Abstract
Hypersaline habitats are among the most polyextreme habitats on Earth, but they contain a rather large diatom species diversity. A review of the diatom species’ richness was made on three scales: 1. a separate lake in Crimea; 2. all hypersaline waters in Crimea; and 3. the world totality as a whole. In total, 51 species were found in Lake Chersonesskoye during sampling from 2004 to 2018. In ten Crimean hypersaline lakes, 91 species were noted in total. All diatom species found in the studied hypersaline water bodies of the world belong to 458 species, which is 2.7% of the total number of known species of Bacillariophyta. In all three scales, the similarity of the species’ composition between water bodies as well as studied periods was not found. Most of all the identified species were found only in one of the studied water bodies, and only 59 species (13% of the total list) were found in more than three water bodies. An analysis showed that no more than 40% of the species that exist in hypersaline waters have been identified on a global scale. The diatom shell nanostructure from hypersaline waters has specific peculiarities that may be valuable for some technological applications. The knowledge of diatoms in hypersaline waters not only has pure scientific importance but also covers some needs of bio- and nanotechnologies.
1. Introduction
Phylum Bacillariophyta Karsten, 1928, which is diverse and has a long evolutionary history, according to Algabase [1], currently includes more than 17 thousand taxa of diatoms inhabiting a wide variety of habitats, including extreme ones [2,3,4,5]. Hypersaline environments that exist on all continents of our planet are among the most polyextreme habitats on the planet; however, they contain a rather large species diversity of organisms in different taxa [6,7]. Diatoms are no exception, many species of which can inhabit this inhospitable environment on different continents, which has been noted for a long time [7,8,9,10,11].
Life in a hypersaline environment requires specific adaptations; in unicellular organisms, including Bacillariophyta, this is primarily due to the accumulation of high concentrations of organic osmolytes, compatible, metabolic, and counteracting cytoprotectants in cells [12,13,14,15], and/or the creation of an exopolysaccharide matrix around cells [16,17,18]. For example, experiments with Phaeodactylum tricornutum Bohlin, 1898, showed that the species produced more exopolysaccharides in a hypersaline environment than in a less salty environment [16]. The adaptation of diatoms to high salinity occurs as a complex up-regulation of the metabolome, and specific secondary metabolites are produced [19,20]. Living in an extreme environment and being primary producers, diatoms also synthesize unique compounds that not only affect the metabolism of ecosystems as a whole but are also of undoubted interest to various industrial sectors [18,21,22]. The study of diatom diversity in hypersaline waters is therefore of undoubted value, not only for the development of various branches of biology but also for aquaculture, biotechnology, biodiesel production, pharmacology, and the cosmetics industry [14,18,22]. To solve the problem of a lack of food and other resources for the growing human population, we need to develop aquaculture. However, the development of aquaculture, including diatoms, under the growing shortage of fresh water, makes the task of developing hypersaline aquaculture of current interest [23]. This can contribute to better general environmental management and reduce fresh water use. For the development of the industry of diatom cultivation in hypersaline waters, it is necessary to determine the list of species that are suitable for this.
In addition, the study of diatoms in hypersaline water bodies is important for paleoreconstruction of the history of a particular water body, its origin, and hydrology [9,24,25].
The study of diatoms in hypersaline water bodies has received attention for more than 100 years [8,9,26,27,28], and by now, quite a lot of data on Bacillariophyta from various regions of our planet have been accumulated. However, despite this, there are still no answers to the next questions. How many of their species exist in hypersaline waters in total? What percentage of all the known diatoms are found and able to live in hypersaline water bodies? What factors and how do they influence the formation of the diatom flora in hypersaline habitats? Does their species’ composition fundamentally differ from that of the seas and freshwater reservoirs? What marine and freshwater species can adapt to life in high salinity, or do these water bodies have their own unique flora?
The objectives of this work are as follows: 1. to compile a total list of Bacillariophyta species in hypersaline water bodies in the world based on modern nomenclature as full as possible; 2. to prove or disprove hypotheses that (a) there is a significant quantitative dependence of species richness on salinity but (b) salinity is not the only significant factor determining species richness and composition of diatoms; and 3. to show that the study of diatoms in hypersaline waters has both scientific importance and applications for bio- and nanotechnologies.
2. Materials and Methods
This work reviews the literature and our own data on diatoms from hypersaline water bodies of different regions in the planet including our new, previously unpublished data on the most studied hypersaline lake: Lake Chersonesskoye (Crimea) (Figure 1, Table 1).
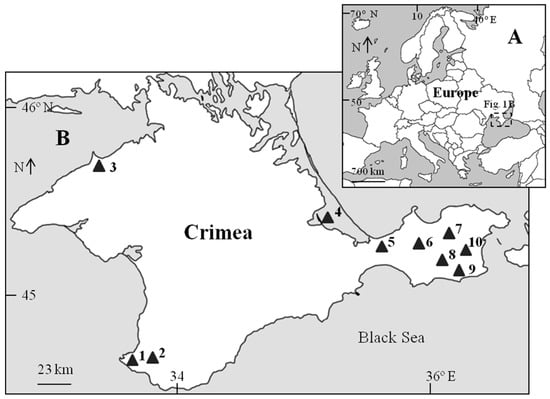
Figure 1.
The hypersaline water bodies in Crimea, data of which were used in this review (1—Lake Chersonesskoye; 2—small lake near Bay Cozachyia; 3—Lake Bakalskoye; 4—Lagoon Sivash (the Sea of Azov); 5—Lake Achi; 6—Lake Marfovskoye; 7—Lake Shimakhanskoye; 8—Lake Kirkoyashskoye; 9—Lake Koyashskoye; 10—Lake Tobechikskoye). (A)—Europe scale; (B)—local scale.

