar] [CrossRef]
- Novosel, M.; Požar-Domac, A.; Pasarić, M. Diversity and Distribution of the Bryozoa along Underwater Cliffs in the Adriatic Sea with Special Reference to Thermal Regime. Mar. Ecol. 2004, 25, 155–170. [Google Scholar] [CrossRef]
- Orlić, M.; Beg Paklar, G.; Dadić, V.; Leder, N.; Mihanović, H.; Pasarić, M.; Pasarić, Z. Diurnal Upwelling Resonantly Driven by Sea Breezes around an Adriatic Island. J. Geophys. Res. 2011, 116, C09025. [Google Scholar] [CrossRef]
- Zavatarelli, M.; Raicich, F.; Bregant, D.; Russo, A.; Artegiani, A. Climatological Biogeochemical Characteristics of the Adriatic Sea. J. Mar. Syst. 1998, 18, 227–263. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik (Towards a perfection of quantitative phytoplankton methodology). Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Babić, I.; Mucko, M.; Petrić, I.; Bosak, S.; Mihanović, H.; Vilibić, I.; Dupčić Radić, I.; Cetinić, I.; Balestra, C.; Casotti, R.; et al. Multilayer Approach for Characterization of Bacterial Diversity in a Marginal Sea: From Surface to Seabed. J. Mar. Syst. 2018, 184, 15–27. [Google Scholar] [CrossRef]
- Becker, S.; Aoyama, M.; Woodward, E.; Bakker, K.; Coverly, S.; Mahaffey, C.; Tanhua, T. GO-SHIP Repeat Hydrography Nutrient Manual: The Precise and Accurate Determination of Dissolved Inorganic Nutrients in Seawater, Using Continuous Flow Analysis Methods. Front. Mar. Sci. 2020, 7, 581790. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Lorenzen, C.J.; Holmes, R.W.; Strickland, J.D.H. Fluorometric Determination of Chlorophyll. ICES J. Mar. Sci. 1965, 30, 3–15. [Google Scholar] [CrossRef]
- Andrews, S. Babraham Bioinformatics— FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 5 December 2022).
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J.; et al. The Protist Ribosomal Reference Database (PR2): A Catalog of Unicellular Eukaryote Small Sub-Unit RRNA Sequences with Curated Taxonomy. Nucleic Acids Res. 2013, 41, D597–D604. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (ITOL) v4: Recent Updates and New Developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Bisanz, J.E. Qiime2R: Importing QIIME2 Artifacts and Associated Data into R Sessions. Available online: https://github.com/jbisanz/qiime2R (accessed on 5 December 2022).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; Rstudio. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. 2022. Available online: https://CRAN.R-project.org/package=ggplot2 (accessed on 22 December 2022).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 22 December 2022).
- Harrel, F.E., Jr.; Dupont, C. Hmisc: Harrell Miscellaneous. 2022. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 22 December 2022).
- Wickham, H.; Rstudio. Tidyverse: Easily Install. and Load. the “Tidyverse”. 2022. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 22 December 2022).
- Müller, K.; Wickham, H.; Francois, R.; Bryan, J.; Rstudio. Tibble: Simple Data Frames. 2022. Available online: https://CRAN.R-project.org/package=tibble (accessed on 22 December 2022).
- Wickham, H.; Hester, J.; Francois, R.; Bryan, J.; Bearrows, S.; RStudio. Readr: Read Rectangular Text Data. 2022. Available online: https://github.com/mandreyel/ (accessed on 22 December 2022).
