An Improved Model for Water Quality Management Accounting for the Spatiotemporal Benthic Flux Rate
Abstract
1. Introduction
2. Materials and Methods
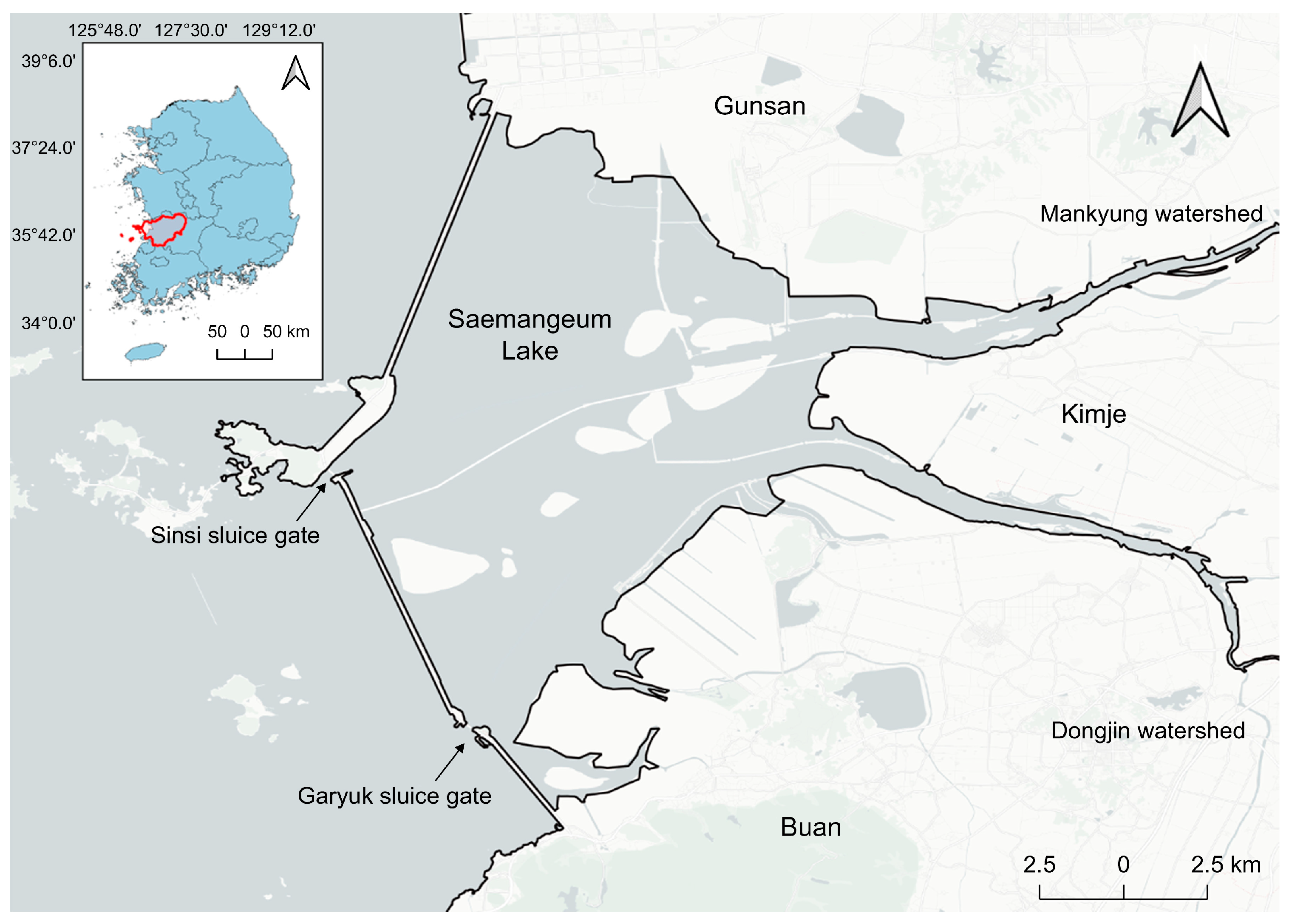
2.1. Study Area
2.2. Model Description
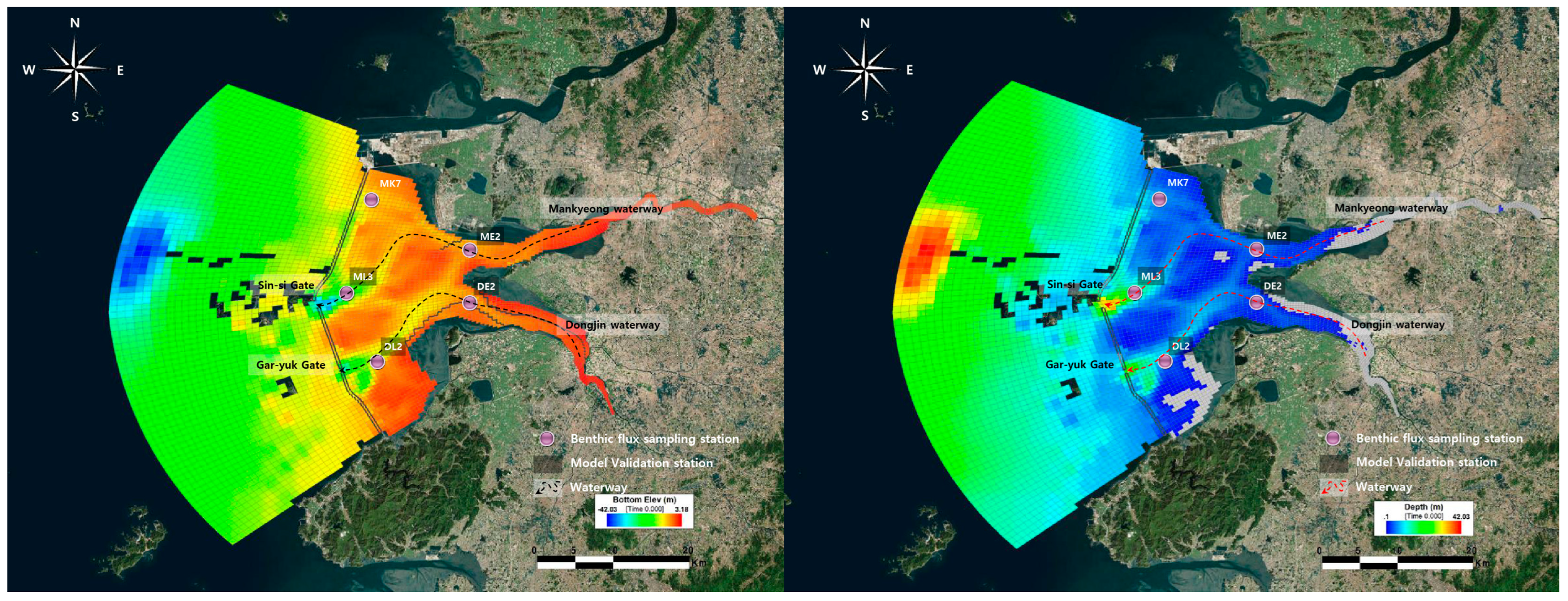
2.3. Model Construction
2.4. Water Body-Benthic Sediment Reaction Simulation Method
2.5. Model Assessment
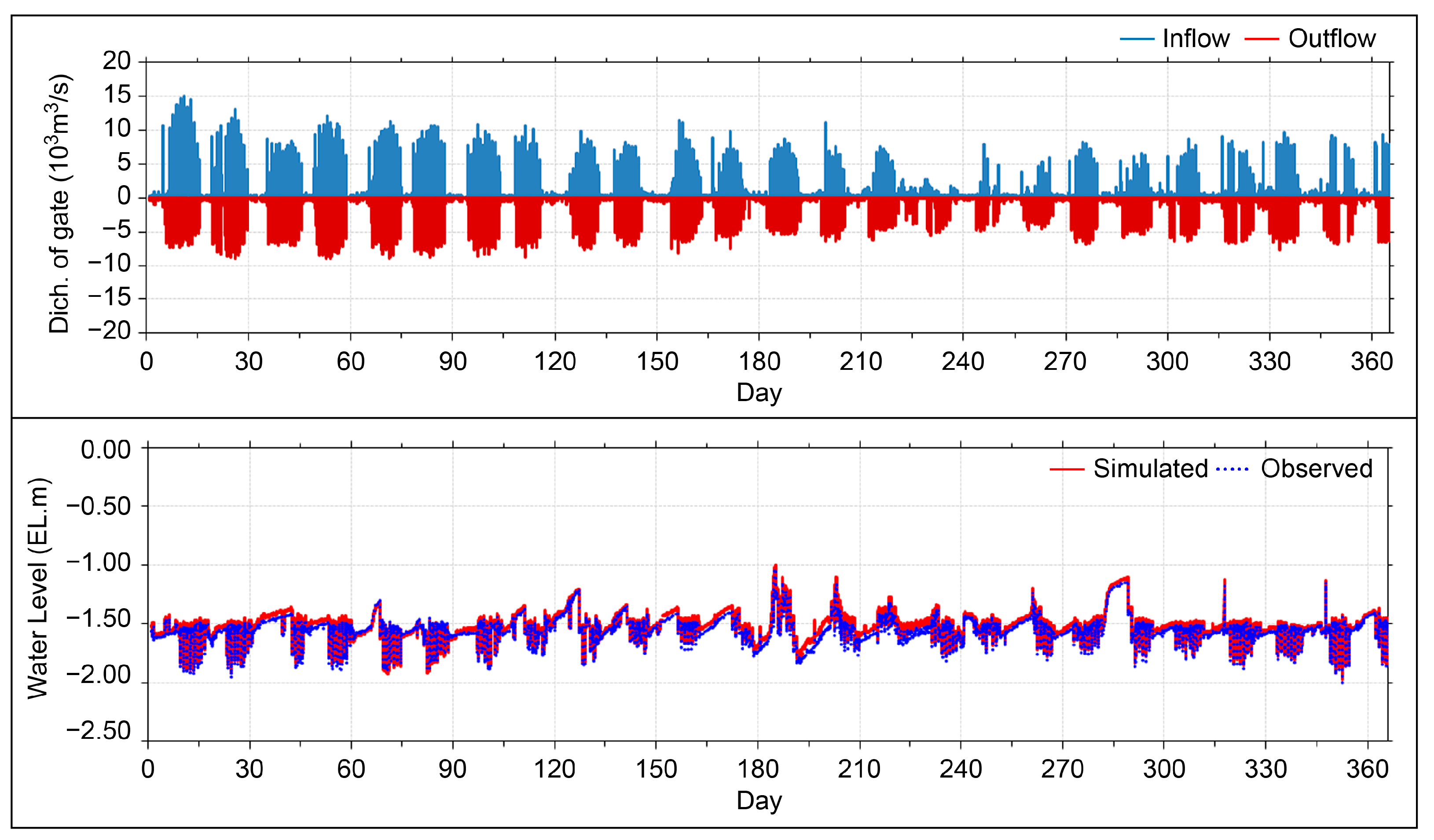
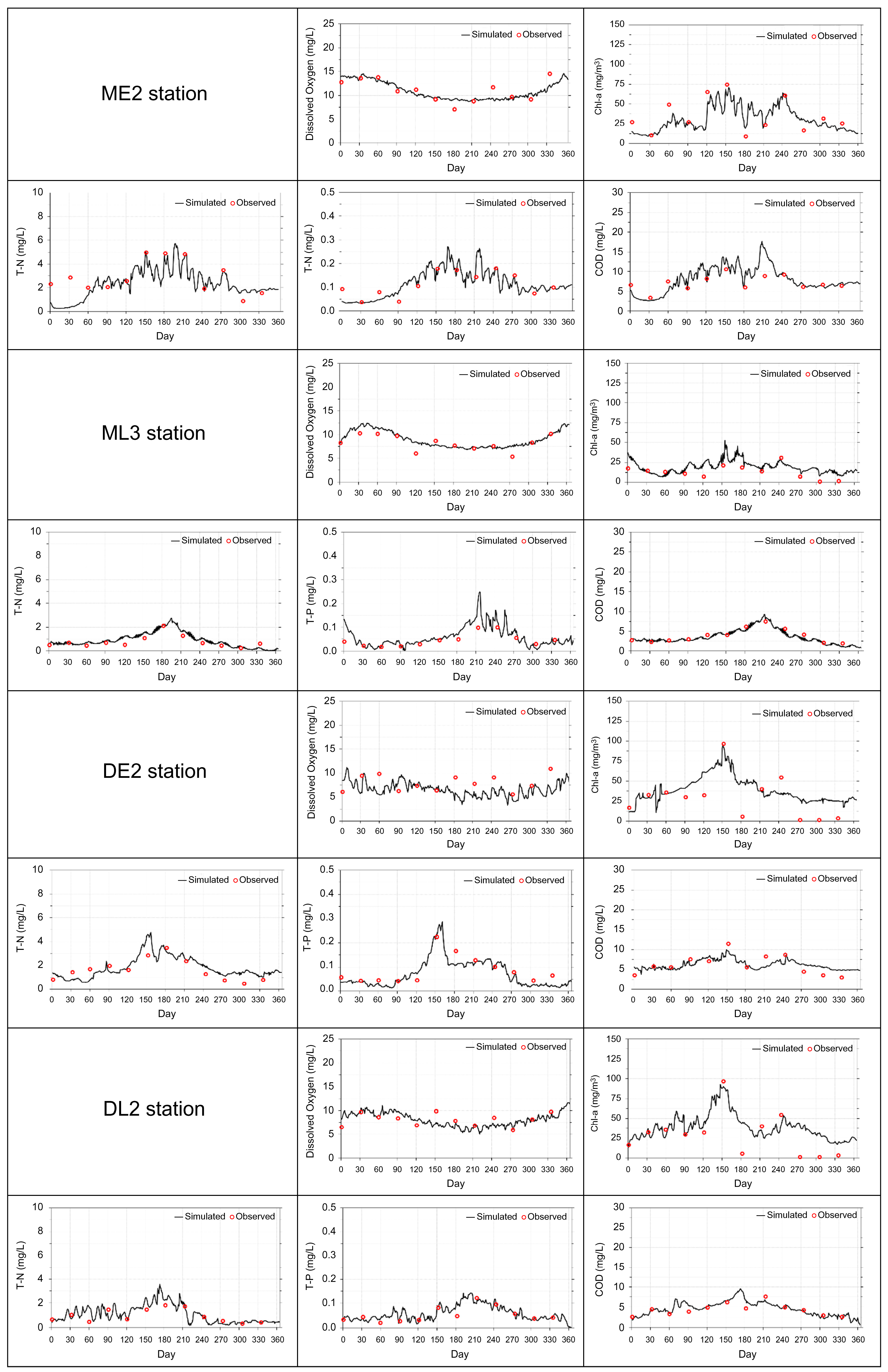
2.6. Model Validation
2.7. Validation of Water Body-Benthic Sediment Reaction Simulation Method
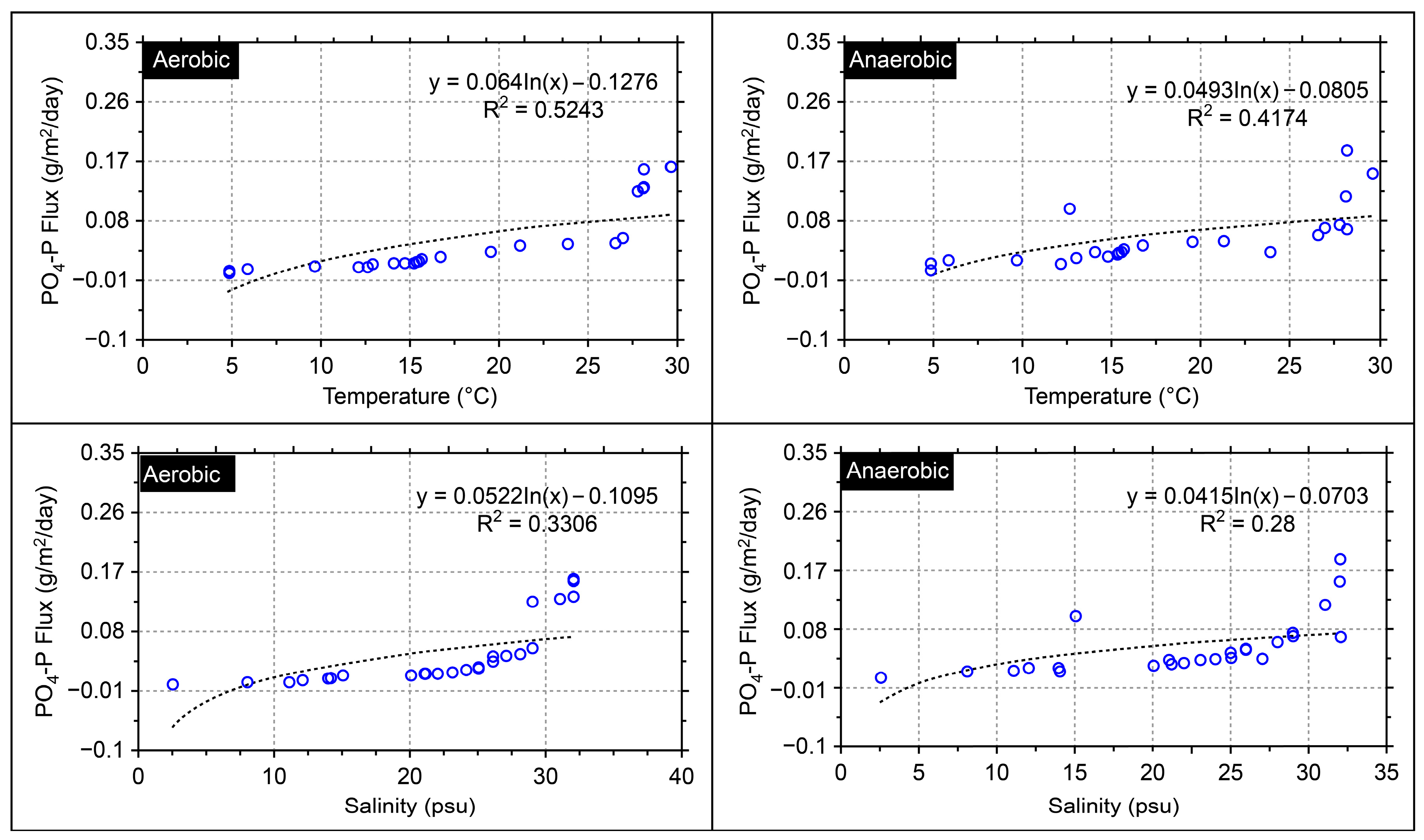
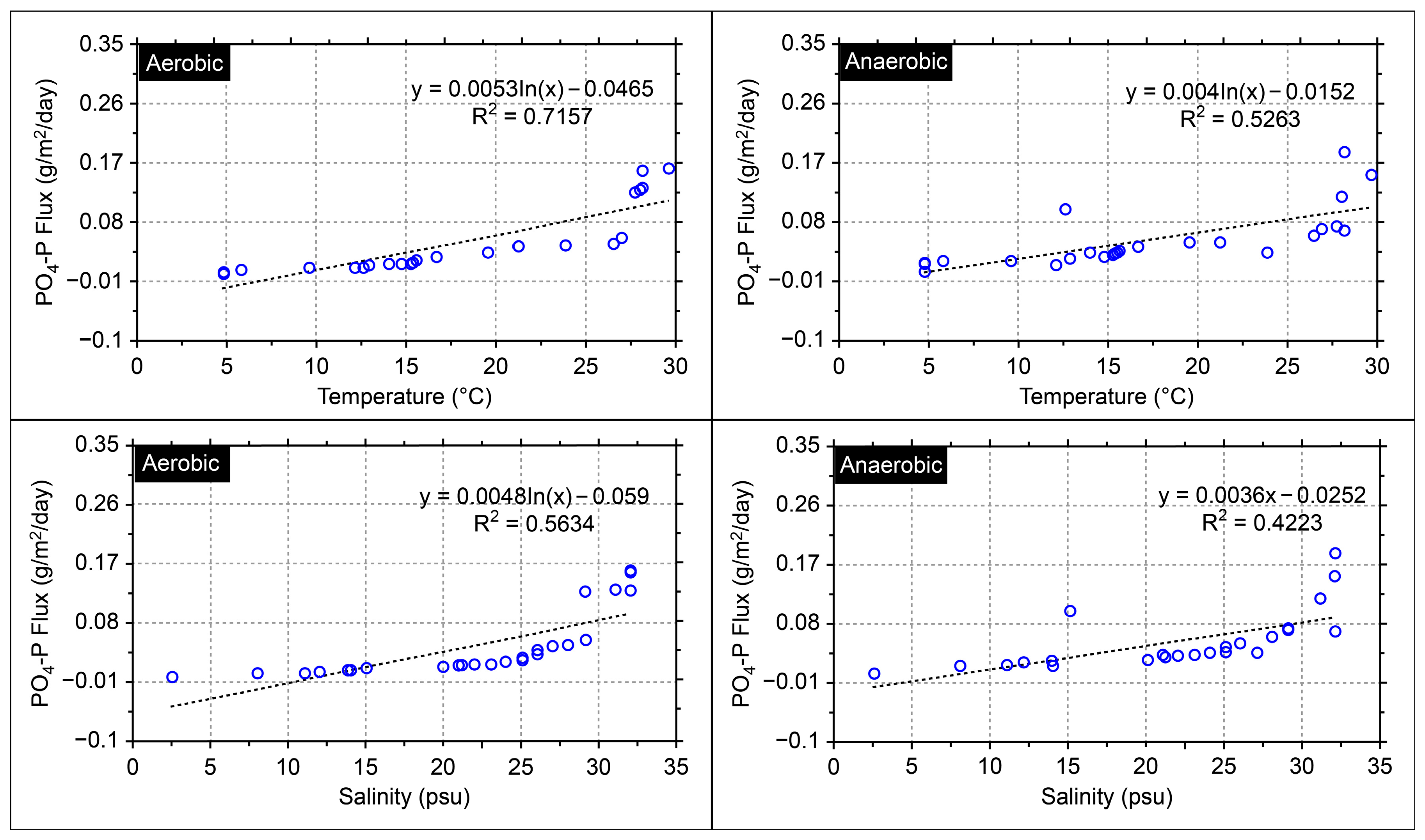
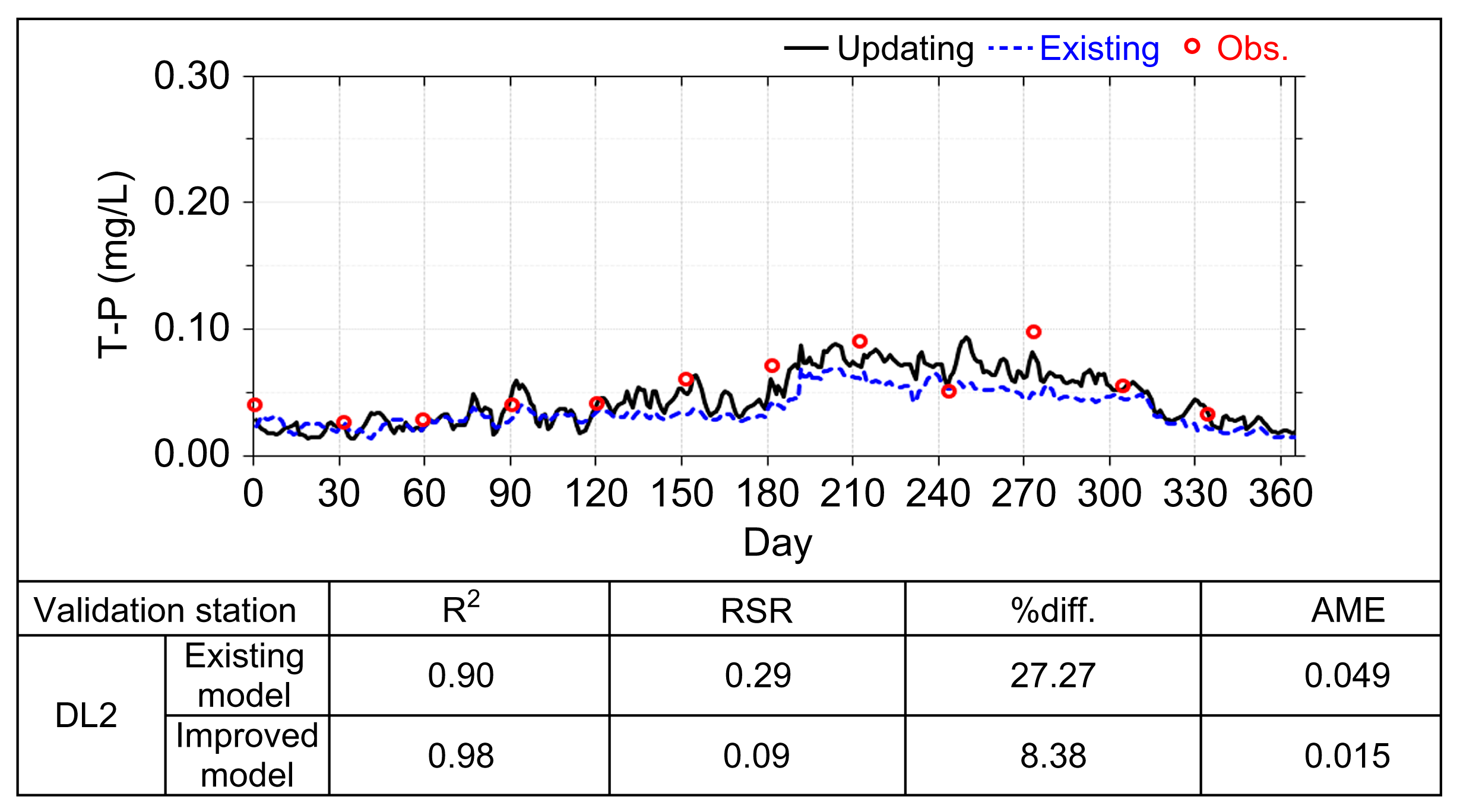
3. Results and Discussion
Application of the Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sahavacharin, A.; Sompongchaiyakul, P.; Thaitakoo, D.J.L. The Effects of Land-Based Change on Coastal Ecosystems. Landsc. Ecol. Eng. 2022, 18, 351–366. [Google Scholar] [CrossRef]
- Korea Environment Institute (KEI). A Study on the Development of Environmental Standards for Waterbed Sediments; Korea Environment Institute (KEI): Sejong, Republic of Korea, 2001. [Google Scholar]
- Lim, J.W.; Kwun, S.K. Development and Application of Multiple Box Water Quality Model for Estuary Reservoirs. J. Korean Soc. Agric. Eng. 1989, 31, 111–122. [Google Scholar]
- Portnoy, J.W. Summer Oxygen Depletion in a Diked New England Estuary. Estuaries 1991, 14, 122–129. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, K.S. A Study on Release Characteristics of Sediment and Its Impacts on Water Quality in Daecheong Dam Reservoir. J. Environ. Impact Assess. 2000, 9, 99–107. [Google Scholar]
- Hsieh, C.D.; Yang, W.F. Optimal Nonpoint Source Pollution Control Strategies for a Reservoir Watershed in Taiwan. J. Environ. Manag. 2007, 85, 908–917. [Google Scholar] [CrossRef]
- Oh, K.; Chung, S.; Cho, Y. Analysis of Physicochemical Characteristics of Suspended Sediments Flowing into the Saemangeum Reservoir in the Summer. J. Korean Soc. Environ. Eng. 2015, 37, 99–106. [Google Scholar] [CrossRef]