Table 1.
The hypersaline water bodies in different regions, data of which were used in this review.
The main results on diatoms in Lake Chersonessus were published previously [10,29,52]. Additionally, to the published data, here the authors have also used their own new data, which were obtained due to a more detailed analysis of microalgae in bottom sediments and the green algae Cladophora mats, which were taken in different seasons of 2017–2018 [29,53]. The methods of collection and processing of samples have been previously published [29,53]; due to this, they are not described here. New analyses of our old samples included the following: microphotography and determination of diatoms were carried out under an Olympus BX53F light microscope using a ×100 immersion lens (Olympus immersion oil n = 1.518) with a Jenoptik ProgRes Gryphax Arktur camera with Gryphax Arktur software. In addition, to analyze the fine structures of diatom shells, they were photographed under a Hitachi SU3500 scanning electron microscope (magnification factor: 5–300,000; resolution: up to 3 nm; depth of field: 0.5 mm). To determine the species of diatoms, their shells were cleaned of organic matter by the “cold” and “hot” methods, and permanent preparations were made according to the method described [36]. The species identification was made using [32,54,55,56,57,58,59,60,61], as well as numerous articles.
A detailed description of Lake Chersonesskoye was also provided before [29,53,62]; due to this, information about it will be briefly given here. It is a hypersaline marine lake with an area of 0.05 km2 and is located on the Black Sea coast (near Sevastopol, Crimea), separated from the sea by a narrow boulder–pebble embankment. Its average depth is about 0.4 m with a maximum of >1.5 m. There is high spatial and temporal salinity variability, ranging from 27 to 340 g L−1.
Published data were searched by various sets of keywords (hypersaline and algae; hyperhaline and algae; hypersaline and Bacillariophyta; hyperhaline and Bacillariophyta; hypersaline/hyperhaline lakes; hypersaline/hyperhaline lagoons) in various search engines such as Scholar Google, ResearchGate, eLibrary, Scopus, Web of Science, and ScienceDirect. This review covers 29 hypersaline water bodies or their groups from different regions (Table 1). The species indicated in the lists as sp. were not taken into account. For species designated in the literature as cf. or sp. aff, this status was saved. Taxonomy and nomenclatural names of microalgae are given following [1]. Ecological and phytogeographic characteristics of microalgae were determined using [1,60,63].
The parameters of the regression equations and the correlation coefficient were calculated in the program MS Excel 2010. The similarity of species’ compositions of microalgal communities between lakes and regions was evaluated by the Jaccard and Czekanowski–Sørensen–Dice similarity indices [64]:
where KJ and KCSD are the Jaccard and Czekanowski–Sørensen–Dice similarity coefficients, respectively; c is the number of species common to both plots or periods; a is the number of species found in the first case; b is the number of species found in the second case.
KJ = c/(a + b − c),
KCSD = 2c/(a + b),
The threshold values used to conclude that the species’ composition is similar/dissimilar are 0.42 (Jaccard) and 0.59 (Czekanowski–Sørensen–Dice) [64].
3. Results and Discussion
3.1. The Diatom Species’ Composition in the Single Lake Chersonesskoye
During 2004–2018, the diatom species’ composition was studied in Lake Chersonesskoye, which is probably the most studied lake on this issue worldwide. According to our own data and the literature data for this lake, we summarized the species’ composition of not only diatoms but also other microalgae. To date, a total of 99 species of microalgae have been found in it: 51 species belong to Bacillariophyta, 20 to Miozoa, 8 to Cyanobacteria, 9 to Chlorophyta, 6 to Haptophyta, 2 to Cryptophyta, and 2 to Euglenozoa. To calculate these values, previously published data [10,29,30,53] were supplemented by 22 new species not included in published studies after their additional identification in earlier collected samples [29,31]. In plankton, 61 species of microalgae were noted, and 62 species in mats of the green filamentous algae Cladophora spp. and bottom sediments were noted. Diatoms are an almost diverse group in the microphytobenthos of Lake Chersonesskoye, including 51 species. Among them, 11 species belong to the genus Nitzschia, 6 species belong to Navicula, and 5 species belong to Cocconeis (Table S1). In plankton, most of the species (19) were dinophytes. The calculated Jaccard and Czekanowski–Sørensen–Dice similarity coefficients between plankton and benthos values were 0.21 and 0.12, respectively. This confirms their difference, despite the shallow depth and strong mixing of the lake. In our studies, 28 species of microalgae were identified only at the genus level [10,29,30,53]. Consequently, not all species existing in the lake have already been identified. The number of species found increased with the number of examined samples. In the case of Lake Chersonesskoye, as shown earlier [31], this relation can be described by a power equation (R = 0.964, p = 0.0005):
where K is the number of species found; N is the number of analyzed samples.
K = 5.520 × N0.612,
The calculation by Equation (3) showed that when processing 500 samples, approximately 142 microalgae species, including 126 diatoms, in total can be found in the lake. 9 species of diatoms were noted in all periods of the study: Achnanthes brevipes C. Agardh, 1824; Cocconeis kujalnitzkensis Gusliakov et Gerasimiuk, 1992; Dickieia subinflata (Grunow) D. G. Mann 1994; Halamphora coffeiformis (C. Agardh) Mereschkowsky, 1903; Halamphora hyalina (Kützing) Rimet et R. Jahn in Rimet et al., 2018; Mastogloia braunii Grunow, 1863; Navicula ramosissima (Agardh) P. T. Cleve, 1895; Nitzschia frustulum (Kützing) Grunow in Cleve et Grunow, 1880; and Nitzschia sigma (Kützing) W. Smith, 1853 (Figure 2).