- Viličić, D.; Vučak, Z.; Škrivanić, A.; Gržetić, Z. Phytoplankton Blooms in the Oligotrophic Open South Adriatic Waters. Mar. Chem. 1989, 28, 89–107. [Google Scholar] [CrossRef]
- Viličić, D. Phytoplankton Taxonomy and Distribution in the Offshore Southern Adriatic. Nat. Croat. 1998, 7, 127–141. [Google Scholar]
- Viličić, D.; Terzić, S.; Ahel, M.; Burić, Z.; Jasprica, N.; Carić, M.; Caput Mihalić, K.; Olujić, G. Phytoplankton Abundance and Pigment Biomarkers in the Oligotrophic, Eastern Adriatic Estuary. Environ. Monit. Assess. 2008, 142, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Jasprica, N.; Caric, M.; Kršinić, F.; Kapetanović, T.; Batistić, M.; Njire, J. Planktonic Diatoms and Their Environment in the Lower Neretva River Estuary (Eastern Adriatic Sea, NE Mediterranean). Nova Hedwig. 2012, 141, 405–430. [Google Scholar]
- Viličić, D.; Jasprica, N.; Carić, M.; Burić, Z. Taxonomic Composition and Seasonal Distribution of Microphytoplankton in Mali Ston Bay (Eastern Adriatic). Acta Bot. Croat. 1998, 57, 29–48. [Google Scholar]
- Drakulović, D.; Krivokapić, S.; Mandić, M.; Redžic, A. Phytoplankton Community in Boka Kotorska Bay (South-Eastern Adriatic). Rapp. Comm. Int. Mer. Médit. 2013, 40, 428. [Google Scholar]
- La Ferla, R.; Maimone, G.; Azzaro, M.; Conversano, F.; Brunet, C.; Cabral, A.S.; Paranhos, R. Vertical Distribution of the Prokaryotic Cell Size in the Mediterranean Sea. Helgol. Mar. Res. 2012, 66, 635–650. [Google Scholar] [CrossRef]
- Viličić, D.; Marasović, I.; Mioković, D. Checklist of Phytoplankton in the Eastern Adriatic Sea. Acta Bot. Croat. 2002, 61, 57–91. [Google Scholar]
- Viličić, D.; Leder, N.; Gržetić, Z.; Jasprica, N. Microphytoplankton in the Strait of Otranto (Eastern Mediterranean). Mar. Biol. 1995, 123, 619–630. [Google Scholar] [CrossRef]
- Turchetto, M.; Bianchi, F.; Boldrin, A.; Malaguti, A.; Rabitti, S.; Socal, G.; Strada, L. Nutrients, Phytoplankton and Primary Production Processes in Oligotrophic Areas (Southern Adriatic and Northern Ionian). Atti AIOL 2000, 13, 269–278. [Google Scholar]
- Socal, G.; Boldrin, A.; Bianchi, F.; Civitarese, G.; De Lazzari, A.; Rabitti, S.; Totti, C.; Turchetto, M.M. Nutrient, Particulate Matter and Phytoplankton Variability in the Photic Layer of the Otranto Strait. J. Mar. Syst. 1999, 20, 381–398. [Google Scholar] [CrossRef]
- Čalić, M.; Carić, M.; Kršinić, F.; Jasprica, N.; Pećarević, M. Controlling Factors of Phytoplankton Seasonal Succession in Oligotrophic Mali Ston Bay (South-Eastern Adriatic). Environ. Monit. Assess. 2013, 185, 7543–7563. [Google Scholar] [CrossRef] [PubMed]
- Krivokapić, S.; Bosak, S.; Viličić, D.; Kušpilić, G.; Drakulović, D.; Pestorić, B. Algal Pigments Distribution and Phytoplankton Group Assemblages in Coastal Transitional Environment—Boka Kotorska Bay (South Eastern Adriatic Sea). Acta Adriat. 2018, 59, 35–49. [Google Scholar] [CrossRef]
- Viličić, D. A Study of Phytoplankton in the Adriatic Sea after the July 1984 Bloom. Int. Rev. Ges. Hydrobiol. 1991, 76, 197–211. [Google Scholar] [CrossRef]
- Decembrini, F.; Caroppo, C.; Azzaro, M. Size Structure and Production of Phytoplankton Community and Carbon Pathways Channelling in the Southern Tyrrhenian Sea (Western Mediterranean). Deep. Sea Res. Part. II Top. Stud. Oceanogr. 2009, 56, 687–699. [Google Scholar] [CrossRef]