- Yoo, S.C.; Suh, W.S.; Lee, H.Y. Impacts on Residence Time and Water Quality of the Saemangeum Reservoir Caused by Inner Development. J. Korean Soc. Mar. Environ. Energy 2012, 15, 186–197. [Google Scholar] [CrossRef]
- Korea Rural Community Corporation (KRC). Prediction Study on Water Quality Associated with the Changing of Saemangeum Project Condition; Korea Rural Community Corporation (KRC): Najusi, Republic of Korea, 2015. [Google Scholar]
- Wei, H.; Zhao, L.; Zhang, H.; Lu, Y.; Yang, W.; Song, G. Summer Hypoxia in Bohai Sea Caused by Changes in Phytoplankton Community. Anthr. Coasts 2021, 4, 77–86. [Google Scholar] [CrossRef]
- Hao, A.; Kobayashi, S.; Yan, N.; Xia, D.; Zhao, M.; Iseri, Y. Improvement of Water Quality Using a Circulation Device Equipped with Oxidation Carriers and Light Emitting Diodes in Eutrophic Pond Mesocosms. J. Environ. Chem. Eng. 2021, 9, 105075. [Google Scholar] [CrossRef]
- Malecki, L.M.; White, J.R.; Reddy, K.R. Nitrogen and Phosphorus Flux Rates from Sediment in the Lower St. Johns River Estuary. J. Environ. Qual. 2004, 33, 1545–1555. [Google Scholar] [CrossRef]
- Tufford, D.L.; McKellar, H.N. Spatial and Temporal Hydrodynamic and Water Quality Modeling Analysis of a Large Reservoir on the South Carolina (USA) Coastal Plain. Ecol. Modell. 1999, 114, 137–173. [Google Scholar] [CrossRef]
- Kung, H.T.; Ying, L.G. A Study of Lake Eutrophication in Shanghai, China. Geogr. J. 1991, 157, 45–50. [Google Scholar] [CrossRef]
- National Institute of Environmental Research (NIER). A Model Construction for Quantification on Water Quality Improvement Effect of Saeamangeum Area (II), Incheon, Korea. 2014; Environmental Digital Library (MOE). Available online: https://library.me.go.kr (accessed on 2 June 2023).
- Shin, S.; Her, Y.; Muñoz-Carpena, R.; Yu, X. Quantifying the Contribution of External Loadings and Internal Hydrodynamic Processes to the Water Quality of Lake Okeechobee. Sci. Total Environ. 2023, 883, 163713. [Google Scholar] [CrossRef] [PubMed]