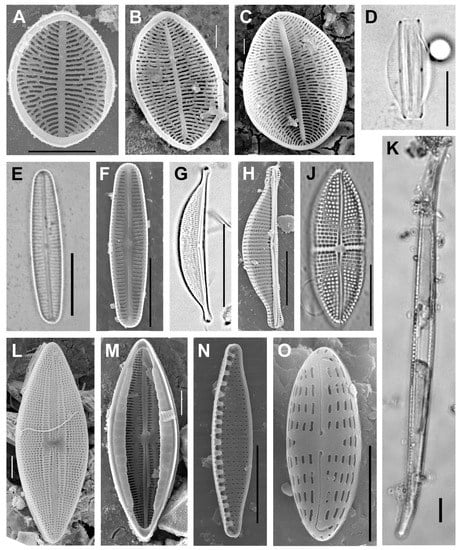
Figure 2.
The most common diatom species in Lake Chersonnesskoye (SEM photos): (A–C)—Cocconeis kujalnitzkensis; (D)—Halamphora hyalina; (E,F)—Dickieia subinflata; (G,H)—Halamphora coffeiformis; (J)—Achnanthes brevipes; (K)—Nitzschia sigma; (L,M)—Mastogloia braunii; ((L)—external valve view; (M)—internal valve view); (N)—Nitzschia frustulum; (O)—Navicula ramosissima. Scale bar: (B,C,L)—2 µm; (O)—3 µm; (A,H,M,N)—5 µm; (D–F), K—10 µm; (G,J)—20 µm.
Among them, only three species, A. brevipes, C. kujalnitzkensis, and H. coffeiformis, were found in all the samples. These species were also encountered by us in the microphytobenthos of Lagoon Sivash [53] and other saline lakes in the Crimea [10]. Even though the nine species mentioned above were found in all the periods, the total species’ composition varied greatly in different periods of research; the calculated values of the coefficients of species’ similarities (Equations (1) and (2)) were below the critical ones. Such high variability in the algae species’ composition in the lake, as well as in other hypersaline water bodies, can probably be explained by the fact that the species’ composition is determined by the interaction of many environmental factors, among which the random factor also plays an important role [28,46,49,53].
In Lake Chersonesskoye, according to [1,60], marine microalgae species predominate (40%), and marine brackish water species account for 17%. The predominance of marine species can be easily explained by the fact that the lake is constantly fed by seawater due to its filtration through the boulder barrage and the splash of seawater into the lake during storms. In total, in the Black Sea, the salinity of which is 17–19 g L−1, more than 1000 species of benthic microalgae have been found to date [63], and 47 of them are common with Lake Chersonesskoye, i.e., accounting for almost half of the total species found in the lake. There are more than 200 species of microalgae in Bay Cozachyia (the Black Sea), which is closest to the lake [63], 33 of which are common with the lake. Only 15–16% of species that can enter lakes from adjacent marine waters can exist in the lake. The presence in the hypersaline lake of a sufficiently large number of species (27% of all the species found), which are considered freshwater [1,60], probably requires a revision of their status. The authors think that, at least, it is incorrect to attribute to freshwater species those that are massively found in hypersaline waters. In general, the ecological classification of species concerning salinity cannot be considered adequately due to this classification, which was practically made up without taking into account data on hypersaline waters.
3.2. The Diatom Species’ Composition in the Crimean Hypersaline Lakes
In the Crimean Peninsula (Figure 1), the largest in the Black Sea (area 27,000 km2), there are a large number of hypersaline lakes (more than 50) and the world’s largest hypersaline lagoon, Sivash [31,65,66], but the diatom species’ composition was studied in only 10 lakes and the lagoon (Table 1). In total, 91 species of diatoms were found (Table S1), while 33 species were not recorded in any other hypersaline water bodies in the world. The highest diatom species’ richness among the Crimean water bodies was found in Lake Bakalskoye (57 species), Lake Chersonesskoye (51 species), and Lagoon Sivash (27 species). Only in Lake Achi, diatoms were not found in 3 taken samples. When comparing the diatom species’ composition in the Crimean lakes using Jaccard and Czekanowski–Sørensen–Dice coefficients, no similarity was found, and the values of both coefficients were less than critical values. Despite the geographical proximity of the Crimean lakes, the diatom species’ composition differs between them; it is unique for each lake. However, there is a complex of species that are found in half or more of the Crimean lakes (Table S1). The following species were recorded in six lakes: A. brevipes, H. coffeiformis, Navicula pennata var. pontica Mereschkowsky, 1902; in five lakes, Rhopalodia musculus (Kützing) O. Müller, 1900; in four lakes, C. kujalnitzkensis, C. placentula var. euglypta (Ehrenberg) Cleve, 1895; C. scutellum Ehrenberg, 1838; Hantzschia petitiana (Grunow) Grunow in Cleve et Grunow, 1880; M. braunii, M. pumila (Cleve et Möller) P.T. Cleve, 1895; Nitzschia sigma (Kützing) W. Smith, 1853; and Tabularia tabulata (C. Agardh) Snoeijs, 1992.
3.3. Diatoms in the World’s Hypersaline Waters
The complete list of diatom species noted in the studied hypersaline water bodies of the world (Table 1) includes 458 species (Table S1), which is 2.7% of the total number of known species of Bacillariophyta [1]. This list does not cover all the existing diatom species’ richness, if only because it does not include species defined only to the genus level [67], as well as dubious taxon names [68]. It can be concluded that the diatom flora in the hypersaline water bodies is still insufficiently studied. Let us assume that Lake Chersonesskoye is one of the most studied hypersaline water bodies in the world in terms of diatom species’ richness, and only about 40% of species have been identified in it, as shown above. It is logical to conclude that no more than 40% of the species that exist in hypersaline waters have been identified on a global scale. Consequently, we can assume that no more than 7% of the total Bacillariophyta species existing in nature can exist in hypersaline habitats.
