Continuous Flow Experimental Study on Ozonation of Ibuprofen Catalyzed by Silicate-Based Microfiltration Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
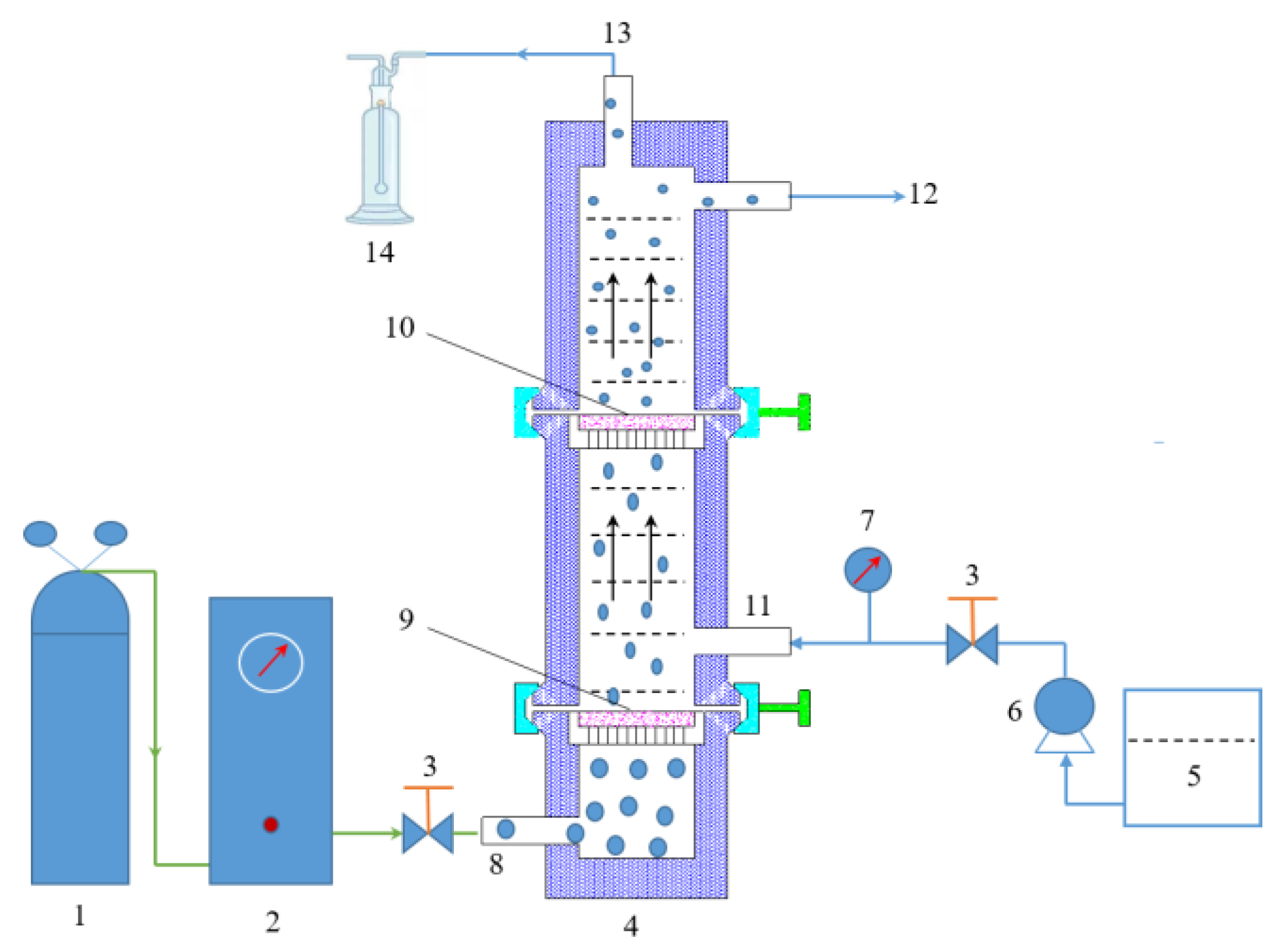
2.2. Experimental Equipment
2.3. Analysis
3. Results and Discussions
3.1. Influence of Ibuprofen Concentration
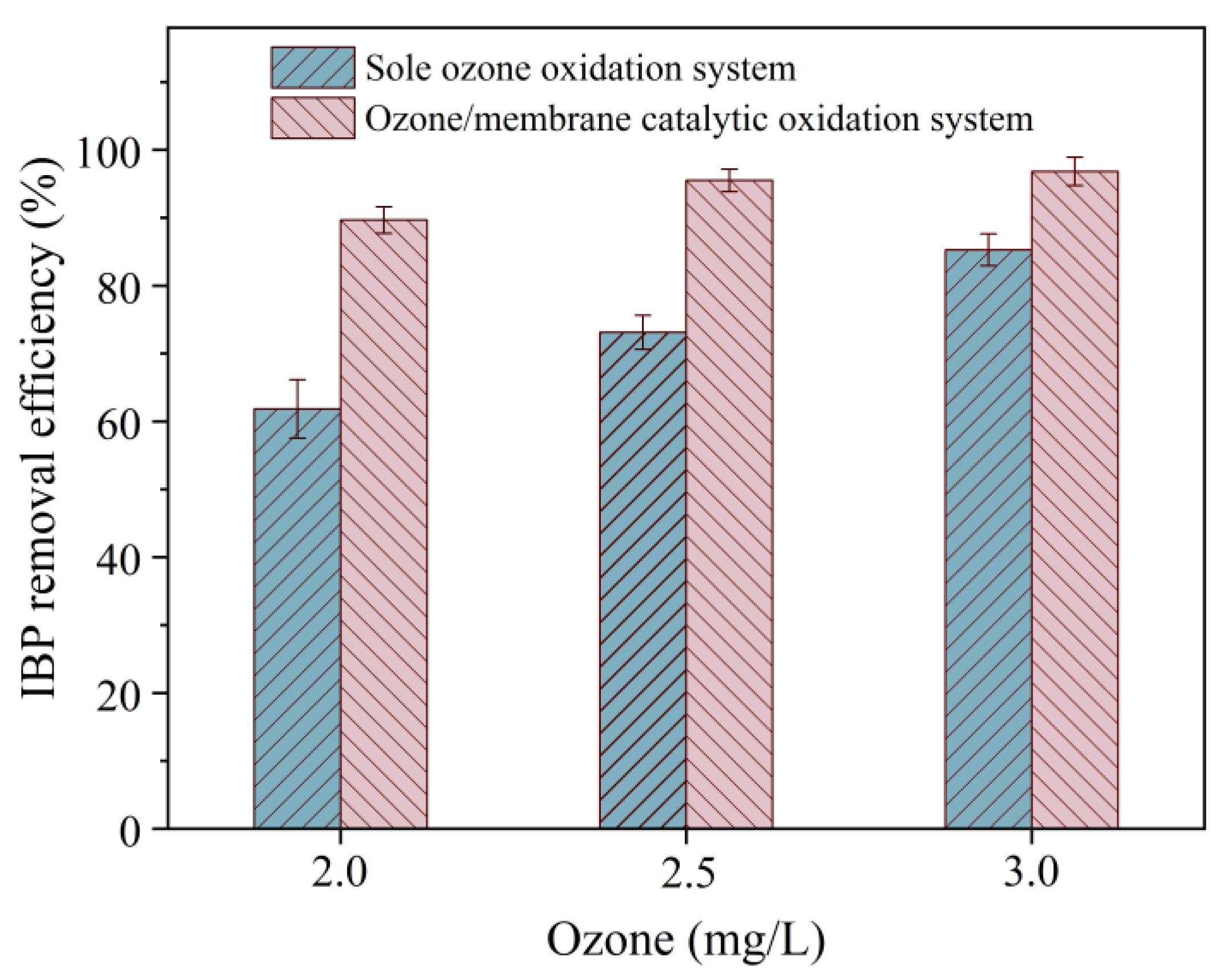
3.2. Influence of Ozone Concentration
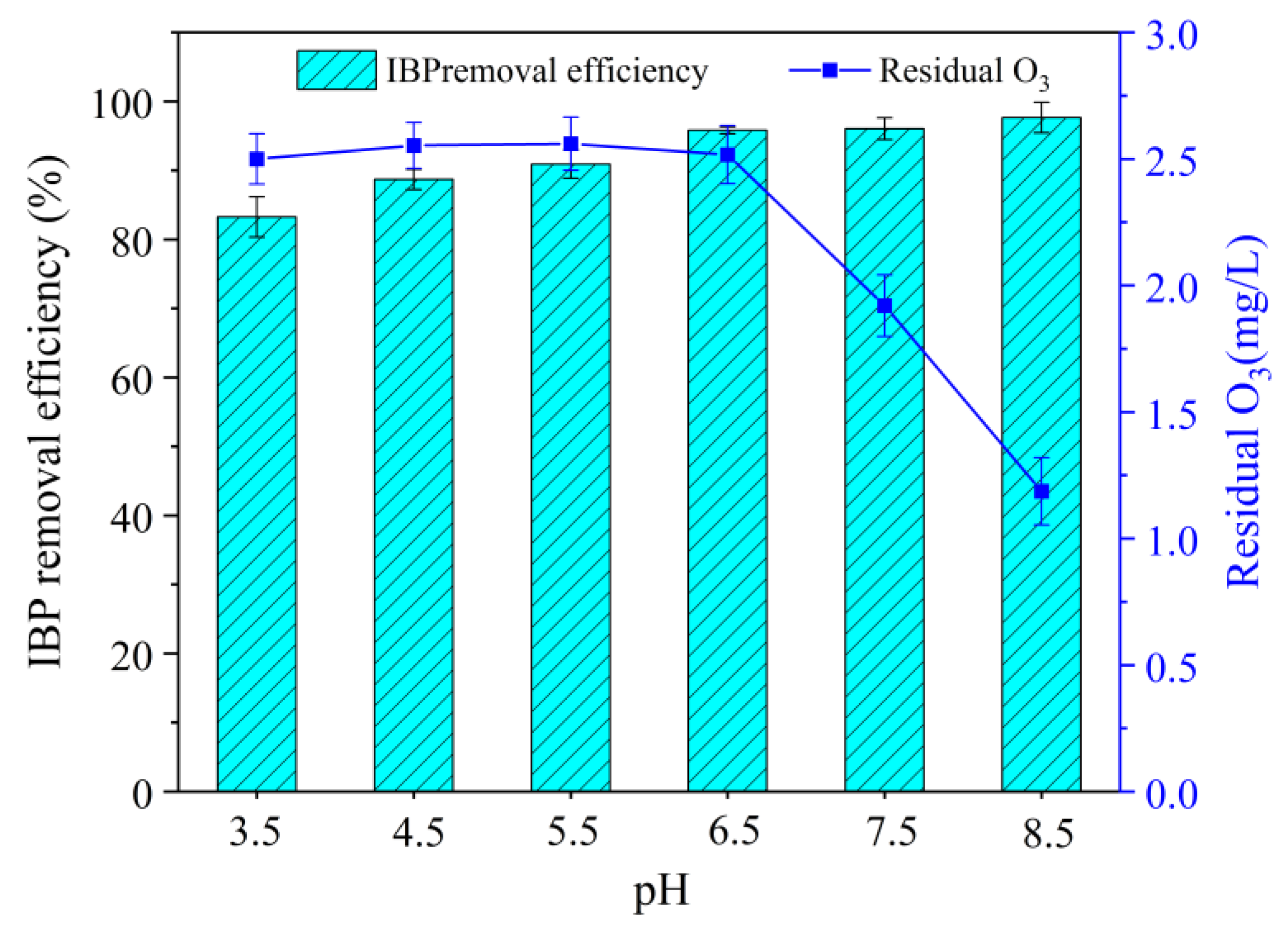
3.3. Influence of Solution pH
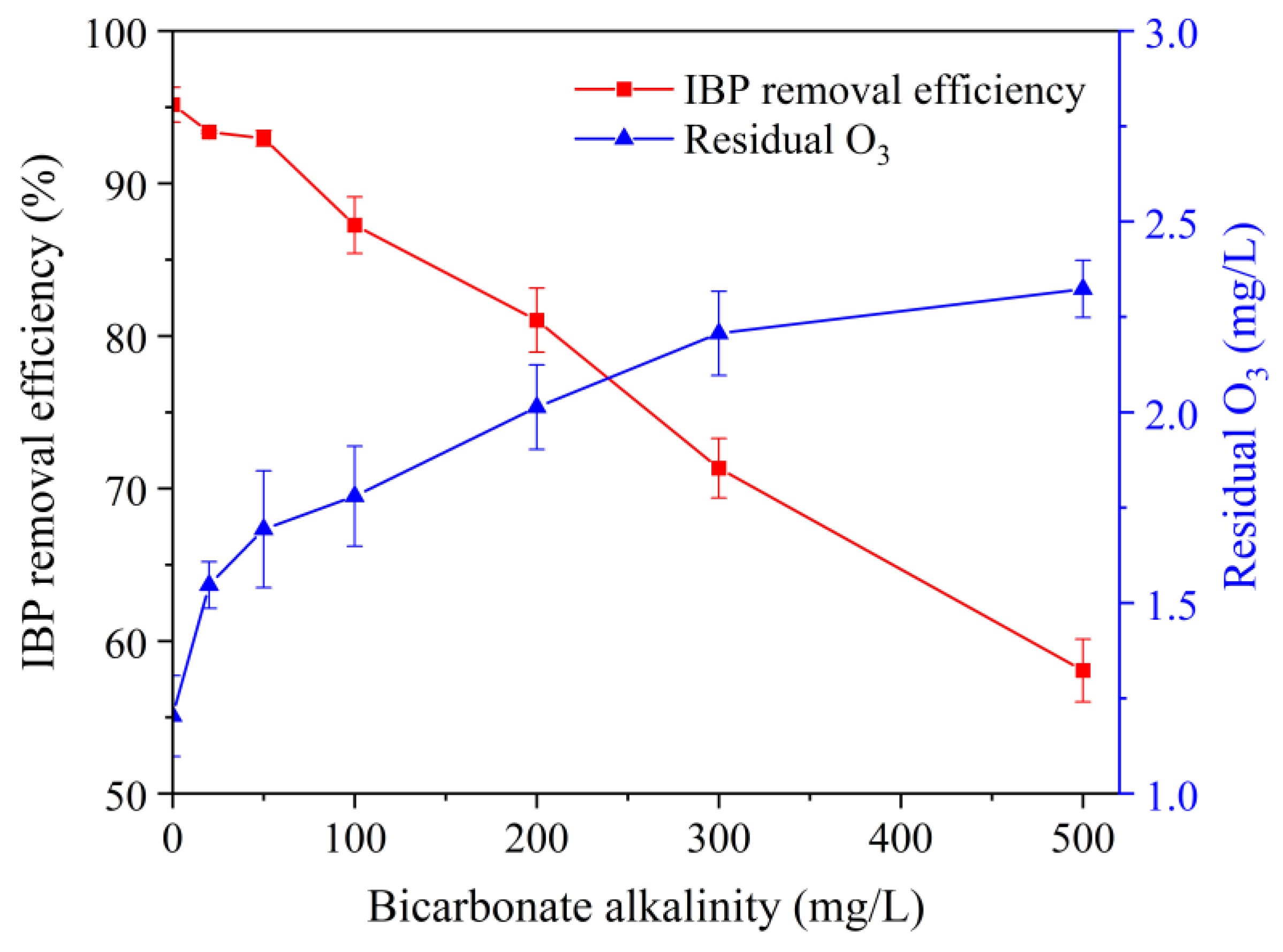
3.4. Influence of Alkalinity
3.5. Influence of Humic Acid Concentration
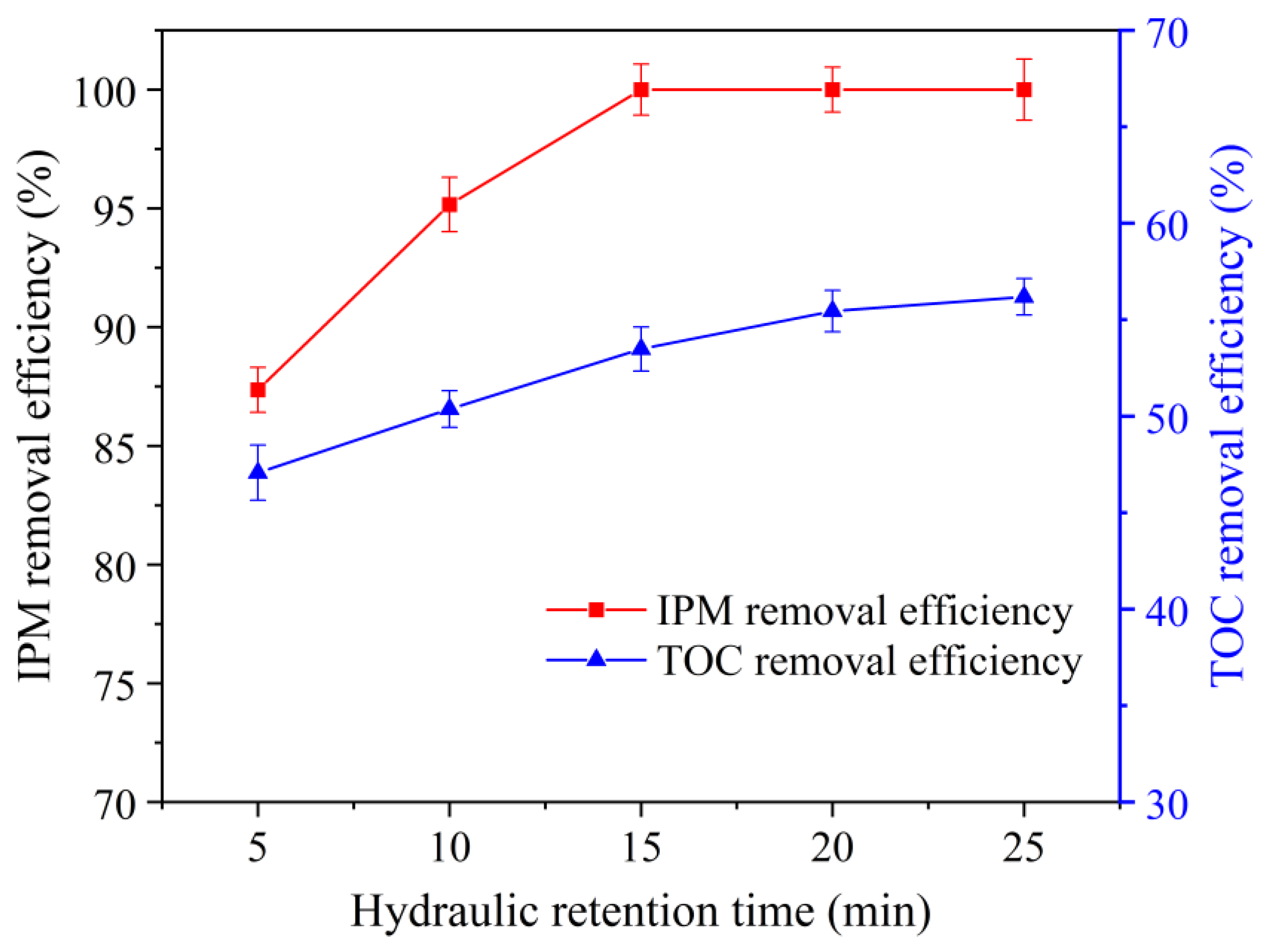
3.6. Influence of Hydraulic Residence Time
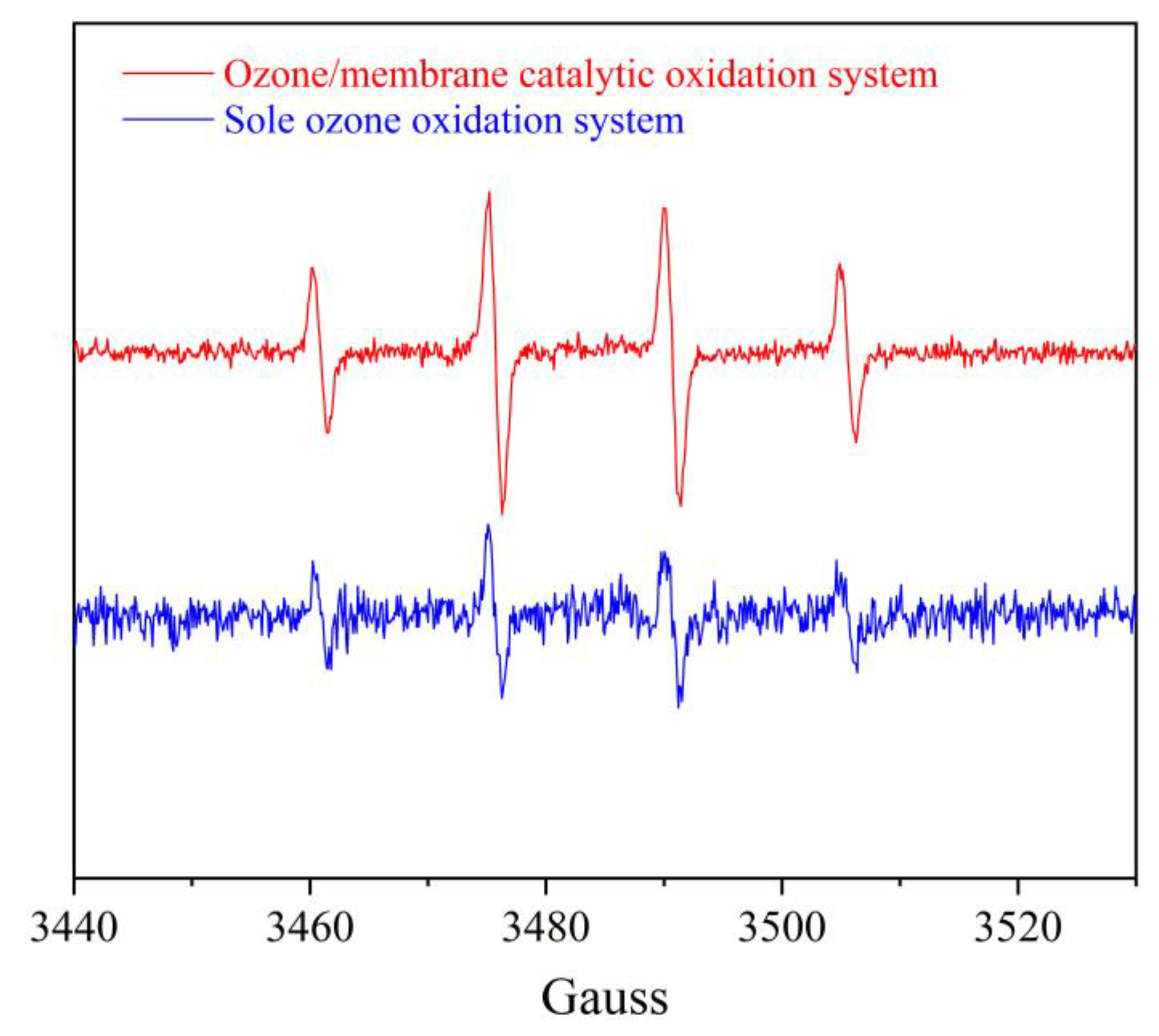
3.7. Formation of ∙OH in the Reaction System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brillas, E. A critical review on ibuprofen removal from synthetic waters, natural waters, and real wastewaters by advanced oxidation processes. Chemosphere 2022, 286, 131849. [Google Scholar] [CrossRef]