- North Carolina Department of Environment and Natural Resources, Falls Lake Nutrient Response Model Final Report, North Carolina. 2009; North Carolina Environmental Quality. Available online: https://www.deq.nc.gov (accessed on 2 June 2023).
- Wang, Y.; Peng, Z.; Liu, G.; Zhang, H.; Zhou, X.; Hu, W. A Mathematical Model for Phosphorus Interactions and Transport at the Sediment-Water Interface in a Large Shallow Lake. Ecol. Model. 2023, 476, 110254. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Sun, B.; Liu, Y. Developing a 3D Hydrodynamic and Water Quality Model for Floating Treatment Wetlands to Study the Flow Structure and Nutrient Removal Performance of Different Configurations. Sustainability 2022, 14, 7495. [Google Scholar] [CrossRef]
- Qin, Z.; He, Z.; Wu, G.; Tang, G.; Wang, Q. Developing Water-Quality Model for Jingpo Lake Based on EFDC. Water 2022, 14, 2596. [Google Scholar] [CrossRef]
- Rosario-Llantín, J.A.; Zarillo, G.A. Flushing Rates and Hydrodynamical Characteristics of Mosquito Lagoon (Florida, USA). Environ. Sci. Pollut. Res. Int. 2021, 28, 30019–30034. [Google Scholar] [CrossRef]
- Yildiz, K.; Karakaya, N.; Kilic, S.; Evrendilek, F. Interaction Effects of the Main Drivers of Global Climate Change on Spatiotemporal Dynamics of High Altitude Ecosystem Behaviors: Process-Based Modeling. Environ. Monit. Assess. 2020, 192, 457. [Google Scholar] [CrossRef]
- Khadka, P.; Sharma, S.; Mathis, T. Monitoring an Ungagged Coastal Marsh to Analyze the Salinity Interaction of the Marsh with Lake Erie. Environ. Monit. Assess. 2021, 193, 645. [Google Scholar] [CrossRef]
- Luo, X.; Li, X. Using the EFDC Model to Evaluate the Risks of Eutrophication in an Urban Constructed Pond from Different Water Supply Strategies. Ecol. Modell. 2018, 372, 1–11. [Google Scholar] [CrossRef]
- Choi, J.; Oh, C.; Kweon, Y.; Ahn, C. Consideration on the Operation of water level management and Environmental Change Associated with Inner Dike Constructions in Saemangeum Reservoir. J. Korean Soc. Mar. Environ. Energy 2013, 16, 290–298. [Google Scholar] [CrossRef]