The largest number of diatom species was found in the hypersaline water bodies of Russia (218 species), of which 91 species were in Crimea (Table S1). In the Aral Sea, 139 species were found; in the USA, 81 species were found, including in the lakes of the Great Salt Plains, where 31 species were found; in Mono Lake, where 25 species were found; and in the Great Salt Lake, where 56 species were found; in Brazil, 60 species were found, including in the Araruama Lagoon, where 45 species were found, and in Lagoon Vermelha, where 23 species were found. In general, the number of species found in the studied water bodies varies from 1 (Lake Kirkoyashskoye) to 139 (Aral Sea). The following genera prevailed in the general list by the number of species: Nitzschia, 52 species; Navicula, 37; Halamphora, 22; and Amphora, 21 (Table S1).
In general, for hypersaline water bodies, a trend toward a decrease in the number of species with an increase in salinity was noted [6,12,28,48,49,69]. The data from Table S1 made it possible to quantitatively analyze the total species’ richness depending on salinity. The number of species monotonously decreases with an increase in salinity (Figure 3). The dependence is highly significant (R = −0.980, p = 0.0001).
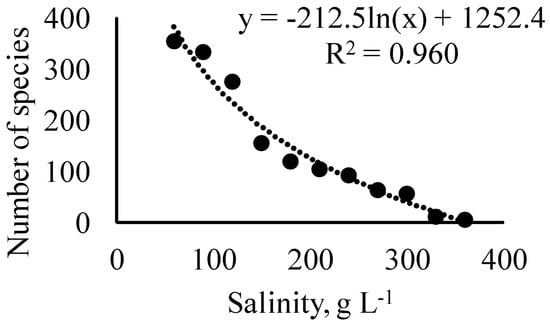
Figure 3.
Dependence of diatom species’ richness on their tolerated upper salinity limit at the global scale.
In a global comparison of the diatom species’ composition between water bodies in different regions, the similarity of the species’ composition was also not found. Such results of comparative analysis are expected due to the fact that most of all the identified species were only found in one of the studied water bodies (Table S1). Only 59 species of diatoms (13% of the total list) were found in more than three water bodies. In seven water bodies, C. placentula var. euglypta, Mastogloia lanceolata Thwaites ex W. Smith, 1856; N. ramosissima, Nitzschia scalpelliformis Grunow in Cleve et Grunow, 1880; and Pleurosigma elongatum W. Smith, 1852, were found; in seven, Cylindrotheca closterium (Ehrenberg) Reimann et Lewin, 1964; M. pumila (Cleve et Möller) P. T. Cleve, 1895; and Tryblionella punctata W. Smith, 1853, were found; in nine, A. brevipes, C. scutellum, Navicula salinarum Grunow in Cleve et Grunow, 1880, were found; and in ten, C. placentula Ehrenberg 1838 var. placentula, N. frustulum, N. sigma, Rhopalodia musculus (Kützing) O. Müller, 1900, were found. Among the species found in more than three lakes, the most represented were the genera Navicula, Nitzschia (seven species), Cocconeis (five species), Mastogloia, Rhopalodia, and Tryblionella (three species). Thus, the species’ composition of each water body can be considered unique. From this follows the obvious conclusion that salinity, up to a certain critical value, is not the main factor in the formation of the species structure of hypersaline water bodies. This has already been shown by the example of the spatiotemporal variability of the species’ composition of diatoms for the individual water bodies, Sivash Bay [27] and Lake Chersonesskoye [49]. At the same time, salinity acts as an important ecological filter that limits the possibility of the existence of species in water bodies with different salinity levels. The realization of this possibility and the formation of the species structure of an individual water body depends on a whole variety of abiotic and biotic factors, including the factor of chance, the position in the landscape, and the geological history of the water body [9,28,31,46,49,52,63].
There is currently growing interest in the use of diatoms for various applications [21]. One of the reasons for this is the value of secondary metabolites that are synthesized by diatoms for various goals. The different biologically active compounds are very valuable, among them are PUFA, antibiotics, enzyme inhibitors (to treat various diseases caused); other pharmacology active compounds, and toxins [70]. The nanostructured silica of diatom shells is also a very promising source of material for different technological applications [71,72]. The diatom cells are surrounded by a porous biosilica microshell of nanostructured morphology with pores sized in ten hundreds of nanometers and a large specific surface area of up to 200 m2 g−1 [71]. These characteristics are very similar to those of the technologically produced porous silica used in different nanotechnologies. Some other valuable properties of the diatom biosilica are also biocompatibility, tailored surface chemistry, chemical inertness, and thermal stability [71]. The worldwide diatoms’ availability combined with their low cost make their nanostructured silica an attractive source for new materials in the fields of photonics, nanoengineering, biosensing, drug delivery, regenerative medicine, and others [71,72,73,74]. As shown above, in hypersaline waters, there is a rather high species diversity of diatoms, while individual species can reach very high biomass, up to more than 200 g m−2 of the bottom [10,29,31]. Existence in a harsh hypersaline environment requires a wide range of adaptations in diatoms, and not only biochemical ones, as mentioned above. Salinity is a very important factor influencing pore sizes and other silica shell properties, and, as an example, some diatom species have the double frustule in hypersaline media [26,27]. There are optimal shell pore sizes for different technological applications [75,76,77,78]. So, a diatom’s shell nanostructure from hypersaline waters has specific peculiarities that may be valuable for some technological applications. Summarizing all the above, the authors concluded that knowledge of diatoms in hypersaline waters has not only pure scientific importance but also for diatom cultivation in hypersaline waters to cover some needs of bio- and nanotechnologies. Diatoms are a promising and valuable component of hypersaline aquaculture, the development of which can contribute to more sustainable water use [23]. Due to this, the ecology and biotechnological potential of high halotolerant diatoms must be studied deeper. For the transition to sustainable management of aquatic ecosystems, not only fundamental knowledge of biodiversity and ecosystem functioning is required, but also opportunities for the practical use of their bioresources to solve the problems of developing humanity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15122252/s1, Table S1: Diatoms of hypersaline water bodies of the world.