- Buser, H.-R.; Poiger, T.; Müller, M.D. Occurrence and Environmental Behavior of the Chiral Pharmaceutical Drug Ibuprofen in Surface Waters and in Wastewater. Environ. Sci. Technol. 1999, 33, 2529–2535. [Google Scholar] [CrossRef]
- Ternes, T.A.; Herrmann, N.; Bonerz, M.; Knacker, T.; Siegrist, H.; Joss, A. A rapid method to measure the solid–water distribution coefficient (Kd) for pharmaceuticals and musk fragrances in sewage sludge. Water Res. 2004, 38, 4075–4084. [Google Scholar] [CrossRef]
- Méndez-Arriaga, F.; Maldonado, M.I.; Gimenez, J.; Esplugas, S.; Malato, S. Abatement of ibuprofen by solar photocatalysis process: Enhancement and scale up. Catal. Today 2009, 144, 112–116. [Google Scholar] [CrossRef]
- Wang, L.; Ying, G.-G.; Zhao, J.-L.; Yang, X.-B.; Chen, F.; Tao, R.; Liu, S.; Zhou, L.-J. Occurrence and risk assessment of acidic pharmaceuticals in the Yellow River, Hai River and Liao River of north China. Sci. Total Environ. 2010, 408, 3139–3147. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Ying, G.-G.; Wang, L.; Yang, J.-F.; Yang, X.-B.; Yang, L.-H.; Li, X. Determination of phenolic endocrine disrupting chemicals and acidic pharmaceuticals in surface water of the Pearl Rivers in South China by gas chromatography–negative chemical ionization–mass spectrometry. Sci. Total Environ. 2009, 407, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Adityosulindro, S.; Barthe, L.; González-Labrada, K.; Jáuregui Haza, U.J.; Delmas, H.; Julcour, C. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste) water. Ultrason. Sonochem. 2017, 39, 889–896. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. Photocatalytic degradation of ibuprofen in water using TiO2/UV and g-C3N4/visible light: Study of intermediate degradation products by liquid chromatography coupled to high-resolution mass spectrometry. Chemosphere 2019, 215, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Carballa, M.; Omil, F.; Alder, A.C.; Lema, J.M. Comparison