- Korea Rural Community Corporation (KRC). Ecological Changes in the Saemangeum Water and Reclaimed Land Areas (IV); Korea Rural Community Corporation (KRC): Najusi, Republic of Korea, 2009. [Google Scholar]
- Hamrick, J.M. A Three-Dimensional Environmental Fluid Dynamics Computer Code: Theoretical and Computational Aspects. 63 Williamsburg; Special Report 317; College of William and Mary, Virginia Institute of Marine Science: Williamsburg, VA, USA, 1992. [Google Scholar]
- Tetra Tech, Inc. The Environmental Fluid Dynamics Code Theory and Computation Volume 1: Hydrodynamics and Mass Transport; Tetra Tech Inc.: Fairfax, VA, USA, 2007. [Google Scholar]
- Tetra Tech, Inc. The Environmental Fluid Dynamics Code Theory and Computation Volume 3: Water Quality Module; Tetra Tech Inc.: Fairfax, VA, USA, 2007. [Google Scholar]
- Ministry of Land, Infrastructure and Transport (MLIT). Preliminary Investigation on Securing and Procurement of Saemangeum Landfill; Ministry of Land, Infrastructure and Transport (MLIT): Sejong, Republic of Korea, 2011.
- Matsumoto, K.; Takanezawa, T.; Ooe, M. Ocean Tide Models Developed by Assimilating TOPEX/POSEIDON Altimeter Data into Hydrodynamical Model: A Global Model and a Regional Model Around Japan. J. Oceanogr. 2000, 56, 567–581. [Google Scholar] [CrossRef]
- Makinde, O.O.; Edun, O.M.; Akinrotimi, O.A. Comparative Assessment of Physical and Chemical Characteristics of Water in Ekerekana and Buguma Creeks, Niger Delta, Nigeria. J. Environ. Prot. Sustain. Dev. 2015, 1, 126–133. [Google Scholar]
- Ejigu, M.T. Overview of Water Quality Modeling. Cogent Eng. 2021, 8, 1891711. [Google Scholar] [CrossRef]
- Chapra, S.C. Surface Water Quality Modeling; Tufts University: Medford, MA, USA, 1996. [Google Scholar]
- Wang, C.; Liu, S.; Shi, X.; Cui, G.; Wang, H.; Jin, X.; Fan, K.; Hu, S. Numerical Modeling of Contaminant Advection Impact on Hydrodynamic Diffusion in a Deformable Medium. J. Rock Mech. Geotech. Eng. 2022, 14, 994–1004. [Google Scholar] [CrossRef]
- Ministry of the Environment (MOE). Study on Master Plan for Water Quality Improvement in Saemangeum Basin; Ministry of the Environment (MOE): Sejong, Republic of Korea, 2011.