- Turley, C.M. The Changing Mediterranean Sea—A Sensitive Ecosystem? Prog. Oceanogr. 1999, 44, 387–400. [Google Scholar] [CrossRef]
- Yahia-Kefi, D.O.; Souissi, S.; Gomez, F.; Daly Yahia, M.N. Spatio-Temporal Distribution of the Dominant Diatom and Dinoflagellate Species in the Bay of Tunis (SW Mediterranean Sea). Medit. Mar. Sci. 2005, 6, 17. [Google Scholar] [CrossRef]
- Becacos-Kontos, T. Primary Production and Environmental Factors in an Oligotrophic Biome in the Aegean Sea. Mar. Biol. 1977, 42, 93–98. [Google Scholar] [CrossRef]
- Berman, T.; Townsend, D.; Elsayed, S.; Trees, C.; Azov, Y. Optical Transparency, Chlorophyll and Primary Productivity in the Eastern Mediterranean near the Israeli Coast. Oceanol. Acta 1984, 7, 367–372. [Google Scholar]
- Thingstad, T.F.; Sakshaug, E. Control of Phytoplankton Growth in Nutrient Recycling Ecosystems. Theory and Terminology. Mar. Ecol. Prog. Ser. 1990, 63, 261–272. [Google Scholar] [CrossRef]
- Estrada, M.; Blasco, D. Two Phases of the Phytoplankton Community in the Baja California Upwelling1. Limnol. Oceanogr. 1979, 24, 1065–1080. [Google Scholar] [CrossRef]
- Viličić, D. Phytoplankton Communities of the South Adriatic in the Greater Vicinity of Dubrovnik. Acta Bot. Croat. 1984, 43, 175–189. [Google Scholar]
- Pestorić, B.; Drakulović, D.; Mandić, M.; López Abbate, C. Distribution Changes of Plankton Communities in the Harbour Porto Montenegro (South Adriatic Sea). Stud. Mar. 2018, 31, 5–31. [Google Scholar] [CrossRef]
- Siokou-Frangou, I.; Christaki, U.; Mazzocchi, M.G.; Montresor, M.; Ribera d’Alcalá, M.; Vaqué, D.; Zingone, A. Plankton in the Open Mediterranean Sea: A Review. Biogeosciences 2010, 7, 1543–1586. [Google Scholar] [CrossRef]
- Ignatiades, L.; Gotsis-Skretas, O.; Pagou, K.; Krasakopoulou, E. Diversification of Phytoplankton Community Structure and Related Parameters along a Large-Scale Longitudinal East-West Transect of the Mediterranean Sea. J. Plankton Res. 2009, 31, 411–428. [Google Scholar] [CrossRef]
- Ninčević Gladan, Ž.; Matić, F.; Arapov, J.; Skejić, S.; Bužančić, M.; Bakrač, A.; Straka, M.; Dekneudt, Q.; Grbec, B.; Garber, R.; et al. The Relationship between Toxic Phytoplankton Species Occurrence and Environmental and Meteorological Factors along the Eastern Adriatic Coast. Harmful Algae 2020, 92, 101745. [Google Scholar] [CrossRef] [PubMed]
- Massana, R.; Pernice, M.; Bunge, J.A.; Campo, J. del Sequence Diversity and Novelty of Natural Assemblages of Picoeukaryotes from the Indian Ocean. ISME J. 2011, 5, 184–195. [Google Scholar] [CrossRef]
- Clarke, L.J.; Bestley, S.; Bissett, A.; Deagle, B.E. A Globally Distributed Syndiniales Parasite Dominates the Southern Ocean Micro-Eukaryote Community near the Sea-Ice Edge. ISME J. 2019, 13, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Piredda, R.; Tomasino, M.P.; D’Erchia, A.M.; Manzari, C.; Pesole, G.; Montresor, M.; Kooistra, W.H.C.F.; Sarno, D.; Zingone, A. Diversity and Temporal Patterns of Planktonic Protist Assemblages at a Mediterranean Long Term Ecological Research Site. FEMS Microbiol. Ecol. 2017, 93, fiw200. [Google Scholar] [CrossRef]
- Mucko, M.; Bosak, S.; Casotti, R.; Balestra, C.; Ljubešić, Z. Winter Picoplankton Diversity in an Oligotrophic Marginal Sea. Mar. Genom. 2018, 42, 14–24. [Google Scholar] [CrossRef]