Author Contributions
Conceptualization, E.A. and D.B.; methodology, N.S.; formal analysis, E.A., N.S. and A.P.; investigation, D.B., E.A., R.L. and A.P.; writing—original draft preparation, N.S.; writing—review and editing, D.B., E.A., R.L., A.P. and N.S.; project administration, E.A.; funding acquisition, E.A. All authors have read and agreed to the published version of the manuscript.
Funding
Sample processing, data analysis, and manuscript writing were supported by the Russian Science Foundation (grant 18-16-00001). The long-term field studies of lakes and works on scanning electron microscope were conducted in the frameworks of the state assignments of A.O. Kovalevsky Institute of Biology of the Southern Seas of RAS (No. 121041500203-3 and 121041400077-1, respectively).
Data Availability Statement
All data used in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank S.N. Shadrina and L.A. Kartceva for providing SEM micrographs B, C, L, and M for Figure 2, which were created on the equipment of The Core Facilities Center “Cell and Molecular Technologies in Plant Science” at the Komarov Botanical Institute RAS (Russia) and B. Datson (Australia) for her help in improving the English.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guiry, M.D.; Guiry, G.M. AlgaeBase. In World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2023; Available online: https://www.algaebase.org (accessed on 6 February 2023).
- Krembs, C.; Eicken, H.; Junge, K.; Deming, J.W. High concentrations of exopolymeric substances in Arctic winter sea ice: Implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep Sea Res. Part I Oceanogr. Res. Pap. 2002, 49, 2163–2181. [Google Scholar] [CrossRef]
- Hobbs, W.O.; Wolfe, A.P.; Inskeep, W.P.; Amskold, L.; Konhauser, K.O. Epipelic diatoms from an extreme acid environment. Beowulf Spring, Yellowstone National Park, USA. Nova Hedwig. 2009, 135, 71–83. [Google Scholar]
- Fazlutdinova, A.; Gabidullin, Y.; Allaguvatova, R.; Gaysina, L. Diatoms in Kamchatka’s Hot Spring Soils. Diversity 2020, 12, 435. [Google Scholar] [CrossRef]
- Heine-Fuster, I.; López-Allendes, C.; Aránguiz-Acuña, A.; Véliz, D. Differentiation of diatom guilds in extreme environments in the Andean Altiplano. Front. Environ. Sci. 2021, 9, 266. [Google Scholar] [CrossRef]
- Shadrin, N.; Anufriieva, E. Ecosystems of hypersaline waters: Structure and trophic relations. Zh. Obshch. Biol. 2018, 79, 418–427. (In Russian) [Google Scholar]
- Sacco, M.; White, N.E.; Harrod, C.; Salazar, G.; Aguilar, P.; Cubillos, C.F.; Meredith, K.; Baxter, B.K.; Oren, A.; Anufriieva, E.; et al. Salt to conserve: A review on the ecology and preservation of hypersaline ecosystems. Biol. Rev. 2021, 96, 2828–2850. [Google Scholar] [CrossRef]
- Daniels, L.L. On the Flora of Great Salt Lake. Am. Nat. 1917, 51, 499–506. [Google Scholar] [CrossRef]
- Patrick, R. Some Diatoms of Great Salt Lake. Bull. Torrey Bot. Club 1936, 63, 157–166. [Google Scholar] [CrossRef]
- Nevrova, E.L.; Shadrin, N.V. Benthic diatoms in Crimean saline lakes. Mar. Ecol. J. 2005, 4, 61–71. (In Russian) [Google Scholar]
- Häusler, S.; Weber, M.; de Beer, D.; Ionescu, D. Spatial distribution of diatom and cyanobacterial mats in the Dead Sea is determined by response to rapid salinity fluctuations. Extremophiles 2014, 18, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Clavero, E.; Hernández-Mariné, M.; Grimalt, J.O.; Garcia-Pichel, F. Salinity tolerance of diatoms from thalassic hypersaline environments. J. Phycol. 2000, 36, 1021–1034. [Google Scholar] [CrossRef]
- Oren, A. Diversity of organic osmotic compounds and osmotic adaptation in cyanobacteria and algae. In Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 639–655. [Google Scholar]
- Shadrin, N.V.; Anufriieva, E.V.; Shadrina, S.N. Brief review of phototrophs in the Crimean hypersaline lakes and lagoons: Diversity, ecological role, the possibility of using. Mar. Biol. J. 2017, 2, 80–85. [Google Scholar]
- Nakov, T.; Judy, K.J.; Downey, K.M.; Ruck, E.C.; Alverson, A.J. Transcriptional Response of Osmolyte Synthetic Pathways and Membrane Transporters in a Euryhaline Diatom During Long-term Acclimation to a Salinity Gradient. J. Phycol. 2020, 56, 1712–1728. [Google Scholar] [CrossRef]
- Abdullahi, A.S.; Underwood, G.J.; Gretz, M.R. Extracellular matrix assembly in diatoms (Bacillariophyceae). V. Environmental effects on polysaccharide synthesis in the model diatom, Phaeodactylum tricornutum. J. Phycol. 2006, 42, 363–378. [Google Scholar] [CrossRef]