- Haas, A.F.; Smith, J.E.; Thompson, M.; Deheyn, D.D. Effects of Reduced Dissolved Oxygen Concentrations on Physiology and Fluorescence of Hermatypic Corals and Benthic Algae. PeerJ 2014, 2, e235. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, X.; Yao, Y.; Li, L.; Wu, F. Effects of Biological Activity, Light, Temperature and Oxygen on Phosphorus Release Processes at the Sediment and Water Interface of Taihu Lake, China. Water Res. 2008, 42, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Y.; Wang, H.; Yu, Y.; Liu, J.; Qiao, R.; Liu, M.; Ma, S.; Wang, H. Decreased Internal Phosphorus Loading from Eutrophic Sediment After Artificial Light Supplement: Preliminary Evidence from a Microcosm Experiment. Front. Environ. Sci. 2022, 10, 148. [Google Scholar] [CrossRef]
- Jiang, H.Z.; Shen, Y.M.; Wang, S.D. Numerical Study on Salinity Stratification in the Oujiang River Estuary. J. Hydrodyn. 2009, 21, 835–842. [Google Scholar] [CrossRef]
- Huang, F.; Lin, X.; Yin, K.J.E.I. Effects of Algal-Derived Organic Matter on Sediment Nitrogen Mineralization and Immobilization in a Eutrophic Estuary. Ecol. Indic. 2022, 138, 108813. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, S.; Zhou, J.; Chen, Z.; Shen, J.; Xie, X.; Wu, F.; Chen, P. Sediment Geochemistry of Lake Daihai, North-Central China: Implications for Catchment Weathering and Climate Change During the Holocene. J. Paleolimnol. 2010, 43, 75–87. [Google Scholar] [CrossRef]
- Miranda, L.S.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Adsorption-Desorption Behavior of Heavy Metals in Aquatic Environments: Influence of Sediment, Water and Metal Ionic Properties. J. Hazard. Mater. 2022, 421, 126743. [Google Scholar] [CrossRef]
- Bai, J.; Ye, X.; Jia, J.; Zhang, G.; Zhao, Q.; Cui, B.; Liu, X. Phosphorus Sorption-Desorption and Effects of Temperature, pH and Salinity on Phosphorus Sorption in Marsh Soils from Coastal Wetlands with Different Flooding Conditions. Chemosphere 2017, 188, 677–688. [Google Scholar] [CrossRef]
- Zhang, H.; Elskens, M.; Chen, G.; Chou, L. Phosphate Adsorption on Hydrous Ferric Oxide (HFO) at Different Salinities and pHs. Chemosphere 2019, 225, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, J.; Ekholm, P.; Pitkänen, H. Coastal eutrophication thresholds: A matter of sediment microbial processes. AMBIO A J. Hum. Environ. 2009, 38, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Hansel, C.M.; Lentini, C.J.; Tang, Y.; Johnston, D.T.; Wankel, S.D.; Jardine, P.M. Dominance of Sulfur-Fueled Iron Oxide Reduction in Low-Sulfate Freshwater Sediments. ISME J. 2015, 9, 2400–2412. [Google Scholar] [CrossRef]