- Rowena, S.; Picard, K.; Clarke, J.; Walker, C.; Martins, C.; Marshall, C.; Amorim, A.; Woodward, M.; Widdicombe, C.; Tarran, G.; et al. Composition and Patterns of Taxa Assemblages in the Western Channel Assessed by 18S Sequencing, Microscopy and Flow Cytometry. J. Mar. Sci. Eng. 2023, 11, 480. [Google Scholar]
- Piredda, R.; Claverie, J.-M.; Decelle, J.; de Vargas, C.; Dunthorn, M.; Edvardsen, B.; Eikrem, W.; Forster, D.; Kooistra, W.H.C.F.; Logares, R.; et al. Diatom Diversity through HTS-Metabarcoding in Coastal European Seas. Sci. Rep. 2018, 8, 18059. [Google Scholar] [CrossRef]
- Ujević, I.; Ninčević-Gladan, Z.; Roje, R.; Skejić, S.; Arapov, J.; Marasović, I. Domoic Acid—A New Toxin in the Croatian Adriatic Shellfish Toxin Profile. Molecules 2010, 15, 6835–6849. [Google Scholar] [CrossRef] [PubMed]
- Mochemadkar, S.; Gauns, M.; Pratihary, A.; Thorat, B.; Roy, R.; Pai, I.K.; Naqvi, S. Response of Phytoplankton to Nutrient Enrichment with High Growth Rates in a Tropical Monsoonal Estuary—Zuari Estuary, India. Indian. J. Geo-Mar. Sci. 2013, 42, 314–325. [Google Scholar]
- Revelante, N.; Gilmartin, M. Microplankton Diversity Indices as Indicators of Eutrophication in the Northern Adriatic Sea. Hydrobiologia 1980, 70, 277–286. [Google Scholar] [CrossRef]
- Pucher-Petković, T.; Marasović, I. Développement Des Populations Phytoplanctoniques Caractéristiques Pour Un Milieu Eutrophisé (Baie de Kaštela, Adriatique Centrale). Acta Adriat. 1980, 21, 79–93. [Google Scholar]
- Boldrin, A.; Miserocchi, S.; Rabitti, S.; Turchetto, M.M.; Balboni, V.; Socal, G. Particulate Matter in the Southern Adriatic and Ionian Sea: Characterisation and Downward Fluxes. J. Mar. Syst. 2002, 33–34, 389–410. [Google Scholar] [CrossRef]
- Berland, B.; Burlakova, Z.P.; Georgieva, L.; Izmestieva, M.; Kholodov, V.; Krupatkina, D.K.; Maestrini, S.; Zaika, V.E. Phytoplancton Estival de La Mer Du Levant, Biomasse et Facteurs Limitants. Prod. Relat. Trophique Ecosystèmes Mar. 1987, 2, 61–83. [Google Scholar]
- Gotsis-Skretas, O.; Pagou, K.; Moraitou-Apostolopoulou, M.; Ignatiades, L. Seasonal Horizontal and Vertical Variability in Primary Production and Standing Stocks of Phytoplankton and Zooplankton in the Cretan Sea and the Straits of the Cretan Arc (March 1994–January 1995). Prog. Oceanogr. 1999, 44, 625–649. [Google Scholar] [CrossRef]
- Latasa, M.; Estrada, M.; Delgado, M. Plankton-Pigment Relationships in the Northwestern Mediterranean during Stratification. Mar. Ecol. Prog. Ser. 1992, 88, 61–73. [Google Scholar] [CrossRef]
- Kimor, B. Distinctive Features of the Plankton of the Eastern Mediterranean. Annls. Inst. Oceanogr. 1983, 59, 97–106. [Google Scholar]
- Kimor, B.; Berman, T.; Schneller, A. Phytoplankton Assemblages in the Deep Chlorophyll Maximum Layers off the Mediterranean Coast of Israel. J. Plankton Res. 1987, 9, 433–443. [Google Scholar] [CrossRef]
- Drakulović, D.; Vuksanović, N.; Joksimović, D. Dynamics of Phytoplankton in Boka Kotorska Bay. Stud. Mar. 2010, 25, 1–20. [Google Scholar]
- Burić, Z.; Cetinić, I.; Viličić, D.; Mihalić, K.C.; Carić, M.; Olujić, G. Spatial and Temporal Distribution of Phytoplankton in a Highly Stratified Estuary (Zrmanja, Adriatic Sea). Mar. Ecol. 2007, 28, 169–177. [Google Scholar] [CrossRef]
- Vidussi, F.; Claustre, H.; Manca, B.B.; Luchetta, A.; Marty, J.-C. Phytoplankton Pigment Distribution in Relation to Upper Thermocline Circulation in the Eastern Mediterranean Sea during Winter. J. Geophys. Res. Ocean. 2001, 106, 19939–19956. [Google Scholar] [CrossRef]