between the conventional anaerobic digestion of sewage sludge and its combination with a chemical or thermal pre-treatment concerning the removal of pharmaceuticals and personal care products. Water Sci. Technol. 2006, 53, 109–117. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, L.; Chen, Z.; Shen, B.; Wei, J.; Zeng, P.; Wen, X. Catalytic ozonation for metoprolol and ibuprofen removal over different MnO2 nanocrystals: Efficiency, transformation and mechanism. Sci. Total Environ. 2021, 785, 147328. [Google Scholar] [CrossRef]
- Quero-Pastor, M.J.; Garrido-Perez, M.C.; Acevedo, A.; Quiroga, J.M. Ozonation of ibuprofen: A degradation and toxicity study. Sci. Total Environ. 2014, 467, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Arriaga, F.; Torres-Palma, R.A.; Pétrier, C.; Esplugas, S.; Gimenez, J.; Pulgarin, C. Ultrasonic treatment of water contaminated with ibuprofen. Water Res. 2008, 42, 4243–4248. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Tang, W.-T. Photodecomposition of ibuprofen over g-C3N4/Bi2WO6/rGO heterostructured composites under visible/solar light. Sci. Total Environ. 2020, 731, 139172. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Qiu, Q. A state-of-the-art review on the carbonation process in cementitious materials: Fundamentals and characterization techniques. Constr. Build. Mater. 2020, 247, 118503. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Chang, J.; Shen, J.; Kang, J.; Chen, Q. Fabrication of a low-cost cementitious catalytic membrane for p-chloronitrobenzene degradation using a hybrid ozonation-membrane filtration system. Chem. Eng. J. 2015, 262, 904–912. [Google Scholar] [CrossRef]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Xu, L.; He, Z.; Wei, X.; Shang, Y.; Shi, J.; Jin, X.; Bai, X.; Shi, X.; Jin, P. Facile-prepared Fe/Mn co-doped biochar is an efficient catalyst for mediating the degradation of aqueous ibuprofen via catalytic ozonation. Chem. Eng. J. 2023, 461, 142028. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, M.; Ma, L.; Wang, Y.; Zhang, D.; Li, L. Ozonation catalysed by ferrosilicon for the degradation of ibuprofen in water. Environ. Pollut. 2021, 268, 115722. [Google Scholar] [CrossRef]
- Almeida, V.M.; Orge, C.A.M.; Pereira, F.R.; Soares, O.S.G.P. O3 based advanced oxidation for ibuprofen degradation. Chin. J. Chem. Eng. 2022, 42, 277–284. [Google Scholar] [CrossRef]
- Buxton, G.V.; Elliot, A.J. Rate constant for reaction of hydroxyl radicals with bicarbonate ions. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1986, 27, 241–243. [Google Scholar] [CrossRef]
- Goslan, E.H.; Fearing, D.A.; Banks, J.; Wilson, D.; Hills, P.; Campbell, A.T.; Parsons, S.A. Seasonal variations in the disinfection by-product precursor profile of a reservoir water. J. Water Supply Res. Technol. 2002, 51, 475–482. [Google Scholar] [CrossRef]
- Buettner, G.R.; Oberley, L.W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem. Biophys. Res. Commun. 1978, 83, 69–74. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual au- thor(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |




| Project | Technical Parameters |
|---|---|
| Diameter (mm) | 50 |
| Thickness (mm) | 3 |
| Mean pore size (μm) | 2.2 |
| Porosity (%) | 31 |
| Effective contact area (cm2) | 7.0 |
| Catalysts | IBP (mg/L) | Ozone | Degradation Efficiency | TOC Removal | References |
|---|---|---|---|---|---|
| α-MnO2 | 10.0 | 0.5 mg/min | 99.5% | 32.3% | [10] |
| Fe/Mn co-doped biochar | 50.0 | 4.93 mg/min | 95.4% | 80.5% | [18] |
| Ferrosilicon | 10.0 | 9.0 mg/L | 75.0% | 68.0% | [19] |
| Fe/CNT | 20.0 | 50.0 mg/L | 100% | 50.0% | [20] |
| Membrane | 10.0 | 2.5 mg/L | 87.6% | 43.6% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Chen, Z.; Shen, J.; Yan, P.; Wang, B.; Yuan, L.; Kang, J.; Zhao, S.; Liu, Y. Continuous Flow Experimental Study on Ozonation of Ibuprofen Catalyzed by Silicate-Based Microfiltration Membrane. Water 2023, 15, 2184. https://doi.org/10.3390/w15122184
Wang W, Chen Z, Shen J, Yan P, Wang B, Yuan L, Kang J, Zhao S, Liu Y. Continuous Flow Experimental Study on Ozonation of Ibuprofen Catalyzed by Silicate-Based Microfiltration Membrane. Water. 2023; 15(12):2184. https://doi.org/10.3390/w15122184
Chicago/Turabian StyleWang, Weiqiang, Zhonglin Chen, Jimin Shen, Pengwei Yan, Bingyuan Wang, Lei Yuan, Jing Kang, Shengxin Zhao, and Yue Liu. 2023. "Continuous Flow Experimental Study on Ozonation of Ibuprofen Catalyzed by Silicate-Based Microfiltration Membrane" Water 15, no. 12: 2184. https://doi.org/10.3390/w15122184
APA StyleWang, W., Chen, Z., Shen, J., Yan, P., Wang, B., Yuan, L., Kang, J., Zhao, S., & Liu, Y. (2023). Continuous Flow Experimental Study on Ozonation of Ibuprofen Catalyzed by Silicate-Based Microfiltration Membrane. Water, 15(12), 2184. https://doi.org/10.3390/w15122184