- Reed, D.C.; Slomp, C.P.; Gustafsson, B.G.J.L. Sedimentary Phosphorus Dynamics and the Evolution of Bottom--Water Hypoxia: A Coupled Benthic–Pelagic Model of a Coastal System. Limnol. Oceanogr. 2011, 56, 1075–1092. [Google Scholar] [CrossRef]
- Boström, B.; Andersen, J.M.; Fleischer, S.; Jansson, M. Exchange of Phosphorus across the Sediment-Water Interface. Hydrobiologia 1988, 170, 229–244. [Google Scholar] [CrossRef]
- Coelho, J.P.; Flindt, M.R.; Jensen, H.S.; Lillebø, A.I.; Pardal, M.A. Phosphorus Speciation and Availability in Intertidal Sediments of a Temperate Estuary: Relation to Eutrophication and Annual P-Fluxes. Estuar. Coast. Shelf Sci. 2004, 61, 583–590. [Google Scholar] [CrossRef]
- Ministry of the Environment (MOE). Sediment Monitoring of the Saemangeum Lake; Ministry of the Environment (MOE): Sejong, Republic of Korea, 2018.
- Moriasi, D.N.; Arnold, J.G.; Van Liew, M.W.; Bingner, R.L.; Harmel, R.D.; Veith, T.L. Model Evaluation Guidelines for Systematic Quantification of Accuracy in Watershed Simulations. Trans. ASABE 2007, 50, 885–900. [Google Scholar] [CrossRef]
- Tasnim, B.; Fang, X.; Hayworth, J.S.; Tian, D. Simulating Nutrients and Phytoplankton Dynamics in Lakes: Model Development and Applications. Water 2021, 13, 2088. [Google Scholar] [CrossRef]
- Chapra, S.C.; Dobson, H.F.H. Quantification of the Lake Trophic Typologies of Naumann (Surface Quality) and Thienemann (Oxygen) with Special Reference to the Great Lakes. J. Gr. Lakes Res. 1981, 7, 182–193. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; in press. [Google Scholar]
- Lee, H.; Romero, J. IPCC, 2023: Climate Change 2023: Synthesis Report. A Report of the Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Ed.; IPCC: Geneva, Switzerland, 2023; in press. [Google Scholar]
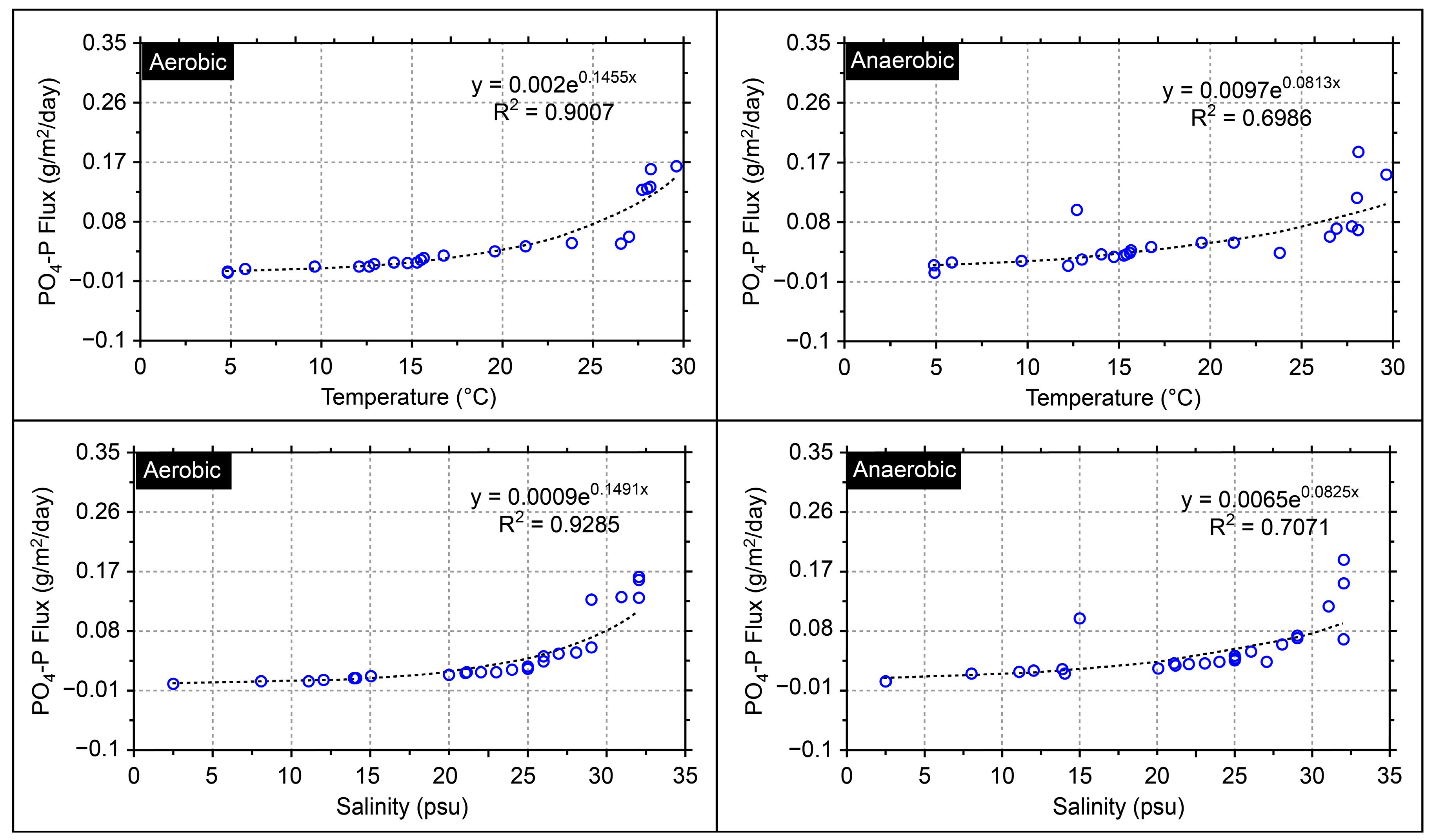
| Parameters | Unit | Definition | Calibration |
|---|---|---|---|
| PMc, PMd, PMs | /day | maximum growth rate under optimal conditions for algal group x | 2.8 |
| KHNx | /gNm3 | half-saturation constant for nitrogen uptake for algal group x | 0.01 |
| KHPx | /gPm3 | half-saturation constant for phosphorus uptake for algal group x | 0.001 |
| KHS | /gSim3 | half-saturation constant for silica uptake for diatoms | 0.05 |
| CCHlx | gC/mgChl | carbon-to-chlorophyll ratio in algal group x | 0.03 |
| TMc1, TMd1, TMg1 | °C | optimal temperature for algal growth for algal group x | 20.0 |
| TMc2 | °C | optimal temperature for algal growth for algal group x | 27.5 |
| TMd2, TMg2 | 25.0 | ||
| BFPO4d | - | sediment-water exchange flux of phosphate (g P/m2/day) | 0.009 |
| WSS | m/day | settling velocity of particulate metal | 1.0 |
| KRP | /day | minimum hydrolysis rate of refractory particulate organic phosphorus | 0.15 |