- Marty, J.-C.; Chiavérini, J. Seasonal and Interannual Variations in Phytoplankton Production at DYFAMED Time-Series Station, Northwestern Mediterranean Sea. Deep. Sea Res. Part. II Top. Stud. Oceanogr. 2002, 49, 2017–2030. [Google Scholar] [CrossRef]
- Viličić, D. A Phytoplankton Study of Southern Adriatic Waters near Dubrovnik for the Period from June 1979 to July 1980. CENTRO 1985, 1, 35–36. [Google Scholar]
- Estrada, M. Phytoplankton Assemblages across a NW Mediterranean Front: Changes from Winter Mixing to Spring Stratification. Oecol. Aquatic. 1991, 10, 157–185. [Google Scholar]
- Giaccone, G.; Geraci, R. Biogeografia Delle Alghe Del Mediterráneo. An. Jard. Bot. Madr. 1989, 1, 27–34. [Google Scholar]
- Gómez, F. Endemic and Indo-Pacific Plankton in the Mediterranean Sea: A Study Based on Dinoflagellate Records. J. Biogeogr. 2006, 33, 261–270. [Google Scholar] [CrossRef]
- Skejić, S.; Arapov, J.; Kovačević, V.; Bužančić, M.; Bensi, M.; Giani, M.; Bakrač, A.; Mihanović, H.; Gladan, Ž.N.; Urbini, L.; et al. Coccolithophore Diversity in Open Waters of the Middle Adriatic Sea in Pre- and Post-Winter Periods. Mar. Micropaleontol. 2018, 143, 30–45. [Google Scholar] [CrossRef]
- Cros, L.; Fortuño, J.M. Atlas of Northwestern Mediterranean Coccolithophores. Sci. Mar. 2002, 66, 1–182. [Google Scholar] [CrossRef]
- Estrada, M.; Marrase, C.; Latasa, M.; Berdalet, E.; Delgado, M.; Riera, T. Variability of Deep Chlorophyll Maximum Characteristics in the Northwestern Mediterranean. Mar. Ecol. Prog. Ser. 1993, 92, 289–300. [Google Scholar] [CrossRef]
- Gran-Stadniczeñko, S.; Egge, E.; Hostyeva, V.; Logares, R.; Eikrem, W.; Edvardsen, B. Protist Diversity and Seasonal Dynamics in Skagerrak Plankton Communities as Revealed by Metabarcoding and Microscopy. J. Eukaryot. Microbiol. 2019, 66, 494–513. [Google Scholar] [CrossRef] [PubMed]
- Lohrenz, S.E.; Arnone, R.A.; Wiesenburg, D.A.; DePalma, I.P. Satellite Detection of Transient Enhanced Primary Production in the Western Mediterranean Sea. Nature 1988, 335, 245–247. [Google Scholar] [CrossRef]
- Magazzù, G.; Decembrini, F. Primary Production, Biomass and Abundance of Phototrophic Picoplankton in the Mediterranean Sea: A Review. Aquat. Microb. Ecol. 1995, 9, 97–104. [Google Scholar] [CrossRef]
- Wang, F.; Huang, B.; Xie, Y.; Cai, S.; Wang, X.; Mu, J. Diversity, Composition, and Activities of Nano- and Pico-Eukaryotes in the Northern South China Sea With Influences of Kuroshio Intrusion. Front. Mar. Sci. 2021, 8, 658233. [Google Scholar] [CrossRef]
- Mena, C.; Reglero, P.; Hidalgo, M.; Sintes, E.; Santiago, R.; Martín, M.; Moyà, G.; Balbín, R. Phytoplankton Community Structure Is Driven by Stratification in the Oligotrophic Mediterranean Sea. Front. Microbiol. 2019, 10, 1698. [Google Scholar] [CrossRef]
- De Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, Ć.; Le Bescot, N.; Probert, I.; et al. Eukaryotic Plankton Diversity in the Sunlit Ocean. Science 2015, 348, 1261605. [Google Scholar] [CrossRef]
- Margalef, R. Composición Específica Del Fitoplancton de La Costa Catalano-Levantina (Mediterráneo Occidental) En 1962–1967. Geography 1969, 33, 345–380. [Google Scholar]