- Steele, D.J.; Franklin, D.J.; Underwood, G.J. Protection of cells from salinity stress by extracellular polymeric substances in diatom biofilms. Biofouling 2014, 30, 987–998. [Google Scholar] [CrossRef]
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- Abid, O.; Sellami-Kammoun, A.; Ayadi, H.; Drira, Z.; Bouain, A.; Aleya, L. Biochemical adaptation of phytoplankton to salinity and nutrient gradients in a coastal solar saltern, Tunisia. Estuar. Coast. Shelf Sci. 2008, 80, 391–400. [Google Scholar] [CrossRef]
- Nikitashina, V.; Stettin, D.; Pohnert, G. Metabolic adaptation of diatoms to hypersalinity. Phytochemistry 2022, 4, 113267. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, T.; Robert, J.M. Diatom cultivation and biotechnologically relevant products. Part I: Cultivation at various scales. Appl. Microbiol. Biotechnol. 2003, 60, 612–623. [Google Scholar] [CrossRef] [PubMed]
- de Jesús Paniagua-Michel, J.; Olmos-Soto, J.; Morales-Guerrero, E.R. Algal and microbial exopolysaccharides: New insights as biosurfactants and bioemulsifiers. Adv. Food Nutr. Res. 2014, 73, 221–257. [Google Scholar]
- Anufriieva, E.V. How can saline and hypersaline lakes contribute to aquaculture development? A review. J. Oceanol. Limnol. 2018, 36, 2002–2009. [Google Scholar] [CrossRef]
- Wilson, S.E.; Cumming, B.F.; Smol, J.P. Diatom-salinity relationships in 111 lakes from the Interior Plateau of British Columbia, Canada: The development of diatom-based models for paleosalinity reconstructions. J. Paleolimnol. 1994, 12, 197–221. [Google Scholar] [CrossRef]
- Taukulis, F.E. Diatom Communities in Lakes and Streams of Varying Salinity from South-West Western Australia: Distribution and Predictability. Ph.D. Thesis, Curtin University of Technology, Perth, Australia, 2007. [Google Scholar]
- Round, F.E. Benthic marine diatoms. Oceanogr. Mar. Biol. Annu. Rev. 1971, 9, 83–139. [Google Scholar]
- Siqueiros-Beltrones, D. Benthic diatoms from Laguna Figueroa, Baja California. Cienc. Mar. 1988, 14, 85–112. [Google Scholar] [CrossRef]
- Ehrlich, A. Atlas of the Inland-Water Diatom Flora of Israel; Geological Survey of Israel, Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1995; 166p. [Google Scholar]
- Prazukin, A.; Shadrin, N.; Balycheva, D.; Firsov, Y.; Lee, R.; Anufriieva, E. Cladophora spp. (Chlorophyta) modulate environment and create a habitat for microalgae in hypersaline waters. Eur. J. Phycol. 2021, 56, 231–243. [Google Scholar] [CrossRef]
- Senicheva, M.I.; Gubelit, Y.I.; Prazukin, A.V.; Shadrin, N.V. Phytoplankton of hypersaline lakes of Crimea. In Microalgae of the Black Sea: Problems of Biodiversity Preservation and Biotechnological Use; Tokarev, Y.N., Finenko, Z.Z., Shadrin, N.V., Eds.; EKOSI-Gidrophyzika: Sevastopol, Russia, 2008; pp. 93–99. (In Russian) [Google Scholar]
- Shadrin, N.; Balycheva, D.; Anufriieva, E. Microphytobenthos in the Hypersaline Water Bodies, the Case of Bay Sivash (Crimea): Is Salinity the Main Determinant of Species Composition? Water 2021, 13, 1542. [Google Scholar] [CrossRef]
- Guslyakov, N.E.; Zakordonets, O.A.; Gerasimyuk, V.P. Atlas of Diatoms in the Benthos of the Northwestern Part of the Black Sea and Adjacent Water Bodies; Naukova Dumka: Kyiv, Ukraine, 1992; 112p. (In Russian) [Google Scholar]
- Shichalyeyeva, G.N.; Gerasimiuk, V.P.; Kiryushkina, A.N.; Ennan, A.A.; Tsarenko, P. Algofloristic studies of the Kuyalnik Estuary and temporary water bodies of its vicinities (Northwestern Black Sea Coast, Ukraine). Algologia 2017, 27, 277–298. [Google Scholar] [CrossRef]
- Bulatov, S.A. Summary data on the algoflora of the Kara-Bogaz-Gol Bay of the Caspian Sea. In VIII Scientific-Practical Conference with International Participation. “Problems of Preserving the Caspian Ecosystem in the Conditions of Development of Oil and Gas Fields”; VNIRO: Astrakhan, Russia, 2021; pp. 48–56. (In Russian) [Google Scholar]
- Nemtseva, N.V.; Selivanova, E.A.; Yatsenko-Stepanova, T.N.; Ignatenko, M.E. The structure of algoplankton in the Sol-Iletsk lakes with different levels of salinity. Izv. Penz. Gos. Pedagog. Univ. Im. V. G. Belinskogo Yestestvennyye Nauk. 2011, 25, 535–541. (In Russian) [Google Scholar]
- Proshkina-Lavrenko, A.I.; Glezer, Z.I.; Jouse, A.P.; Makarova, I.P.; Sheshukova-Poretskaya, V.S. Diatoms of the USSR: Fossil and Modern; Nauka: Leningrad, Russia, 1974; Volume 1, 403p. (In Russian) [Google Scholar]