| KLP | /day | minimum hydrolysis rate of labile particulate organic phosphorus | 0.0175 |
| KDP | /day | minimum mineralization rate of dissolved organic phosphorus | 0.001 |
| Kcd | /day | oxidation rate of chemical oxygen demand at TRCOD | 20 |
| BFCOD | - | benthic flux rate of chemical oxygen demand | 0.12 |
| KRN | /day | minimum hydrolysis rate of refractory particulate organic nitrogen | 0.075 |
| KLN | /day | minimum hydrolysis rate of labile particulate organic nitrogen | 0.175 |
| KDN | /day | minimum mineralization rate of dissolved organic nitrogen | 0.001 |
| BFNH4 | - | benthic flux rate of ammonia nitrogen | 0.009 |
| WSRP | m/day | settling velocity of refractory particulate organic matter | 0.55 |
| WSLP | m/day | settling velocity of labile particulate organic matter | 0.55 |
| KRC | /day | minimum dissolution rate of refractory particulate organic carbon | 0.075 |
| KLC | /day | minimum dissolution rate of labile particulate organic carbon | 0.175 |
| KDC | /day | minimum respiration rate of dissolved organic carbon | 0.01 |
| ASCd | gSi/gC | silica-to-carbon ratio of diatoms | 0.05 |
| KSU | /day | dissolution rate of particulate biogenic silica | 0.05 |
| Classification | Station | Water Temperature (°C) | Salinity (psu) | ||
|---|---|---|---|---|---|
| Aerobic | Anaerobic | ||||
| 1st | ME2 | 0.1317 | 0.1528 | 26.5 | 2.80 |
| ML3 | 0.0191 | 0.0216 | 23.8 | 23.8 | |
| MK7 | 0.1311 | 0.1884 | 21.2 | 21.2 | |
| DE2 | 0.0561 | 0.0504 | 26.9 | 1.20 | |
| DL2 | 0.0178 | 0.0302 | 19.5 | 19.5 | |
| 2nd | ME2 | 0.0280 | 0.0382 | 15.3 | 9.40 |
| ML3 | 0.0176 | 0.0270 | 15.2 | 25.5 | |
| MK7 | 0.0048 | 0.0230 | 14.0 | 24.0 | |
| DE2 | 0.0115 | 0.0053 | 16.7 | 6.6 | |
| DL2 | 0.0433 | 0.0744 | 14.7 | 22.8 | |
| 3rd | ME2 | 0.047 | 0.0332 | 15.5 | 12.1 |
| ML3 | 0.1273 | 0.0248 | 12.6 | 30.5 | |
| MK7 | 0.0069 | 0.0346 | 12.1 | 30.7 | |
| DE2 | 0.0145 | 0.0247 | 15.6 | 17.0 | |
| DL2 | 0.0112 | 0.0491 | 12.9 | 22.6 | |
| 4th | ME2 | 0.0458 | 0.0710 | 28.1 | 3.4 |
| ML3 | 0.0223 | 0.0223 | 28.1 | 17.3 | |
| MK7 | 0.0119 | 0.0159 | 29.6 | 12.1 | |
| DE2 | 0.0178 | 0.0601 | 27.7 | 0.8 | |
| DL2 | 0.0046 | 0.0178 | 28.0 | 19.4 | |
| 5th | ME2 | 0.1589 | 0.330 | 9.6 | 7.4 |
| ML3 | 0.0244 | 0.06898 | 4.8 | 30.7 | |
| MK7 | 0.0344 | 0.0447 | 4.8 | 30.7 | |
| DE2 | 0.0009 | 0.0285 | 5.8 | 18.3 | |
| DL2 | 0.1635 | 0.1184 | 4.8 | 29.2 | |
| Criteria | Equation | Optimal Value |
|---|---|---|
| RSR | 0 | |
| % Difference | 0 | |
| AME | 0 |
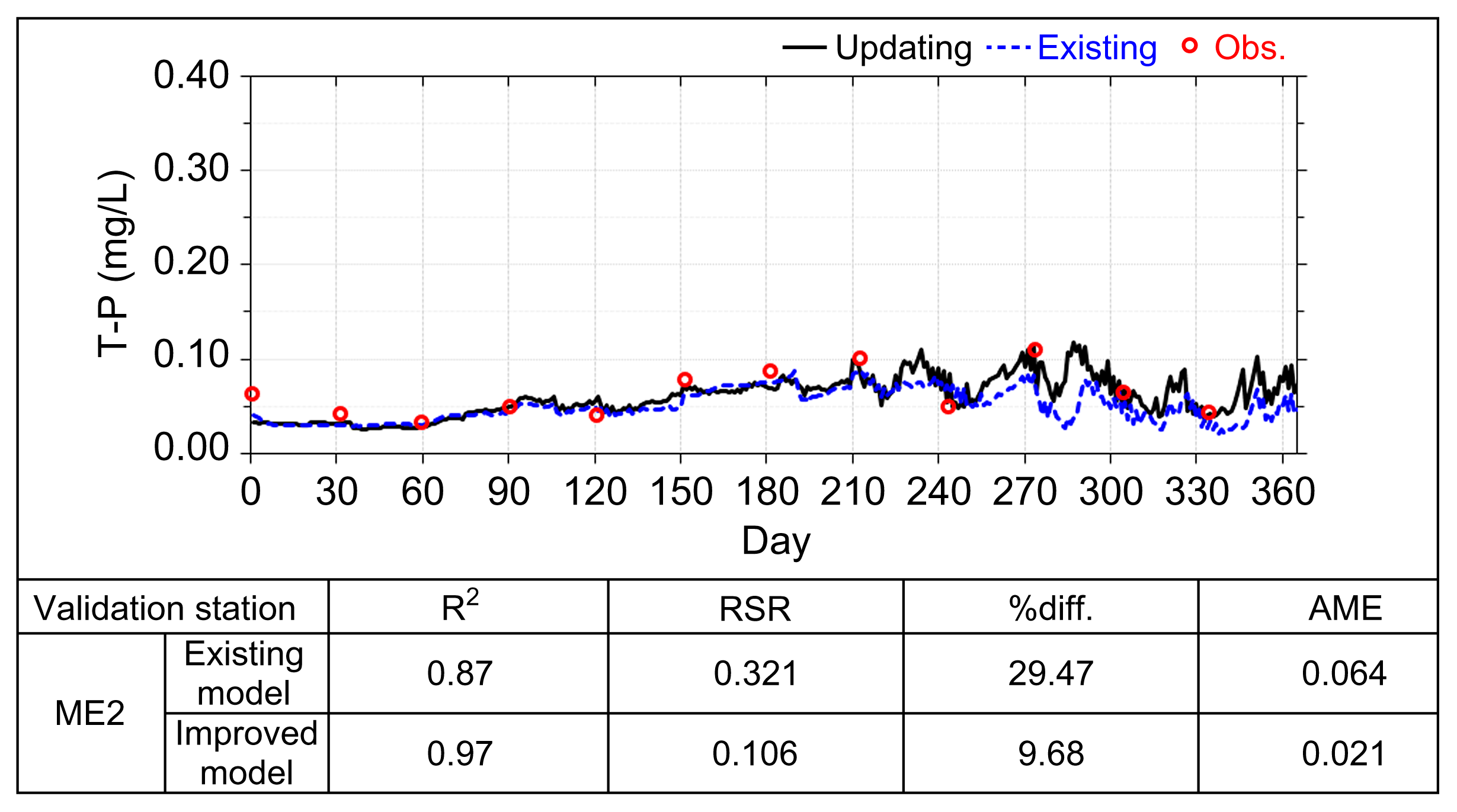
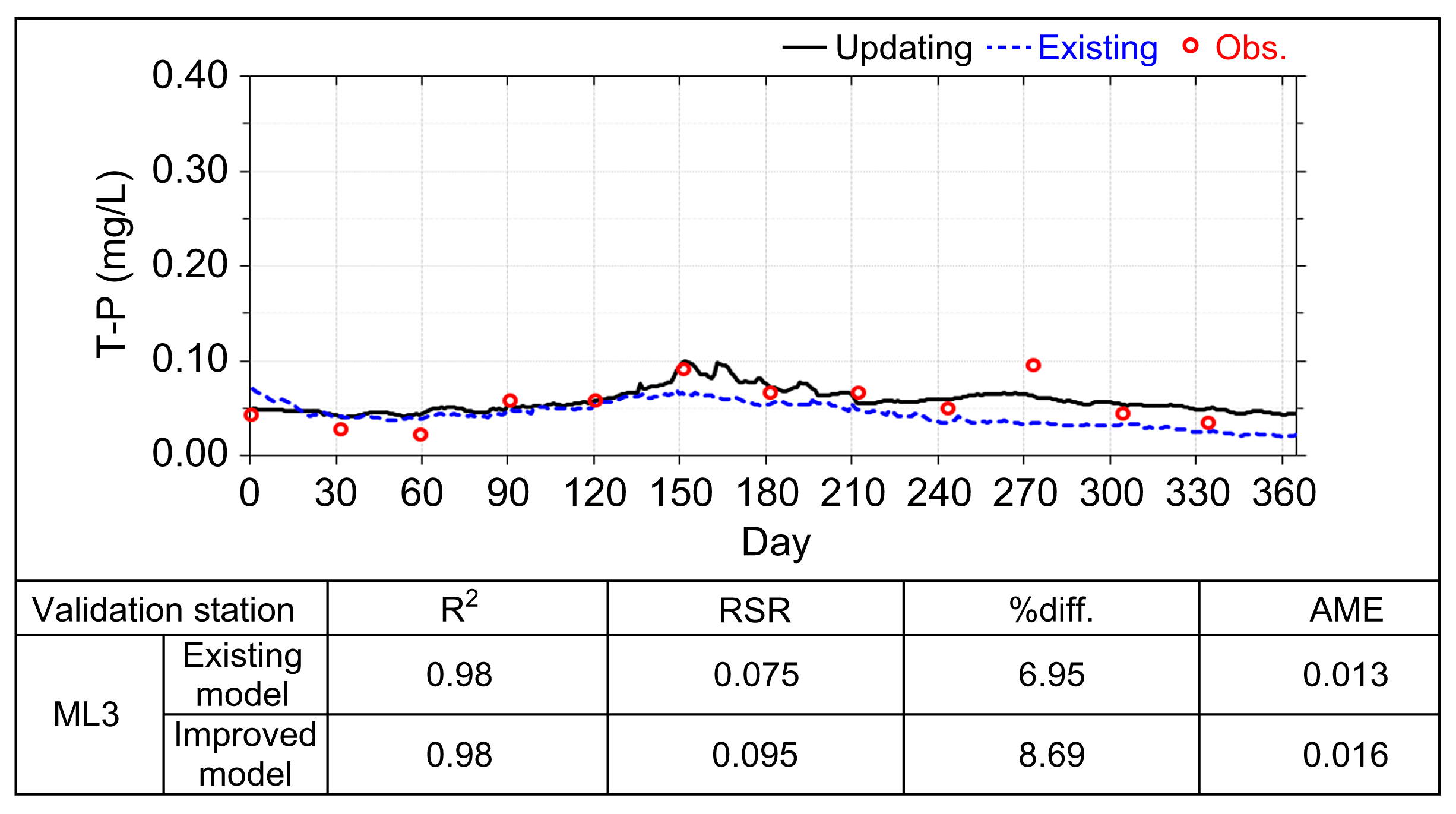
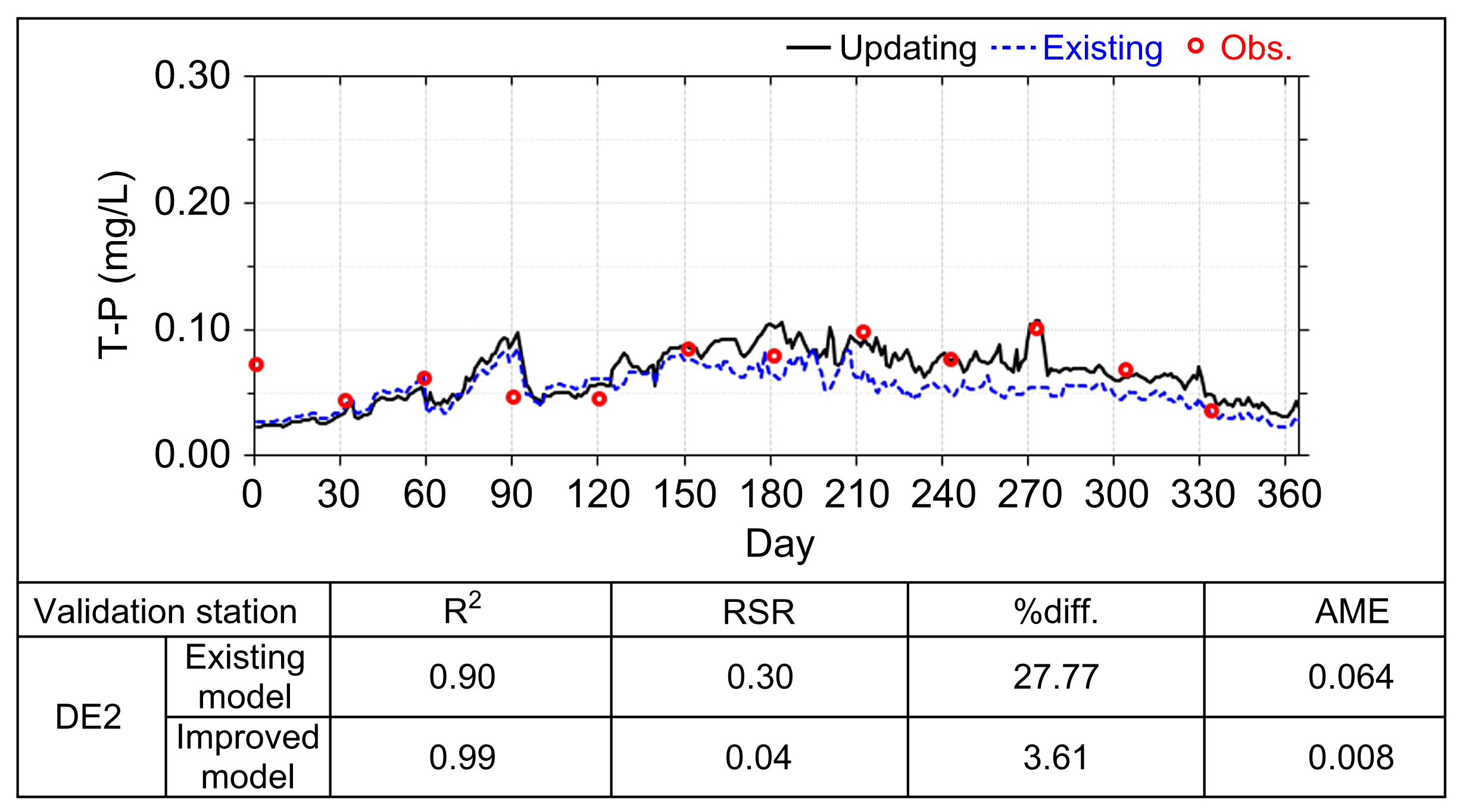
| Validation Stations | RSR | %diff. | AME | |
|---|---|---|---|---|
| DO | ME2 | 0.029 | 2.7 | 0.241 |
| ML3 | 0.066 | 6.0 | 0.501 | |