- Šantić, D.; Piwosz, K.; Matić, F.; Vrdoljak Tomaš, A.; Arapov, J.; Dean, J.L.; Šolić, M.; Koblížek, M.; Kušpilić, G.; Šestanović, S. Artificial Neural Network Analysis of Microbial Diversity in the Central and Southern Adriatic Sea. Sci. Rep. 2021, 11, 11186. [Google Scholar] [CrossRef]
- Šantić, D.; Vrdoljak Tomaš, A.; Lušić, J. Spatial and Temporal Patterns of Picoplankton Community in the Central and Southern Adriatic Sea. In The Montenegrin Adriatic Coast: Marine Biology; Joksimović, A., Đurović, M., Zonn, I.S., Kostianoy, A.G., Semenov, A.V., Eds.; The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2020; pp. 29–51. ISBN 978-3-030-77513-1. [Google Scholar]
- Mackey, K.R.M.; Paytan, A.; Caldeira, K.; Grossman, A.R.; Moran, D.; McIlvin, M.; Saito, M.A. Effect of Temperature on Photosynthesis and Growth in Marine Synechococcus Spp. Plant. Physiol. 2013, 163, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Hafner, D.; Car, A.; Jasprica, N.; Kapetanović, T.; Radić, I.D. Relationship between Marine Epilithic Diatoms and Environmental Variables in Oligotrophic Bay, NE Mediterranean. Mediterr. Mar. Sci. 2018, 19, 223–239. [Google Scholar] [CrossRef]
- Civitarese, G.; Crise, A.; Crispi, G.; Mosetti, R. Circulation Effects on Nitrogen Dynamics in the Ionian Sea. Oceanol. Acta 1996, 19, 609–622. [Google Scholar]
- Dolan, J.R.; Vidussi, F.; Claustre, H. Planktonic Ciliates in the Mediterranean Sea: Longitudinal Trends. Deep. Sea Res. Part I Oceanogr. Res. Pap. 1999, 46, 2025–2039. [Google Scholar] [CrossRef]
- Šantić, D.; Krstulović, N.; Šolić, M.; Ordulj, M.; Kušpilić, G. Dynamics of Prokaryotic Picoplankton Community in the Central and Southern Adriatic Sea (Croatia). Helgol. Mar. Res. 2013, 67, 471–481. [Google Scholar] [CrossRef]
- Larkin, A.A.; Blinebry, S.K.; Howes, C.; Lin, Y.; Loftus, S.E.; Schmaus, C.A.; Zinser, E.R.; Johnson, Z.I. Niche Partitioning and Biogeography of High Light Adapted Prochlorococcus across Taxonomic Ranks in the North Pacific. ISME J. 2016, 10, 1555–1567. [Google Scholar] [CrossRef]
- Mella-Flores, D.; Mazard, S.; Humily, F.; Partensky, F.; Mahé, F.; Bariat, L.; Courties, C.; Marie, D.; Ras, J.; Mauriac, R.; et al. Is the Distribution of Prochlorococcus and Synechococcus Ecotypes in the Mediterranean Sea Affected by Global Warming? Biogeosciences 2011, 8, 2785–2804. [Google Scholar] [CrossRef]
- Zwirglmaier, K.; Jardillier, L.; Ostrowski, M.; Mazard, S.; Garczarek, L.; Vaulot, D.; Not, F.; Massana, R.; Ulloa, O.; Scanlan, D.J. Global Phylogeography of Marine Synechococcus and Prochlorococcus Reveals a Distinct Partitioning of Lineages among Oceanic Biomes. Env. Microbiol. 2008, 10, 147–161. [Google Scholar] [CrossRef]
- Coello-Camba, A.; Agustí, S. Picophytoplankton Niche Partitioning in the Warmest Oligotrophic Sea. Front. Mar. Sci. 2021, 8, 651877. [Google Scholar] [CrossRef]
- Schmoker, C.; Hernández-León, S. Stratification Effects on the Plankton of the Subtropical Canary Current. Prog. Oceanogr. 2013, 119, 24–31. [Google Scholar] [CrossRef]
- Šilović, T.; Mihanović, H.; Batistić, M.; Radić, I.D.; Hrustić, E.; Najdek, M. Picoplankton Distribution Influenced by Thermohaline Circulation in the Southern Adriatic. Cont. Shelf Res. 2018, 155, 21–33. [Google Scholar] [CrossRef]
- Moore, L.R.; Post, A.F.; Rocap, G.; Chisholm, S.W. Utilization of Different Nitrogen Sources by the Marine Cyanobacteria Prochlorococcus and Synechococcus. Limnol. Oceanogr. 2002, 47, 989–996. [Google Scholar] [CrossRef]