- Sapozhnikov, F.V.; Ivanishcheva, P.S.; Simakova, U.V. Modern assemblage changes of benthic algae as a result of hypersalinization of the Aral Sea. J. Mar. Syst. 2009, 76, 343–358. [Google Scholar] [CrossRef]
- Zavyalov, P.O.; Arashkevich, E.G.; Bastida, I.; Ginzburg, A.I.; Dikarev, S.N.; Zhitina, J.C.; Izhitsky, A.C.; Ishniyazov, D.P.; Kostyanoy, A.G.; Kravtsova, V.I.; et al. The Large Aral Sea in the Beginning of Century 21: Physics, Biology, Chemistry; Nauka: Moscow, Russia, 2012; 252p. (In Russian) [Google Scholar]
- Sapozhnikov, F.V.; Kalinina, O.Y.; Kurbaniyazov, A.K.; Yusupov, B.; Mukhitdinova, S.; Abdimutalip, N.A. About the condition of the microphytobenthos of reservoirs of system of the Aral Sea on researches of the complex international expedition. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Biol. Med. Sci. 2017, 3, 171–176. (In Russian) [Google Scholar]
- Reed, J. A diatom-conductivity transfer function for Spanish salt lakes. J. Paleolimnol. 1998, 19, 399–416. [Google Scholar] [CrossRef]
- Moreno, D.F.; Sánchez-Castillo, P.M.; Delgado, C.; Almeida, S.F. Diatom Species That Characterize Saline Ponds (South of Spain) with the Description of a New Navicula Species. Res. Sq. 2021, preprint. [Google Scholar] [CrossRef]
- Siqueiros-Beltrones, D. Association Structure of Benthic Diatoms in A Hypersaline Environment. Cienc. Mar. 1990, 16, 101–127. [Google Scholar] [CrossRef]
- Dor, I.; Ehruch, A. The Effect of Salinity and Temperature Gradients on the Distribution of Littoral Microalgae in Experimental Solar Ponds, Dead Sea Area, Israel. Mar. Ecol. 1987, 8, 193–205. [Google Scholar] [CrossRef]
- Laut, L.; Figueiredo, M.S.; Lorini, M.L.; Belart, P.; Clemente, I.; Martins, M.V.A.; Filho, J.G.M.; Laut, V. Diatoms from the most hypersaline lagoon in Brazil: Vermelha lagoon. Cont. Shelf Res. 2019, 181, 111–123. [Google Scholar] [CrossRef]
- Sylvestre, F.; Beck-Eichler, B.; Duleba, W.; Debenay, J.-P. Modern benthic diatom distribution in a hypersaline coastal lagoon: The Lagoa de Araruama (R.J.), Brazil. Hydrobiologia 2001, 443, 213–231. [Google Scholar] [CrossRef]
- Kirkwood, A.E.; Henley, W.J. Algal community dynamics and halotolerance in a terrestrial, hypersaline environment. J. Phycol. 2006, 42, 537–547. [Google Scholar] [CrossRef]
- Kociolek, J.P.; Herbst, D.B. Taxonomy and Distribution of Benthic Diatoms from Mono Lake, California, U.S.A. Trans. Am. Micros. Soc. 1992, 111, 338–355. [Google Scholar] [CrossRef]
- Nagasathya, A.; Thajuddin, N. Diatom Diversity in Hypersaline Environment. J. Fish. Aquat. Sci. 2008, 3, 328–333. [Google Scholar] [CrossRef]
- Bae, H.; Park, J.; Ahn, H.; Khim, J.S. Shift in Benthic Diatom Community Structure and Salinity Thresholds in a Hypersaline Environment of Solar Saltern, Korea. Algae 2020, 35, 361–373. [Google Scholar] [CrossRef]
- Dolapsakis, N.P.; Tafas, T.; Abatzopoulos, T.J.; Ziller, S.; Economou-Amilli, A. Abundance and growth response of microalgae at Megalon Embolon solar saltworks in northern Greece: An aquaculture prospect. J. Appl. Phycol. 2005, 17, 39–49. [Google Scholar] [CrossRef]
- Wilson, S.E.; Cumming, B.F.; Smol, J.P. Assessing the reliability of salinity inference models from diatom assemblages: An examination of a 219-lake data set from western North America. Can. J. Fish. Aquat. Sci. 1996, 53, 1580–1594. [Google Scholar] [CrossRef]
- Hodgson, D.A.; Vyverman, W.; Sabbe, K. 2001. Limnology and biology of saline lakes in the Rauer Islands, Eastern Antarctica. Antarct. Sci. 2001, 13, 255–270. [Google Scholar] [CrossRef]
- Shadrin, N.; Balycheva, D.; Anufriieva, E. Spatial and temporal variability of microphytobenthos in a marine hypersaline lake (Crimea): Are there some general patterns? J. Sea Res. 2021, 177, 102121. [Google Scholar] [CrossRef]
- Krishtofovich, A.N. Diatom Analysis. Volume. 3. Key to Fossil and Modern Diatoms; Gosgeolizdat: Leningrad, Russia, 1950; 398p. (In Russian) [Google Scholar]
- Proshkina-Lavrenko, A.I. Planktonic Diatoms of the Black Sea; AN USSR: Moscow-Leningrad, Russia, 1955; 222p. (In Russian) [Google Scholar]
- Proshkina-Lavrenko, A.I. Diatoms of the Black Sea benthos; AN USSR: Moscow-Leningrad, Russia, 1963; 243p. (In Russian) [Google Scholar]
- Witkowski, A.; Lange-Bertalot, H.; Metzeltin, D. Diatom Flora of Marine Coasts I. Iconongraphia Diatomologica, 7; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2000; 950p. [Google Scholar]