| DE2 | 0.204 | 18.7 | 1.491 | |
| DL2 | 0.003 | 0.3 | 0.022 | |
| Chl-a | ME2 | 0.123 | 11.2 | 3.955 |
| ML3 | 0.480 | 44.0 | 5.909 | |
| DE2 | 0.361 | 33.1 | 9.697 | |
| DL2 | 1.596 | 146.3 | 22.549 | |
| T-N | ME2 | 0.134 | 12.3 | 0.353 |
| ML3 | 0.295 | 27.0 | 0.214 | |
| DE2 | 0.139 | 12.7 | 0.209 | |
| DL2 | 0.115 | 10.6 | 0.101 | |
| T-P | ME2 | 0.027 | 2.5 | 0.003 |
| ML3 | 0.471 | 43.2 | 0.020 | |
| DE2 | 0.157 | 14.4 | 0.013 | |
| DL2 | 0.083 | 7.6 | 0.004 | |
| COD | ME2 | 0.118 | 10.9 | 0.773 |
| ML3 | 0.110 | 10.1 | 0.394 | |
| DE2 | 0.146 | 13.4 | 0.833 | |
| DL2 | 0.009 | 0.8 | 0.035 | |
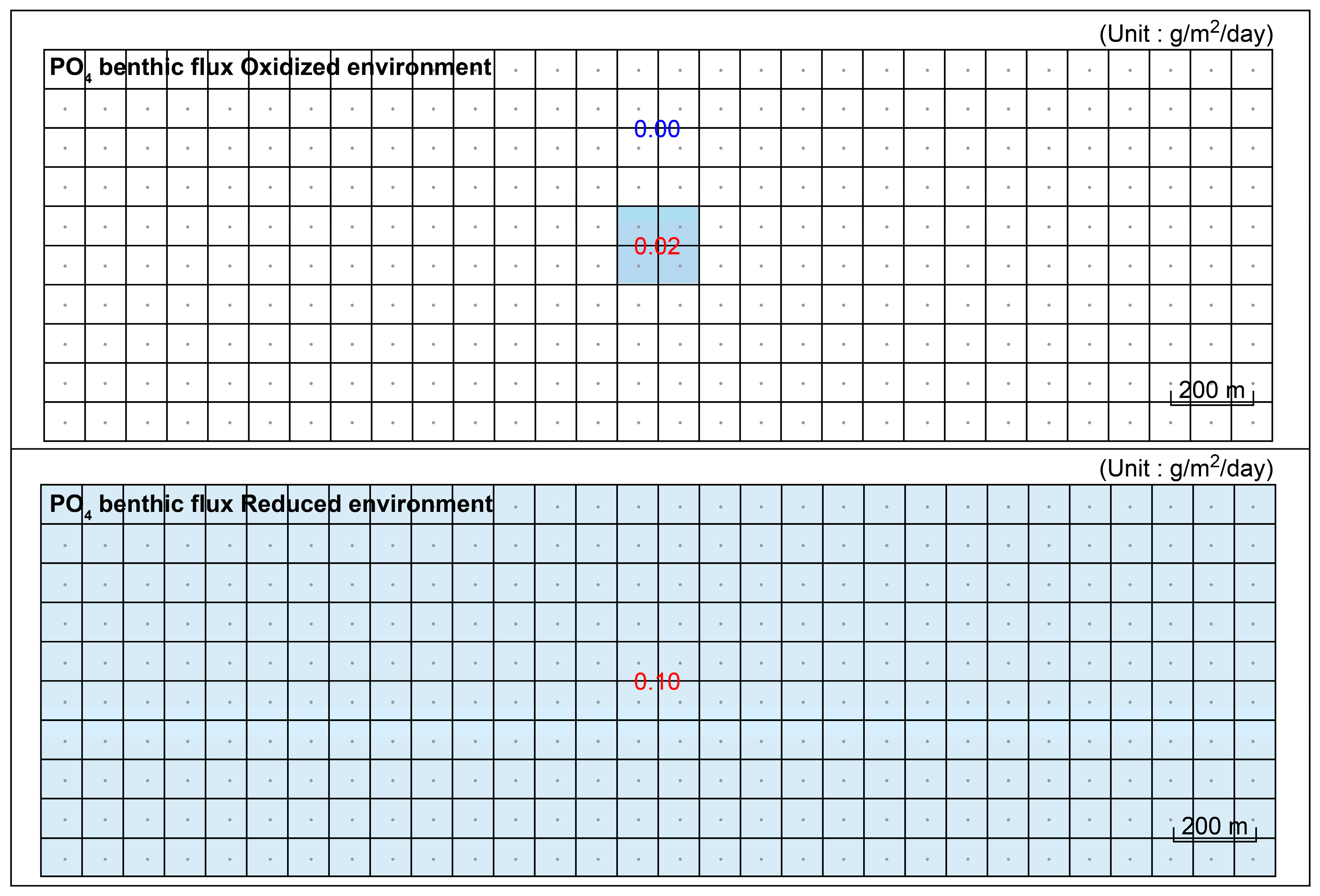
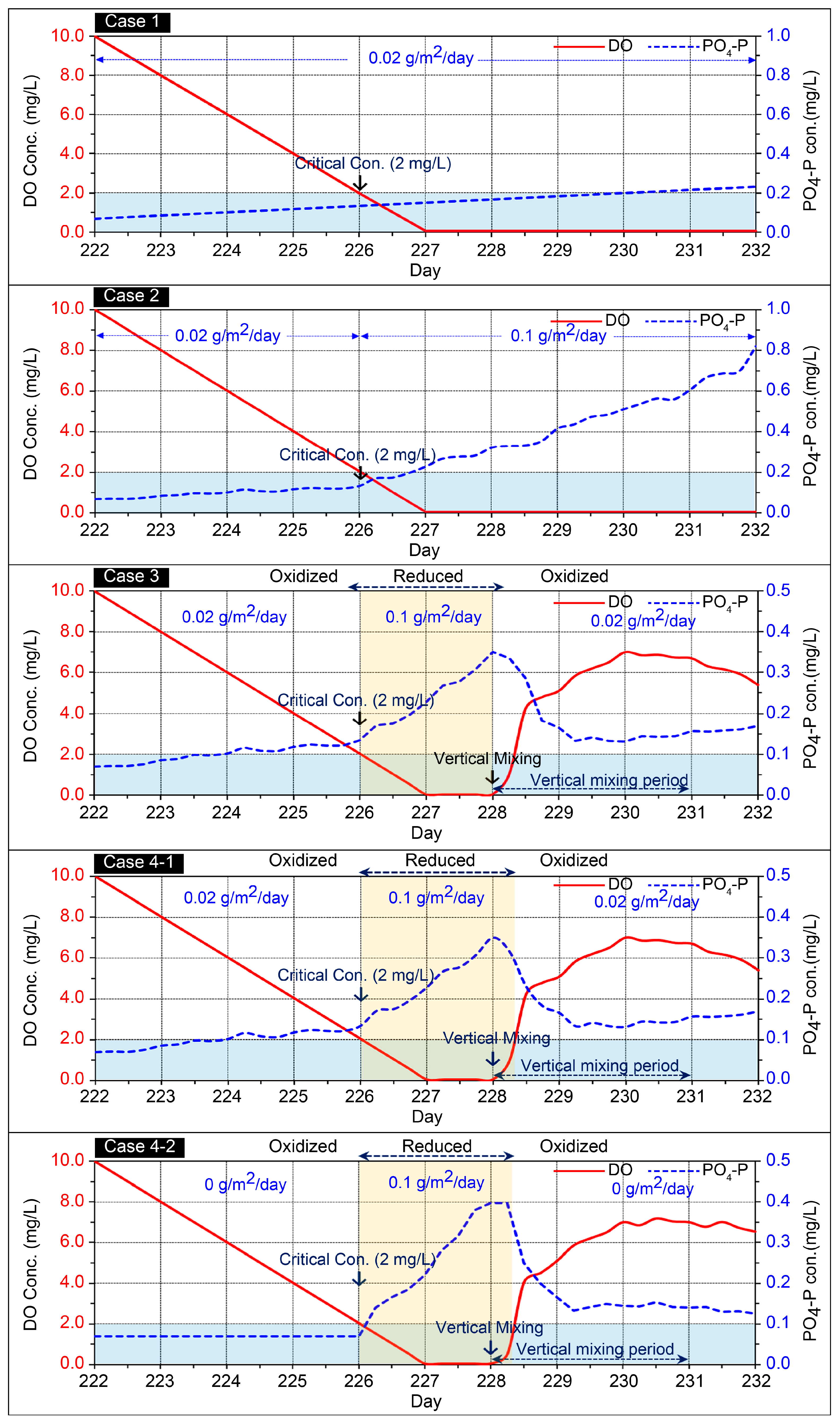
| Experiment Cases | Applied Model | Vertical Mixing | Release Zone | Flux Rate (g/m2/day) | ||
|---|---|---|---|---|---|---|
| SOD | PO4 | |||||
| Aerobic (Oxidized) | Anaerobic (Reduced) | |||||
| Case 1 | Existing | × | × | −2.0 | 0.02 | |
| Case 2 | Improved | × | × | −2.0 | 0.02 | 0.10 |
| Case 3 | Improved | ○ | × | −2.0 | 0.02 | 0.10 |
| Case 4-1 Case 4-2 | Improved | ○ | ○ | −2.0 | (Zone 1) 0.02 (Zone 2) 0.00 | (Zone 1) 0.10 (Zone 2) 0.10 |
| Item | Description | |
|---|---|---|
| Purpose of experiment | Simulation of total phosphorus changes according to improved method of applying the flux rate | |
| Scope of the model | 64 km in the east-west direction, and 54 km in the south-north direction | |
| Model configuration | Grid configuration | Orthogonal Curvilinear Coordinate |
| Number of grids | 5897 | |
| Experimental conditions | Open boundary | Composite tides of the four main sub-tidal currents (M2, S2, K1, O1) |
| Calculation interval | △t = 6 s | |
| Experiment period | 1 January 2016–31 December 2016 | |
| Experimental condition | (Existing model) flux rate: 0.003 g/m2/day(Improved model) flux rate: spatiotemporal flux rate according to water temperature-salinity changes | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Park, Y. An Improved Model for Water Quality Management Accounting for the Spatiotemporal Benthic Flux Rate. Water 2023, 15, 2219. https://doi.org/10.3390/w15122219
Kim S, Park Y. An Improved Model for Water Quality Management Accounting for the Spatiotemporal Benthic Flux Rate. Water. 2023; 15(12):2219. https://doi.org/10.3390/w15122219
Chicago/Turabian StyleKim, Semin, and Youngki Park. 2023. "An Improved Model for Water Quality Management Accounting for the Spatiotemporal Benthic Flux Rate" Water 15, no. 12: 2219. https://doi.org/10.3390/w15122219
APA StyleKim, S., & Park, Y. (2023). An Improved Model for Water Quality Management Accounting for the Spatiotemporal Benthic Flux Rate. Water, 15(12), 2219. https://doi.org/10.3390/w15122219