- Duarte, C.M.; Agustí, S.; Gasol, J.M.; Vaqué, D.; Vazquez-Dominguez, E. Effect of Nutrient Supply on the Biomass Structure of Planktonic Communities: An Experimental Test on a Mediterranean Coastal Community. Mar. Ecol. Prog. Ser. 2000, 206, 87–95. [Google Scholar] [CrossRef]
- Jardillier, L.; Boucher, D.; Personnic, S.; Jacquet, S.; Thénot, A.; Sargos, D.; Amblard, C.; Debroas, D. Relative Importance of Nutrients and Mortality Factors on Prokaryotic Community Composition in Two Lakes of Different Trophic Status: Microcosm Experiments. FEMS Microbiol. Ecol. 2005, 53, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Pittera, J.; Humily, F.; Thorel, M.; Grulois, D.; Garczarek, L.; Six, C. Connecting Thermal Physiology and Latitudinal Niche Partitioning in Marine Synechococcus. ISME J. 2014, 8, 1221–1236. [Google Scholar] [CrossRef]
- Korlević, M.; Pop RIstova, P.; Garić, R.; Amann, R.; Orlić, S. Bacterial Diversity in the South Adriatic Sea during a Strong Deep Winter Convection Year. Appl. Environ. Microbiol. 2015, 81, 1715–1726. [Google Scholar] [CrossRef] [PubMed]
- Šantić, D.; Kovačević, V.; Bensi, M.; Giani, M.; Vrdoljak Tomaš, A.; Ordulj, M.; Santinelli, C.; Šestanović, S.; Šolić, M.; Grbec, B. Picoplankton Distribution and Activity in the Deep Waters of the Southern Adriatic Sea. Water 2019, 11, 1655. [Google Scholar] [CrossRef]
- Henley, W.J.; Hironaka, J.L.; Guillou, L.; Buchheim, M.A.; Buchheim, J.A.; Fawley, M.W.; Fawley, K.P. Phylogenetic Analysis of the ‘Nannochloris-like’ Algae and Diagnoses of Picochlorum Oklahomensis Gen. et Sp. Nov. (Trebouxiophyceae, Chlorophyta). Phycologia 2004, 43, 641–652. [Google Scholar] [CrossRef]
- Claustre, H.; Sciandra, A.; Vaulot, D. Introduction to the Special Section Bio-Optical and Biogeochemical Conditions in the South East Pacific in Late 2004: The BIOSOPE Program. Biogeosciences 2008, 5, 679–691. [Google Scholar] [CrossRef]
- Shi, X.L.; Marie, D.; Jardillier, L.; Scanlan, D.J.; Vaulot, D. Groups without Cultured Representatives Dominate Eukaryotic Picophytoplankton in the Oligotrophic South East Pacific Ocean. PLoS ONE 2009, 4, e7657. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F. Bacterivory and Herbivory: Key Roles of Phagotrophic Protists in Pelagic Food Webs. Microb. Ecol. 1994, 28, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Turk, V.; Lučić, D.; Njire, J.; Terzić, S.; Tinta, T.; Benović, A.; Malej, A. The Epiplankton Community in the Southern Adriatic: Multiple Trophic Levels along the South–North and Inshore–Offshore Gradients. Acta Adriatica 2012, 53, 263–276. [Google Scholar]
- Gallina, A.A.; Celussi, M.; Del Negro, P. Large-Scale Distribution and Production of Bacterioplankton in the Adriatic Sea. J. Sea Res. 2011, 66, 1–8. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Fuhrman, J.A. Contrasting Diversity Patterns of Prokaryotes and Protists over Time and Depth at the San-Pedro Ocean Time Series. ISME Commun. 2022, 2, 36. [Google Scholar] [CrossRef]
- Campbell, L.; Gaonkar, C.C.; Henrichs, D.W. Chapter 5—Integrating Imaging and Molecular Approaches to Assess Phytoplankton Diversity. In Advances in Phytoplankton Ecology; Clementson, L.A., Eriksen, R.S., Willis, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 159–190. ISBN 978-0-12-822861-6. [Google Scholar]

| Dominant Taxa/Group | Max (Cell L−1) | Fr (%) |