- Taylor, J.C.; Harding, W.R.; Archibald, C.G. An Illustrated Guide to Some Common Diatom Species from South Africa; Water Research Commission: Pretoria, South Africa, 2007; 188p. [Google Scholar]
- Krakhmalny, A.F. Dinophyta of Ukraine (Illustrated Book for Identification); Alterpres: Kiev, Ukraine, 2011; 444p. (In Russian) [Google Scholar]
- Ryabushko, L.I.; Begun, A.A. Diatoms of the Microphytobenthos of the Sea of Japan. Book 2; PK «KIA» Publishers: Sevastopol, Russia, 2016; 324p. (In Russian) [Google Scholar]
- Cristóbal, G.; Blanco, S.; Bueno, G. Modern Trends in Diatom Identification; Springer: Cham, Switzerland, 2020; 294p. [Google Scholar]
- Prazukin, A.V.; Anufriieva, E.V.; Shadrin, N.V. Cladophora mats in a Crimean hypersaline lake: Structure, dynamics, and inhabiting animals. J. Oceanol. Limnol. 2018, 36, 1930–1940. [Google Scholar] [CrossRef]
- Ryabushko, L.I. Microphytobenthos of the Black Sea; EKOSI-Gidrofizica: Sevastopol, Russia, 2013; 416p. (In Russian) [Google Scholar]
- Semkin, B.I. On the relation between mean values of two measures of inclusion and measures of similarity. Bull. Bot. Sada-Inst. DVO RAN 2009, 3, 91–101. (In Russian) [Google Scholar]
- Shadrin, N.; Kolesnikova, E.; Revkova, T.; Latushkin, A.; Chepyzhenko, A.; Drapun, I.; Dyakov, N.; Anufriieva, E. Do separated taxa react differently to a long-term salinity increase? The meiobenthos changes in Bay Sivash, largest hypersaline lagoon worldwide. Knowl. Manag. Aquat. Ecosyst. 2019, 420, 36. [Google Scholar] [CrossRef]
- Shadrin, N.; Kolesnikova, E.; Revkova, T.; Latushkin, A.; Chepyzhenko, A.; Dyakov, N.; Anufriieva, E. Macrostructure of benthos along a salinity gradient: The case of Sivash Bay (the Sea of Azov), the largest hypersaline lagoon worldwide. J. Sea Res. 2019, 154, 101811. [Google Scholar] [CrossRef]
- Hotos, G.N. A Preliminary Survey on the Planktonic Biota in a Hypersaline Pond of Messolonghi Saltworks (W. Greece). Diversity 2021, 13, 270. [Google Scholar] [CrossRef]
- Servant-Vildary, S.; Roux, M. Multivariate analysis of diatoms and water chemistry in Bolivian saline lakes. Hydrobiologia 1990, 197, 267–290. [Google Scholar] [CrossRef]
- Nübel, U.; Garcia-Pichel, F.; Clavero, E.; Muyzer, G. Matching molecular diversity and ecophysiology of benthic cyanobacteria and diatoms in communities along a salinity gradient. Environ. Microbiol. 2000, 2, 217–226. [Google Scholar] [CrossRef]
- Lebeau, T.; Robert, J.M. Diatom cultivation and biotechnologically relevant products. Part II: Current and putative products. Appl. Microbiol. Biotechnol. 2003, 60, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.; De Stefano, L. Special Issue on New Frontiers in Diatom Nanotechnology. Appl. Sci. 2022, 12, 10332. [Google Scholar] [CrossRef]
- Roychoudhury, P.; Bose, R.; Dabek, P.; Witkowski, A. Photonic Nano-/Microstructured Diatom Based Biosilica in Metal Modification and Removal—A Review. Materials 2022, 15, 6597. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, C.; Chianese, G.; Terracciano, M.; De Stefano, L.; Rea, I. Nanostructured biosilica of diatoms: From water world to biomedical applications. Appl. Sci. 2020, 10, 6811. [Google Scholar] [CrossRef]
- Tramontano, C.; De Stefano, L.; Terracciano, M.; Chianese, G.; Rea, I. Diatomite-based nanoparticles: Fabrication strategies for medical applications. In Algae Biotechnology: Integrated Algal Engineering for Bioenergy, Bioremediation, and Biomedical Applications; Ahmad, A., Banat, F., Taher, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 427–446. [Google Scholar]
- Aizdaicher, N.A.; Markina, Z.V. The effect of decrease in salinity on the dynamics of abundance and the cell size of Corethron hystrix (Bacillariophyta) in laboratory culture. Ocean Sci. J. 2010, 45, 1–5. [Google Scholar] [CrossRef]
- Yung, M.M.; Wong, S.W.; Kwok, K.W.; Liu, F.Z.; Leung, Y.H.; Chan, W.T.; Li, X.Y.; Djurišić, A.B.; Leung, K.M. Salinity-dependent toxicities of zinc oxide nanoparticles to the marine diatom Thalassiosira pseudonana. Aquat. Toxicol. 2015, 165, 31–40. [Google Scholar] [CrossRef]
- Su, Y.; Lundholm, N.; Ellegaard, M. Effects of abiotic factors on the nanostructure of diatom frustules—Ranges and variability. Appl. Microbiol. Biotechnol. 2018, 102, 5889–5899. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, B.; Duan, D.; Pan, K. Salinity-dependent nanostructures and composition of cell surface and its relation to Cd toxicity in an estuarine diatom. Chemosphere 2019, 215, 807–814. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).