|---|---|---|
| Dinoflagellates indet. (<20 µm) | 23,430 | 91.67 |
| Penatae indet. | 1135 | 85.00 |
| Pseudo-nitzschia delicatissima (Cleve) Heiden | 14,060 | 81.67 |
| Proboscia alata (Brightwell) Sundström | 1325 | 80.00 |
| Rhizosolenia imbricata Brightwell | 755 | 75.00 |
| Gyrodinium fusiforme Kofoid & Swezy | 570 | 58.33 |
| Coccolitophorids indet. (<10 µm) | 5680 | 55.00 |
| Cylindrotheca closterium (Ehrenberg) Reimann & J.C. Lewin | 945 | 53.33 |
| Leptocylindrus danicus Cleve | 3220 | 53.33 |
| Guinardia flaccida (Castracane) Peragallo | 570 | 51.67 |
| Guinardia striata (Stolterfoth) Hasle | 2270 | 51.67 |
| Hemiaulus chinensis Greville | 1135 | 51.67 |
| Dinoflagellates indet. (micro) | 970 | 51.67 |
| Thalassionema frauenfeldii (Grunow) Tempère & Peragallo | 1515 | 50.00 |
| Cryptophyta | 8510 | 50.00 |
| S and T (δ/σ; Σδ%) | S and DCM (δ/σ; Σδ%) | T and DCM (δ/σ; Σδ%) | |
|---|---|---|---|
| Dominant taxa/groups | Av. Diss% = 36.53 | Av. Diss% = 48.86 | Av. Diss% = 46.47 |
| Cryptophyta | 1.10; 10.2 | 0.99; 7.49 | 1.41; 10.58 |
| Coccolitophorids indet. (<10 µm) | 1.02; 8.66 | 1.16; 8.64 | 1.21; 9.03 |
| Leptocylindrus danicus | 1.18; 8.85 | 1.07; 6.83 | 1.16; 7.65 |
| Thalassionema frauenfeldi | 0.97; 6.54 | 1.41; 8.09 | 1.34; 6.99 |
| Cylindrotheca closterium | 1.11; 6.45 | 1.09; 6.05 | 1.20; 6.61 |
| Guinardia striata | 1.30; 7.08 | 1.45; 7.76 | |
| Dinoflagellates indet. (<20 µm) | 0.78; 6.71 | 0.86; 7.04 | |
| Proboscia alata | 1.19; 6.81 | 1.15; 6.20 | |
| Pseudo-nitzschia delicatissima | 1.13; 8.67 | 1.11; 7.74 | |
| Combried | 1.20; 7.58 | 1.32; 7.35 | |
| Gyrodinium fusiforme | 1.20; 6.11 | 1.57; 7.16 | |
| Hemiaulus chinensis | 1.21; 6.14 | 1.14; 5.71 | |
| Rhizosolenia imbricate | 1.00; 5.96 |
| NO2 | NO3 | PO4 | SiO4 | Temp | Chl a | |
|---|---|---|---|---|---|---|
| NO2 (µM) | 1.00 | |||||
| NO3 (µM) | 0.45 | 1.00 | ||||
| PO4 (µM) | 0.63 | 0.65 | 1.00 | |||
| SiO4 (µM) | 0.37 | 0.85 | 0.56 | 1.00 | ||
| Temperature (°C) | 0.53 | −0.42 | 1.00 | |||
| Chlorophyll a (µgL−1) | 0.57 | 0.50 | −0.74 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matek, A.; Mucko, M.; Casotti, R.; Trano, A.C.; Achterberg, E.P.; Mihanović, H.; Čižmek, H.; Čolić, B.; Cuculić, V.; Ljubešić, Z. Phytoplankton Diversity and Co-Dependency in a Stratified Oligotrophic Ecosystem in the South Adriatic Sea. Water 2023, 15, 2299. https://doi.org/10.3390/w15122299
Matek A, Mucko M, Casotti R, Trano AC, Achterberg EP, Mihanović H, Čižmek H, Čolić B, Cuculić V, Ljubešić Z. Phytoplankton Diversity and Co-Dependency in a Stratified Oligotrophic Ecosystem in the South Adriatic Sea. Water. 2023; 15(12):2299. https://doi.org/10.3390/w15122299
Chicago/Turabian StyleMatek, Antonija, Maja Mucko, Raffaella Casotti, Anna Chiara Trano, Eric P. Achterberg, Hrvoje Mihanović, Hrvoje Čižmek, Barbara Čolić, Vlado Cuculić, and Zrinka Ljubešić. 2023. "Phytoplankton Diversity and Co-Dependency in a Stratified Oligotrophic Ecosystem in the South Adriatic Sea" Water 15, no. 12: 2299. https://doi.org/10.3390/w15122299
APA StyleMatek, A., Mucko, M., Casotti, R., Trano, A. C., Achterberg, E. P., Mihanović, H., Čižmek, H., Čolić, B., Cuculić, V., & Ljubešić, Z. (2023). Phytoplankton Diversity and Co-Dependency in a Stratified Oligotrophic Ecosystem in the South Adriatic Sea. Water, 15(12), 2299. https://doi.org/10.3390/w15122299








